Abstract
Growth factors such as platelet-derived growth factor (PDGF) exert potent effects on wound healing including the regeneration of tooth-supporting structures. This investigation examined the effect of the local delivery of PDGF-BB when combined with reconstructive periodontal surgery on local wound fluid (WF) levels of PDGF-AB, vascular endothelial growth factor (VEGF), and bone collagen telopeptide (ICTP) in humans with advanced periodontitis. Sixteen patients exhibiting localized periodontal osseous defects were randomized to one of three groups (β-TCP carrier alone, β-TCP + 0.3 mg/mL of recombinant human PDGF-BB [rhPDGF-BB], or β-TCP + 1.0 mg/mL of rhPDGF-BB) and monitored for 6 months. WF was harvested and analyzed for PDGF-AB, VEGF, and ICTP WF levels. Teeth contralateral to the target lesions served as controls. Increased levels of VEGF in the WF was observed for all surgical treatment groups with the 1.0 mg/mL rhPDGF-BB group showing the most pronounced difference at 3 weeks in the AUC analysis versus control (p < 0.0001). PDGF-AB WF levels were increased for the carrier alone group compared to both rhPDGF-BB groups. Low-dose rhPDGF-BB application elicited increases in ICTP at days 3–5 in the wound healing process, suggesting a promotion of bone turnover at early stages of the repair process (p < 0.02). These results demonstrate contrasting inducible expression patterns of PDGF-AB, VEGF, and ICTP during periodontal wound healing in humans.
INTRODUCTION
Periodontitis, one of the most common oral inflammatory infectious diseases and the leading cause of tooth loss, is characterized by the destruction of tooth-supporting tissues.1 Growth factors are natural biological molecules that mediate and regulate key cellular events during tissue repair, including cell proliferation, chemotaxis, differentiation, and matrix synthesis by binding to specific cell-surface receptors.2-4 Platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and pyridinoline cross-linked carboxyterminal telopeptide of Type I collagen (ICTP) are mediators involved in mitogenesis, angiogenesis, and bone turnover. These mediators are essential for wound regeneration of periodontal tissues. Growth factors such as PDGF can stimulate cells involved in periodontal regeneration and enhance periodontal wound healing and regeneration.5,6 Some of these inducible growth factors such as PDGF and VEGF can work coordinately to stimulate osteogenesis and vasculogenesis.7-9 However, little is known regarding the release of these molecules into local periodontal wound fluid (WF) or gingival crevicular fluid (GCF) during periodontal wound repair.
PDGF has shown strong effects in promoting bone and soft tissue healing.5,6,10 The effect of exogenous PDGF-BB on bone healing was tested in a pilot study using a unilateral tibial osteotomy model in rabbits. PDGF-treated tibiae displayed a more florid and advanced state of osteogenic differentiation, both endosteally and periosteally, than the control defects. Data suggest that exogenous PDGF has a stimulatory effect on fracture healing, and systemic administration of PDGF increases bone density and strength throughout the skeleton.11,12
Studies have clearly demonstrated the mechanism of action of PDGF, showing the presence of cell-surface receptors for PDGF on periodontal ligament and alveolar bone cells, and signal proliferative and chemotactic responses by these cells.13,14 Studies indicate that gingival epithelium may be a source of PDGF A and B chains and that the A chain may have a more prominent role to play during early stages of healing.15,16 Additionally, recombinant human PDGF-BB (rhPDGF-BB) has been shown to promote the regeneration of periodontal tissue including bone, cementum, and periodontal ligament in numerous animal and human studies.5,13,17-21 rhPDGF-BB is approved by the FDA under the trade name Regranex® for the treatment (improved healing) of chronic neuropathic, diabetic cutaneous ulcers.
Gingival crevicular fluid (GCF) is an exudate that can be harvested from the gingival crevice surrounding the tooth, one that contains many biochemical factors including growth factors.22-24 It is a complex mixture of substances derived from serum, leukocytes, structural cells of the periodontium, and oral bacteria.25 In health, GCF represents a transudate of gingival tissue interstitial fluid; however, as gingivitis and periodontitis develop, GCF becomes true inflammatory exudate.26
The aim of this trial is to better understand the mechanisms of wound healing by evaluating pyridinoline cross-linked carboxyterminal telopeptide of Type I collagen (ICTP), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) measured from periodontal wound fluid (WF) during wound healing after reconstructive therapy after local PDGF-BB application. The results of this study demonstrate the sequential release and inducible expression patterns of these wound healing mediators during periodontal tissue repair.
MATERIALS AND METHODS
This investigation evaluated 16 human subjects from the University of Michigan School of Dentistry. Patients involved in the study possessed localized severe periodontal disease requiring periodontal regeneration surgery. The patients in this study were a subset of research subjects participating in a pivotal clinical trial designed to evaluate the safety and effectiveness of rhPDGF-BB to promote soft and hard tissue engineering of the periodontium.27 The subjects provided GCF (or periodontal WF) after the delivery of grafts containing beta-tricalcium phosphate (β-TCP) (VitOss®, Orthovita, Malvern, PA) with or without rhPDGF-BB (combined product: GEM21S™, BioMimetic Therapeutics, Inc., Franklin, TN) to severe periodontal osseous defects. Informed consent was obtained at the initial visit prior to administration of any research-related treatment or procedure. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in obtainment of approval by the University of Michigan's human subjects research review committee.
Inclusion/exclusion criteria
Subjects were entered into the study if they were between 25 and 75 years of age and had no evidence of localized aggressive periodontitis. Patients qualified for the study if one tooth exhibited a soft tissue probing pocket depth measuring 7 mm or greater, and after surgical debridement, a 4 mm or greater vertical bone defect was present with at least one bony wall. Patients were excluded if they were unable to maintain the health of the site or were pregnant. The following were also exclusionary factors at baseline: diagnosis of oral cancer or human immunodeficiency virus within 6 months, previous periodontal surgery within 1 year on the study tooth, tooth mobility greater than grade II, radiographic signs of untreated acute infection at the surgical site, or recent history of smoking more than 20 cigarettes/day.
Randomization and surgical treatment
Subjects were randomized in equal proportions into one of three treatment groups: β-TCP carrier alone (active control), β-TCP + 0.3 mg/mL of rhPDGF-BB, or β-TCP + 1.0 mg/mL of rhPDGF-BB. Sites contralateral to the target lesions served as nonsurgical controls.
Before surgical treatment, each subject received nonsurgical therapy consisting of scaling and root planing to control the disease process and prepare the defect site for surgical treatment. Surgical treatment consisted of full-thickness mucoperiosteal flaps to allow adequate visualization of the osseous lesion. After debridement of the test site, the bone defect was measured and if found to be ≥4 mm vertically, final subject eligibility was confirmed and the root surfaces were decontaminated with a tetracycline paste. The test sites were then treated with β-TCP ± buffer containing rhPDGF-BB, and the flaps were secured with interdental sutures to achieve primary closure. Subjects were instructed to utilize an oral antimicrobial rinse of chlorhexidine (0.12%) twice daily for 6 weeks. Amoxicillin 500 mg three times daily for a minimum of 10 days (or another appropriate antibiotic regimen) was prescribed.
Wound healing score
During the first four follow-up visits, the healing condition of the soft tissues at the surgical site was examined by visual inspection. A secondary endpoint, wound healing (WH) score, was recorded to reflect the extent of healing (WH) at the surgical site.28 Wound Healing Scale: 0 = Absence of inflammation, normal healthy appearance to the tissues superficial to the graft, closed surgical wound; 1 = Mild inflammation; slight marginal change in color (e.g., redness), little change in texture of any portion of the marginal or papillary gingival unit superficial to the graft, closed surgical wound; 2 = Mild inflammation; criteria as above but involving the entire marginal or papillary unit, closed surgical wound; 3 = Moderate inflammation; glazing, redness, edema, and/or hypertrophy of the marginal or papillary unit, bleeding upon gentle palpation, gingival wound open <2 mm; and 4 = Severe inflammation; marked redness, edema, and/or hypertrophy of the marginal or papillary gingival unit, spontaneous bleeding, congestion, gingival wound open >2 mm.
Periodontal wound fluid collection
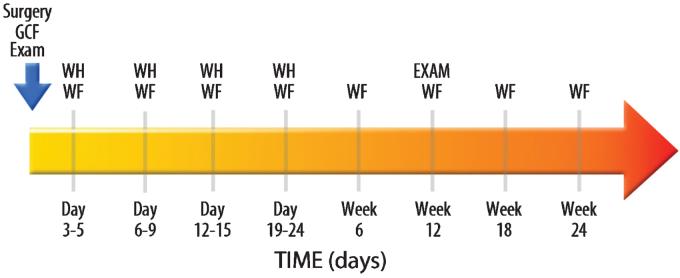
Four teeth were used as WF collection sites in each subject. Three contralateral teeth served as the nonsurgical control sites, and one tooth received the surgery + device: β-TCP with buffer alone (active control), β-TCP, with buffer containing 0.3 mg/mL of rhPDGF-BB, or β-TCP, with buffer containing 1.0 mg/mL of rhPDGF-BB. The WF samples were taken from the mesiobuccal aspect of each tooth except for the defect site where samples were directly taken. The area around each sample site was air-dried and the supragingival plaque biofilm was removed. A sterile methylcellulose strip (Pro Flow, Inc., Amityville, NY) was gently inserted into the gingival sulcus or periodontal pocket until slight resistance was felt. The fluid sample was then collected for 10 seconds and the strips were then immediately placed into Eppendorf tubes. The samples were subsequently kept on ice for transport to the laboratory, where they were stored at −80°C until needed for analysis of ICTP, VEGF, and PDGF-AB. The collection of WF occurred at nine different time points (Fig. 1): Baseline; between Days 3–5, 6–9, 12–15, 19–24; and at weeks 6, 12, 18, and 24 after reconstructive surgery.
FIG. 1.
Study timeline. GCF, gingival crevicular fluid; WF, periodontal wound fluid; WH, wound healing score.
Biomarker analysis
ICTP evaluation
Frozen samples were thawed at room temperature and the proteins were then eluted through centrifugation 5× in 12 × 75 mL polypropylene tubes at 3000 RPM for 5 min with 20 μL phosphate-buffered saline (pH 7.4) containing 15 nM aprotinin, 1 mM polymethyl sulfonyl fluoride (PMSF), and 0.1% of human serum albumin as previously described.29 GCF/WF ICTP levels were quantified using radioimmunoassay (RIA) (DiaSorin Inc., Stillwater, MN) as previously described.30 ICTP was determined as total amount/10-second time collection (pg/site).
VEGF evaluation
Frozen samples were thawed at room temperature and then the proteins were eluted using a rocker for 2 h at 4°C in 200 μL of buffer as previously described.31 The buffer contained antipain, aprotinin, leupeptin, n-ethyl malemide, and zwittergent. GCF/WF VEGF levels were quantified using enzyme-linked immunosorbent assay (ELISA) according to the manufacture's protocol (R&D Systems Inc., Minneapolis, MN). VEGF was determined as total amount/10-second collection (pg/site).
PDGF-AB evaluation
Frozen samples were thawed at room temperature and then the proteins were eluted using a rocker for 2 h at 4°C in diluent from the ELISA kit (R&D Systems, Inc.). GCF/WF PDGF-AB levels were quantified using ELISA according to the manufacturer's protocol. PDGF-AB was determined as total amount/10-second collection (pg/site). The assay is specific for PDGF-AB that would presumably be released endogenously, not detecting the applied rhPDGF-BB. No significant cross-reaction between the isoforms occurs, according to the manufacturer's instructions.
STATISTICAL ANALYSIS
The standard statistics and statistical modeling procedures using analysis of variance (ANOVA) Fishers protected least significant difference (PLSD) post hoc test at the 5% level were used to examine the differences in mediator values between the different treatment groups. A longitudinal analysis was used to take into account non-independence. Each model was run with the mediator level area under the curve (AUC) analysis as the dependent variable, and each of the surgery groups as the independent variables. AUC analysis was performed for two different time points: baseline–3 weeks and baseline–24 weeks. The evaluation over the first 3 weeks is the time frame in which initial healing is complete and complete closure of the wound occurs in the sites that received periodontal grafting surgery. The baseline–24-week AUC analysis accounts for the observation period that includes bone repair and maturation. The following formulae were used to determine the AUC:
Baseline–3-week AUC:
Baseline-24 week AUC:
Statistical analysis using AUC was performed using ANOVA Fishers post hoc test at the 5% level.
RESULTS
The mean age for all patients was 49.8 years with a range of 26–68 years. Half of the patients involved in the study were male (8) and half were female (8). A total of 6 patients in the study were considered smokers and 10 were nonsmokers. The β-TCP carrier alone group contained 2 current smokers, the 0.3 mg/mL of rhPDGF-BB group had 1 current smoker, and the 1.0 mg/mL of rhPDGF-BB group had 3 current smokers. For the test groups, baseline mean probing depth (PD) was 8.1 mm, baseline clinical attachment level (CAL) was 8.8 mm, and the mean bone defect depth was 5.25 mm. The non-surgical control mean PD was 3.3 mm and the mean CAL was 3.5 mm. No statistical differences were found between surgical treatment groups and pretreatment measurements at baseline.
Figure 1 illustrates the timeline for the WF collection. The collection of WF occurred at nine different time points: Baseline; between Days 3–5, 6–9, 12–15, 19–24; and at Week 6, 12, 18, and 24 after reconstructive surgery. Clinical and radiographic examinations occurred at the baseline time point presurgically and at Week 12. A wound healing score was recorded during the first four postsurgical visits. As expected, the wound healing scores decreased linearly over the first four time points (data not shown). With this relatively small sample size, no differences were found between groups regarding wound healing scores.
Figure 2A is a graph of VEGF (pg/site) over time for all groups. The nonsurgical control shows little change in the amount of VEGF released in the PWF throughout the study. For the test groups (0, 0.3, and 1.0 mg/mL rhPDGF-BB) there is an immediate increase in the amount of VEGF released over the first 12–15 days compared to the nonsurgical control group (p < 0.05), but not for carrier alone (NS). The increase of VEGF release peaked at day 12–15 and gradually decreased to baseline values over the remainder of the study. Among the treatment groups, the 1.0 mg/mL PDGF-BB group had the greatest amount of VEGF released with a maximum average of 38.4 pg/site at days 12–15, although this difference was not statistically significant. The 0.3 mg/mL PDGF-BB group and β-TCP carrier alone group had similar levels of VEGF release throughout the study. Their levels also peaked at days 12–15, although with slightly lower, nonsignificant values than the 1.0 mg/mL PDGF-BB group. Figure 2B is an AUC analysis for VEGF over the first 3 weeks. The AUC analysis estimates the amount of the mediator released over a period of time. Statistically significant differences were observed between the non-surgical control and all treatment groups. Figure 2C is an AUC analysis of VEGF for all groups over 24 weeks. Again, statistical differences were observed between the nonsurgical control and all treatment groups (p < 0.05). Over the 24-week time frame, the AUC for the different treatment groups leveled out to similar AUC values.
FIG. 2.
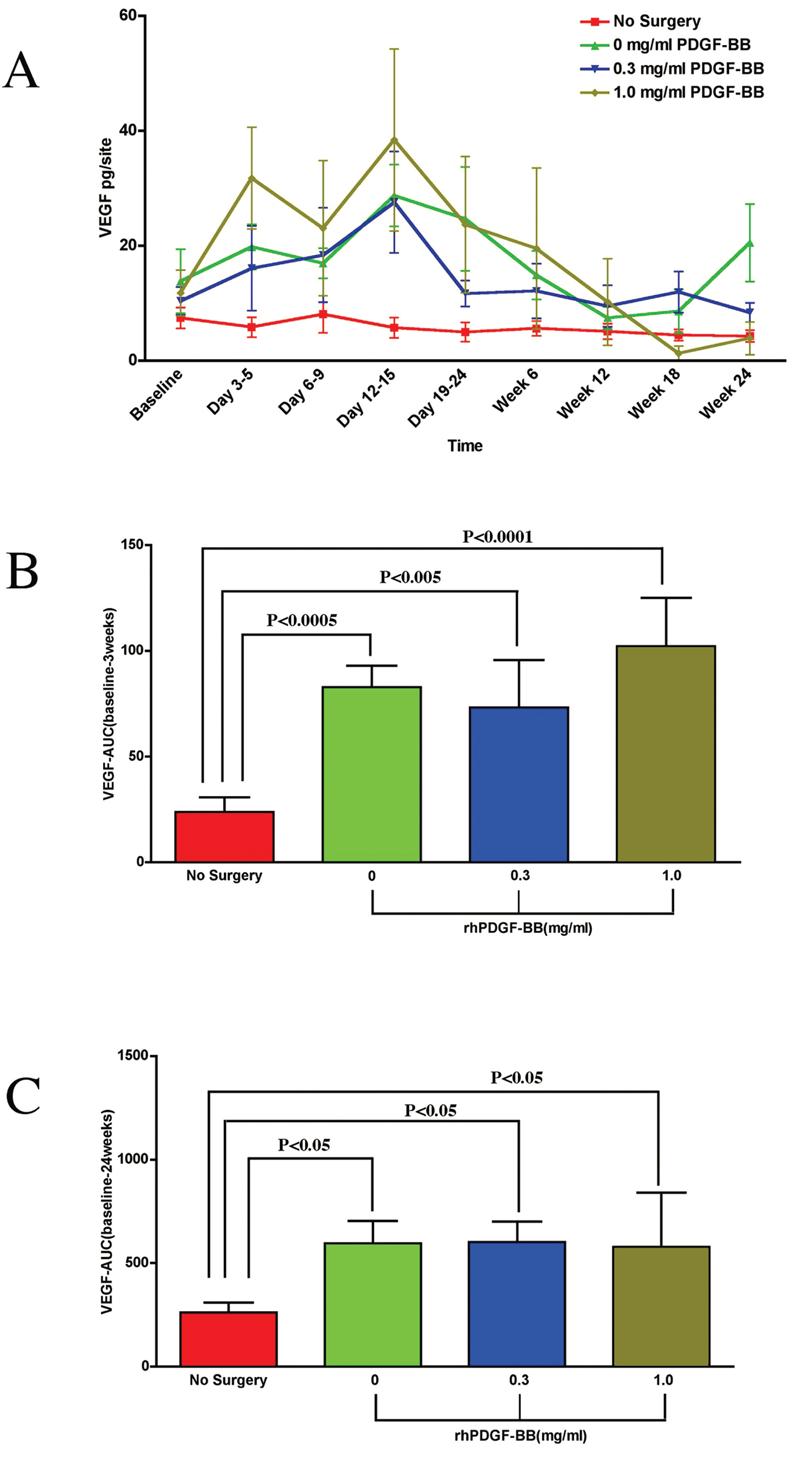
PDGF tissue engineering of periodontal defects promotes local release of vascular endothelial growth factor. (A) Graph of VEGF (pg/site) versus timepoint for all groups: β-TCP carrier alone, 0.3mg/mL PDGF, 1.0 mg/mL PDGF, and the nonsurgical control. Statistically significant differences were found between all treatment groups and the nonsurgical control (p < 0.05) except for Days 6–9 and Week 12. A statistically significant difference was also found between the 0.3 mg/mL PDGF group and the 1.0 mg/mL PDGF group at Days 3–5 (p < 0.05). (B) Area under the curve (AUC) analysis for VEGF up to 3 weeks. The most pronounced statistically significant difference was found between the nonsurgical control and the 1.0 mg/mL PDGF group (p < 0.0001). Statistically significant differences were also observed between the nonsurgical control and the β-TCP carrier alone group (p < 0.0005) and the 0.3 mg/mL PDGF group (p < 0.005). The AUC for VEGF was highest for the 1.0 mg/mL PDGF group compared to the other surgical treatment groups, although there were no statistically significant differences between these treatment groups. (C) Area under the curve (AUC) analysis for VEGF of all time-points. Statistically differences were observed between the nonsurgical control and all treatment groups (p < 0.05). No statistically significant differences were found between treatment groups. Statistical analysis was performed using ANOVA Fisher test at the 5% level. A total of 15 patients were analyzed for VEGF. Bars indicate standard error measurements (SEM).
Figure 3A shows endogenous PDGF-AB release (pg/site) over time for all groups. The amount of PDGF-AB released remained low for all groups at all time points. The β-TCP carrier alone group showed an increase of PDGF-AB production at Days 3–5 and 6–9. No statistical differences were observed between treatment groups or between the 0.3 mg/mL PDGF-BB group and the 1.0 mg/mL PDGF-BB group versus the nonsurgical control. Figure 3B is an AUC analysis for PDGF-AB release over the first 3 weeks. A statistically significant difference was observed between the nonsurgical control and the β-TCP carrier alone group (p < 0.05). No statistical differences were observed between treatment groups or between the 0.3 mg/mL PDGF-BB group and the 1.0 mg/mL PDGF-BB group versus the nonsurgical control over the first 3 weeks. Figure 3C is an AUC analysis of PDGF-AB for all groups over 24 weeks. The AUC for the 0.3 mg/mL PDGF-BB group and the 1.0 mg/mL PDGF-BB group were similar to that of the nonsurgical control.
FIG. 3.
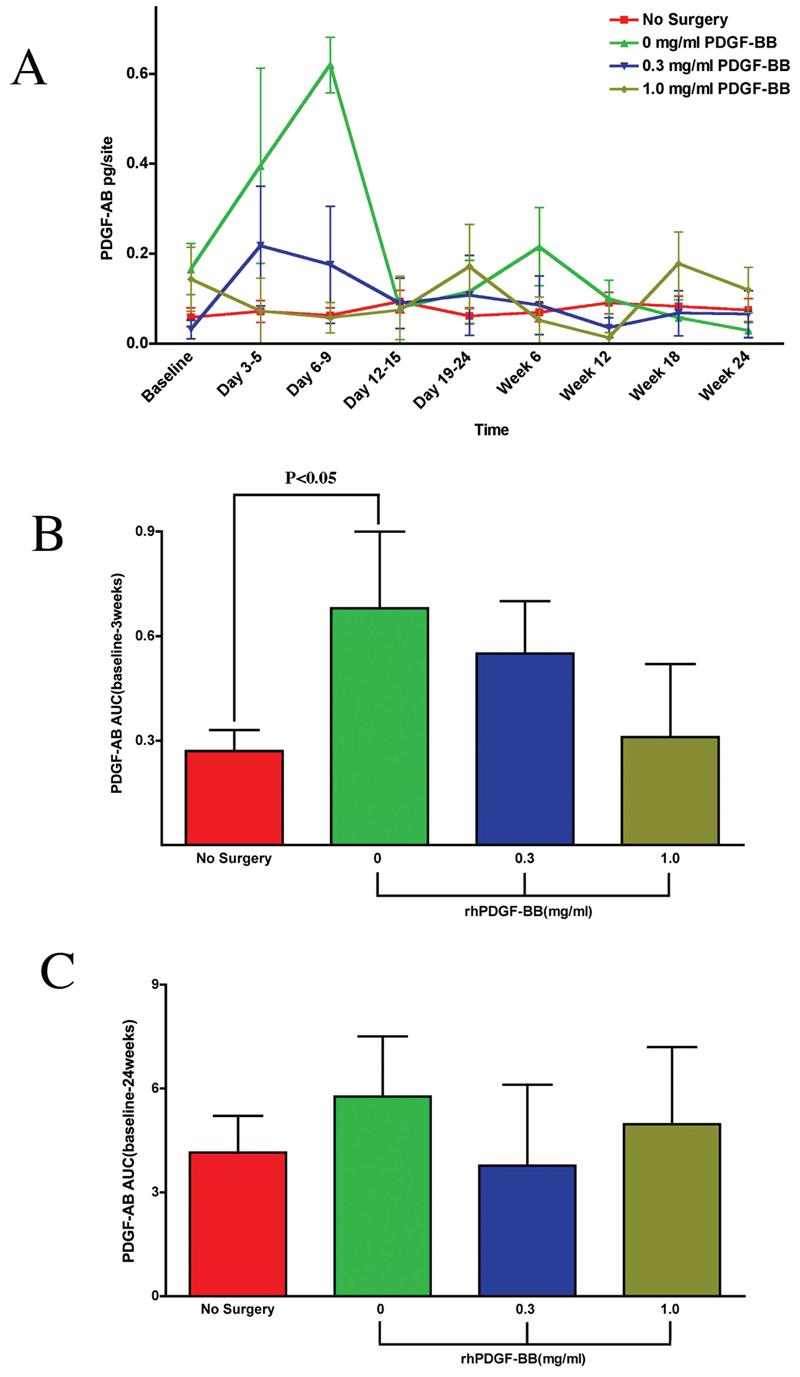
Effect of PDGF-BB-mediated tissue engineering of periodontal defects on local PDGF-AB release. (A) Graph of PDGF-AB release (pg/site) versus time point for all groups: β-TCP carrier alone, 0.3 mg/mL PDGF-BB, 1.0 mg/mL PDGF-BB, and the nonsurgical control. A statistically significant difference was observed between the β-TCP carrier alone group and the nonsurgical control at Days 3–5 (p < 0.04). The 0.3 mg/mL PDGF-BB group and the 1.0 mg/mL PDGF-BB group had similar levels of PDGF-AB released throughout the study. (B) Area under the curve (AUC) analysis for PDGF-AB up to 3 weeks. A statistically significant difference is observed only between the nonsurgical control and the β-TCP carrier alone group (p < 0.05). (C) Area under the curve (AUC) analysis for PDGF-AB release at all time points. Statistically significant differences were not observed when evaluating over 24 weeks for any groups. Statistical analysis was performed using ANOVA Fisher test at the 5% level. A total of 14 patients were analyzed for PDGF-AB (endogenous). Bars indicate standard error measurements (SEM).
Figure 4A is a graph of ICTP release (pg/site) over time for all groups. The nonsurgical control shows little change in the amount of ICTP released in the WF throughout the study. The 0.3 mg/mL PDGF-BB group and the 1.0 mg/mL PDGF-BB group had an increase in the amount of ICTP released at the Day 3–5 time point, with the average amount of ICTP released from the 0.3 mg/mL PDGF-BB group (335.6 pg/site) being more pronounced than the 1.0 mg/mL PDGF-BB group (161 pg/site), although not statistically significant. Figure 4B is an AUC analysis of ICTP over the first 3 weeks. The AUC for the surgical treatment groups was higher than the nonsurgical control, although not statistically significant. The 0.3 mg/mL PDGF-BB group had a higher AUC compared to the other treatment groups, but it was not statistically significant. Figure 4C is an AUC analysis of ICTP for all groups for 24 weeks. The AUC was similar for the 0.3 mg/mL PDGF-BB group and the 1.0 mg/mL PDGF-BB group, and was similar for the β-TCP carrier alone group and the nonsurgical control.
FIG. 4.
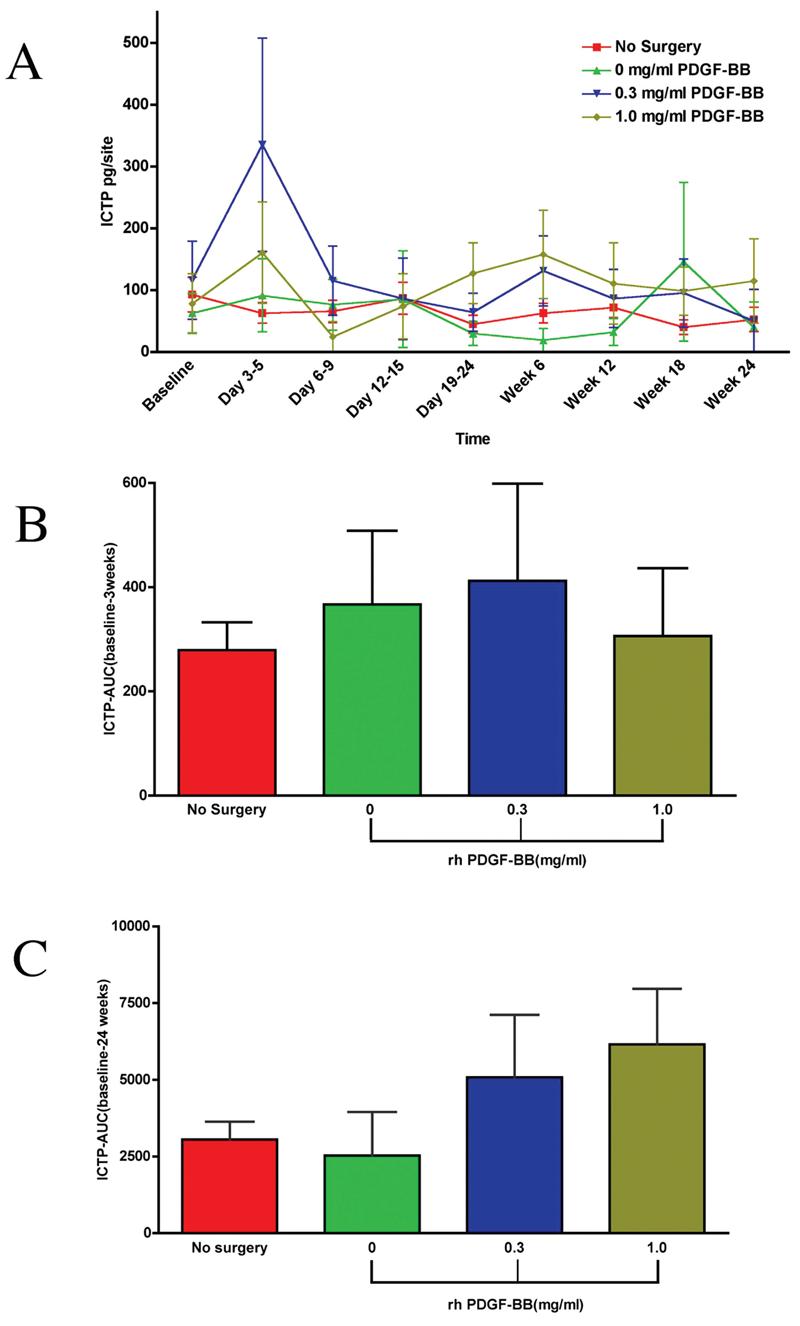
Effect of PDGF-mediated tissue engineering of periodontal defects on local pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) release. (A) Graph of ICTP (pg/site) versus time point for all groups: β-TCP carrier alone, 0.3 mg/mL PDGF-BB, 1.0 mg/mL PDGF-BB, and the nonsurgical control. Statistically significant differences were observed between the 0.3 mg/mL PDGF-BB group and the nonsurgical control (p < 0.003) and between the 0.3 mg/mL PDGFBB group and the β-TCP carrier alone group (p < 0.02) at the Day 3–5 timepoint. At 6 weeks, statistically significant differences were observed for the following groups: β-TCP carrier alone versus the 0.3 mg/mL PDGF-BB (p < 0.03), β-TCP carrier alone versus the 1.0 mg/mL PDGF-BB (p < 0.01), and the 1.0 mg/mL PDGF-BB versus nonsurgical control (p < 0.03). (B) Area under the curve (AUC) analysis for ICTP up to 3 weeks. No statistically significant differences were found between treatment groups. (C) AUC analysis for ICTP of all timepoints. No statistically significant differences were observed between any groups. Statistical analysis was performed using ANOVA Fisher test at the 5% level. A total of 14 patients were analyzed for ICTP. Bars indicate standard error measurements (SEM).
DISCUSSION
PDGF is a mitogen and chemoattractant for cells of mesenchymal origin including osteoblasts, and gingival and periodontal fibroblasts.32 The PDGF molecule consists of two disulfide-polypeptide chains that are encoded by two genes, pdgf-a and pdgf-b. Cells such as degranulating platelets, osteoblasts, smooth muscle fibroblasts, endothelial cells, macrophages, and keratinocytes produce PDGF.33 PDGF is an important stimulator of angiogenesis and wound healing in tissues such as skin, bone, and periodontium.33 It has been shown that in dermal wounds, the epidermis is a primary source of PDGF.34 Gingival epithelium may be a source of both PDGF protein and PDGF receptors, because both are induced at the wound site after injury and possibly regulate wound healing events.15
VEGF possesses strong mitogenic, angiogenic, and vascular permeability-enhancing activities. Angiogenesis is a major component of wound healing that begins by disrupting the continuity of pre-existing blood vessels. Endothelial cells migrate and proliferate from the area of the degraded basement membrane to form new capillaries. VEGF promotes angiogenesis by stimulating migration of endothelial cells through the extracellular matrix via a cascade of ligand/receptor interactions.35 In addition to its own receptor interactions, VEGF upregulates the expression of other surface molecules including intercellular adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1), which are molecules involved in cell–cell signaling.36 VEGF has also been recently assayed from the GCF derived from healthy and diseased periodontal patients.37 Higher levels of VEGF were found in chronically diseased sites than in healthy sites. In addition to its role in the regulation of angiogenesis, VEGF has been recently suggested to play an important role in the regulation of bone remodeling by attracting endothelial cells and osteoclasts, and stimulating osteoblast differentiation.38-41 The role of VEGF related to neovascularization in periodontal wounds has not been studied to date.
Yao et al.42 compared reepithelialization, contraction rates, and growth factor profiles in full-thickness porcine wounds. The results showed that endogenous VEGF concentrations peaked on day 4, reaching levels of 420 to 482 pg/mL. A comparison of PDGF-BB concentrations showed similar patterns (peaks of 77–91 pg/mL on days 2 and 3). On day 11, wound contraction in 2-month-old pigs was about 10% faster than in 24-month-old pigs (p < 0.05).42 Early healing events in periodontal and epidermal wounds can be compared and contrasted to identify those stages that are critical to the outcome of healing after periodontal regeneration.43,44
Pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) is a member of collagen degradative molecules that include pyridinoline, deoxypyridinoline, N-telopeptides, and C-telopeptides.23 Pyridinoline (Pyr) and deoxypyridinoline (Dpy) are nonreducible crosslinks present in the mature form of collagen.45 Pyr and Dpy form extracellularly and stabilize collagen molecules during maturation of the matrix of bone and cartilage.46 Before osteoclastic bone resorption and collagen matrix degradation, Pyr, Dpy, and amino-, and the carboxy-terminal crosslinked telopeptides of type I collagen are released into the tissue fluid. They are considered specific molecules found after the earliest bone resorption, and these molecules are not reused for new collagen synthesis.23 For this reason, pyridinoline cross-links have emerged as promising biomarkers of bone resorption and turnover. Recent studies have used ICTP levels to evaluate the response to periodontal therapy, and have demonstrated significant correlations between local ICTP levels and clinical measures of periodontal disease.46,47
The growth factors evaluated in this study are known to mediate and regulate cellular events during wound healing such as cell proliferation, chemotaxis, differentiation, and matrix synthesis.2-4,10 Specifically, this study evaluated the release of the growth factors PDGF-AB and VEGF as well as ICTP in the periodontal WF after a periodontal bone grafting procedure.
This study reveals contrasting inducible expression patterns of PDGF-AB, VEGF, and ICTP during periodontal wound healing in humans. Specifically, the levels of VEGF show increased levels in the surgical test sites for all test groups peaking at Days 12–15. The high-dose PDGF-BB (1.0 mg/mL) group promoted the highest levels of VEGF release over the 24-week observation period, although not significantly different from the other test groups. The use of an AUC analysis allows for a total estimate of mediator release over the course of the study rather than at specific time points. Using an AUC analysis, statistically significant differences were found for the total amount of VEGF released between the treatment versus nonsurgical groups over the first 3 weeks and at the 24-week time point. Of interest, several studies have demonstrated the coordinated effects of PDGF and VEGF on the promotion of vasculogenesis. PDGF promotes blood vessel smooth muscle pericyte formation, whereas VEGF promotes endothelial cell proliferation.8,9 Together, the two factors work to potently promote angiogenesis, a key determinant in stimulating bone regeneration. The result found here that periodontal injury induces VEGF release is suggestive that it may have promoted a greater clinical response in patients receiving rhPDGF-BB as shown in the pivotal clinical trial results.27
The level of PDGF-AB released from the WF between groups showed a statistically significant difference only at the Day 3–5 time point for β-TCP carrier alone versus nonsurgical control (p < 0.04). However, there was an increase in the amount of PDGF-AB released at Days 3–5 and 6–9 for the β-TCP carrier alone group. The AUC analysis for PDGF-AB release into PWF only showed a statistically significant difference over the first 3 weeks for the β-TCP carrier alone group versus the nonsurgical group (p < 0.05). No statistically significant differences were observed over the 24-week time frame. These data are suggestive that exogenous PDGF-BB does little to affect increases and tends to decrease endogenous release of PDGF-AB. This result could be explained by a feedback inhibition of PDGF expression due to bolus delivery of exogenous PDGF-BB. An expanded study would be necessary to better understand this potential mechanism.
The amount of ICTP released from the WF showed an apparent increase in the amount of ICTP released at Days 3–5 for the 0.3 mg/mL PDGF group that was statistically significant versus the β-TCP carrier alone group (p < 0.02) and nonsurgical control groups (p < 0.003). The AUC analysis for ICTP released into the WF did not show any statistically significant differences at the 3-week or 24-week time points. A recent report describes longer-term release of ICTP from periodontal wounds treated by PDGF.48
Final clinical outcomes from the multicenter parent study show a mean increase in linear bone growth of 2.6 mm for the 0.3 mg/mL PDGF-BB group, 1.5 mm for the 1.0 mg/mL PDGF group, and 0.9 mm for the β-TCP carrier alone group. Mean percent bone fill was reported to be 57% for the 0.3 mg/mL PDGF-BB group, 34% for the 1.0 mg/mL PDGF-BB group, and 18% for the β-TCP carrier alone group.27 Comparisons of both concentrations of rhPDGF-BB demonstrated statistically significant improvements compared to the β-TCP carrier alone group.
In conclusion, data from this study show that when PDGF-BB is delivered to promote periodontal tissue engineering of tooth-supporting osseous defects, there is a clinical and biological effect on growth factors released from the wound. VEGF is induced during wound repair, whereas exogenous PDGF-BB may reduce the release of endogenous PDGF-AB from the wound site after several days of healing. It also appears that there may be a marked increase in bone turnover during the first few days of wound healing when PDGF-BB is added to the osteoconductive scaffold, because the amount of ICTP release from the wound was increased for the 0.3 mg/mL PDGF-BB group. Future studies with expanded patient populations will be needed to better understand the effects of rhPDGF-BB on periodontal wound healing.
ACKNOWLEDGMENTS
This study was supported by NIH/NIDCR Grants T-35DE07101, R01-DE13397, and by BioMimetic Therapeutics, Inc. The authors thank Mary Gilson Layher and Sarah Webb for their technical assistance and contributions to the project.
REFERENCES
- 1.Williams RC. Periodontal disease. N. Engl. J. Med. 1990;322:373. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- 2.Heldin CH. Platelet-derived growth factor—an introduction. Cytokine Growth Factor Rev. 2004;15:195. doi: 10.1016/j.cytogfr.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim. Biophys. Acta. 1998;1378:F79. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 4.Rosenkranz S, Kazlauskas A. Evidence for distinct signaling properties and biological responses induced by the PDGF receptor alpha and beta subtypes. Growth Factors. 1999;16:201. doi: 10.3109/08977199909002130. [DOI] [PubMed] [Google Scholar]
- 5.Lynch SE, de Castilla GR, Williams RC, Kiritsy CP, Howell TH, Reddy MS, Antoniades HN. The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing. J. Periodontol. 1991;62:458. doi: 10.1902/jop.1991.62.7.458. [DOI] [PubMed] [Google Scholar]
- 6.Parkar MH, Kuru L, Giouzeli M, Olsen I. Expression of growth-factor receptors in normal and regenerating human periodontal cells. Arch. Oral Biol. 2001;46:275. doi: 10.1016/s0003-9969(00)00099-6. [DOI] [PubMed] [Google Scholar]
- 7.Giannobile WV, Whitson SW, Lynch SE. Non-coordinate control of bone formation displayed by growth factor combinations with IGF-I. J. Dental Res. 1997;76:1569. doi: 10.1177/00220345970760090901. [DOI] [PubMed] [Google Scholar]
- 8.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery [see comment] Nature Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 9.Rolny C, Lu L, Agren N, Nilsson I, Roe C, Webb GC, Welsh M. Shb promotes blood vessel formation in embryoid bodies by augmenting vascular endothelial growth factor receptor-2 and platelet-derived growth factor receptor-beta signaling. Exp. Cell Res. 2005;308:381. doi: 10.1016/j.yexcr.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol. Ther. 2004;9:519. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitlak BH, Finkelman RD, Hill EL, Li J, Martin B, Smith T, D'Andrea M, Antoniades HN, Lynch SE. The effect of systemically administered PDGF-BB on the rodent skeleton. J. Bone Miner. Res. 1996;11:238. doi: 10.1002/jbmr.5650110213. [DOI] [PubMed] [Google Scholar]
- 12.Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone. 1994;15:203. doi: 10.1016/8756-3282(94)90709-9. [DOI] [PubMed] [Google Scholar]
- 13.Lynch SE, Williams RC, Polson AM, Howell TH, Reddy MS, Zappa UE, Antoniades HN. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J. Clin. Periodontol. 1989;16:545. doi: 10.1111/j.1600-051x.1989.tb02334.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda N, Lin WL, Kumar NM, Cho MI, Genco RJ. Mitogenic, chemotactic, and synthetic responses of rat periodontal ligament fibroblastic cells to polypeptide growth factors in vitro. J. Periodontol. 1992;63:515. doi: 10.1902/jop.1992.63.6.515. [DOI] [PubMed] [Google Scholar]
- 15.Green RJ, Usui ML, Hart CE, Ammons WF, Narayanan AS. Immunolocalization of platelet-derived growth factor A and B chains and PDGF-alpha and beta receptors in human gingival wounds. J. Periodontal Res. 1997;32:209. doi: 10.1111/j.1600-0765.1997.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 16.Pinheiro ML, Feres-Filho EJ, Graves DT, Takiya CM, Elsas MI, Elsas PP, Luz RA. Quantification and localization of platelet-derived growth factor in gingiva of periodontitis patients. J. Periodontol. 2003;74:323. doi: 10.1902/jop.2003.74.3.323. [DOI] [PubMed] [Google Scholar]
- 17.Rutherford RB, Niekrash CE, Kennedy JE, Charette MF. Platelet-derived and insulin-like growth factors stimulate regeneration of periodontal attachment in monkeys. J. Periodontal Res. 1992;27:285. doi: 10.1111/j.1600-0765.1992.tb01679.x. [DOI] [PubMed] [Google Scholar]
- 18.Cho MI, Lin WL, Genco RJ. Platelet-derived growth factor-modulated guided tissue regenerative therapy. J. Periodontol. 1995;66:522. doi: 10.1902/jop.1995.66.6.522. [DOI] [PubMed] [Google Scholar]
- 19.Park JB, Matsuura M, Han KY, Norderyd O, Lin WL, Genco RJ, Cho MI. Periodontal regeneration in class III furcation defects of beagle dogs using guided tissue regenerative therapy with platelet-derived growth factor. J. Periodontol. 1995;66:462. doi: 10.1902/jop.1995.66.6.462. [DOI] [PubMed] [Google Scholar]
- 20.Giannobile WV, Hernandez RA, Finkelman RD, Ryan S, Kiritsy CP, D'Andrea M, Lynch SE. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J. Periodontal Res. 1996;31:301. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 21.Howell TH, Fiorellini JP, Paquette DW, Offenbacher S, Giannobile WV, Lynch SE. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J. Periodontol. 1997;68:1186. doi: 10.1902/jop.1997.68.12.1186. [DOI] [PubMed] [Google Scholar]
- 22.Embery G, Waddington RJ, Hall RC, Last KS. Connective tissue elements as diagnostic aids in periodontology. Periodontology. 2000;24:193. doi: 10.1034/j.1600-0757.2000.2240109.x. [DOI] [PubMed] [Google Scholar]
- 23.Giannobile WV, Al-Shammari KF, Sarment DP. Matrix molecules and growth factors as indicators of periodontal disease activity. Periodontology. 2003;31:125. doi: 10.1034/j.1600-0757.2003.03108.x. [DOI] [PubMed] [Google Scholar]
- 24.Goodson JM. Gingival crevice fluid flow. Periodontology. 2003;31:43. doi: 10.1034/j.1600-0757.2003.03104.x. [DOI] [PubMed] [Google Scholar]
- 25.Uitto VJ. Gingival crevice fluid—an introduction. Periodontology 2000. 2003;31:9. doi: 10.1034/j.1600-0757.2003.03101.x. [DOI] [PubMed] [Google Scholar]
- 26.Alfano MC. The origin of gingival fluid. J. Theor. Biol. 1974;47:127. doi: 10.1016/0022-5193(74)90103-9. [DOI] [PubMed] [Google Scholar]
- 27.Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, McAllister BS, Murphy KS, McClain PK, Nevins ML, Paquette DW, Han TH, Reddy MS, Lavin PT, Genco RJ, Lynch SE. Platelet-derived growth factor (rhPDGF-BB) stimulates bone fill and rate of attachment level gain: Results of a large multi-center randomized controlled trial. J. Periodontol. 2005;76:2205. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- 28.Lobene RR, Weatherford T, Ross NM, Lamm RA, Menaker L. A modified gingival index for use in clinical trials. Clin. Prev. Dent. 1986;8:3. [PubMed] [Google Scholar]
- 29.Giannobile WV, Lynch SE, Denmark RG, Paquette DW, Fiorellini JP, Williams RC. Crevicular fluid osteocalcin and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) as markers of rapid bone turnover in periodontitis. A pilot study in beagle dogs. J. Clin. Periodontol. 1995;22:903. doi: 10.1111/j.1600-051x.1995.tb01793.x. [DOI] [PubMed] [Google Scholar]
- 30.Risteli J, Elomaa I, Niemi S, Novamo A, Risteli L. Radioimmunoassay for the pyridinoline cross-linked carboxyterminal telopeptide of type I collagen: a new serum marker of bone collagen degradation. Clin. Chem. 1993;39:635. [PubMed] [Google Scholar]
- 31.Payne JB, Johnson GK, Reinhardt RA, Dyer JK, Maze CA, Dunning DG. Nicotine effects on PGE2 and IL-1 beta release by LPS-treated human monocytes. J. Periodontal Res. 1996;31:99. doi: 10.1111/j.1600-0765.1996.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 32.Anderson TJ, Lapp CA, Billman MA, Schuster GS. Effects of transforming growth factor-beta and platelet-derived growth factor on human gingival fibroblasts grown in serum-containing and serum-free medium. J. Clin. Periodontol. 1998;25:48. doi: 10.1111/j.1600-051x.1998.tb02363.x. [DOI] [PubMed] [Google Scholar]
- 33.Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996;19:23S. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 34.Antoniades HN, Galanopoulos T, Neville-Golden J, Kiritsy CP, Lynch SE. Injury induces in vivo expression of platelet-derived growth factor (PDGF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts. Proc. Natl. Acad. Sci. U S A. 1991;88:565. doi: 10.1073/pnas.88.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taub PJ, Silver L, Weinberg H. Plastic surgical perspectives on vascular endothelial growth factor as gene therapy for angiogenesis. Plast. Reconstr. Surg. 2000;105:1034. doi: 10.1097/00006534-200003000-00031. [DOI] [PubMed] [Google Scholar]
- 36.Melder RJ, Koenig GC, Witwer BP, Safabakhsh N, Munn LL, Jain RK. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat. Med. 1996;2:992. doi: 10.1038/nm0996-992. [DOI] [PubMed] [Google Scholar]
- 37.Booth V, Young S, Cruchley A, Taichman NS, Paleolog E. Vascular endothelial growth factor in human periodontal disease. J. Periodontal Res. 1998;33:491. doi: 10.1111/j.1600-0765.1998.tb02349.x. [DOI] [PubMed] [Google Scholar]
- 38.Deckers MM, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, Lowik CW. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. 2000;141:1667. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- 39.Ferraro JW. Experimental evaluation of ceramic calcium phosphate as a substitute for bone grafts. Plast. Reconstr. Surg. 1979;63:634. doi: 10.1097/00006534-197905000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Johnson RB, Serio FG, Dai X. Vascular endothelial growth factors and progression of periodontal diseases. J. Periodontol. 1999;70:848. doi: 10.1902/jop.1999.70.8.848. [DOI] [PubMed] [Google Scholar]
- 41.Bouletreau PJ, Warren SM, Spector JA, Peled ZM, Gerrets RP, Greenwald JA, Longaker MT. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast. Reconstr. Surg. 2002;109:2384. doi: 10.1097/00006534-200206000-00033. [DOI] [PubMed] [Google Scholar]
- 42.Yao F, Visovatti S, Johnson CS, Chen M, Slama J, Wenger A, Eriksson E. Age and growth factors in porcine full-thickness wound healing. Wound Repair Regen. 2001;9:371. doi: 10.1046/j.1524-475x.2001.00371.x. [DOI] [PubMed] [Google Scholar]
- 43.Wikesjo UM, Nilveus RE, Selvig KA. Significance of early healing events on periodontal repair: a review. J. Periodontol. 1992;63:158. doi: 10.1902/jop.1992.63.3.158. [DOI] [PubMed] [Google Scholar]
- 44.Wikesjo UM, Selvig KA. Periodontal wound healing and regeneration. Periodontology. 1999;19:21. doi: 10.1111/j.1600-0757.1999.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 45.Talonpoika JT, Hamalainen MM. Type I collagen carboxyterminal telopeptide in human gingival crevicular fluid in different clinical conditions and after periodontal treatment. J. Clin. Periodontol. 1994;21:320. doi: 10.1111/j.1600-051x.1994.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 46.Golub LM, Lee HM, Greenwald RA, Ryan ME, Sorsa T, Salo T, Giannobile WV. A matrix metal-loproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm. Res. 1997;46:310. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]
- 47.Al-Shammari KF, Giannobile WV, Aldredge WA, Iacono VJ, Eber RM, Wang HL, Oringer RJ. Effect of non-surgical periodontal therapy on C-telopeptide pyridinoline cross-links (ICTP) and interleukin-1 levels. J. Periodontol. 2001;72:1045. doi: 10.1902/jop.2001.72.8.1045. [DOI] [PubMed] [Google Scholar]
- 48.Sarment DP, Cooke JW, Miller SE, Jin Q, McGuire MK, Kao RT, McClain PK, McAllister BS, Lynch SE, Giannobile WV. Effect of rhPDGF-BB on bone turnover following periodontal repair. J. Clin. Periodontol. 2006;33:135. doi: 10.1111/j.1600-051X.2005.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]