Abstract
The epidermal growth factor receptor family (EGFR) has been implicated in a number of cancers, including breast, and its members have become the target of novel cancer therapies. In this report, we show a novel link between erlotinib, a potent EGFR inhibitor, DNA damage, and homology-directed recombinational repair (HDR) in human breast cancer cells. Erlotinib suppresses HDR. This is not secondary to erlotinib-mediated changes in cell cycle and is associated with increased γ-H2AX foci, which is an in situ marker of chromosomal double strand breaks (DSBs). Both Rad51 and BRCA1 are essential components of the HDR machinery. Consistent with decreased HDR in erlotinib-treated cells, erlotinib also attenuates DNA damage-induced Rad51 foci and results in cytoplasmic retention of BRCA1. As BRCA1 is a shuttling protein and its nuclear function of promoting HDR is controlled by its subcellular localization, we further demonstrate that targeted translocation of BRCA1 to the cytoplasm enhances erlotinib sensitivity. These findings suggest a novel mechanism of action of erlotinib through its effects on the BRCA1/HDR pathway. Furthermore, BRCA1/HDR status may be an innovative avenue to enhance the sensitivity of cancer cells to erlotinib.
Keywords: Tarceva, erlotinib, epidermal growth factor receptor (EGFR), breast cancer, BRCA1, H2AX, DNA damage, homologous recombination, radiation
Introduction
The epidermal growth factor receptor (EGFR) family plays an essential role in modulating proliferation, differentiation, and survival and has become a heavily targeted novel cancer therapeutic strategy(1). Aberrant expression and dysregulation of any EGFR can be found in a number of cancers, including lung, pancreas, head and neck, brain, and breast(1). In approximately 20-30% of breast cancers, overexpression of HER2/ErbB2 is seen and portends a more aggressive phenotype and poorer prognosis(1). However, with the development of molecular targeted therapies against HER2 such as trastuzumab (Herceptin), improved outcomes are being achieved. This success has initiated the pursuit of other compounds which can suppress HER2 activity. In particular, drugs which inhibit EGFR subtype HER1 have been shown to be active against HER2(2, 3). One such drug is erlotinib (Tarceva), an orally bioavailable small molecular weight inhibitor of HER1.
Erlotinib treatment results in blockade of multiple signal transduction pathways involved in cell proliferation and survival. In particular, erlotinib has been shown to inhibit growth and cause cells to accumulate in the G1 phase with a concomitant decrease in S phase of the cell cycle. Interestingly, erlotinib also decreases radiation-induced Rad51 expression and enhances radiation-induced apoptosis, suggesting a potential role of erlotinib in altering the DNA damage response(4). Similarly, gefitinib, a selective EGFR tyrosine kinase inhibitor, has been reported to elicit radiosensitization by suppressing cellular capacity to repair radiation induced DNA damage(5). Furthermore, blockade of EGFR signaling with a monoclonal antibody to the EGFR can inhibit radiation-induced activation of DNA-PK and increase radiosensitivity of treated cells(6). These studies thus support a role of EGFR in the DNA damage response.
The DNA damage response is commonly triggered upon generation of DNA double strand breaks (DSBs), which can be repaired via two principal pathways, homology-directed recombinational repair (HDR) and nonohomologous end joining (NHEJ)(7, 8). NHEJ, which occurs in all phases of the cell cycle, is an error prone process that rejoins the ends of DSB with no or few homology. In contrast, HDR predominantly takes place in S phase of the cell cycle and is a high fidelity process which requires an intact homologous DNA sequence as the template. Multiple proteins, including the Rad51 recombinase and BRCA1, are involved in this intricate process. Rad51 catalyzes the DNA strand invasion and exchange reaction in HDR while BRCA1 promotes HDR through mechanisms involving chromatin remodeling and controlling the function of several other HDR proteins through protein-protein interactions(7). Recent studies also show that the function of BRCA1 in DNA repair is regulated by its nuclear shuttling(9).
In this study, we investigated the effects of erlotinib on DNA damage and repair in human breast cancer cells. We demonstrate for the first time that erlotinib treatment itself results in decreased HDR events and increased chromosomal DSBs in human breast cancer cells. Consistent with decreased HDR, DNA damage-induced Rad51 foci are attenuated by erlotinib. As BRCA1's function in promoting HDR-mediated DSB repair is dependent on its nuclear localization, we also find that erlotinib reduces nuclear BRCA1 levels with a concomitant sequestration of BRCA1 to the cytosol. It is worth to note that these effects are not indirect events due to erlotinib-mediated cell cycle changes. Furthermore, we show that targeted translocation of BRCA1 to the cytoplasm in breast cancer cells enhances erlotinib-mediated cytotoxicity. Taken together, these novel findings suggest that, in addition to its effects on cell cycle, the action of erlotinib may also involve BRCA1 and the HDR machinery.
Materials and Methods
Cell culture
Previously established MCF7DRGFP cells(9), which were kindly provided by Dr. Simon Powell (Washington University School of Medicine) were maintained as previously described. MCF7DRGFP cells carry a chromosomally integrated HDR substrate. All transfections were performed using FuGene6 according to the manufacturer's recommendations (Roche).
Immunofluorescence
MCF7DRGFP cells were cultured and mounted onto sterile glass slides. Cells were then treated with DMSO vehicle, 1μM erlotinib, 4Gy radiation, or 1μM erlotinib followed by 4Gy radiation. Following the treatment period, immunohistochemistry was performed as previously described(9). Primary antibodies include 200μg/μl mouse anti-phospho-γ-H2AX antibody (Upstate, Cat#07-164), 1:500 rabbit anti-Rad51 antibody (Calbiochem, Cat#PC130), or 1:100 mouse anti-BRCA1 (Ab-1) (Calbiochem, mouse mAb MS110). Secondary antibodies include 1:1000 anti-mouse Alexa488 conjugated antibody (Molecular Probe, Cat#A-11008) or 1:1000 anti-Rabbit Alexa594 conjugated antibody (Molecular Probe, Cat#A-11005). Staining patterns were visualized via fluorescence microscopy (Carl Zeiss, Thornwood, NY). A total of 50 cells were counted per field, and a total of 10 fields were assessed. For foci analysis, cells with >10 foci were counted. For BRCA1 localization, cells were assessed as having nuclear staining only, cytoplasmic staining only, or both nuclear/cytoplasmic staining(8).
Chromosomal HDR analysis
HDR was assessed as previously described(10). Briefly, MCF7DRGFP cells were treated with either DMSO vehicle, 1μM, or 6μM erlotinib for 16 hours. Two days after transfection with I-SceI-expression plasmid or empty vector (total exposure to erlotinib for 64 hours), cells were subjected to two-color fluorescence analysis which revealed the percentage of green fluorescent cells relative to the total cell number. For each analysis, 100,000 cells were processed. All transfections were performed using FuGene 6 (Roche).
Cell cycle analysis
MCF7DRGFP cells were treated with DMSO vehicle, 1μM, 6 μM erlotinib, or serum starved for the indicated times. Following the treatment period, cell cycle distribution was determined using standard ethanol fixation and propidium iodide staining followed by flow cytometry as previously described(10).
Clonogenic survival assay
The colony-forming ability was evaluated in MCF7DRGFP cells transiently expressing either vector control or a peptide containing truncated BRCA1 (trBRCA1), which has been shown to effectively shift BRCA1 to the cytoplasm(11). Briefly, cells were transfected with either vector control or trBRCA1. Twenty four hours after transfection, cells were subjected to the treatment with either DMSO or the indicated dose of erlotinib. Colonies were stained and counted as positive if >50 cells were seen. Survival fraction was calculated as: (number of colonies for erlotinib/number of cells plated)/(number of colonies for corresponding control/number of cells plated).
Statistical Analysis
The data were analyzed via either t-test (HDR analysis) or analysis of variance (ANOVA) followed by a Bonferroni post test (remaining data) using GraphPad Prism version 4.02 for Windows (GraphPad Software, San Diego, CA).
Results
Erlotinib increases γ-H2AX foci
The downstream actions of erlotinib are mediated by inhibition of the mitogen-activated protein kinase and phosphatidylinositol-3-kinase/Akt pathways. As these effects can result in altered expression and localization of proteins involved in the DNA damage response and enhance ionizing radiation-induced cytotoxicity(4-6, 12, 13), we investigated whether erlotinib alone can increase nuclear γ-H2AX foci, markers of DNA DSBs in the breast cancer cell line MCF-7DRGFP derived from the well-established MCF-7 cell line and carries a single copy of a chromosomally integrated HDR substrate(10).
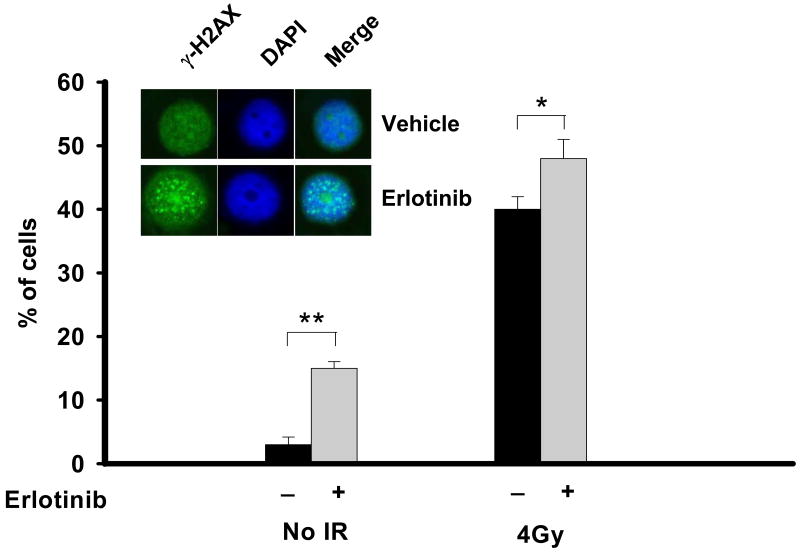
As seen in figure 1, treatment of MCF-7DRGFP cells for 16 hours with 1μM erlotinib leads to a 5 fold increase (15% versus 3%, p<0.001) in γ-H2AX foci compared to vehicle treated cells. Foci can also be seen in 40% of cells 30 minutes following exposure to radiation, which is a potent inducer of DNA DSB and γ-H2AX foci. The combination of erlotinib and radiation enhances γ-H2AX foci compared to radiation alone (48% versus 40%, p<0.01). Consistent with previous reports(4-6), these results further substantiate a link between EGFR inhibition and the DNA damage response.
Figure 1. Erlotinib and radiation increases γ-H2AX foci in human breast cancer cells.
MCF7DRGFP cells were treated with DMSO vehicle, 1μM erlotinib for 16 hours, 4 Gray (Gy) radiation alone, or 1μM erlotinib for 16 hours followed by 4Gy radiation. Following the erlotinib treatment period or 30 minutes after radiation, cells were assessed for γ-H2AX foci. Shown is the % of foci-containing cells with >10 foci. The inset shows a representative staining of increased γ-H2AX foci (green) in erlotinib-treated cells (bottom panels) compared to untreated cells (top panels). Cell nuclei were also stained with DAPI (blue). Merge of the two stains is also shown. *p<0.01, **p<0.001
Erlotinib decreases homology-directed recombinational repair (HDR)
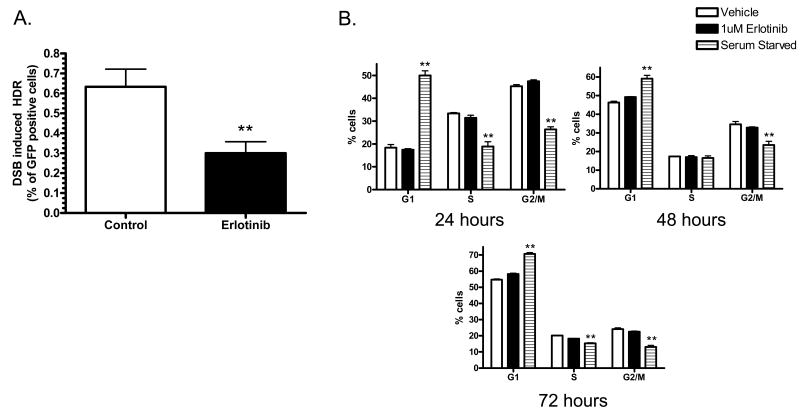
As a result of increased DNA DSB, cells trigger DNA damage checkpoints and activate the DNA repair machinery(9, 14). Since erlotinib increases γ-H2AX foci, which are indicative of DNA DSBs, and has been previously shown to decrease the expression of the HDR protein Rad51 after radiation, we investigated the effects of erlotinib on cellular capacity to repair DSBs via HDR. In these experiments, site specific chromosomal DSBs were induced in MCF-7DRGFP cells by the transient expression of the I-SceI endonuclease. Only HDR utilizing the homology between the two mutated founder GFP repeats can restore GFP function. Expression of GFP in these cells was then quantified using flow cytometry. As shown in figure 2A, 1μM erlotinib significantly decreases the percentage of GFP positive cells compared to control cells (0.63% versus 0.3%, p<0.001), resulting in a 2 fold decrease in HDR efficiency. This concentration of erlotinib has been shown to induce cytotoxic effects in breast cancer cells and correspond to clinically relevant doses (15). In addition, these HDR effects are not due to differences in cell viability or transfection efficiency, which are included as controls in all HDR assays.
Figure 2. Effects of erlotinib on (A) HDR events and (B) cell cycle distribution.
(A) MCF7DRGFP cells were treated for 16 hours with DMSO vehicle or 1μM erlotinib, followed by transfection of a pISceI or control vector. After 48 hours, cells were harvested for GFP expression analysis via flow cytometry. Cells were exposed to erlotinib for a total of 64 hours (B) Cell cycle distribution was analyzed 24-72 hours after 1μM of erlotinib treatment or serum starvation as a positive control for G1 accumulation. *p<0.01, **p<0.001
It is known that one of the mechanisms for erlotinib-mediated cytotoxicity is via induced accumulation of cells at the G1 phase of cell cycle with a concomitant decrease of cells in S & G2/M phases. Given that HDR predominantly takes place in S-phase when DNA replication occurs, we next examined whether our observed decrease in HDR by erlotinib is an indirect result of erlotinib-mediated changes in cell cycle, such as a dramatic reduction of cells in S-phase or accumulation in G1 phase. When analyzed at 24, 48, and 72 hours following 1 μM erlotinib treatment, there is no significant decrease of cells in S phase or accumulation of cells in G1 phase (figure 2B) to account for the erlotinib-mediated 2-fold decrease in HR. However, in agreement with previous reports, 6μM erlotinib robustly induces a significant G1 accumulation similar to that of serum starvation as early as 24 hours (46% cells in G1, supplemental figure 1A). These observations suggest that the effects of erlotinib may involve the down-regulation of HDR and is not secondary to erlotinib-mediated cell cycle effects.
Erlotinib attenuates radiation-induced Rad51 foci and sequesters BRCA1 in the cytoplasm
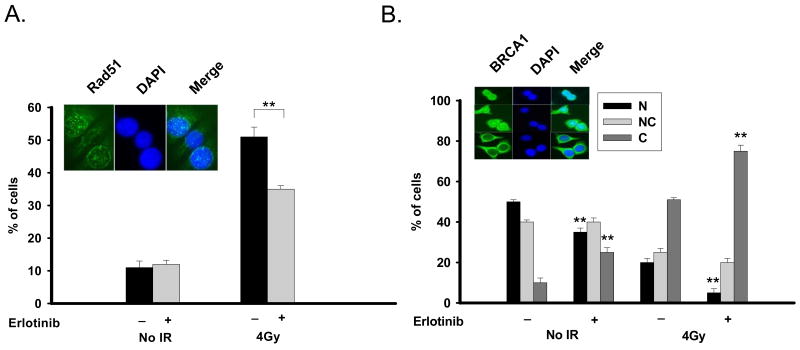
To further explore erlotinib-mediated decrease in HDR, we investigated Rad51 foci, a well characterized in vivo functional marker of HDR. MCF-7DRGFP cells were exposed to 1μM erlotinib for 16 hours, and Rad51 foci were examined. As seen in figure 3A, erlotinib alone does not significantly affect Rad51 foci (11% versus 12%). However, the sensitivity of this assay may not be sufficient to detect erlotinib-mediated effects on Rad51 foci. To amplify this DNA damage response as well as to obtain a reference control, radiation was utilized, which results in robust induction of Rad51 foci (figure 3A). Consistent with the decreased HDR seen with erlotinib, radiation-induced Rad51 foci is significantly attenuated by erlotinib (51% versus 35% of cells, p<0.001). This is in agreement with previously observed reduction of radiation-induced Rad51 expression by erlotinib in human lung and head and neck cancers(4).
Figure 3. Effect of erlotinib on Rad51 foci and BRCA1 subcellular location.
(A) Erlotinib decreases radiation-induced Rad51 foci. MCF7DRGFP cells were treated with DMSO vehicle, 1μM erlotinib for 16 hours, 4Gy radiation alone, or 1μM erlotinib for 16 hours followed by 4Gy radiation. Following the erlotinib treatment period or 8 hours after radiation, cells were assessed for Rad51 foci. Shown is the % of foci-containing cells with >10 foci. The inset shows a representative staining of Rad51 foci (green). Cell nuclei were also stained with DAPI (blue). Merge of the two stains is also shown. (B) Erlotinib sequesters BRCA1 in the cytoplasm. MCF7DRGFP cells were treated with DMSO vehicle, 1μM erlotinib for 16 hours, 4Gy radiation alone, or 1μM erlotinib for 16 hours followed by 4Gy radiation. Following the erlotinib treatment period or 24 hours after radiation, cells were assessed for BRCA1 subcellular localization. The inset is representative of the various BRCA1 staining patterns (green) as indicated. Cell nuclei were also stained with DAPI (blue). Merge of the two stains is also shown. **p<0.001
BRCA1 plays a critical role in the DNA damage response pathway and promotes error-free HDR. Recent studies show that BRCA1 is a shuttling protein which is retained in the nucleus during S-phase and accumulates in the cytosol during mitosis(9). In response to radiation-induced DNA damage, nuclear export of BRCA1 to the cytoplasm is observed in a p53-dependent manner (9). Specifically, the functions of BRCA1 in promoting HDR are dictated by its nuclear localization(9, 16). We thus sought to determine whether the effects of erlotinib on HDR inhibition involve changes in BRCA1 localization, which controls its nuclear function of repair. MCF-7 DRGFP cells with functional p53 exhibit a robust radiation-induced BRCA1 cytoplasmic translocation (figure 3B), which is consistent with previous reports(9). Interestingly, after 16 hours of 1μM erlotinib treatment, a significant cytoplasmic shift of BRCA1 is observed in MCF-7DRGFP cells when compared to untreated cells (25% versus 10%, p<0.001, figure 3B). It is worth to note that there is no cell cycle effect observed from erlotinib at this time and dose (figure 2B). With the combination of erlotinib and radiation, this effect is further enhanced (51% versus 75% of cells, p<0.001 versus radiation alone). These findings suggest a potential link between erlotinib-mediated effects on BRCA1 localization and downregulation of HDR.
Nuclear repair function of BRCA1 is critical for sensitivity to erlotinib
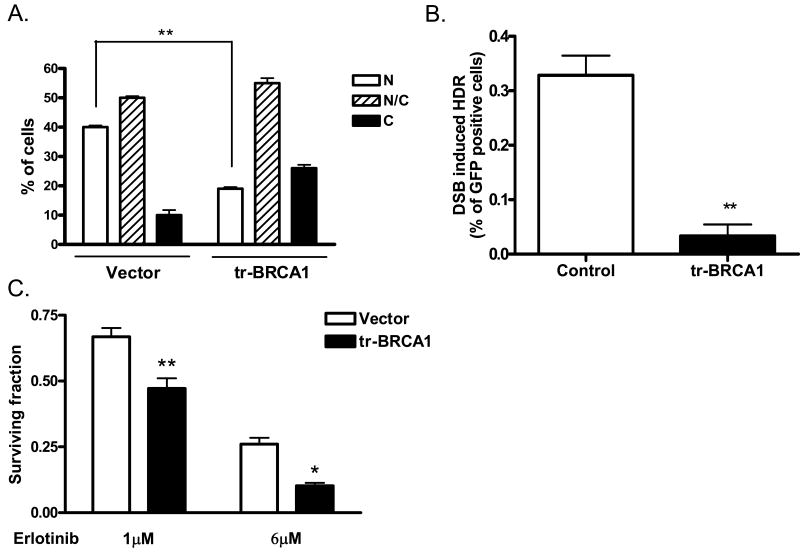
Given that the critical function of nuclear BRCA1 involves promoting HDR-mediated repair of DSB, we next assessed the role of BRCA1 in erlotinib-mediated cytotoxic effects. As HDR depends on BRCA1 nuclear localization, we reasoned that depletion of nuclear BRCA1 would inhibit HDR and thus augment erlotinib's cytotoxic actions through HDR suppression. To achieve this, we utilized a peptide containing truncated BRCA1 (trBRCA1), which contains the BRCA1 nuclear export sequence (NES) and BARD1 binding domain and has been shown to effectively shift BRCA1 to the cytoplasm(11, 16). Ectopic expression of trBRCA1 in MCF7 cells results in a 2-fold reduction (40% versus 20%) in nuclear BRCA1 (figure 4A) and 10-fold inhibition (0.33% versus 0.03%) of HDR activity (figure 4B).
Figure 4. Nuclear repair function of BRCA1 is critical for sensitivity to erlotinib.
(A) tr-BRCA1 drives BRCA1 to the cytoplasm. Twenty-four hours following transient transfection of tr-BRCA1 or control vector, MCF7 cells were subjected to immunohistochemical analysis for BRCA1. BRCA1 localization was scored as strictly nuclear (N), strictly cytoplasmic (C), or both nuclear and cytoplasmic (N/C) (inset of figure 3B). (B) Expression of tr-BRCA1 suppresses DNA homology-directed repair. MCF7DRGFP cells were co-transfected with tr-BRCA1 or vector control and I-SceI-expression plasmid or empty vector. Forty-eight hours later, cells were subjected to flow cytometric analysis for GFP expression. (C) Targeted translocation of BRCA1 to the cytoplasm with trBRCA1 enhances erlotinib-mediated cytotoxicity. MCF7 cells transiently expressing either control vector or trBRCA1 were treated with 1 or 6μM erlotinib or DMSO control for 72 hours and clonogenic survival was assessed. *p<0.01, **p<0.001
Having established that trBRCA1 can deplete nuclear BRCA1 and inhibit HDR capacity, we next assessed the effect of altering BRCA1 localization (and hence HDR) on erlotinib-mediated cytotoxicity. trBRCA1 was transiently expressed in MCF7 cells. Twenty four hours after transfection, cells were treated with erlotinib for 72 hours, and clonogenic survival following erlotinib treatment was assessed. As shown in figure 4C, 1μM erlotinib induces a cytoxic effect. Furthermore, targeted translocation of BRCA1 to the cytosol enhances this cytotoxic response of MCF7 DRGFP breast cancer cells to erlotinib. We also examined the cytotoxic effect of a higher dose (6μM) of erlotinib, which induces more than 4 fold decrease of HDR (supplemental figure 1B) and 2-fold increase accumulation of cells in G1 phase (supplemental figure 1A). As seen in figure 4C, the survival fraction of cells at this concentration is reduced compared to 1μM, likely due to the combined effects of erlotinib-mediated cell cycle redistribution and suppression of HDR at 6μM. Interestingly, an enhanced cytotoxicity by erlotinib continues to be observed following BRCA1 translocation to the cytosol (and hence reduced HDR) by trBRCA1. Together, our findings in this study suggest a potential role for the BRCA1-HDR pathway in the cytotoxic effects of erlotinib beyond its cell cycle effects.
Discussion
In this study, we demonstrate that erlotinib treatment of human breast cancer cells results in suppression of HDR and increased basal levels of γ-H2AX foci, which are indicative of accumulation of DNA DSBs. Inhibition of HDR is also associated with sequestration of BRCA1 to the cytoplasm and attenuation of DNA damage-induced Rad51 foci, but is not an indirect result of erlotinib-mediated cell cycle effects. Furthermore, disruption of the repair function by targeted translocation of BRCA1 to the cytoplasm enhances erlotinib-mediated cytotoxicity. These findings suggest a novel link between erlotinib-mediated cytotoxicity, DNA damage, and the HDR machinery in human breast cancer cells.
HDR is a complex process which is intricately tied to the cell cycle. We and others have previously reported a dependence of HDR and HDR protein subcellular localization on cell cycle distribution(9). Because erlotinib has been reported to induce G1 accumulation (4), it is important that the effect on HDR by erlotinib is not secondary to erlotinib-mediated changes in cell cycle distribution. As shown in figure 2B, low doses (1μM) of erlotinib does not result in significant changes in cell cycle distribution, specifically no S-phase reduction, to account for a 2 fold reduction in HDR capacity. In addition, higher doses of erlotinib (6μM) robustly induces G1 accumulation at similar magnitudes as serum starvation as early as 24 hours following treatment. However, a further enhanced cytotoxicity can still be observed by manipulation of HDR through changing BRCA1 localization. Thus, the erlotinib-mediated reduction of HDR appears to be independent of, and in addition to, erlotinib's cell cycle effects.
Recent reports suggest that sensitivity to erlotinib is dependent on regulation of the phosphatidylinositol-3 kinase (PI3K)/Akt pathway, which can alter the DNA damage response (17). Downstream Akt targets such as the FoxO subfamily of Forkhead transcription factors and MDM2 have been shown to stimulate the DNA damage and repair pathways (12, 13). In addition, radiation-induced Rad51 expression is reduced following erlotinib exposure in human lung and head and neck cancers(4).
Our results indicate that the underlying mechanisms by which erlotinib down-regulates HDR may involve BRCA1. Recent studies have shown that BRCA1 function is dictated by its subcellular localization(9, 16). In particular, BRCA1-mediated HDR occurs in the nucleus(16). We find that after 16 hours of erlotinib treatment, a time at which no significant changes in cell cycle are observed, nuclear BRCA1 levels are reduced. This suggests that erlotinib-mediated suppression of HDR may occur at least in part via sequestration of BRCA1 in the cytoplasm. Consistent with this notion is that forced shift of BRCA1 to the cytoplasm enhances erlotinib-mediated cytotoxicity in BRCA1-expressing cells. Alternatively, erlotinib may cause DSBs which could induce BRCA1 cytoplasmic redistribution similar to our previous report of radiation-induced BRCA1 nuclear export(9). This change in BRCA1 localization would then alter HDR capacity such that erlotinib-induced DSBs cannot be repaired and thus lead to cytotoxicity.
Inhibition of EGFR signaling has also been implicated in radiation-induced activation of the DNA damage response. Radiation-induced nuclear import of the EGFR has been associated with induction of DNA-PK-dependent repair of DSBs through nonhomologous end joining (NHEJ), and blockade of EGFR with a monoclonal antibody prevents this activation leading to radiosensitization (6). It is possible that erlotinib may also have effects on the NHEJ DNA repair pathway. Our preliminary studies do not show a significant difference in erlotinib-mediated activation of DNA-PK foci (data not shown). However, further in depth investigation is warranted to determine if erlotinib affects NHEJ.
In summary, our findings indicate that the cytotoxic effects of erlotinib occur, in part, via downregulation of HDR and sequestration of BRCA1 to the cytoplasm away from its role in HDR in the nucleus. This is not secondary to erlotinib-mediated cell cycle effects. Furthermore, targeting of BRCA1 away from the nucleus enhances erlotinib-mediated cytotoxicity. These results suggest the potential use of BRCA1/HDR status as a molecular marker to predict patient response to erlotinib. Additionally, the BRCA1/HDR pathway may be a potential target to sensitize cancer cells to erlotinib.
Supplementary Material
Supplemental Fig. 1
Supplemental Fig. 2
Acknowledgments
This work was supported by grants R01 CA118158-02 from the National Institute of Health (to F.X.), RR0725 from the Radiological Society of North America (to E.S.Y.), and Grant Number P50CA095103 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or NIH. We would like to thank Dr. Simon Powell for generously providing MCF-7DRGFP cells.
References
- 1.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–65. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 2.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61:8887–95. [PubMed] [Google Scholar]
- 3.Schaefer G, Shao L, Totpal K, Akita RW. Erlotinib Directly Inhibits HER2 Kinase Activation and Downstream Signaling Events in Intact Cells Lacking Epidermal Growth Factor Receptor Expression. Cancer Res. 2007;67:1228–38. doi: 10.1158/0008-5472.CAN-06-3493. [DOI] [PubMed] [Google Scholar]
- 4.Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva) Cancer Res. 2005;65:3328–35. doi: 10.1158/0008-5472.CAN-04-3547. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T, Munshi A, Brooks C, Liu J, Hobbs ML, Meyn RE. Gefitinib radiosensitizes non-small cell lung cancer cells by suppressing cellular DNA repair capacity. Clin Cancer Res. 2008;14:1266–73. doi: 10.1158/1078-0432.CCR-07-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dittmann K, Mayer C, Fehrenbacher B, et al. Radiation-induced Epidermal Growth Factor Receptor Nuclear Import Is Linked to Activation of DNA-dependent Protein Kinase. J Biol Chem. 2005;280:31182–9. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- 7.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–54. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 8.van Gent DC, Hoeijmakers JH, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet. 2001;2:196–206. doi: 10.1038/35056049. [DOI] [PubMed] [Google Scholar]
- 9.Feng Z, Kachnic L, Zhang J, Powell SN, Xia F. DNA damage induces p53-dependent BRCA1 nuclear export. J Biol Chem. 2004;279:28574–84. doi: 10.1074/jbc.M404137200. Epub 2004 Apr 15. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Willers H, Feng Z, et al. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol Cell Biol. 2004;24:708–18. doi: 10.1128/MCB.24.2.708-718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown MA, Nicolai H, Howe K, et al. Expression of a truncated Brca1 protein delays lactational mammary development in transgenic mice. Transgenic Res. 2002;11:467–78. doi: 10.1023/a:1020348025139. [DOI] [PubMed] [Google Scholar]
- 12.Tran H, Brunet A, Grenier JM, et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–4. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 13.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 2001;98:11598–603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–62. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 15.Akita R, Sliwkowski M. Preclinical studies with Erlotinib (Tarceva) Seminars in Oncology. 2003;30:15–24. [PubMed] [Google Scholar]
- 16.Fabbro M, Schuechner S, Au WW, Henderson BR. BARD1 regulates BRCA1 apoptotic function by a mechanism involving nuclear retention. Exp Cell Res. 2004;298:661–73. doi: 10.1016/j.yexcr.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Bianco R, Shin I, Ritter CA, et al. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene. 2003;22:2812–22. doi: 10.1038/sj.onc.1206388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1
Supplemental Fig. 2