Abstract
Background and Purpose
Exercise and rehabilitative training each have been implicated in the promotion of restorative neural plasticity after cerebral injury. Because motor skill training induces synaptic plasticity and exercise increases plasticity-related proteins, we asked if exercise could improve the efficacy of training on a skilled motor task after focal cortical lesions.
Methods
Female young and middle-aged rats were trained on the single-pellet retrieval task and received unilateral ischemic sensorimotor cortex lesions contralateral to the trained limb. Rats then received both, either, or neither voluntary running and/or rehabilitative training for 5 weeks beginning 5 days postlesion. Motor skill training consisted of daily practice of the impaired forelimb in a tray-reaching task. Exercised rats had free access to running wheels for 6 h/day. Reaching function was periodically probed using the single-pellet retrieval task.
Results
In young adults, motor skill training significantly enhanced skilled reaching recovery compared to controls. However, exercise did not significantly enhance performance when administered alone or in combination with skill training. There was also no major benefit of exercise in older rats. Additionally, there were no effects of exercise in a measure of coordinated forelimb placement (the foot-fault test) or in immunocytochemical measures of several plasticity-related proteins in the motor cortex.
Conclusions
In young and middle-aged animals, exercise did not improve motor skill training efficacy following ischemic lesions. Practicing motor skills more effectively improved recovery of these skills than did exercise. It remains possible that an alternative manner of administering exercise would be more effective.
Keywords: Exercise, Rehabilitation, Skilled reaching, Experience-dependent plasticity
Behavioral experience can enhance function after brain injury. For example, rats exposed to complex housing environments pre- and/or postinjury typically have improved functional outcome compared to animals in standard housing.1,2 Another source of activity-dependent plasticity is general exercise such as wheel running, which influences neuronal circuitry of brain and spinal cord when initiated pre- and postinjury (reviewed in3). In both young and aged intact animals, exercise increases synaptic plasticity-related proteins within the hippocampus and enhances cognitive function, as measured using hippocampal-dependent tasks, including the Morris water maze.4,5 In models of injury, exercise is beneficial for increasing axonal regeneration in sensory neurons,6 restoring plasticity-related proteins after spinal cord injury,7,8 and protecting from damage caused by cerebral ischemia.9
Although exercise can be beneficial, learning motor skills results in neuronal structural and functional plasticity in the motor cortex and cerebellum that is not found with simple exercise or repetition of previously learned skills.10-12 For example, intact rats trained on a skilled reaching task exhibited a significant increase in the representation area of the wrist and digits detected using intracortical microstimulation mapping, compared to animals trained on an unskilled, bar-pressing task.13 Similarly, in the cerebellum, acrobatic training, but not voluntary or forced exercise, induced synaptogenesis.11,14 Thus, learning may more effectively induce activity-dependent plasticity than mere use, at least in some of the brain regions that mediate motor movements. After brain damage, motor skill training has also been found to drive apparently adaptive neural plasticity. For example, training monkeys or rats on skilled reaching tasks after focal lesions of the sensorimotor cortex (SMC) prevents the further loss and promotes functional reorganization of movement representations,15-17 suggesting that mechanisms of plasticity involved in learning a new task in intact animals also play a role in maintenance and reorganization of remaining cortex postinjury.
This study focuses on the possibility that exercise could be used as a positive modulator of motor skill “re-learning” after brain damage. Training animals in skilled reaching after unilateral cerebral infarcts has been found to improve function in the injury-impaired forelimb,18-20 and therefore we refer to such training as “rehabilitative.” However, skill training is often insufficient to return animals to preinjury levels of performance. This study examined whether general exercise could augment the efficacy of rehabilitative training on a skilled motor task after a unilateral focal cortical lesion. After acquisition of the single-pellet retrieval task, groups of young adult female rats received unilateral ischemic damage to the forelimb representation region of the SMC. The animals then received rehabilitative training on a tray-reaching task, voluntary exercise on a running wheel, or a combination of the two. We expanded this study to include middle-aged female rats undergoing rehabilitation to test whether exercise would be more beneficial in older, presumably less spontaneously active, animals. We also investigated the possible effects of exercise and motor training on immunohistochemical measures of the plasticity-related proteins, brain-derived neurotrophic factor (BDNF), spinophillin, microtubule associated protein-2 (MAP-2), and N-methyl-D-aspartate receptor subunit 1 (NMDA-1), in the perilesion tissue because of previous studies indicating that exercise or postoperative experience can increase these proteins.5,6,18,21,22
Materials and Methods
Subjects
Twenty-eight 4-month-old and twelve 1- to 1.6-year-old female Long-Evans hooded rats that were bred at the University of Texas at Austin were pair housed and kept on a 12/12 h light/dark cycle with water access ad libitum. Animals were frequently handled 2 to 3 weeks prior to the experiment, and all behavioral procedures were carried out in the same room in which the animals were housed. Prior to the start of behavioral methods, animals were placed on scheduled feeding of 15 g of rat chow given once per day (to ensure rats were not sated at the time of training).
Weights were monitored throughout the study, and, within ages, there were no group differences in weight change over the course of the experiment. In young females, the mean ± SEM starting and end weights (g) were 278.41 ± 4.7 and 294.8 ± 6.1, respectively. In the middle-aged females, starting and end weights were 419.1 ± 17.4 and 339.2 ± 9.9. Due to studies finding an influence of estrogen on dendritic outgrowth23 and injury volume,24 rats in this study were monitored for their estrous state throughout the experimental procedure using cytological characterization of vaginal smears (as described in22). The effects of estrous stage at the time of the twice-weekly probe trials could not be adequately assessed due to an insufficient sampling of estrous stages within subjects over days. Previous studies have found that moderate food deprivation has no effect on the estrous cycle.25 Animals were randomly placed into different groups matched for preoperative reaching performance. Young rats (4 months) were divided into exercise and rehabilitation (Rehab+Run, n = 8), exercise alone (Cont+Run, n = 7), rehabilitation alone (Rehab+Cont, n = 7), and neither manipulation (Cont+Cont, n = 6). Aged rats (1-1.6 years) were divided into groups, matched for age, receiving either exercise and rehabilitation (Rehab+Run, n = 6) or rehabilitation alone (Rehab+Cont, n = 6).
Surgery
All animals were administered a focal unilateral lesion aimed at the forelimb representation area of the SMC contralateral to the preferred limb. Animals were anesthetized using intraperitoneal (i.p.) injections of Equithesin (140 mg/kg chloral hydrate and 35 mg/kg sodium pentobarbital) and placed in a stereotaxic apparatus. After a midsagittal incision, a craniectomy was made between 1.5 mm anterior, 0.5 mm posterior, and 3.0 to 4.5 mm lateral to Bregma. Pia mater was exposed by removal of dura in the area underlying the craniectomy. Endothelin-1 (ET-1, 80 μM, 0.2 μg/μl; 4 μl total volume administered), a vasospasm-inducing peptide, was then topically administered with a Hamilton microsyringe and left undisturbed for 10 minutes before the skin was sutured, as described previously.26,27 Atropine (0.25 mg/kg) was administered i.p. to counter the respiratory depressive effects of Equithesin. Rats were allowed 4 days of recovery before postoperative behavioral manipulations began.
Training and Testing on the Single-Pellet Retrieval Task
The single-pellet retrieval task (Figure 1a) was used as a sensitive measure of deficits and recovery in skilled forelimb reaching behavior, and this required that rats be proficient in this task prior to the infarct. Training was conducted in a Plexiglas reaching chamber (length: 26 cm, height: 34 cm, width: 16 cm) consisting of a 1 cm by 23 cm window in the center of one 16 cm wide wall. Animals were first acclimatized to the chamber as previously described22 with 4.5 mg of banana-flavored food pellets (Bioserve, Frenchtown, NJ) placed on the floor. Rats have been found to have a limb preference when performing skilled tasks,28,29 and this preference was determined in all animals by shaping them to the task in 10-minute sessions administered for 2 to 10 days. Animals were first allowed to reach with either forelimb for centrally placed food pellets on a 3 cm high reaching shelf. When 20 consecutive reach attempts were made with one limb during a 10-minute session, this limb was identified as the preferred limb. For subsequent days of preoperative training, a wall was placed 0.5 cm from the window within the chamber and ipsilateral to the preferred limb. This enabled the animal to reach with only the preferred limb for pellets placed, one at a time, in a shallow well located contralateral to this limb at a distance of 1 cm from the reaching window.
Figure 1.

Rats were trained to proficiency on the single-pellet retrieval task preoperatively, and this test was used to probe reaching function after the ischemic lesions (a). The tray-reaching task was used for rehabilitative training (b). Exercised rats (c) had access to a running wheels (via a chicken wire tube) for 6 h/day, whereas no-exercise controls were given access to a novel metal box (d).
Reaching success was chosen as the primary outcome measure because it is a highly sensitive measure of recovery of skilled motor function in rats.18,22,30,31 A successful reach occurred when the animal extended its forelimb through the window, grasped the pellet, moved the pellet to its mouth, and ate it. If the pellet was displaced from the well or if the animal dropped the pellet before consuming it, the trial was recorded as a “miss” or “drop,” respectively. In the presence of a pellet on the shelf, each extension of the trained limb through the reaching window was considered a reach attempt. Performance was measured as the total number of successes divided by the total number of reach attempts during a session. Young animals were trained preoperatively until they achieved a criterion of 30% successful retrievals per session. Older animals (which we had not previously investigated in our lab using this task) were preoperatively trained to a higher criterion (50% success) to ensure sensitivity in detecting postoperative deficits and recovery patterns. To ensure equivalency in the recency of training experience, all rats also received a “refresher” session of training on the day prior to surgery.
Postoperative Training and Behavioral Measures
Postoperative training consisted of exercise, rehabilitation, or both that was initiated on postoperative day 5 and continued for 5 weeks. The delay in onset of the manipulations was to minimize possible influences of the behavioral manipulations on lesion size, which have been found in previous studies.5,32 Probe trials of reaching performance on the single-pellet retrieval task were performed after every fifth day of the rehabilitative training period for 2 successive days. Middle-aged animals received one additional probe trial 4 days after surgery. On probe trial days, animals did not receive rehabilitation, but the regular exercise regimen was provided every day of the 5 weeks. All reaching tasks were performed during the animal's light period. Reaching was then followed (within 3 hours) with voluntary access to a running wheel or novel cage. Access was initiated with 3 hours remaining in the light period and continuing for 3 hours of the dark cycle (total of 6 hours).
Rehabilitative Training: Tray-Reaching Task
The tray-reaching task (Figure 1b, adapted from20,33) was used for motor rehabilitative training. This task differs from the single-pellet retrieval task in that a greater variety of reaching distances and trajectories is required and grasping more than one pellet at a time is permitted. Animals receiving rehabilitation (Rehab+Run and Rehab+Cont) were allowed to reach for 20 minutes for ∼100 food pellets (4.5 g of 45 mg pellets) placed on an inclined tray. Young animals received an inclined tray made from 3 glass microscope slides (2.5 cm wide × 7.5 cm long) constructed into a trough with a 25-degree incline toward the reaching window. The tray was elevated so that the height of the lower point of the trough was 3 cm from the table top. Aged animals received a tray made from an aluminum block constructed to the same specifications of the glass tray. A plastic lip was attached to the base of the incline to prevent the pellets from rolling into the reaching chamber. A wall was placed ipsilateral to the impaired forelimb (the preoperatively preferred limb) to force the animals to reach with this limb. No-rehab control groups (Cont+Run and Cont+Cont) were placed in identical chambers for 20 minutes but received 4.5 g of pellets on the floor of the chamber. The experimenter recorded the number of pellets consumed by each animal.
Exercise: Running Wheel
Each day, exercised animals (Rehab+Run and Cont+Run) were individually placed for a 6-hour period (3 hours each in the light and dark cycles) in cages that were connected via wire-mesh tubes to metal running wheels (Figure 1c). The distance run in kilometers (calculated by a cycling computer, CatEye Enduro 8, attached to each running wheel) was recorded at the end of every 6-hour period. No-exercise controls (Rehab+Cont and Cont+Cont) were individually placed in cages for 6 hours (3:3 hours, light:dark) that were identical to those used for the exercised groups except that they had wire-mesh tubes connected to a novel metal box rather than the running wheels (Figure 1d). All animals had access to food (15 g) and water (ad libitum) during the 6 hours in the individual cages. After each session, rats were returned to their home cage with their cage-mate.
Coordinated Forelimb Use During Locomotion
Rats were administered the foot-fault test to measure coordinated forelimb placement during locomotor movements.34 Animals were placed on an elevated grid platform (33 cm × 30 cm; grid openings: 8.4 and 6.25 cm2) and were videotaped exploring the surface for 2 minutes. As rats moved across the platform, placing their paws on the rungs of the grid openings, the experimenter counted the number of slips with either forelimb through the grid openings. Performance was evaluated as the number of errors (“foot faults”) that were executed in ratio to the total number of steps accomplished in the same time period during slow-motion video playback. Following unilateral lesions, the forelimb opposite the lesion frequently has an increase in the number of foot faults through the grid openings.35,36 Animals were administered the foot-fault test preoperatively and 5 to 6 time points postoperatively using an apparatus and testing protocol that has previously revealed significant deficits in male rats with similar lesions.26,31
Histology
Six weeks postlesion, all animals were given a lethal dose of sodium pentobarbital and transcardially perfused with 0.1 M sodium phosphate buffer (PB) followed by 4% paraformaldehyde in PB. Brains were isolated, placed in 4% paraformaldehyde solution, and stored at 4°C for 2 weeks. Six rostral to caudal sets of 50 μm thick coronal sections throughout the cerebrum were then made using a vibratome. One set was stained with toluidine blue to be used for reconstructions of the lesions, estimation of the volume of remaining cortex, and perilesion neuron counts. Adjacent sets were utilized for immunocytochemistry of plasticity-related proteins.
Analyses of Cortical Damage
Lesion size was indirectly estimated by measures of remaining cortical volume in the SMC region. The perimeter of 50 μm thick Nissl-stained coronal sections, 600 μm apart, was traced using Neurolucida software (Microbrightfield Inc, Williston, VT) at a final magnification of ×17. The appearance of the corpus callosum was used as a landmark for the most rostral section to be traced (approximately 2.7 mm anterior to bregma). The area of nonnecrotic/nongliotic cortex was measured in the next 5 sections, moving caudally (ie, the caudal-most section was approximately 0.3 mm posterior to bregma). Volume was then estimated using the Cavalieri method37 as the product of summed area and the distance between section planes (600 μm). To visualize the extent of each lesion, they were reconstructed onto templates of cortical coronal sections (adapted from38). For each group, reconstructions were overlaid to determine the representative and common regions of all lesions.
Given that neurons in peri-infarct tissue are vulnerable to use-dependent excitotoxicity,39 we also assessed neuronal density in this region. For each brain, 3 sections each had 2 samples (1 medial, 1 lateral to the lesion) in each of layers II/III and layer V analyzed (total of 12 samples per brain per layer). Necrotic/gliotic tissue directly adjacent to the lesion was excluded. All samples were observed at 100× oil objective with a final magnification of ×1680. The optical dissector method and an unbiased sampling frame were utilized in obtaining the number of neurons per unit volume in an unbiased fashion40 using Neurolucida software (Microbrightfield Inc). Neuron number per unit volume was calculated as the total neurons counted divided by the volume sampled (sample number × sample frame area × section thickness).
Immunocytochemistry for Plasticity-Related Proteins
We investigated expression of plasticity-related proteins BDNF and NMDAR-1, as well as the dendritic proteins spinophilin (a spine-enriched protein) and MAP-2 (a dendritic cytoskeletal protein), in young adults and BDNF in both young and older rats in layers II/III and layer V of remaining SMC near the lesion. BDNF optical density was also analyzed in hippocampal subregions. A free-floating immunocytochemistry method similar to that previously described22,26 was utilized for anti-NMDAR-1 (1:200; Chemicon, Temecula, CA), MAP-2 (1:500; clone AP-20, Sigma, St Louis, MO), spinophilin (1:500; Upstate Cell Signaling Solutions, Charlottesville, VA), and BDNF (1:1000; AB1779, Chemicon) using the avidin-biotinalyted complex and 3-3′ diaminobenzidine tetrahydrochloride visualization method. Immunoreactivity (IR) was analyzed using the cycloid grid intersection method (MAP-2; described in22) optical densitometry (NMDAR-1, BDNF, and spinophilin, described in31), and semiautomated puncta quantification using an Image J program (WCIF 1.34p; spinophilin).
Statistical Analyses
To analyze reaching performance across probe days, 3-way analysis of variance (ANOVA) for Exercise condition by Rehabilitative training condition by Day was performed using the SPSS program (SPSS Inc, Chicago, IL). Young and middle-aged rats, which were tested in separate experiments conducted at different times, were analyzed separately. To further analyze reaching performance between groups, simple mean comparisons or 1-way ANOVAs were utilized. One-way ANOVAs were also used to examine the effect of estrous stage at time of surgery on remaining cortical volume as well as the effects of treatment group on remaining cortical volume. To investigate the relationships between average distance run, reaching performance, and remaining cortical volume, bivariate correlations were employed.
Results
Lesion Extent
Young Adults
Lesion reconstructions revealed that the damage consistently involved the forelimb representational region of the SMC defined by cytoarchitecture (Figure 2). The remaining cortical volume in the SMC region was not significantly different between groups. The average volume (means ± SEM in mm3) was 71.8 ± 1.6 in the Rehab+Run, 71.7 ± 2.5 in the Cont+Run, 73.8 ± 2.2 in the Rehab+Cont, and 70.0 ± 2.8 in the Cont+Cont group (P > .5; F[3, 27] = .43).
Figure 2.

Ischemic sensorimotor cortex lesions. Schematic representations of the damage common to all lesions, the range, and a representative lesion within each group. Numbers to the right indicate the distance from bregma of the coronal sections.
When animals were separated by estrous stage at the time of surgery, a significant difference was found in remaining cortical volume (P < .05, F[2, 27] = 3.4). As shown in Figure 3a, animals in proestrus (Rehab+Run: n = 4, Cont+Run: n = 3, Rehab+Cont: n = 3, Cont+Cont: n = 3) were found to have significantly more cortical volume remaining than animals in either diestrus (Rehab+Run: n = 2, Cont+Run: n = 3, Rehab+Cont: n = 4, Cont+Cont: n = 2) or estrus stage (Rehab+Run: n = 2, Cont+Run: n = 1, Rehab+Cont: n = 0, Cont+Cont: n = 1). Neuronal densities assessed in the perilesion cortex were found to not be significantly different between groups in either layer II/III or layer V. In comparing remaining cortical volume and reaching performance (averaged over the 5 weeks of testing), no significant correlation was found, but it should also be noted that the correlation is limited by the restricted range of lesion sizes and the differential effects of training. With a median split of remaining cortical volume, reaching performance averaged 25.2% ± 2.4% versus 28.6% ± 2.4% in rats with smaller versus larger lesions, respectively. Power analysis indicates that 222 rats would be needed to detect a significant difference (P < .05) in these lesion size–dependent effects at 80% power.
Figure 3.

Estrous stage effects at the time of lesion induction in young adults. Rats in proestrus (when estrogen and progesterone are at their peak) had (a) significantly more remaining cortical volume in the sensorimotor cortex region and (b) significantly fewer errors on the foot-fault test (a measure of coordinated forelimb use during locomotion) 10 days postinfarct, compared to animals in diestrus and estrous. Estrous stage distributions were approximately equal among experimental groups (see text for details). Most of the older rats were in anestrus at the time of lesion induction. Data are means ± SEM. *P < .05 compared with proestrus.
Middle-Aged Animals
As with the younger rats, lesions were consistently placed in the forelimb representation region of the SMC (Figure 2). No significant differences in cortical volume remaining between groups or significant correlation between cortical volume remaining and reaching performance were found. Average remaining volumes were (means ± SEM in mm3) 77.9 ± 2.9 in the Rehab+Run and 79.2 ± 5.1 in the Rehab+Cont. Vaginal smears of these animals at the time of surgery revealed that most (n = 10/12) of the subjects were in anestrus, an indication of cessation of the rat's reproductive cycle. No significant difference in neuronal density in the perilesion cortex was found.
Exercise: Voluntary Wheel Running
Young Adults
Over days, both groups increased the distance run (P < .001, F[34, 442] = 8.1; Figure 4a). When pooled across the first 3 days of running, animals averaged 0.86 km, and by the last 3 days averaged 6.7 km distance run per day. No differences occurred between groups in the distance run (P > .05, F[1, 13] = 2.1) or in group-by-day interaction (P > .05, F[34, 442] = 0.84). The cumulative distance run for groups Rehab+Run and Cont+Run was 177.8 ± 28.3 and 124.29 ± 22.7 mean ± SEM km. No relationship was found between the cumulative distance run across 5 weeks and performance on the reaching probe trials (R = .115, P > .05). However, a significant positive correlation was present between the cumulative distance run and the remaining cortical volume in the SMC region (R = .6, P < .05). Rats with larger SMC lesions tended to run less.
Figure 4.
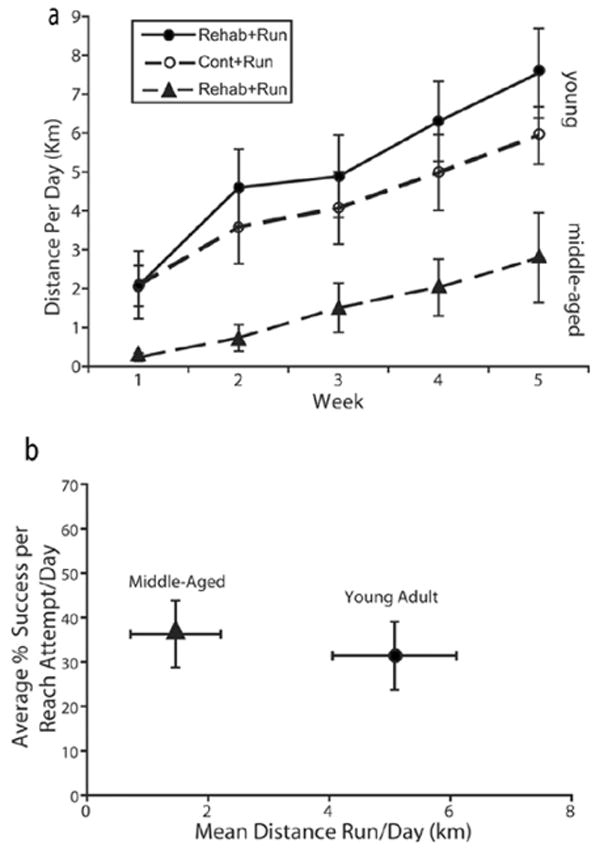
Voluntary exercise. By the fifth week, both young adults and middle-aged adults increased the distance run compared to the first week (a). Although the average distance run in the middle-aged animals was less than the young adult rats, the performance in the skilled reach task averaged across all 5 weeks was similar (b). There were no significant correlations between distance run and reaching task performance (see text for details).
Middle-Aged Animals
Like the younger rats, middle-aged rats significantly increased in the distance run over days (P < .05, F[1, 5] = 4.04; Figure 4a), but they ran less than younger rats (Figure 4b). The average distance run in the first 3 days was 0.18 km, and by the last 3 days of the study, animals averaged 3.1 km. There was no correlation between cumulative distance run over 5 weeks and the reaching performance on the probe trials. In addition, no correlation was found for cumulative distance run and the remaining cortical volume in the SMC region.
Rehabilitation: Tray-Reaching Task
Young Animals
After the first day, all rats retrieved most or all pellets (∼100 pellets; 4.5 g) with the impaired forelimb with no difference between groups (Rehab+Run and Rehab+Cont) in the average weight of pellets consumed during each day of the tray-reaching task. Averaged across groups, rats consumed 3.5 ± 1.1 g in the first 3 days of rehabilitation and 4.5 ± 0.1 g in the last 3 days.
Middle-Aged Animals
Although there was no difference between Rehab+Run and Rehab+Cont groups in the performance of the tray-reaching task, rats did show marked improvement of the task by having an increase in the amount of pellets consumed across the days of the study. Rats ate 1.5 ± 0.7 g in the first 3 days of training, and this gradually improved to 3.5 ± 1.0 g in the last 3 days of rehabilitation.
Skilled Reaching Performance
Young Adults
Twice-weekly probe trials of reaching performance on the single-pellet retrieval task were analyzed with a 3-way ANOVA for Rehab condition by Exercise condition by Day of testing. As shown in Figure 5a, rehabilitative training on the tray-reaching task had a major facilitatory effect on recovery of skilled reaching performance as measured using the single-pellet retrieval task. There was a significant main effect of Rehab condition (P < .001 F[1, 24] = 49.2) as well as a Rehab-by-Day effect (P < .05, F[1, 24] = 2.8). Post hoc comparisons revealed that the Rehab (Rehab+Run and Rehab+Cont) groups performed significantly better on the probe trials than the No-Rehab groups (Cont+Run and Cont+Cont) beginning in week 2 and thereafter. Exercise did not significantly affect performance in reaching probe trials or further enhance the effects of rehabilitative training on reaching performance. No main (P > .05 F[1, 24] = 0.67) or interaction effects of exercise condition (exercise by day: P > .05 F[9, 16] = 0.34, exercise by rehab: P > .05 F[1, 24] = 2.51, exercise by day by rehab: P > .05 F[9, 16] = 0.54) were found. When animals were separated by estrous stage at time of lesion application, there was no significant difference in reaching performance on the first probe days (postoperative days 9 and 10). Pooled across groups, on these days, the average percent success per reach attempt was 19.5 ± 3.4, 20.8 ± 2.9, and 17.1 ± 3.8 for rats in diestrus, proestrus, and estrus at the time of surgery.
Figure 5.

Single-pellet retrieval task. Young adult rats (a) receiving rehabilitation on the tray-reaching task had a significant improvement in reaching performance in probe trials after lesions, whereas there were no significant main or interaction effects of exercise. In middle-aged animals (b), exercise influenced the pattern of recovery, but not the overall outcome (note that middle-aged animals were trained to a higher preoperative criterion than younger rats and received an additional probe trial prior to rehabilitation training onset). Data are means ± SEM. *P < .05 and **P < .005 rehab significantly different versus no-rehab.
Middle-Aged Animals
In repeated-measures ANOVA, no overall group effect was present, but a group-by-day effect was present (P < .001, F[1, 10] = 3.5). Rehab+Run performed significantly worse in the first week, significantly better in the second week, and was not significantly different from Rehab for the remainder of the study compared with Rehab+Cont (Figure 5b). Thus, the pattern of recovery was significantly different between groups, but there was no consistent improvement in performance as a result of exercise.
Foot-Fault Test Performance
Young Animals
Compared to preoperative performance, no consistent mean decline in performance on the foot-fault task occurred, as first assessed 10 days after the ischemic lesions. (This is in contrast to effects in male rats with similarly sized lesions,26,31 and this task is potentially less challenging for the lighter weight female rats of this age.) When the animals were separated according to estrous stage at the time of surgery, however, a 1-way ANOVA revealed an overall significant difference in the number of errors per stage (Figure 3b). Animals in proestrus at the time of surgery made significantly fewer errors compared to those in diestrus (P < .05, F[1, 23] = 5.13) and those in estrous (P < .05, F[1, 16] = 6.24). Furthermore, the diestrus and estrus, but not proestrus, subgroups had a significant postoperative impairment in foot-fault test performance (t = −2.5, P < .025 prelesion versus day 10). No significant effects of exercise or training condition on this measure occurred when all animals were included in the analysis or when the proestrus-at-lesion subgroup of rats were excluded from the analysis. There were no significant differences between the diestrus and estrus subgroups.
Middle-Aged Animals
In middle-aged rats, lesions resulted in an increased error rate on the foot-fault task. We found no significant effect of exercise on the recovery pattern (Figure 6). A repeated-measures ANOVA revealed no significant difference between Rehab+Run and Rehab+Cont groups, but a significant effect of day was present, reflecting the improvement of performance during the 5 weeks of testing.
Figure 6.

Foot-fault task. Middle-aged animals were found to have a significant increase of errors in the task 4 days after ischemic injury (post-op). However, after 5 weeks, animals were capable of completing the task with fewer errors. There were no significant effects of the exercise condition on this test.
Immunocytochemistry for Plasticity-Related Proteins
With the exception of the spinophilin puncta quantification, no analyses revealed significant group differences, and these data are therefore not shown. Young adult rats receiving rehabilitation alone had significantly more spinophilin-IR puncta (eg, layer II/III mean ± SEM spinophilin-IR puncta per mm2, 197,759 ± 37,864) compared to the combination of exercise and rehabilitation (Rehab+Run 108,449 ± 10,239, P < .05, F[1, 13] = 5.8) and Cont+Cont (67,974 ± 5,712, P < .05, F[1, 11] = 9.7); this approached significance compared to exercised animals (Cont+Run 119,332 ± 16,526, P = .082, F[1, 12] = 3.6). However, when insular granular cortex of the same hemisphere was examined in Rehab+Cont and Cont+Cont, the Rehab+Cont group spinophillin-IR puncta was increased (P < .05, F[1, 13] = 6.2).
Discussion
In summary, young and middle-aged rats receiving 5 weeks of rehabilitative reach training had a significant enhancement of performance on a skilled reaching task after unilateral ischemic lesions compared to rats without training. Voluntary running had no major effect when administered alone or in combination with skill training. Furthermore, we found no correlation between distance run and recovery of skilled reaching performance. Exercise also failed to improve recovery of coordinated forelimb placement during locomotion, as assessed by the foot-fault test in middle-aged rats.
The beneficial effects of motor skills training are consistent with previous findings.2 For example, Biernaskie and Corbett18 found that task-specific rehabilitation in combination with enriched environment housing improved performance on the Montoya staircase task after unilateral middle cerebral artery occlusion compared to untrained rats. Behavioral improvements resulting from motor skill training after unilateral cerebral injury have been linked with reorganization of cortical movement representations.17,41-43 Nudo and colleagues44 trained animals on a skilled reaching task that increased intracortical microstimulation-evoked movement representations within the motor cortex corresponding to fine digit movements used to perform the task. When the same animals were later trained on a wrist-dependent task, the representation of the digits decreased and wrist representational areas expanded. These findings support the specificity of behavioral effects on neural connections in the motor cortex. These previous findings also suggest that the tray-reaching task of the present study may have improved function because it engaged remaining cortical tissue sufficiently to reorganize it in a functionally beneficial manner.
In contrast to skill training alone, exercise failed to have major effects on skilled reaching recovery. Previously, Komitova et al45 found that rats given voluntary running after cortical ischemic damage performed at the same level of deficit as control animals in traversing a rotating rod. Similarly, voluntary exercised animals performed worse in a battery of locomotor behavioral tasks (eg, traversing a beam or rotating pole) after ischemic damage to the cortex compared to animals given social interaction or an enriched environment.46 Apparent benefits of physical activity, however, have been repeatedly found in other injury models (reviewed in3), including an increase in cognitive function after traumatic brain injury.5 Neuroplastic effects of exercise have predominately been examined in the hippocampus and cerebellum, where cellular proliferation and angiogenic effects are found.3,11,47,48 Exercise also increases expression of neurotrophins, especially BDNF, which mediate its effects on hippocampal-dependent tasks.49 Exercise induces angiogenesis in the motor cortex,50 but it is slow to show these effects. Swain and colleagues51 found that exercise induced angiogenesis in the primary motor cortex only after chronic (30 days) exposure to voluntary running and not at any time point earlier. Although the 5 weeks of exercise used in the present study was substantial, an even longer exercise regime may be necessary to facilitate motor recovery after ischemic damage. In contrast to our failure to find that skills training can be augmented by exercise, previous studies have reported that the efficacy of rehabilitative motor skill training can be improved by its combination with other treatments, including pharmacotherapies and facilitative stimulation.10,52,53
With the exception of spinophilin-immunoreactive puncta, we saw no effect of either rehabilitation or exercise on immunoreactivity for plasticity-related proteins (BDNF, MAP-2, and NMDAR1), as assayed in layers II/III and V of the remaining SMC of the infarcted hemisphere of young rats. Furthermore, no differential immunoreactivity for BDNF in hippocampal subregions was found. There are several possible reasons for this. Brains were assayed at the end of the training/exercise period (6 weeks postlesion), and it is likely that earlier time points of analyses are needed to sensitively detect changes in at least some of these plasticity-related proteins. In intact animals, increased hippocampal BDNF mRNA21 and protein54 have been found after 1 and 4 weeks of exercise, respectively. Elevated levels of BDNF protein in the motor cortex have been found after 14 days of exercise, but not later.55 On the other hand, hippocampal BDNF protein was not significantly increased by exercise provided during the first 7 days after a fluid percussion injury56 nor cortical BDNF protein with exercise given 5 to 12 days after an ischemic cerebral lesion.57 It is also possible that our scheduled feeding procedures created a ceiling effect in the production of plasticity-related proteins, given the major influences of dietary manipulations on levels of neuroplasticity-related molecules.58,59 Finally, the nonexercised controls in this study were given access to a novel environment during the running period, and it is possible that this experience itself was sufficient to elevate these proteins.
We did find a significant effect of rehabilitative training alone for spinophilin, a dendritic spine protein60,61 in the perilesion motor cortex, which may indicate that tray reaching produced synaptogenesis in this region. However, the elevated levels also seen in the insular granular cortex, which was intended to serve as a control region, must be taken into consideration. This area is responsive to thermal stimulation of the tongue62 and has not previously been found to undergo dendritic structural change in response to reach training in the contralesional cortex.30 It is also possible that these data indicate a nonlocalized increase in spine density in the remaining cortex of the damaged hemisphere. If so, the failure to find this effect in the Rehab+Run group suggests that running reduces or alters the time course of this effect.
Another consideration across models of brain injury is the time after injury at which a physical intervention starts.63,64 In a traumatic brain injury model, animals receiving voluntary exercise early postinjury (days 0-6) were found to have reduced expression of plasticity-related proteins in the hippocampus and worsened water maze performance compared to animals exercised between 14 and 20 days after injury.5 Although it is unknown whether such sensitive periods exist for exercise after SMC lesions, a high level of overuse of the impaired forelimb too early after unilateral electrolytic lesions can exaggerate lesion size and limb use deficits.65,66 Other time-dependent effects for skilled rehabilitative tasks after unilateral cerebral infarcts have been shown. Greater behavioral improvements67 and greater influences on cortical reorganization68 were found with earlier onset of training. In the present study, each session of exercise followed each day's training on the reaching task. Many studies finding beneficial effects of exercise after injury used a regimen in which the animal experienced the exercise before the application of the injury (eg,6,47,69). Exercise might also have a priming effect if administered before each session of rehabilitative training, although the possibility of fatigue during the performance of the reaching task would also need to be considered. It should be noted that, although there was no overall benefit of exercise in the middle-aged animals, they were capable of performing at preinfarct levels a week earlier than the rehabilitated-alone animals, supporting the possibility of subtle benefits of this manipulation that might be enhanced by an alternative administration regime.
Although lesion size, as assessed by remaining cortical volume, was similar between experimental conditions, rats with larger lesions tended to run less, which could be a result of either motor and motivation deficits resulting from larger lesions or a tendency for running to reduce lesion size. The latter possibility seems less likely given the 5-day delay in onset and the low initial rates of running. Consistent with the well-established neuroprotective roles of progesterone and estrogen,70 we also found smaller lesions and better postischemic foot-fault task performance in those animals that were in proestrus, a period when estrogen and progesterone are at their peak, at the time of infarct induction. Also, animals in proestrus at the time of surgery had a significantly better postischemic performance on the foot fault test.
This study further supports the efficacy of motor skill training in improving functional outcome after a unilateral cerebral infarct. In the context of abundant evidence for beneficial effects of exercise in other behavioral measures and injury models,71 the present findings also provide important information about exercise parameters that are unlikely to be particularly beneficial for improving motor skills after cortical ischemia. Further investigations of the time of onset, task specificity, and duration of postischemic physical activity are needed to determine whether exercise can augment task-specific motor skills training.
Acknowledgments
These studies were funded by grants from the NIH (MH 64586, MH 65728, RR020700). We would like to thank the entire Dr Jones lab for their ongoing assistance, Dr T. Schallert for loaning the running wheels and providing the idea for the chicken wire tubes, and Martin Woodlee for the idea of using the CatEye system as a monitoring and recording system for the running wheels.
References
- 1.Kolb B, Gibb R. Environmental enrichment and cortical injury: behavioral and anatomical consequences of frontal cortex lesions. Cereb Cortex. 1991;1(2):189–198. doi: 10.1093/cercor/1.2.189. [DOI] [PubMed] [Google Scholar]
- 2.Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990-2002) Prog Neurobiol. 2004;72(3):167–182. doi: 10.1016/j.pneurobio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19(4):283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- 4.Ding Q, et al. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140(3):823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 5.Griesbach GS, Hovda DA, Molteni R, Wu A, Gómez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125(1):129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 6.Molteni R, Zheng JQ, Ying Z, Gómez-Pinilla F, Twiss JL. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci U S A. 2004;101(22):8473–8478. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ying Z, Roy RR, Edgerton VR, Gómez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193(2):411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Hutchinson KJ, Gómez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127(Pt 6):1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- 9.Wang RY, Yang YR, Yu SM. Protective effects of treadmill training on infarction in rats. Brain Res. 2001;922(1):140–143. doi: 10.1016/s0006-8993(01)03154-7. [DOI] [PubMed] [Google Scholar]
- 10.Adkins DL, Boychuk A, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101(6):1776–1782. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- 11.Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87(14):5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monfils MH, Plautz EJ, Kleim JA. In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. Neuroscientist. 2005;11(5):471–483. doi: 10.1177/1073858405278015. [DOI] [PubMed] [Google Scholar]
- 13.Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80(6):3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 14.Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol. 2004;1(4):351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75(5):2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 16.Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46(2):173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Ramanathan D, Conner JM, Tuszynski MH. A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc Natl Acad Sci U S A. 2006;103(30):11370–11375. doi: 10.1073/pnas.0601065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21(14):5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBow SB, Davies MLA, Clarke HL, Colbourne F. Constraint-induced movement therapy and rehabilitation exercises lessen motor deficits and volume of brain injury after striatal hemorrhagic stroke in rats. Stroke. 2003;34(4):1021–1026. doi: 10.1161/01.STR.0000063374.89732.9F. [DOI] [PubMed] [Google Scholar]
- 20.Gharbawie OA, Gonzalez CL, Whishaw IQ. Skilled reaching impairments from the lateral frontal cortex component of middle cerebral artery stroke: a qualitative and quantitative comparison to focal motor cortex lesions in rats. Behav Brain Res. 2005;156(1):125–137. doi: 10.1016/j.bbr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122(3):647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage in female rats: forelimb behavioral effects and dendritic structural plasticity in the contralateral homotopic cortex. Exp Neurol. 2004;190(2):433–445. doi: 10.1016/j.expneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336(2):293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Chopp M, Li Y. Neuroprotective effects of progesterone after transient middle cerebral artery occlusion in rat. J Neurol Sci. 1999;171(1):24–30. doi: 10.1016/s0022-510x(99)00247-6. [DOI] [PubMed] [Google Scholar]
- 25.Tropp J, Markus EJ. Effects of mild food deprivation on the estrous cycle of rats. Physiol Behav. 2001;73(4):553–559. doi: 10.1016/s0031-9384(01)00487-5. [DOI] [PubMed] [Google Scholar]
- 26.Adkins DL, Voorhies AC, Jones TA. Behavioral and neuroplastic effects of focal endothelin-1 induced sensorimotor cortex lesions. Neuroscience. 2004;128(3):473–486. doi: 10.1016/j.neuroscience.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Fuxe K, Bjelke B, Andbjer B, Grahn H, Rimondini R, Agnati LF. Endothelin-1 induced lesions of the frontoparietal cortex of the rat. A possible model of focal cortical ischemia. Neuroreport. 1997;8(11):2623–2629. doi: 10.1097/00001756-199707280-00040. [DOI] [PubMed] [Google Scholar]
- 28.Peterson GM, McGiboney DR., Jr Reeducation of handedness in the rat following cerebral injuries. J Comp Physiol Psychol. 1951;44(2):191–196. doi: 10.1037/h0060555. [DOI] [PubMed] [Google Scholar]
- 29.Whishaw IQ. Lateralization and reaching skill related: results and implications from a large sample of Long-Evans rats. Behav Brain Res. 1992;52(1):45–48. doi: 10.1016/s0166-4328(05)80323-7. [DOI] [PubMed] [Google Scholar]
- 30.Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22(19):8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu JE, Jones TA. Contralesional neural plasticity and functional changes in the less-affected forelimb after large and small cortical infarcts in rats. Exp Neurol. 2006;201(2):479–494. doi: 10.1016/j.expneurol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Kozlowski DA, James DC, Schallert T. Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J Neurosci. 1996;16(15):4776–4786. doi: 10.1523/JNEUROSCI.16-15-04776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whishaw IQ, O'Connor WT, Dunnett SB. The contributions of motor cortex, nigrostriatal dopamine and caudate-putamen to skilled forelimb use in the rat. Brain. 1986;109(Pt 5):805–843. doi: 10.1093/brain/109.5.805. [DOI] [PubMed] [Google Scholar]
- 34.Barth TM, Jones TA, Schallert T. Functional subdivisions of the rat somatic sensorimotor cortex. Behav Brain Res. 1990;39(1):73–95. doi: 10.1016/0166-4328(90)90122-u. [DOI] [PubMed] [Google Scholar]
- 35.Jones TA. Multiple synapse formation in the motor cortex opposite unilateral sensorimotor cortex lesions in adult rats. J Comp Neurol. 1999;414(1):57–66. [PubMed] [Google Scholar]
- 36.Schmanke TD, Avery RA, Barth TM. The effects of amphetamine on recovery of function after cortical damage in the rat depend on the behavioral requirements of the task. J Neurotrauma. 1996;13(6):293–307. doi: 10.1089/neu.1996.13.293. [DOI] [PubMed] [Google Scholar]
- 37.Gundersen HJ, Bendtsen TF, Korbo L, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis. 1988;96(5):379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos CWG. The Rat Brain in Stereotaxic Coordinates. 2nd. Sydney: Academic Press; 1986. [Google Scholar]
- 39.Woodlee MT, Schallert T. The interplay between behavior and neurodegeneration in rat models of Parkinson's disease and stroke. Restor Neurol Neurosci. 2004;22(35):153–161. [PubMed] [Google Scholar]
- 40.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 41.Dancause N, Barbay S, Frost SB, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25(44):10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friel KM, Nudo RJ. Recovery of motor function after focal cortical injury in primates: compensatory movement patterns used during rehabilitative training. Somatosens Mot Res. 1998;15(3):173–189. doi: 10.1080/08990229870745. [DOI] [PubMed] [Google Scholar]
- 43.Friel KM, Barbay S, Frost SB, et al. Effects of a rostral motor cortex lesion on primary motor cortex hand representation topography in primates. Neurorehabil Neural Repair. 2007;21(1):51–61. doi: 10.1177/1545968306291851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16(2):785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komitova M, Zhao LR, Gidö G, Johansson BB, Eriksson P. Postischemic exercise attenuates whereas enriched environment has certain enhancing effects on lesion-induced subventricular zone activation in the adult rat. Eur J Neurosci. 2005;21(9):2397–2405. doi: 10.1111/j.1460-9568.2005.04072.x. [DOI] [PubMed] [Google Scholar]
- 46.Johansson BB, Ohlsson AL. Environment, social interaction, and physical activity as determinants of functional outcome after cerebral infarction in the rat. Exp Neurol. 1996;139(2):322–327. doi: 10.1006/exnr.1996.0106. [DOI] [PubMed] [Google Scholar]
- 47.Ding YH, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res. 2006;3(1):15–23. doi: 10.2174/156720206775541787. [DOI] [PubMed] [Google Scholar]
- 48.Ding YH, Xia-Dong L, Jie L, et al. Exercise-induced overexpression of angiogenic factors and reduction of ischemia/reperfusion injury in stroke. Curr Neurovasc Res. 2004;1(5):411–420. doi: 10.2174/1567202043361875. [DOI] [PubMed] [Google Scholar]
- 49.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 50.Kleim JA, Cooper NR, VandenBerg PM. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002;934(1):1–6. doi: 10.1016/s0006-8993(02)02239-4. [DOI] [PubMed] [Google Scholar]
- 51.Swain RA, Harris AB, Wiener EC, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117(4):1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 52.Adkins DL, Jones TA. D-amphetamine enhances skilled reaching after ischemic cortical lesions in rats. Neurosci Lett. 2005;380(3):214–218. doi: 10.1016/j.neulet.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 53.Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25(8):780–788. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- 54.Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363(1):43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 55.Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004;1028(1):92–104. doi: 10.1016/j.brainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004;1016(2):154–162. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- 57.Ploughman M, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience. 2005;136(4):991–1001. doi: 10.1016/j.neuroscience.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 58.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16(3):129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 59.Mattson MP. Energy intake, meal frequency, and health: a neurobiological perspective. Annu Rev Nutr. 2005;25:237–260. doi: 10.1146/annurev.nutr.25.050304.092526. [DOI] [PubMed] [Google Scholar]
- 60.Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci U S A. 1997;94(18):9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng J, Yan Z, Ferreira A, et al. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci U S A. 2000;97(16):9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res. 1986;379(2):329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- 63.Griesbach GS, Gomez-Pinilla F, Hovda DA. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma. 2007;24(7):1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- 64.Ploughman M, Granter-Button S, Chernenko G, et al. Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Res. 2007;1150:207–216. doi: 10.1016/j.brainres.2007.02.065. [DOI] [PubMed] [Google Scholar]
- 65.Leasure JL, Schallert T. Consequences of forced disuse of the impaired forelimb after unilateral cortical injury. Behav Brain Res. 2004;150(12):83–91. doi: 10.1016/S0166-4328(03)00254-7. [DOI] [PubMed] [Google Scholar]
- 66.Humm JL, Kozlowski DA, James DC, Gotts JE, Schallert T. Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain Res. 1998;783(2):286–292. doi: 10.1016/s0006-8993(97)01356-5. [DOI] [PubMed] [Google Scholar]
- 67.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci. 2004;24(5):1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbay S, Plautz EJ, Friel KM, et al. Behavioral and neurophysiological effects of delayed training following a small ischemic infarct in primary motor cortex of squirrel monkeys. Exp Brain Res. 2006;169(1):106–116. doi: 10.1007/s00221-005-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J, Luan X, Clark JC, Rafols JA, Ding Y. Neuroprotection against transient cerebral ischemia by exercise pre-conditioning in rats. Neurol Res. 2004;26(4):404–408. doi: 10.1179/016164104225016038. [DOI] [PubMed] [Google Scholar]
- 70.Stein DG. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 2001;24(7):386–391. doi: 10.1016/s0166-2236(00)01821-x. [DOI] [PubMed] [Google Scholar]
- 71.Kleim JA, Jones TA, Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochem Res. 2003;28(11):1757–1769. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]


