Abstract
Mammalian ageing is associated with reduced regenerative capacity in tissues that contain stem cells1,2. It has been proposed that this is at least partially caused by the senescence of progenitors with age3,4; however, it has not yet been tested whether genes associated with senescence functionally contribute to physiological declines in progenitor activity. Here we show that progenitor proliferation in the subventricular zone and neurogenesis in the olfactory bulb, as well as multipotent progenitor frequency and self-renewal potential, all decline with age in the mouse forebrain. These declines in progenitor frequency and function correlate with increased expression of p16INK4a, which encodes a cyclin-dependent kinase inhibitor linked to senescence5. Ageing p16INK4a-deficient mice showed a significantly smaller decline in subventricular zone proliferation, olfactory bulb neurogenesis, and the frequency and self-renewal potential of multipotent progenitors. p16INK4a deficiency did not detectably affect progenitor function in the dentate gyrus or enteric nervous system, indicating regional differences in the response of neural progenitors to increased p16INK4a expression during ageing. Declining subventricular zone progenitor function and olfactory bulb neurogenesis during ageing are thus caused partly by increasing p16INK4a expression.
Stem cells must persist throughout adult life in numerous tissues, including the central nervous system (CNS)6, in order to replace the mature cells that are lost to turnover, injury, or disease. However, the function of stem cells and other progenitors declines with age in diverse tissues including the haematopoietic system7–9, muscle10,11 and brain6,12,13. Consistent with this, ageing tissues exhibit reduced repair capacity and an increased incidence of degenerative disease1,4. However, the mechanisms responsible for the age-related decline in the function of stem cells and other progenitors remain uncertain.
p16INK4a gene expression increases with age in a variety of tissues14–16. Although induction of p16INK4a expression can cause the senescence of a variety of cell types in culture5,17 and in vivo18, some cells (including some neural progenitors) are unaffected by increased p16INK4a expression or p16INK4a deletion19–21. It is thus unclear whether increased p16INK4a expression causes declines in progenitor function during ageing in vivo. We have addressed this question by examining progenitor frequency, proliferation and neurogenesis in the forebrain lateral ventricle subventricular zone (SVZ) of ageing wild-type and p16INK4a-deficient mice. The SVZ contains a mixed population of stem cells and other progenitors that engage in neurogenesis throughout adult life6,13,22. The rate of neurogenesis is known to decline in ageing mammals13, but the physiological mechanisms responsible for this decline have not been identified.
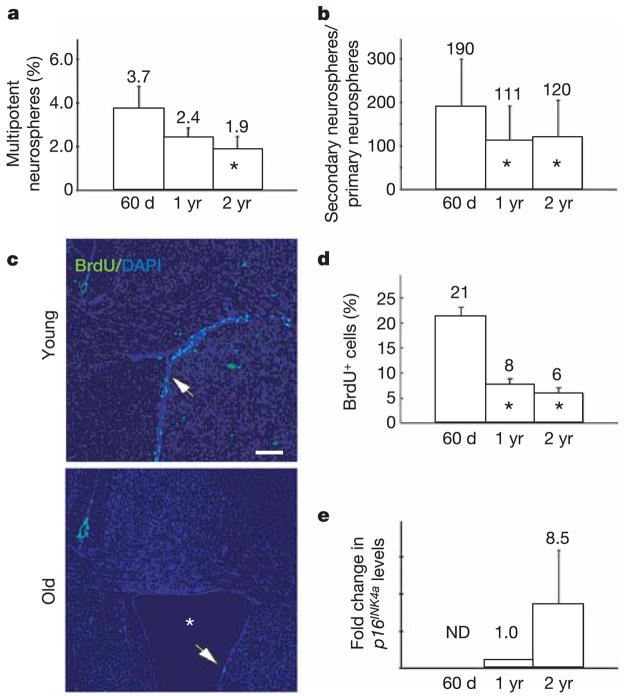
We compared various measures of progenitor function in the SVZ of 60-day-old, 1-yr-old and 2-yr-old mice to determine the effects of ageing. Consistent with a previous study6, we found a twofold reduction with age in the frequency of SVZ cells that formed multipotent neurospheres in culture (Fig. 1a; asterisk, P < 0.05). This was associated with an approximately twofold reduction in the self-renewal potential of these multipotent neurospheres (Fig. 1b; asterisk, P < 0.05). In vivo, we observed an approximately threefold reduction in the rate of proliferation in the SVZ with age (Fig. 1c, d; asterisk, P < 0.05). These data suggest that stem cell frequency and self-renewal potential, as well as overall proliferation rate, decline with age in the SVZ.
Figure 1. Neural progenitor function declines with age.
a, The percentage of SVZ cells that formed multipotent neurospheres in culture declined with age (asterisk, P < 0.05 relative to 60-day-old mice; three independent experiments; 5–6 mice per age; error bars for all panels are ±s.d.). b, The self-renewal potential of these primary neurospheres also declined with age (asterisk, P < 0.05; three independent experiments; 5–6 mice per age). c, d, Proliferation in the SVZ (percentage of BrdU+ cells after a 2-h pulse) also declined significantly with age (three mice per age; 5–7 sections per mouse). The SVZ thinned in old mice (c, arrows), and the lateral ventricle expanded (asterisk) due to cortical atrophy (c, lateral ventricle is not visible at this magnification in young mice; scale bar, 200μm). e, p16INK4a mRNA expression increased with age as detected by quantitative (real-time) PCR in uncultured SVZ cells (three independent samples per age). Note that 1-yr-old samples were set to 1.0 as the reference sample. ND, not detected.
Adult Bmi1-deficient mice also exhibit reduced stem cell frequency and self-renewal potential, as well as reduced proliferation in the SVZ20. These effects are largely caused by increased p16INK4a and Arf expression in the absence of Bmi1 (refs 19–21). We therefore wondered whether the age-related changes observed in wild-type mice (Fig. 1) were associated with increased p16INK4a or Arf expression in neural progenitors. p16INK4a and Arf expression increase with age in some tissues15,16, although these studies did not examine neural progenitors. We did not detect any age-related increase in Arf expression in uncultured SVZ cells at the RNA or protein levels (data not shown). In contrast, p16INK4a expression increased substantially with age in uncultured SVZ cells (Fig. 1e). p16INK4a expression was not detectable by polymerase chain reaction (PCR) in the SVZ of 60-day-old mice, but became detectable by 1 yr of age and further increased by 2 yr of age. We were not able to detect p16INK4a protein in the SVZ by western blot, consistent with previous studies that have also not been able to detect this protein in most uncultured mouse tissues that express p16INK4a mRNA15,16, presumably due to its low expression level and the limited sensitivity of available antibodies.
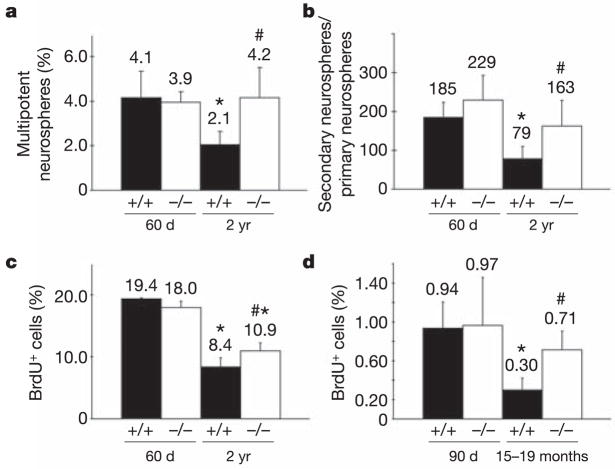
To test the function of p16INK4a in ageing neural progenitors we examined young (60-day-old) and old (2-yr-old) p16INK4a-deficient mice as well as littermate controls. p16INK4a deficiency did not grossly affect the composition of the SVZ, with similar proportions of SVZ cells staining positively for the glia marker GFAP or the neuroblast marker doublecortin in wild-type and p16INK4a-deficient mice irrespective of age (data not shown). Similarly, apoptotic SVZ cells (activated caspase-3+) were rare in all treatments (data not shown). Deletion of p16INK4a in young mice did not significantly affect the frequency of SVZ cells that formed multipotent neurospheres in culture (Fig. 2a), or the self-renewal potential of these cells upon subcloning (Fig. 2b). This is consistent with our failure to detect p16INK4a expression in SVZ cells from young mice in vivo, as well as with previous studies19,20. In contrast, p16INK4a deficiency in old mice significantly increased the frequency of SVZ cells that formed multipotent neurospheres in culture (Fig. 2a; hash, P < 0.05 relative to old wild-type mice), as well as the self-renewal potential of these cells (Fig. 2b; hash, P < 0.05 relative to old wild-type mice). Consistent with this finding, the rate of proliferation among SVZ cells in vivo was also significantly increased by p16INK4a deficiency in old but not young mice (Fig. 2c; hash, P < 0.05 relative to old wild-type mice). Nonetheless, the percentage of proliferating cells in old p16INK4a-deficient mice was still significantly less than observed in normal or p16INK4a-deficient young mice (Fig. 2c; asterisk, P < 0.05 relative to young mice). Like old wild-type mice, old p16INK4a-deficient mice also exhibited enlarged lateral ventricles due to cortical atrophy (Fig. 1c, data not shown). These observations indicate that p16INK4a deficiency partially rescued the age-related declines in progenitor activity in the SVZ but did not prevent cortical atrophy.
Figure 2. p16INK4a causes age-related declines in stem and progenitor cell function in the SVZ.
a, The percentage of SVZ cells that formed multipotent neurospheres in culture significantly declined in 2-yr-old wild-type mice as compared with 60-day-old mice (asterisk, P < 0.01) but significantly increased in old mice with p16INK4a deficiency (hash, P < 0.01 relative to old wild-type mice). b, Self-renewal potential (the number of secondary neurospheres generated per subcloned primary neurosphere) significantly declined in old wild-type mice as compared to young mice (asterisk, P < 0.05). p16INK4a deficiency significantly increased the self-renewal of neurospheres from old but not young mice (hash, P < 0.05 relative to old wild-type mice). c, The percentage of SVZ cells that incorporated a 2-h pulse of BrdU significantly declined in old as compared to young mice (asterisk, P < 0.01), but significantly increased in old mice with p16INK4a deficiency (hash, P < 0.01 relative to old wild-type mice). d, The frequency of BrdU label-retaining cells in the SVZ significantly declined in old wild-type mice as compared with young wild-type mice (asterisk, P < 0.05), but significantly increased in old mice with p16INK4a deficiency (hash, P < 0.05 relative to old wild-type mice). All values are mean ± s.d. for at least three independent experiments. All mice were histologically negative for intracranial neoplasms.
p16INK4a deficiency seemed to rescue completely the age-related decline in cells that can form stem cell colonies in culture (Fig. 2a), while only partially rescuing the overall decline in SVZ proliferation (Fig. 2c). One possibility that would be consistent with previous studies of p16INK4a in neural progenitors19–21 is that p16INK4a expression had a greater effect on SVZ stem cells than on downstream progenitors. A number of studies have argued that stem cells can be identified within the SVZ based on their ability to retain the DNA replication label 5-bromodeoxyuridine (BrdU; stem cells divide infrequently and are retained within the SVZ while other progenitors divide continuously before migrating out of the SVZ)22–25. Therefore, we examined the effects of age and p16INK4a deficiency on the frequency of BrdU label-retaining cells. We administered BrdU for 8 days, followed by a 4-week chase with no BrdU, then killed the mice. p16INK4a deficiency had no effect on the frequency of label-retaining cells in the young adult SVZ (Fig. 2d). The frequency of label-retaining cells declined significantly in old wild-type mice but not in old p16INK4a-deficient mice (Fig. 2d). Thus, the frequencies of label-retaining cells in vivo exhibited similar trends as observed for the frequencies of cells that could form multi-lineage colonies in culture. These data suggest that p16INK4a deficiency can largely rescue the age-related decline in the frequency of early progenitors within the SVZ.
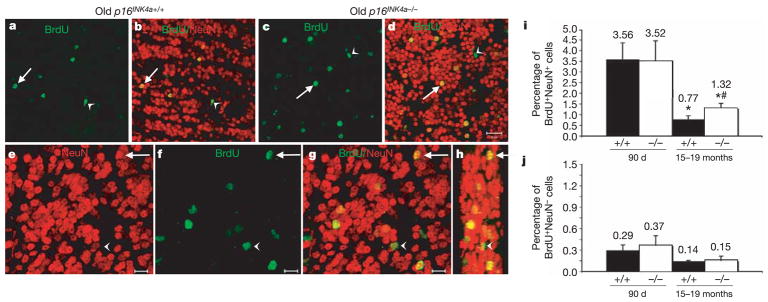
SVZ progenitors form neuroblasts throughout life that migrate into the olfactory bulb and differentiate into neurons13,22. To test whether the increase in p16INK4a expression within the SVZ also affects neurogenesis, we examined the effects of age and p16INK4a deficiency on the generation of new neurons in the olfactory bulb. We administered BrdU to mice for 8 days to mark dividing progenitors, followed by a 4-week chase period with no BrdU (during which neurons could migrate and differentiate), then killed the mice to analyse sections through the olfactory bulb by confocal microscopy. As we observed for progenitor function, p16INK4a deficiency had no effect on neurogenesis in young mice, but neurogenesis significantly decreased with age, and p16INK4a deficiency significantly increased the frequency of newly generated olfactory bulb neurons in 15–19-month-old mice (Fig. 3i). Notably, p16INK4a deficiency had no effect on the frequency of non-neuronal cells within the olfactory bulb (Fig. 3j). This may at least partially reflect our previous obserpvation that changes in p16INK4a expression do not appear to affect gliarestricted progenitors20. p16INK4a deficiency thus partially rescued the age-related decline in neurogenesis in the olfactory bulb in addition to rescuing partially the decline in progenitor function in the SVZ.
Figure 3. p16INK4a causes age-related declines in olfactory bulb neurogenesis.
a–d, Low-magnification images (scale bar, 20μm) from sagittal sections of the olfactory bulb of old wild-type (a, b; same field of view) and p16INK4a-deficient (c, d; same field of view) mice. Arrows point to new neurons in the granular layer (BrdU+NeuN+) whereas arrowheads point to new non-neuronal cells (BrdU+NeuN−). e–h, Higher magnification images (scale bar, 10μm) from one field of view from an old p16INK4a-deficient mouse. Arrow indicates BrdU+NeuN+ neuron; arrowhead indicates BrdUþNeuN2 non-neuronal cell. Panel h is a three-dimensional, reconstructed side view (80° turn in the z-axis) of panel g. i, Neurogenesis significantly (asterisk, P < 0.05) declined with age (BrdU+NeuN+ neurons as a percentage of all NeuN+ neurons). p16INK4a deficiency did not affect the level of neurogenesis in young mice, but significantly (hash, P = 0.02 relative to old wild-type mice) increased neurogenesis in old mice. j, The frequency of BrdU+NeuN− non-neuronal cells was not significantly affected by p16INK4a deficiency (also as a percentage of NeuN+ neurons). The same trends were observed when the counts were expressed per unit area (not shown). Values are mean ± s.d. from 25 to 30 fields of view per mouse, three mice per treatment.
To test whether these effects of p16INK4a occurred throughout the CNS, we also examined the effect of p16INK4a deficiency on progenitor activity and neurogenesis in the dentate gyrus26. The rates of progenitor proliferation and neurogenesis in the dentate gyrus decline markedly with age12. To test whether this is affected by p16INK4a, we administered BrdU for 8 days to mark proliferation in the subgranular layer, followed by a 4-week chase period without BrdU to examine neurogenesis in the granular layer. p16INK4a deficiency did not significantly affect the rate of proliferation among progenitors in the subgranular layer, or the frequency of BrdU+NeuN+ newly generated neurons or BrdU+NeuN− non-neuronal cells in the granular layer of the dentate gyrus (Supplementary Fig. 1). Thus, although p16INK4a deficiency consistently increased all measures of progenitor activity and neurogenesis in the ageing subventricular zone/olfactory bulb, it did not detectably affect proliferation or neurogenesis in the dentate gyrus.
We also examined the effect of age and p16INK4a deficiency on the neural crest stem cells that persist throughout adult life in the enteric nervous system (in the gut wall)19,20,27. The frequency of these neural crest stem cells declined with age, and p16INK4a expression increased with age in p75+ (neurotrophin-receptor expressing) gut cells enriched for neural crest stem cells (Supplementary Fig. 2). However, p16INK4a deficiency had no effect on neural crest stem cell frequency in young or old mice. These results indicate that although the age-related increase in p16INK4a expression causes a decline in SVZ progenitor function and neurogenesis, other mechanisms are more important in the ageing of stem and progenitor cells in the hippocampus and peripheral nervous system.
The mechanisms that account for the increase in p16INK4a expression with age remain unclear. In principle, p16INK4a expression could be developmentally programmed to increase with age to counter the increasing incidence of cancer that is observed in the ageing nervous system. Alternatively, p16INK4a expression may reflect the induction of senescence in ageing cells in response to damage that accumulates from oxidative metabolism or other stresses. Although Bmi1 is important for p16INK4a repression19,20,28, we did not detect any change in Bmi1 expression with age at the RNA or protein levels in SVZ cells (Supplementary Fig. 3). Nonetheless, subtle reductions in Bmi1 expression or activity cannot be excluded and could be functionally important, as even the twofold reduction in Bmi1 expression in Bmi1−/+ mice leads to increased p16INK4a expression (data not shown). Additional work will be required to resolve the potentially multifactorial mechanisms that regulate age-related changes in p16INK4a expression.
The simplest interpretation of our data is that p16INK4a acts cell autonomously within neural stem cells, because p16INK4a deficiency increased the self-renewal of single neural stem cells in culture. However, we cannot exclude the possibility of non-cell-autonomous effects by p16INK4a in vivo. In addition to investigating this issue further, it will also be of interest to determine whether circulating humoral factors11 influence p16INK4a expression during ageing.
One study found that moderately increased expression of p16INK4a and p19ARF in transgenic mice does not detectably accelerate gross measures of ageing or change lifespan, despite reducing cancer incidence29. However, that study did not examine neural progenitor function or neurogenesis. We also examined p16INK4a bacterial artificial chromosome (BAC) transgenic mice that exhibited a moderate increase in p16INK4a expression and found no effect of p16INK4a overexpression on stem cell frequency or self-renewal potential, although old transgenic mice did exhibit a modest reduction in SVZ proliferation (data not shown). It seems that the levels of p16INK4a that are required to deplete stem cells are qualitatively higher than the levels that are required to inhibit carcinogenesis. Together, the data suggest that although physiological increases in p16INK4a expression during ageing reduce neural progenitor function and neurogenesis in the SVZ/olfactory bulb, modest overexpression of p16INK4a does not substantially amplify these effects or accelerate gross measures of ageing.
Although physiological p16INK4a expression reduces stem/progenitor cell function and neurogenesis with age, how this relates to overall ageing or lifespan remains unclear. It is difficult to examine the effect of p16INK4a deficiency on mouse lifespan because p16INK4a deficiency increases cancer incidence30 in addition to affecting age-related changes in stem/progenitor cell function. Declining stem/progenitor cell function may be a major cause of the decline in regenerative capacity and the increase in degenerative disease that is observed in ageing tissues. On the other hand, increases in the death or dysfunction of mature cells in ageing tissues also contribute to age-related morbidity. It is similarly unknown whether physiological differences in the rate at which stem/progenitor cell activity declines with age has a detectable impact on longevity. The fact that p16INK4a did not affect progenitors in certain regions of the nervous system suggests that p16INK4a is not likely to promote generically the ageing of all cells. Indeed, it is likely that there will be important differences between tissues, and perhaps between progenitor cells and differentiated cells, in terms of the genes that regulate the ageing process. Although these questions remain unanswered, it may be possible to gain important new insights into the ageing process at a cellular level by studying individual cell types that have known physiological functions in vivo, such as haematopoietic or neural stem cells.
Our data suggest that stem cell function is regulated by a balance between proto-oncogenes, like Bmi1 (that promote stem cell maintenance and regenerative capacity but can also contribute to neoplastic proliferation), and tumour suppressors, like p16INK4a (that reduce regenerative capacity and promote ageing but also reduce cancer incidence). This balance changes with age and is influenced by other proto-oncogenes and tumour suppressors that also regulate p16INK4a expression. The networks of proto-oncogenes and tumour suppressors that regulate stem cell self-renewal, cancer cell proliferation and stem cell ageing may have evolved to balance the need for regenerative capacity while guarding against the increasing risk of neoplasms with age. p16INK4a thus reduces cancer incidence5,30 but also contributes to ageing by reducing progenitor function and neurogenesis in at least certain regions of the nervous system.
METHODS
Mice
p16INK4a+/− mice were backcrossed at least six times onto a C57BL background. p16INK4a genotyping was performed by PCR as described30.
Isolation of CNS progenitors
Adult SVZ was obtained by microdissecting the lateral walls of the lateral ventricles, then dissociating for 20 min at 37 °C in 0.025% trypsin/0.5 mM EDTA (Calbiochem) plus 0.001% DNase1 (Roche). After quenching the enzymatic dissociation with staining medium (L15 medium containing 1 mg ml− BSA (Sigma A-3912), 10 mM HEPES (pH 7.4) and 1% penicillin/streptomycin (BioWhittaker)) containing 0.014% soybean trypsin inhibitor (Sigma) and 0.001% DNase1, the cells were washed and re-suspended in staining medium, triturated, filtered through nylon screen (45μm, Sefar America), counted by haemocytometer, and plated.
Cell culture and self-renewal assay
Cells were plated at clonal density (1.3 cells per μl) on ultra-low-binding plates (Corning) to grow neurospheres. Culture medium was based on a 5:3 mixture of DMEM-low glucose as described previously19: neurobasal medium (Gibco) supplemented with 20 ng ml−1 human bFGF (R&D Systems), 20 ng ml−1 EGF (R&D Systems), 1% N2 supplement (Gibco), 2% B27 supplement (Gibco), 50μM 2-mercaptoethanol, 1% penicillin/streptomycin (Biowhittaker), and 10% chick embryo extract. Cultures were maintained at 37 °C in 6% CO2/balance air. To measure self-renewal, individual neurospheres were dissociated by trituration then replated at clonal density as above. Secondary neurospheres were counted 5–10 days later to determine the number of secondary neurospheres formed per primary neurosphere. CNS neurospheres were tested for multipotency by replating one neurosphere per well into 48-well plates and then culturing adherently for 3–5 days before triple staining for oligodendrocytes (O4), neurons (TuJ1) and astrocytes (GFAP). See Supplementary Methods for details.
BrdU incorporation/proliferation assays
To quantify SVZ proliferation, mice were injected intraperitoneally with 50 mg kg−1 of BrdU (Sigma), and killed 2 h after BrdU injection. To quantify proliferation in the dentate gyrus, mice received a single injection of 50 mg kg−1 BrdU, followed by 1 mg ml−1 BrdU in their drinking water for 8 days before being killed and analysed. To quantify neurogenesis in the dentate gyrus and the olfactory bulb and BrdU retention in the SVZ, mice were treated with BrdU for 8 days. However, the animals were then taken off of BrdU for 4 weeks before the animals were killed. The brains were dissected, fixed in 4% paraformaldehyde overnight, then cryo-protected in 15% sucrose, embedded in 7.5% gelatin/15% sucrose, and flash frozen. Twelve-micrometre sections were cut on a Leica cryostat.
For detection of BrdU in the tissue sections, DNA was denatured in 2 M HCl for 30 min at room temperature and neutralized with 0.1 M sodium borate. Sections were pre-blocked for 1 h at room temperature in goat serum solution (PBS containing 5% goat serum, 1% BSA and 0.3% Triton X-100 (Sigma)). Primary rat anti-BrdU (1:500, Accurate Chemical) diluted in goat serum solution was incubated overnight at 4 °C, followed by fluorescein isothiocyanate (FITC)-conjugated anti-rat Fc fragment (Jackson Labs) for 3–4 h at room temperature. Slides were counter stained in 2.5μg ml−1 DAPI for 10 min at room temperature, then mounted using ProLong antifade solution (Molecular Probes). Numbers of BrdU-labelled cells were divided by total DAPI+ or NeuN+ nuclei.
To ensure that BrdU incorporation results were not skewed by dividing endothelial or haematopoietic cells, we double-labelled SVZ sections with antibodies against BrdU and either anti-CD45 (to identify blood cells) or anti-VE-cadherin (to identify endothelial cells). We did not detect any BrdU+ haematopoietic or endothelial cells.
Western blots and quantitative RT–PCR were performed as described in Supplementary Information.
Statistical analysis
Experiments that involved multiple treatments were initially analysed by analysis of variance. Statistically significant results were followed up with Student’s t-tests.
Confocal analysis of neurogenesis in the olfactory bulb
A Zeiss LSM 510 confocal laser-scanning microscope was used to obtain 25–30 random fields of view throughout all regions of one entire olfactory bulb of each mouse with a ×40 or ×63 objective lens. For each image, 1-μm-thick optical sections were scanned with three different lasers through a 12-μm sagittal tissue section creating a Z-series stack with three distinct channels of fluorescence. An ultraviolet enterprise laser was used to detect the DAPI signal labelling all nuclei. An argon laser detected FITC (BrdU), and a HeNe laser detected Cy3 (NeuN). Channels were merged together to determine whether DAPI, BrdU and NeuN signals co-labelled at every 1-μm slice of the Z-series. Three-dimensional projections were made using LSM 510 software.
Supplementary Material
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Acknowledgments
This work was supported by the National Institute on Aging (grants to S.J.M. and N.E.S.) and the National Institute of Neurological Disorders and Stroke (to S.J.M.). S.J.M. is an Investigator of the Howard Hughes Medical Institute. N.E.S. is supported by the Sidney Kimmel Foundation for Cancer Research, the Paul Beeson Physician Scholars program, and the Ellison Medical Foundation. A.V.M. and N.M.J. were supported by National Research Service Awards from the National Institutes of Health. We thank K. Yeager for tissue sectioning and C. Mountford for mouse colony management.
Footnotes
Author Contributions A.V.M. studied the effect of age on forebrain progenitors, p16INK4a expression and function during ageing in the subventricular zone (Figs 1 and 2), and Bmi1 expression during ageing (Supplementary Fig. 3). S.G.S. contributed to studies of p16INK4a expression during ageing, and the effect of p16INK4a on proliferation and neurogenesis in the subventricular zone and hippocampus (Figs 2 and 3 and Supplementary Fig. 1). N.M.J. studied neurogenesis in the olfactory bulb and hippocampus (Fig. 3 and Supplementary Fig. 1). S.H. and R.P. examined p16INK4a expression in the ageing enteric nervous system and its effect on neural crest stem cells (Supplementary Fig. 2). J.K. and N.E.S. provided ageing p16INK4a-deficient and control mice for some of the experiments and discussed results throughout the project. S.J.M. helped to design and interpret experiments and wrote the manuscript with help from A.V.M., S.G.S. and N.M.J.
References
- 1.Chien KR, Karsenty G. Longevity and lineages: toward the integrative biology of degenerative diseases in heart, muscle, and bone. Cell. 2005;120:533–544. doi: 10.1016/j.cell.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Geiger H, Van Zant G. The aging of lympho-hematopoietic stem cells. Nature Immunol. 2002;3:329–333. doi: 10.1038/ni0402-329. [DOI] [PubMed] [Google Scholar]
- 3.Lombard DB, et al. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Lowe SW, Sherr CJ. Tumor suppression by Ink4a–Arf: progress and puzzles. Curr Opin Genet Dev. 2003;13:77–83. doi: 10.1016/s0959-437x(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 6.Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nature Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Astle CM, Harrison DE. Genetic regulation of primitive hematopoietic stem cell senescence. Exp Hematol. 2000;28:442–450. doi: 10.1016/s0301-472x(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 9.de Haan G, Nijhof W, Van Zant G. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood. 1997;89:1543–1550. [PubMed] [Google Scholar]
- 10.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 11.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enwere E, et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen GP, et al. Immunohistochemical survey of p16INK4A expression in normal human adult and infant tissues. Lab Invest. 1999;79:1137–1143. [PubMed] [Google Scholar]
- 15.Krishnamurthy J, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zindy F, Quelle DE, Roussel MF, Sherr CJ. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15:203–211. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]
- 17.Sharpless NE, Ramsey MR, Balasubramanian P, Castrillon DH, DePinho RA. The differential impact of p16INK4a or p19ARF deficiency on cell growth and tumorigenesis. Oncogene. 2004;23:379–385. doi: 10.1038/sj.onc.1207074. [DOI] [PubMed] [Google Scholar]
- 18.Michaloglou C, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 19.Molofsky AV, et al. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16INK4a and p19Arf senescence pathways. Genes Dev. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molofsky AV, et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruggeman SWM, et al. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes Dev. 2005;19:1438–1443. doi: 10.1101/gad.1299305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 23.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 24.Chiasson BJ, Tropepe V, Morshead CM, Kooy D. v d Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson CB, et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 26.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruger GM, et al. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs JJL, Kieboom K, Marino S, DePinho RA, Lohuizen M. v The oncogene and polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 29.Matheu A, et al. Increased gene dosage of Ink4a/Arf results in cancer resistance and normal aging. Genes Dev. 2004;18:2736–2746. doi: 10.1101/gad.310304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpless NE, et al. Loss of p16INK4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.