Abstract
Destruction of tooth support due to the chronic inflammatory disease periodontitis is a major cause of tooth loss. There are limitations with available treatment options to tissue engineer soft tissue periodontal defects. The exogenous application of growth factors (GFs) such as platelet-derived growth factor (PDGF) has shown promise to enhance oral and periodontal tissue regeneration. However, the topical administration of GFs has not led to clinically significant improvements in tissue regeneration because of problems in maintaining therapeutic protein levels at the defect site. The utilization of PDGF gene transfer may circumvent many of the limitations with protein delivery to soft tissue wounds. The objective of this study was to test the effect of PDGF-A and PDGF-B gene transfer to human gingival fibroblasts (HGFs) on ex vivo repair in three-dimensional collagen lattices. HGFs were transduced with adenovirus encoding PDGF-A and PDGF-B genes. Defect fill of bilayer collagen gels was measured by image analysis of cell repopulation into the gingival defects. The modulation of gene expression at the defect site and periphery was measured by RT-PCR during a 10-day time course after gene delivery. The results demonstrated that PDGF-B gene transfer stimulated potent (>4-fold) increases in cell repopulation and defect fill above that of PDGF-A and corresponding controls. PDGF-A and PDGF-B gene expression was maintained for at least 10 days. PDGF gene transfer upregulated the expression of phosphatidylinosital 3-kinase and integrin α5 subunit at 5 days after adenovirus transduction. These results suggest that PDGF gene transfer has potential for periodontal soft tissue-engineering applications.
INTRODUCTION
Periodontitis, a major cause of tooth loss, is a chronic inflammatory disease leading to destruction of tooth-supporting structures. Current modes of treatment essentially halt the disease progression with limited success in regeneration of the lost tissue. The use of growth factors (GFs) to reengineer periodontal tissue has been reviewed.1 Application of platelet-derived growth factor BB (PDGF-BB) alone or in combination with insulin-like growth factor I (IGF-I) to treat periodontal defects resulted in partial regeneration of periodontal tissues including periodontal ligament (PDL), cementum, and bone in both animal and human studies.2-5 However, one problem with current growth factor delivery to periodontal wounds is the short half-life of the factors. Topical delivery of GFs remains in periodontal defects for a limited duration, possibly because of proteolytic cleavage, and the solubility of the carrier.6 It has been shown that long-term delivery of GFs by gene transfer provides a more sustained delivery above that of GF protein application.7-9 Thus, gene therapy may achieve greater bioavailability of GFs within periodontal wounds with resultant improvements in periodontal regeneration.
PDGF has been studied for gene delivery to successfully promote soft tissue repair.7,9,10 PDGF is a potent stimulator of fibroblast cell migration, mitogenesis, proliferation, and matrix synthesis important in wound healing.11-13 The biological activities of PDGF are mediated through two intrinsic tyrosine kinase receptors (PDGFαR and PDGFβR) that induce several sets of signaling molecules, such as phosphatidylinositol-3-kinase (PI3 kinase).14,15 Adenovirus encoding GFs is being considered for wound repair in order to achieve high but transient transgene expression. In vitro studies performed by our group have shown that adenovirus encoding PDGF delivered to cells derived from periodontal tissues (gingival and PDL fibroblasts, cementoblasts, and osteoblasts) elicits elevated and prolonged PDGF gene expression for at least 1 week.16,17 Ad/PDGF enhanced in all cell types mitogenic and proliferative activities that were sustained above that of PDGF protein. Furthermore, Ad/PDGF gene transfer resulted in sustained tyrosine kinase phosphorylation and downregulation of the growth arrest-specific (gas) gene product PDGFαR for at least 96 h.18
This study sought to test the effect of gene transfer by adenovirus encoding PDGF-A and PDGF-B on human gingival fibroblast (HGF) repopulation and resultant wound fill in a three-dimensional ex vivo wound-healing model. Furthermore, we evaluated the effect of PDGF gene transfer on the regulation of genes associated with tissue repair such as collagen type I, PI3 kinase, and integrin α5 subunit. This new system may provide a methodology to better determine the role of PDGF transgenes in oral and periodontal repair.
MATERIALS AND METHODS
Cell culture and recombinant adenovirus encoding PDGF constructs
The primary human gingival fibroblasts (HGFs) used in this study were a kind gift from M. Somerman (University of Washington, Seattle, WA). HGFs were derived from healthy gingivae of a human gingival tissue biopsy, and used from passages 4–6. The cells were maintained in Dulbecco's modified Eagle's medium (DMEM; GIBCO-BRL Life Technologies, Grand Island, NY) supplemented with 10% fetal calf serum (FCS; Gemini Bio-Products, Woodland, CA). l-glutamine (2 mM), and antibiotics (penicillin [100 units/mL] and streptomycin [100 μg/mL]; GIBCO-BRL) in a humidified atmosphere of 5% CO2 in air at 37°C. Ad/GFP and Ad/PDGF-A viruses were constructed as previously described16 and Ad/PDGF-B was kindly donated by Selective Genetics (San Diego, CA).
Fabrication of fibroblast-populated collagen lattices
Three-dimensional fibroblast-populated collagen lattices (FPCLs) were fabricated according to a method adapted from Al-Khateeb et al.19 The FPC gel was mixed on ice containing rat tail tendon collagen type I (1.5 mg/mL; BD Biosciences, Bedford, MA), 1% 1 M NaOH, 2× DMEM diluted to 1×, antibiotics (penicillin [100 units/mL] and streptomycin [100 μg/mL]), and 3.5 × 104 HGFs/mL. Three-dimensional FPCLs were fabricated by plating 0.5 mL of FPC gel into individual 22-mm-diameter cell culture plates followed by polymerization at 37°C for 1–2 h. DMEM supplemented with 1% platelet-poor plasma (PPP), l-glutamine (2 mM), and antibiotics (penicillin [100 units/mL] and streptomycin [100 μg/mL]) was added and the lattices were incubated overnight.
Fabrication of wounded bilayered model and HGF gene transfer
The acellular collagen lattices were fabricated in 12-well tissue culture plates according to the above-described protocol 1–2 h before preparing the wounded bilayered model. Each FPCL was “wounded,” using a 6-mm biopsy punch (George Tiemann, Hauppauge, NY), and carefully overlaid onto the acellular collagen lattice, using 80 μL of freshly prepared collagen gel solution as an interposing adhering medium (Fig. 1A). All excess collagen gel in the wound area was removed. Forty microliters of collagen gel alone (NT), or collagen gel containing 3.5 × 106 plaque-forming units (PFU) of Ad/GFP, Ad/PDGF-A, or Ad/PDGF-B per milliliter was applied to each of the tissue defects (20 multiplicities of infection [MOI]/wound; Fig. 1A). The wounded bilayered lattice (WBLL) was incubated for an additional ∼2 h to allow for complete polymerization followed by addition of 1.5 mL of DMEM containing 1% PPP. The medium was changed on days 3, 5, 7, and 10 after wounding. A total of 12 WBLLs were fabricated for each treatment group to allow for multiple analyses including cell counting, image analysis, and RNA harvest for gene expression analysis.
FIG. 1.
Fabrication of the bilayered wound defect model. (A) Fibroblast-populatedcollagen lattices (FPCLs) were fabricated and wounded with a 6-mm-diameter biopsy punch. Acellular collagen lattices were constructed and served as the inferior layer wound bed. Each FPCL was overlaid on the acellular layer followed by application of a collagen gel incorporated with 1% PPP (no treatment, NT) or 3.5 × 106 plaque-forming units (PFU)/ml (20 MOI) of adenovirus encoding either GFP, PDGF-A, or PDGF-B. Images depict HGFs migrating from the defect margin. (B) A representative phase-contrast image depicts the wound margin of a defect treated with Ad/GFP for 7 days (original magnification, ×200). Arrows indicate HGF cells transduced by Ad/GFP near the defect margin. (C) Fluorescence image depicts the corresponding HGF cells expressing green fluorescent protein.
Cell quantification of HGFS in ex vivo gingival defects after PDGF gene delivery
Ten days after gene transfer, each FPCL in the defect area was separated and dissected from the defect periphery, using a 6-mm biopsy punch placed over the original defect (i.e., defect area); cells outside this zone were termed the “defect periphery” (Fig. 1A). HGFs were recovered from the FPCLs via collagenase digestion as previously described.19 Briefly, the FPCLs were digested with collagenase type XI (2 mg/mL; Sigma, St. Louis, MO) at 37°C for 1 h, followed by incubation for 20 min with 0.2% (w/v) trypsin and 5 mM ethylenediaminetetraacetic acid. The cells were then pelleted and resuspended in phosphate-buffered saline (pH 7.4). HGF cell numbers yielded from the wound defect and wound periphery areas were measured separately with a Neubauer hemocytometer; n = 4 samples of each treatment group were measured.
HGF cell repopulation into gingival defects after PDGF gene delivery as measured by computer-assisted image analysis
Standardized 20× images of HGFs that repopulated the defect area were captured on days 3, 5, 7, and 10, using a digital camera (Nikon E990; Nikon, Melville, NY) fitted to an inverted microscope (Nikon TMS) with a fixed aperture. The images were coded for masked computer-assisted image analysis, using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD), to determine HGF wound fill and cell density in the wound area at each time point. A single, calibrated examiner evaluated all the images and demonstrated a pre- and poststudy calibration intraexaminer error of <5%. HGF repopulation (defect fill) was determined by measuring the mean distance of cell repopulation into the wound area from the wound margin compared with the mean diameter of the defect area. Cell density in the defect area was determined by measuring five standardized areas of interest (AOI) at 1 mm2 each directly across the horizontal diameter of the defect. The first two AOIs were measured at the wound margin, then another two AOIs were measured at the areas adjacent to the peripheral regions and one AOI was measured in the direct center of the wound area. The summation of cell density from each AOI was performed and the mean values were generated for each group. The images were uncoded and then analyzed using one-way analysis of variance (ANOVA) and Fisher's protected least significant difference (PLSD) to determine the differences among groups.
Effect of Ad/PDGF-A and -B gene transfer on HGF gene expression
To examine the transgene transduction efficiency and the modulation of gene expression after Ad/PDGF-A and -B delivery to HGFs, reverse transcriptase-polymerase chain reaction (RT-PCR) experiments were performed. On days 5 and 10, the wound areas of two WBLLs from each experiment were harvested with a 9-mm biopsy punch to include the wound site and 1.5-mm zone peripheral to the defect. Total RNA was extracted from HGFs, using TRIzol reagent (GIBCO-BRL). One microgram of RNA was reverse transcribed in a 20-μL RT reaction, using a Retroscript kit (Ambion, Austin, TX). RT-PCR was performed to determine the expression of the following genes: PDGF-A, PDGF-B, collagen type I α1 chain (COLIα1), the α subunit of PI3 kinase (P85α), and integrin α5 subunit (α5). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to assess the relative loading of the mRNA samples. The primer pairs for each gene and the expected PCR products are shown in Table 1. A total volume of 25 μL of the PCR was mixed with 5 μL of RT product, 1×PCR buffer (10 mM Tris-HCl [pH 9.0], 50 mM KCl, and 0.1% Triton X-100), 100 μM dNTPs, 750 μM MgCl2, primers (1 μM each), and 0.5 unit of Taq DNA polymerase (Promega, Madison, WI), using a PerkinElmer thermocycler 9600 (PerkinElmer, Norwalk, CT). The PCR conditions for detecting expression of PDGF-B were 94°C for 2 min, 45 cycles of 94°C for 30 s, 60°C for 1 min, followed by 72°C for 10 min. The forward primer of PDGF-B was located at the 3′ end of the PDGF-B gene and the reverse primer included the Ad/PDGF-B virus backbone. The PCR conditions for the other genes were 94°C for 2 min, 30 cycles of 94°C for 30 s, 57°C for 45 s, and 72°C for 45 s, followed by 72°C for 10 min. Ten microliters of PCR product was separated on 1.5% agarose gels in TBE buffer and visualized by staining with ethidium bromide. The relative ratio of gene expression was normalized with GAPDH, using the NIH Image 1.62 program.
Table 1.
Primers Used for RT-PCR Experiments
| Gene | Primer |
PCR product (bp) |
|---|---|---|
| PDGF-A | Forward: 5′-CCTGCCCATTCGGAGGAAGAG-3 ′ | 224 |
| Reverse: 5′-TTGGCCACCTTGACGCTGCG-3 ′ | ||
| Ad/PDGF-B | Forward: 5′-CAAGCACACGCATGACAAGA-3 ′ | 131 |
| Reverse: 5′-TGTGAAATTTGTGATGCTATTGCTTT-3 ′ | ||
| COLIα1 | Forward: 5′-TCCCAGAACATCACCTACCACTGC-3 ′ | 202 |
| Reverse: 5′-TGTATTCAATCACTGTCTTGCCCC-3 ′ | ||
| PI3 kinase (P85α) | Forward: 5′-GTGGAAGATGATGAAGATTTGCCC-3 ′ | 215 |
| Reverse: 5′-AAGCCATAGCCAGTTGCTGTTTTG-3 ′ | ||
| Integrin α5 (α5) | Forward: 5′-GACTACTTTGCCGTGAACCAGAGC-3 ′ | 171 |
| Reverse: 5′-GCGAGTTGTTGAGATTCTTGCTGAG-3 ′ | ||
| GAPDH | Forward: 5′-AAGTCAGAGGAGACCACCTGG-3 ′ | 437 |
| Reverse: 5′-GACAACAGCCTCAAGATCATCAGC-3 ′ |
RESULTS
In the present study, a three-dimensional collagen lattice wound-healing model was fabricated in vitro, using HGF cells, to examine the effect of PDGF-A and -B adenovirus gene transfer to promote ex vivo gingival repair. A 6-mm wound was created in three-dimensional FPCLs to determine the repopulation of HGFs into the defects for up to 10 days. The expression on day 7 post treatment of a reporter gene, GFP, transduced by Ad/GFP in HGFs is depicted in Fig. 1B and C. A phase-contrast image of the defect margin where HGFs repopulated into the defect area is shown in Fig. 1B. There are a number of HGF cells transduced with Ad/GFP in the defect area and at the wound edge that correspond to the fluorescence image in Fig. 1C.
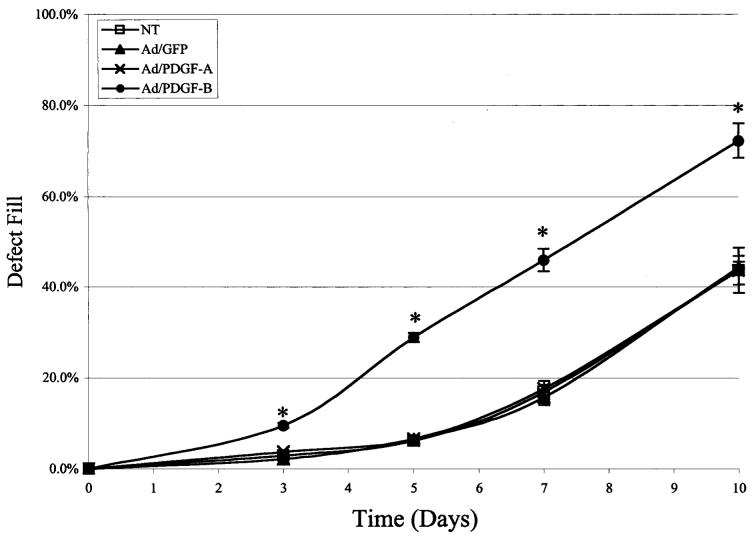
Representative images of HGFs that repopulated the wound area on day 10 from each treatment are depicted in Fig. 2. The images show the greatest density of HGF cells occupying the wound area in the Ad/PDGF-B group as compared with the other treatments. The Ad/PDGF-A treatment did not significantly enhance cell repopulation in the wound area compared with the NT or Ad/GFP-treated defects. Several parameters were determined by image analysis. The rate of defect fill was measured to determine the HGF cell migratory rate over 10 days (Fig. 3). Defect fill progressively increased up to 72% in the Ad/PDGF-B treated wounds over 10 days. The result showed a significantly more rapid defect fill in the Ad/PDGF-B-treated wounds as compared with the other treatments at all time points (p < 0.001). Beyond day 10, many of the lattices began to experience contracture at the peripheral regions, preventing longer term assessment. No significant differences were noted in contracture of the internal wound areas at all time points (p > 0.05).
FIG. 2.
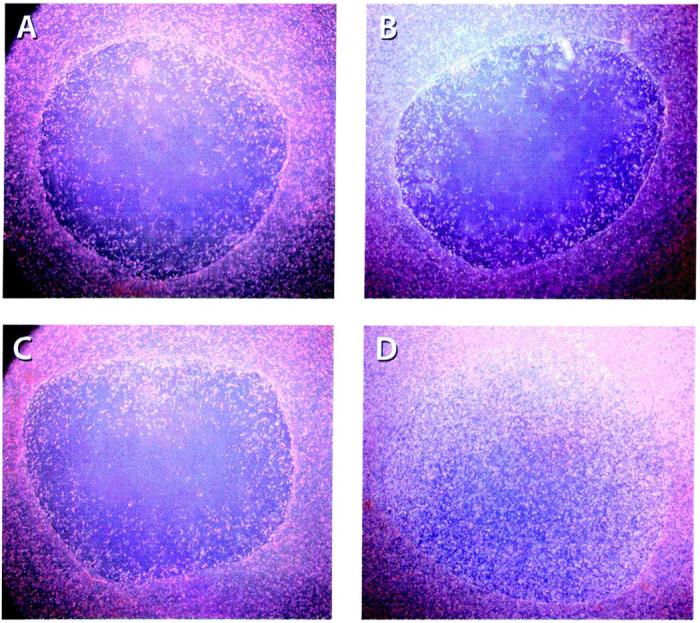
PDGF-B gene transfer stimulates ex vivo gingival defect fill. Images depict HGF repopulation into the wound defect area. Standardized digital images show HGFs filling in the wound area 10 days after no treatment (NT; A) or after treatment with 20 MOI of Ad/GFP (B), Ad/PDGF-A (C), or Ad/PDGF-B (D) per defect (n = 4 defects per group; original magnification, ×20).
FIG. 3.
PDGF-B gene transfer stimulates ex vivo gingival defect fill. HGF repopulation of created defects left untreated (NT) or treated with Ad/GFP, Ad/PDGF-A, or Ad/PDGF-B was measured by computer-assisted image analysis on days 3, 5, 7, and 10 postwounding. The percentage of wound fill was measured by determining the mean distance of cell migration into the wound area compared with the mean diameter of the wound. The data represent mean and standard error of measurement at each time point. Ad/PDGF-B induced significantly higher wound fill over time (*p < 0.001) compared with the other groups 3, 5, 7, and 10 days after gene transfer (n = 4 defects per group).
The effect of Ad/PDGF-A and -B gene transfer on cellular migration and proliferation was determined by measuring total cell density of HGFs that repopulated the defect area, using image analysis (Fig. 4). Significantly greater cell density was detected in the Ad/PDGF-B-treated defects compared with the other treatments at all time points (p < 0.001). In addition, cell counting on day 10 was determined in two separate areas including the defect area, and the defect periphery (Table 2). Compared with all treatment groups, Ad/PDGF-B stimulated the greatest increases in cell repopulation in the defect area and defect periphery (p < 0.001).
FIG. 4.
Density of cell repopulation into collagen wound defects. Total cell density of HGFs that repopulated defects left untreated (NT) or treated with Ad/GFP, Ad/PDGF-A, or Ad/PDGF-B was measured by computer-assisted image analysis on days 3, 5, 7, and 10. The data represent means ± SEM. Ad/PDGF-B induced significantly higher total cell density (*p , 0.001) compared with all other groups 3, 5, 7, and 10 days after gene transfer (n = 4 defects per group).
Table 2.
HGF Cell Counts on Day 10 after Defect Creation
|
Treatment (cells × 103 ± SEM) |
||||
|---|---|---|---|---|
| Cell count (n = 4) | NT | Ad/GFP | Ad/PDGF-A | Ad/PDGF-B |
| Defect area | 2.3 ± 0.4 | 1.3 ± 0.1 | 1.5 ± 0.3 | 4.9 ± 0.6a |
| Defect periphery | 53.1 ± 8.2 | 35.4 ± 5.4 | 49.7 ± 2.7 | 145.0 ± 5.0b |
p < 0.05; significantly different from the other treatments.
p < 0.001; significantly different from the other treatments.
The prolonged transduction of PDGF genes by Ad/PDGF-A and -B gene transfer in HGFs was examined by RT-PCR (Fig. 5). HGF gene expression of both PDGF-A and PDGF-B genes was induced over a period of 10 days in Ad/PDGF-A- and Ad/PDGF-B-treated defects, respectively. No significant change in expression of COLIα1 was observed in both the Ad/PDGF-A- and Ad/PDGF-B-treated defects during the experimental period (Fig. 6A). Upregulation of the PI3 kinase P85α subunit and integrin α5 genes was observed in the Ad/PDGF-B-treated defects on day 5. No changes were observed in the level of expression for these two genes by day 10 in the Ad/PDGF-B-treated wounds (Fig. 6B). No significant changes in PI3 kinase P85α subunit and integrin α5 expression were noted after PDGF-A gene transfer. The results showed that upregulation of PI3 kinase and integrin α5 subunit in response to PDGF-B gene transfer may be involved in the promotion of the early wound-healing process. However, the direct effects of PDGF-B gene transfer on these signaling pathways were not specifically measured.
FIG. 5.
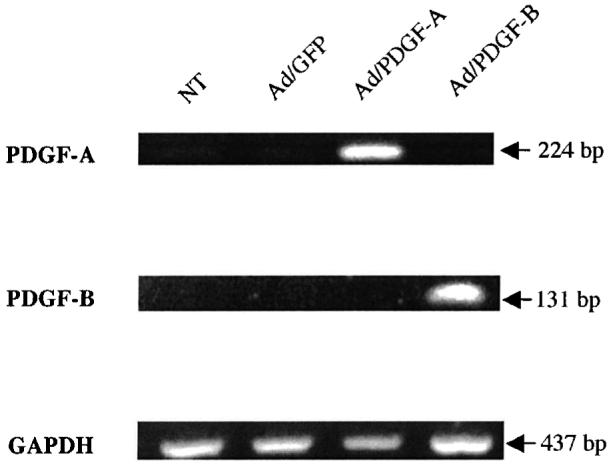
Prolonged induction of PDGF-A and PDGF-B transgenes in HGFs: day 10. RNAs extracted from HGF-populated lattices were subjected to RT-PCR. The PCR products were analyzed on ethidium bromide-stained gels and the expected PCR sizes are indicated. PDGF-A and PDGF-B genes were induced at all time points up to day 10 in the Ad/PDGF-A and Ad/PDGF-B groups, respectively.
FIG. 6.
Effect of PDGF-A and PDGF-B gene transfer on HGF gene expression by RT-PCR. RNAs extracted from HGF-populated lattices were subjected to RT-PCR with various sets of primers (Table 1) to determine the expression of ColIα1, PI3 kinase (P85α), and integrin α5 subunit (α5) compared with GAPDH (A) on day 5 and (B) on day 10. Histograms depict the relative ratio of gene expression normalized to GAPDH on day 5 (A, right) and day 10 (B, right). The PCR products were analyzed on ethidium bromide-stained gels and the expected PCR sizes are indicated. ColIα1 expression levels were not changed appreciably in response to Ad/PDGF-A and Ad/PDGF-B during the 10-day observation period. PI3 kinase (P85α) and integrin α5 subunit (α5) were upregulated in HGFs by PDGF-B induction on day 5, and remained unchanged compared with the other groups on day 10.
DISCUSSION
We have established an artificial three-dimensional wound model to test the effects of Ad/PDGF-A and -B gene transfer to gingival cells derived from healthy periodontal tissue. This model was developed to directly apply growth factor vector immobilized in a soluble matrix to a wound site to serve as a scaffold for invading cells. Subsequent transduction of these cells would then allow for transgene expression and formation of new tissue within the matrix scaffold. This mimicks the in vivo situation in which PDGF is released or incorporated in the wound site and elicits activities toward the surrounding target cell types. The results show the effective prolonged expression of PDGF-A, PDGF-B, and green fluorescent protein genes delivered by adenovirus over a period of 7–10 days in the wound sites (Figs. 1C and 5). It is consistent with our previous work and others showing the sustained production of PDGFs delivered by adenovirus.9,16,17 The GF produced in this wound model is presumably locally produced within the wound and released into the culture medium, affecting neighboring cells in an autocrine, paracrine, and delayed paracrine manner. We have previously shown that PDGF protein production is approximately 200 ng/106 fibroblasts per day.18 Further studies will be necessary to determine differences in expression of PDGF receptors in diseased and regenerating tissues known to be modulated in these situations.20,21
The Ad/PDGF-B-treated wounds exhibited the greatest degree of wound fill by increasing HGF repopulation into the wound defect area. There was a 2-fold increased rate of defect fill in the Ad/PDGF-B group compared with all other groups, including Ad/PDGF-A. Our data are consistent with many in vitro and in vivo studies relating to PDGF and soft tissue repair. PDGF-AB has been shown to be a strong chemotactic factor for gingival fibroblasts in the in vitro Boyden chamber system.22 In addition, it has been demonstrated that PDGF-BB elicited a dramatic increase in wound closure in several animal models.7,9,10 For example, application of PDGF-B encoded by plasmid or adenovirus DNA vector impregnated in collagen matrix induced ∼2.5 times greater wound closure in the 6- or 8-mm ischemic skin wounds in rabbits as compared with the negative control.9,10 Our results using the in vitro model are consistent with the degree of wound closure induced by PDGF-B gene transfer in extraoral soft tissue wounds.
Image analysis showed approximately 4-fold greater cell densities of HGFs that repopulated the defect area in the Ad/PDGF-B-treated sites as compared with the other groups. In addition, Ad/PDGF-B significantly enhanced cellular density in the defect area on day 10 as determined by cell measurement. Mumford and co-workers created a 3-mm defect on tissue culture plastic and showed significantly greater percentages of cell density of HGFs in response to a variety of PDGF-BB concentrations compared with controls over a period of 9 days when PDGF-BB was continuously added to the medium.23 However, they reported that PDGF-BB did not induce higher cellular proliferation of HGFs as compared with control. Thus, the rapid wound fill in their in vitro wound model might occur by activation of cellular migration in response to PDGF-BB rather than cellular proliferation. In addition, it has been shown that HGFs grown in collagen gels exhibit no significant increase cellular proliferation in response to PDGF-BB compared with negative controls on day 14.24 Although we did not directly measure cell migration and proliferation separately, we found that HGF cells transduced with Ad/PDGF-B repopulated the defects more rapidly than the corresponding controls, including Ad/PDGF-A. The stimulatory effects of PDGF on cell migration and proliferation are mediated through two receptors designated as α and β receptors. PDGF-A binds only to PDGF-αR, and PDGF-B binds to both α and β receptors.15 PDGF-A and -B have been shown to be expressed in gingival wounds for up to 3 days postwounding. PDGF-αR was minimally detectable, whereas PDGF-βR was upregulated and became maximal after 7 days.20 Furthermore, PDGF-αR was not measurable in cultured gingival and periodontal ligament fibroblasts, but PDGF-βR appeared to be highly expressed in the 6-week human regenerated periodontal tissues compared with gingival and periodontal ligament tissues.21 This likely explains the limited response of HGFs to Ad/PDGF-A gene transfer in this model.
It has been shown that stimulation of granulation tissue formation by PDGF-A plasmid DNA was comparable to that found with PDGF-B.10 Our in vitro results showed significantly less defect fill induced by Ad/PDGF-A. Possible explanations are as follows: (1) In general, PDGF-BB possesses a more potent stimulatory effect on cellular migration, and induces proliferation greater than PDGF-AA or PDGF-AB;25-27 (2) induction of cell growth by PDGF-AA or Ad/PDGF-A requires additional growth factors present in serum; our system was devoid of progression factors such as IGF-I18,28,29; and (3) PDGF-AA binds selectively to PDGF-α receptors; it has been shown that homodimers of PDGF-α receptor does not mediate chemotaxis in many cell types.30,31 It is possible that PDGF-AA does not induce migration of HGF, especially in this three-dimensional wound model. Although expression of PDGF-αR and -βR was not determined in the present study, these results suggest that PDGF-BB or -AB that bind to PDGF-βRs may play a greater role in periodontal wound healing.
One of the important steps in the wound repair process is synthesis of extracellular matrix proteins, such as collagen type I, by connective tissue cells.32,33 Our results revealed that the level of COLIα1 gene expression in HGFs did not significantly change over a period of 10 days in any treatment. Several in vitro studies have shown that PDGFs do not directly regulate collagen type I gene expression by fibroblasts.34,35 Our result was consistent with that reported by Irwin and coworkers, who found no difference in collagen production between the negative control (2.5% FCS) and PDGF-BB treatment when HGFs were incorporated in the collagen lattice.24 Also, PDGF-BB did not induce COLIα1 mRNA expression in dermal fibroblasts incorporated in collagen gels while increasing collagen type I protein synthesis.36 Although elevated production of collagen has been shown in vivo in response to PDGF treatment,37 this result may be related to the increased number of fibroblasts in the wounds, not the cellular activity per se.34,38
Phosphatidylinositol-3-kinase is an important secondary messenger that regulates cellular migration and proliferation in response to PDGF in many cell types.39-41 The regulatory P85α subunit of PI3 kinase has been shown to directly interact with activated PDGFαR and PDGFβR.42,43 Also, increased levels of phosphorylated P85α subunit of PI3 kinase protein activated by PDGF receptor have been detected.43,44 It has been shown that PDGF-BB induces NIH 3T3 fibroblast cell migration on collagen gels mediated by the P85α subunit of PI3 kinase.45 Our study revealed that PI3 kinase was upregulated on day 5 and remained relatively unchanged after PDGF-B gene transfer as compared with the other groups (Fig. 6). Although the activation state of PI3 kinase P85α subunit was not determined, it is consistent with rapid rates of HGF repopulation in the Ad/PDGF-B-treated defects from days 3–5 with minimal subsequent changes in defect fill rate from days 5 to 10. A serine-threonine protein kinase (Akt) is an important PI3 kinase dependent on downstream signaling molecule for PDGF.15 Our group has shown that Ad/PDGF induces sustained phosphorylation of Akt for up to 96 h in fibroblasts.18 Thus, our data suggest that PI3 kinase is involved in response to exposure of Ad/PDGF-B to HGF migration and proliferation in this three-dimensional artificial wound model at early time points.
It has been reported that expression of integrin α5 was enhanced on fibroblasts adjacent to the wound and in the granulation tissue in a cutaneous wound model.46 In our experimental gingival defect model, HGFs surrounding the defects incorporated with Ad/PDGF-A and -B presumably activated cell surface receptors of the integrin family during migration into the wound and for subsequent matrix biosynthesis. Our study shows that Ad/PDGF-B upregulates integrin α5 subunit gene expression in HGFs on day 5 and that the level of expression was relatively similar to that of other groups at later time points (Fig. 6). Our result is consistent with other studies showing that PDGF-BB enhances the production of integrin α5 at both the mRNA and protein level.46-48 PDGF selectively stimulates fibronectin production in fibroblast cell lines.49,50 Integrin α5β1 binds to fibronectin, which is accumulated in the early phases of wound healing, and plays an important role in modulating fibroblast migration into the wound.51,52 It has been shown in vitro that fibronectin present in the collagen gel and fibrin gel is required for dermal fibroblast cells to transmigrate from the collagen gel into the surrounding fibrin gel.53 Therefore, upregulation of integrin α5 subunit by HGFs in Ad/PDGF-B-treated wounds at early time points correlates with a rapid increase in HGF repopulation into the defect area, especially from days 3 to 5 (Fig. 6).
We noted that Ad/PDGF-B-treated defects did not exhibit wound contraction, suggesting that Ad/PDGF-B incorporated in the collagen scaffold may promote wound healing with minimal scar formation. This is supported by the observation that collagen-formulated Ad/PDGF-B induced complete repair of excisional skin defects, without the development of hypertrophic scar formation.9 Several growth factors work in concert for optimal wound repair and lead to scar formation in most in vivo systems. Application of collagen-formulated Ad/PDGF-B for periodontal wound repair and regeneration awaits further study. A major consideration in using adenovirus for gene transfer is the significant cytotoxic T cell-mediated immune response that occurs when delivered in vivo.54 Despite the inflammatory cell infiltrate that is notable during the early phases of wound repair, delivery of GF genes to periodontal wounds can still result in bone and ligament formation.55 The use of second and third vector systems should aid in decreasing the influence of inflammation when adenovirus is delivered in vivo.
Taken together, we have established a three-dimensional wound model to locally deliver Ad/PDGF-A and Ad/PDGF-B genes to cells derived from periodontal tissue for the promotion of wound healing. The expression of PDGF genes was prolonged for up to 10 days. Ad/PDGF-B enhanced defect fill by induction of HGF migration and proliferation. The upregulation of genes associated with PDGF signaling (PI3 kinase) and fibroblast migration (integrin α5) suggests that several cellular and molecular events are modulated in response to long-term PDGF gene delivery. In vivo delivery of Ad/PDGF-B incorporated in collagen scaffolds as a biomimetic device offers potential for the promotion of periodontal and oral tissue wound repair and regeneration.
ACKNOWLEDGMENTS
We thank Marie Printz from Selective Genetics (San Diego, CA) for assistance with this project. We also thank Chris Jung for preparation of the figures. This study was funded by NIH/NIDCR grants DE 11960 and 13397 and by a University of Michigan Rackham Faculty Award to W.V.G.
REFERENCES
- 1.Anusaksathien O, Giannobile WV. Growth factor delivery to re-engineer periodontal tissues. Curr. Pharm. Biotechnol. 2002;3:129. doi: 10.2174/1389201023378391. [DOI] [PubMed] [Google Scholar]
- 2.Rutherford RB, Niekrash CE, Kennedy JE, Charette MF. Platelet-derivedand insulin-likegrowth factors stimulate regeneration of periodontal attachment in monkeys. J. Periodontal Res. 1992;27:285. doi: 10.1111/j.1600-0765.1992.tb01679.x. [DOI] [PubMed] [Google Scholar]
- 3.Giannobile WV, Finkelman RD, Lynch SE. Comparison of canine and nonhuman primate animal models for periodontal regenerative therapy: Results following a single administration of PDGF/IGF-I. J. Periodontol. 1994;65:1158. doi: 10.1902/jop.1994.65.12.1158. [DOI] [PubMed] [Google Scholar]
- 4.Giannobile WV, Hernandez RA, Finkelman RD, Ryan S, Kiritsy CP, D'Andrea M, Lynch SE. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J. Periodontal Res. 1996;31:301. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 5.Howell TH, Fiorellini JP, Paquette DW, Offenbacher S, Giannobile WV, Lynch SE. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J. Periodontol. 1997;68:1186. doi: 10.1902/jop.1997.68.12.1186. [DOI] [PubMed] [Google Scholar]
- 6.Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996;19:23S. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 7.Liechty KW, Nesbit M, Herlyn M, Radu A, Adzick NS, Crombleholme TM. Adenoviral-mediated over-expression of platelet-derived growth factor-B corrects ischemic impaired wound healing. J. Invest. Dermatol. 1999;113:375. doi: 10.1046/j.1523-1747.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 8.Eming SA, Whitsitt JS, He L, Krieg T, Morgan JR, Davidson JM. Particle-mediated gene transfer of PDGF isoforms promotes wound repair. J. Invest. Dermatol. 1999;112:297. doi: 10.1046/j.1523-1747.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 9.Doukas J, Chandler LA, Gonzalez AM, Gu D, Hoganson DK, Ma C, Nguyen T, Printz MA, Nesbit M, Herlyn M, Crombleholme TM, Aukerman SL, Sosnowski BA, Pierce GF. Matrix immobilization enhances the tissue repair activity of growth factor gene therapy vectors. Hum. Gene Ther. 2001;12:783. doi: 10.1089/104303401750148720. [DOI] [PubMed] [Google Scholar]
- 10.Tyrone JW, Mogford JE, Chandler LA, Ma C, Xia Y, Pierce GF, Mustoe TA. Collagen-embedded platelet-derived growth factor DNA plasmid promotes wound healing in a dermal ulcer model. J. Surg. Res. 2000;93:230. doi: 10.1006/jsre.2000.5912. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan DR, Chao FC, Stiles CD, Antoniades HN, Scher CD. Platelet α granules contain a growth factor for fibroblasts. Blood. 1979;53:1043. [PubMed] [Google Scholar]
- 12.Seppa H, Grotendorst G, Seppa S, Schiffmann E, Martin GR. Platelet-derived growth factor in chemotactic for fibroblasts. J. Cell Biol. 1982;92:584. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heldin P, Laurent TC, Heldin CH. Effect of growth factors on hyaluronan synthesis in cultured human fibroblasts. Biochem. J. 1989;258:919. doi: 10.1042/bj2580919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenkranz S, Kazlauskas A. Evidence for distinct signaling properties and biological responses induced by the PDGF receptor α and β subtypes. Growth Factors. 1999;16:201. doi: 10.3109/08977199909002130. [DOI] [PubMed] [Google Scholar]
- 15.Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim. Biophys. Acta. 1998;1378:F79. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Z, Lee CS, Tejeda KM, Giannobile WV. Gene transfer and expression of platelet-derived growth factors modulate periodontal cellular activity. J. Dent. Res. 2001;80:892. doi: 10.1177/00220345010800030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giannobile WV, Lee CS, Tomala MP, Tejeda KM, Zhu Z. Platelet-derived growth factor (PDGF) gene delivery for application in periodontal tissue engineering. J. Periodontol. 2001;72:815. doi: 10.1902/jop.2001.72.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen QP, Giannobile WV. Adenoviral gene transfer of PDGF downregulates gas gene product PDGFαR and prolongs ERK and Akt/PKB activation. Am. J. Physiol. Cell Physiol. 2002;282:C538. doi: 10.1152/ajpcell.00419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Khateeb T, Stephens P, Shepherd JP, Thomas DW. An investigation of preferential fibroblast wound repopulation using a novel in vitro wound model. J. Periodontol. 1997;68:1063. doi: 10.1902/jop.1997.68.11.1063. [DOI] [PubMed] [Google Scholar]
- 20.Green RJ, Usui ML, Hart CE, Ammons WF, Narayanan AS. Immunolocalization of platelet-derived growth factor A and B chains and PDGF-α and β receptors in human gingival wounds. J. Periodontal Res. 1997;32:209. doi: 10.1111/j.1600-0765.1997.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 21.Parkar MH, Kuru L, Giouzeli M, Olsen I. Expression of growth-factor receptors in normal and regenerating human periodontal cells. Arch. Oral Biol. 2001;46:275. doi: 10.1016/s0003-9969(00)00099-6. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura F, Terranova VP. Comparative study of the chemotactic responses of periodontal ligament cells and gingival fibroblasts to polypeptide growth factors. J. Dent. Res. 1996;75:986. doi: 10.1177/00220345960750041401. [DOI] [PubMed] [Google Scholar]
- 23.Mumford JH, Carnes DL, Cochran DL, Oates TW. The effects of platelet-derived growth factor-BB on periodontal cells in an in vitro wound model. J. Periodontol. 2001;72:331. doi: 10.1902/jop.2001.72.3.331. [DOI] [PubMed] [Google Scholar]
- 24.Irwin CR, Schor SL, Ferguson MW. Effects of cytokines on gingival fibroblasts in vitro are modulated by the extracellular matrix. J. Periodontal Res. 1994;29:309. doi: 10.1111/j.1600-0765.1994.tb01227.x. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda N, Lin WL, Kumar NM, Cho MI, Genco RJ. Mitogenic, chemotactic, and synthetic responses of rat periodontal ligament fibroblastic cells to polypeptide growth factors in vitro. J. Periodontol. 1992;63:515. doi: 10.1902/jop.1992.63.6.515. [DOI] [PubMed] [Google Scholar]
- 26.Lepisto J, Laato M, Niinikoski J, Lundberg C, Gerdin B, Heldin CH. Effects of homodimeric isoforms of platelet-derived growth factor (PDGF-AA and PDGF-BB) on wound healing in rat. J. Surg. Res. 1992;53:596. doi: 10.1016/0022-4804(92)90260-7. [DOI] [PubMed] [Google Scholar]
- 27.Lepisto J, Peltonen J, Vaha-Kreula M, Niinikoski J, Laato M. Platelet-derived growth factor isoforms PDGF-AA, -AB and -BB exert specific effects on collagen gene expression and mitotic activity of cultured human wound fibroblasts. Biochem. Biophys. Res. Commun. 1995;209:393. doi: 10.1006/bbrc.1995.1516. [DOI] [PubMed] [Google Scholar]
- 28.Stiles CD, Capone GT, Scher CD, Antoniades HN, Van Wyk JJ, Pledger WJ. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc. Natl. Acad. Sci. U.S.A. 1979;76:1279. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karenberg TA, Fenn A, Sachinidis A, Hoppe J. The differential activation of phosphatidylinositol-3 kinase and mitogen-activated protein kinases by PDGF-AA and IGF-I might explain the synergistic effect of the two growth factors on the proliferation of AKR-2B fibroblasts. Exp. Cell Res. 1994;213:266. doi: 10.1006/excr.1994.1198. [DOI] [PubMed] [Google Scholar]
- 30.Nister M, Hammacher A, Mellstrom K, Siegbahn A, Ronnstrand L, Westermark B, Heldin CH. A glioma-derived PDGF A chain homodimer has different functional activities from a PDGF AB heterodimer purified from human platelets. Cell. 1988;52:791. doi: 10.1016/0092-8674(88)90421-7. [DOI] [PubMed] [Google Scholar]
- 31.Koyama N, Kinsella MG, Wight TN, Hedin U, Clowes AW. Heparan sulfate proteoglycans mediate a potent inhibitory signal for migration of vascular smooth muscle cells. Circ. Res. 1998;83:305. doi: 10.1161/01.res.83.3.305. [DOI] [PubMed] [Google Scholar]
- 32.Ross R. The fibroblast and wound repair. Biol. Rev. 1968;43:51. doi: 10.1111/j.1469-185x.1968.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 33.Peacock EEJ. Wound Repair. W.B. Saunders; Philadelphia, PA: 1984. [Google Scholar]
- 34.Clark JG, Madtes DK, Raghu G. Effects of platelet-derived growth factor isoforms on human lung fibroblast proliferation and procollagen gene expression. Exp. Lung Res. 1993;19:327. doi: 10.3109/01902149309064350. [DOI] [PubMed] [Google Scholar]
- 35.Tan EM, Qin H, Kennedy SH, Rouda S, Fox JWT, Moore JH., Jr. Platelet-derived growth factors-AA and -BB regulate collagen and collagenase gene expression differentially in human fibroblasts. Biochem. J. 1995;310:585. doi: 10.1042/bj3100585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivarsson M, McWhirter A, Borg TK, Rubin K. Type I collagen synthesis in cultured human fibroblasts: Regulation by cell spreading, platelet-derived growth factor and interactions with collagen fibers. Matrix Biol. 1998;16:409. doi: 10.1016/s0945-053x(98)90014-2. [DOI] [PubMed] [Google Scholar]
- 37.Pierce GF, Tarpley JE, Yanagihara D, Mustoe TA, Fox GM, Thomason A. Platelet-derived growth factor (BB homodimer), transforming growth factor-β1, and basic fibroblast growth factor in dermal wound healing: Neovessel and matrix formation and cessation of repair. Am. J. Pathol. 1992;140:1375. [PMC free article] [PubMed] [Google Scholar]
- 38.Pierce GF, Brown D, Mustoe TA. Quantitative analysis of inflammatory cell influx, procollagen type I synthesis, and collagen cross-linking in incisional wounds: Influence of PDGF-BB and TGF-β1 therapy. J. Lab. Clin. Med. 1991;117:373. [PubMed] [Google Scholar]
- 39.Valius M, Kazlauskas A. Phospholipase C-γ1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor's mitogenic signal. Cell. 1993;73:321. doi: 10.1016/0092-8674(93)90232-f. [DOI] [PubMed] [Google Scholar]
- 40.Choudhury GG, Karamitsos C, Hernandez J, Gentilini A, Bardgette J, Abbound HE. PI-3-kinase and MAPK regulate mesangial cell proliferation and migration in response to PDGF. Am. J. Physiol. 1997;273:F931. doi: 10.1152/ajprenal.1997.273.6.F931. [DOI] [PubMed] [Google Scholar]
- 41.Yamboliev IA, Chen J, Gerthoffer WT. PI 3-kinases and Src kinases regulate spreading and migration of cultured VSMCs. Am. J. Physiol. Cell Physiol. 2001;281:C709. doi: 10.1152/ajpcell.2001.281.2.C709. [DOI] [PubMed] [Google Scholar]
- 42.Heidaran MA, Beeler JF, Uy JC, Ishibashi T, LaRochelle WJ, Pierce JH, Aaronson SA. Differences in substrate specificities of α and β platelet-derived growth factor (PDGF) receptors: Correlation with their ability to mediate PDGF transforming functions. J. Biol. Chem. 1993;268:9287. [PubMed] [Google Scholar]
- 43.Marra F, Gentilini A, Pinzani M, Choudhury GG, Parola M, Herbst H, Dianzani MU, Laffi G, Abboud HE, Gentilini P. Phosphatidylinositol 3-kinase is required for platelet-derived growth factor's actions on hepatic stellate cells. Gastroenterology. 1997;112:1297. doi: 10.1016/s0016-5085(97)70144-6. [DOI] [PubMed] [Google Scholar]
- 44.Hu P, Margolis B, Skolnik EY, Lammers R, Ullrich A, Schlessinger J. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol. Cell. Biol. 1992;12:981. doi: 10.1128/mcb.12.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jimenez C, Portela RA, Mellado M, Rodriguez-Frade JM, Collard J, Serrano A, Martinez AC, Avila J, Carrera AC. Role of the PI3K regulatory subunit in the control of actin organization and cell migration. J. Cell Biol. 2000;151:249. doi: 10.1083/jcb.151.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gailit J, Xu J, Bueller H, Clark RA. Platelet-derived growth factor and inflammatory cytokines have differential effects on the expression of integrins α1β1 and α5β1 by human dermal fibroblasts in vitro. J. Cell Physiol. 1996;169:281. doi: 10.1002/(SICI)1097-4652(199611)169:2<281::AID-JCP7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 47.Kirchberg K, Lange TS, Klein EC, Jungtaubl H, Heinen G, Meyer-Ingold W, Scharfetter-Kochanek K. Induction of β1 integrin synthesis by recombinant platelet-derived growth factor (PDGF-AB) correlates with an enhanced migratory response of human dermal fibroblasts to various extracellular matrix proteins. Exp. Cell Res. 1995;220:29. doi: 10.1006/excr.1995.1288. [DOI] [PubMed] [Google Scholar]
- 48.Harwood FL, Goomer RS, Gelberman RH, Silva MJ, Amiel D. Regulation of αvβ3 integrin receptors by basic fibroblast growth factor and platelet-derived growth factor-BB in intrasynovial flexor tendon cells. Wound Repair Regen. 1999;7:381. doi: 10.1046/j.1524-475x.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- 49.Blatti SP, Foster DN, Ranganathan G, Moses HL, Getz MJ. Induction of fibronectin gene transcription and mRNA is a primary response to growth-factor stimulation of AKR-2B cells. Proc. Natl. Acad. Sci. U.S.A. 1988;85:1119. doi: 10.1073/pnas.85.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen-Hoffmann BL, Schlosser SJ, Brondyk WH, Fahl WE. Fibronectin levels are enhanced in human fibroblasts overexpressing the c-sis protooncogene. J. Biol. Chem. 1990;265:5219. [PubMed] [Google Scholar]
- 51.Gailit J, Pierschbacher M, Clark RA. Expression of functional α4β1 integrin by human dermal fibroblasts. J. Invest. Dermatol. 1993;100:323. doi: 10.1111/1523-1747.ep12470011. [DOI] [PubMed] [Google Scholar]
- 52.Gailit J, Clark RA. Studies in vitro on the role of αv and β1 integrins in the adhesion of human dermal fibroblasts to provisional matrix proteins fibronectin, vitronectin, and fibrinogen. J. Invest. Dermatol. 1996;106:102. doi: 10.1111/1523-1747.ep12328177. [DOI] [PubMed] [Google Scholar]
- 53.Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J. Cell Sci. 1997;110:861. doi: 10.1242/jcs.110.7.861. [DOI] [PubMed] [Google Scholar]
- 54.Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4407. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin Q-M, Anusaksathien O, Webb SA, Rutherford RB, Giannobile WV. BMP gene therapy stimulates periodontal tissue engineering. J. Periodontol. 2003;74:202. doi: 10.1902/jop.2003.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]