Abstract
A body of evidence has indicated that μ-opioid agonists can inhibit DNA synthesis in developing brain. We now report that κ-selective opioid agonists (U69593 and U50488) modulate [3H]thymidine incorporation into DNA in fetal rat brain cell aggregates in a dose- and developmental stage-dependent manner, κ agonists decreased thymidine incorporation by 35% in cultures grown for 7 days, and this process was reversed by the κ-selective antagonist, norbinaltorphimine, whereas in 21-day brain cell aggregates a 3.5-fold increase was evident. Cell labeling by [3H]thymidine was also inhibited by the κ-opioid agonist as shown by autoradiography. In addition, U69593 reduced basal rates of phosphoinositide formation in 7-day cultures and elevated it in 21-day cultures. Control levels were restored by norbinaltorphimine. Pertussis toxin blocked U69593-mediated inhibition of DNA synthesis. The action of κ agonists on thymidine incorporation in the presence of chelerythrine, a protein kinase C (PKC) inhibitor, or in combination with LiCl, a noncompetitive inhibitor of inositol phosphatase, was attenuated in both 7- and 21-day cultures. These results suggest that κ agonists may inhibit DNA synthesis via the phosphoinositide system with a pertussis toxin-sensitive G protein as transducer. In mixed glial cell aggregates, U50488 increased thymidine incorporation into DNA 3.1-fold, and this stimulation was reversed by the opioid antagonist naltrexone.
Keywords: Brain cell aggregates, Opioid peptides, Opioid receptors, Glial cells, DNA synthesis, Phosphoinositide
Substantial evidence exists to support the notion that maternal drug abuse elicits, in addition to fetal growth delay, physiological and behavioral deficits in offspring (McDowell and Kitchen, 1987; Hayford et al., 1988; Farrar and Blumer, 1991). Among the drugs abused are opioid agonists, which appear to modify phenotypic expression of opioid receptors during brain ontogeny (Zagon and McLaughlin, 1987; Tempel et al., 1988; Barg et al., 1989a). In addition, opioids act as DNA synthesis modulators during brain development (Vertes et al., 1982; Bartolome et al., 1986, 1991; Kornblum et al., 1987; Schmahl et al., 1989). Opioid regulation of thymidine incorporation in rat brain has proven transient and dependent on developmental age (Kornblum et al., 1987; Barg et al., 1990). Opioid agonists such as morphine, β-endorphin, [d-Ala2,Me-Phe4,Gly-ol5]enkephalin (DAMGE), and Met-enkephalin decrease thymidine incorporation into DNA in vivo and their actions are reversed by the opioid antagonist naloxone, indicating opioid receptor mediation (Bartolome et al., 1986, 1991; Kornblum et al., 1987; Schmahl et al., 1989). Other reports emphasize the effect of opioids on DNA synthesis in vitro (Davila-Garcia and Azmitia, 1989; Eccleston et al., 1989; Barg et al., 1990; Coscia et al., 1991). Alteration of DNA synthesis by opioids in vitro is not restricted to neural systems, as it occurs in nonneural cells present in human lung and breast cancers and in the immune system as well (Gilmore and Weiner, 1989; Kusnecov et al., 1989; Maneckjee and Minna, 1990; Maneckjee et al., 1990; Taub et al., 1991).
Protein kinase C (PKC) is included among the signal generators that trigger gene activation, leading to DNA synthesis and cell proliferation (Berridge, 1987; Kikkawa et al., 1989; Huang, 1990; Gschwendt et al., 1991). Moreover, modulators of this enzyme, such as phorbol esters, can decrease or increase DNA synthesis. PKC activators facilitate DNA synthesis and differentiation of neurons. Among the signal transduction systems that activate PKC are the phosphoinositide (PtdIns) systems. Implication of PKC in opioid modulation of thymidine incorporation into DNA has yet to be addressed. Furthermore, it is not known whether opioid abatement of thymidine incorporation involves activation of other receptor systems and/or intercellular signaling. Also unresolved are the target cells and opioid receptor types that mediate thymidine incorporation. Previously we have examined the ability of μ opioids to influence DNA synthesis (Coscia et al., 1991; Barg et al., 1992). In this study, the role of κ opioids is addressed and evidence is gained to suggest the intermediacy of κ-opioid receptors in not only neural (neuronal and glial) but also mixed glial cultures. A pertussis toxin-sensitive GTP-binding regulatory protein (G protein) and PtdIns turnover are also implicated.
Materials and Methods
Chemicals
Unless otherwise indicated, all chemicals were purchased from Sigma, St. Louis, MO, U.S.A.
Cultures
Brain cell aggregates were prepared from embryonic day 15 rat brain (Sprague–Dawley rats), as described by Barg et al. (1989a,b), and grown for various periods of time. Brain mixed glial cell aggregates were prepared from postnatal day 1 rat brain and grown for 7 days, as described previously (Guentert-Lauber et al., 1985; Barg et al., 1991). Both cultures were maintained in a chemically defined medium. Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Thymidine incorporation
Culture medium was supplemented with opioids [U69593, U50488, DAMGE, [d-Ala2,d-Leu5]enkephalin (DADLE), β-endorphin, and/or norbinaltorphimine or naltrexone; NIDA, Rockville, MD, U.S.A.] 48 h prior to being harvested. [6-3H]Thymidine (0.1 μCi/ml, 25.5 Ci/mmol; Amersham, Arlington Heights, IL, U.S.A.) was included for the final 23 h. In all experiments, the times indicated represent the total number of days that brain cell aggregates were maintained in culture. [3H]Thymidine incorporation into DNA was determined after aggregates were washed with 10% trichloroacetic acid, 5% trichloroacetic acid, ethanol/ether (1:1), and ether as described previously (Barg et al., 1989a). [3H]Thymidine uptake into cells was estimated by filtering cultures on Whatman GF/B filters (25 mm), washing them with phosphate-buffered saline (20 ml, four times), and measuring the radioactivity on the filter. The difference between the two determinations represents the amount of [3H]thymidine incorporated into cells that is in a non-DNA pool. Amount of labeled DNA was expressed as a function of the original number of seeded cells, which was 3 × 107 cells per plate. In all experiments, when the same radioactivity incorporation data were determined on the basis of protein, comparable results were obtained. Protein content was measured by the method of Lowry et al. (1951).
Autoradiography
Brain cell aggregates, prepared from embryonic day 15 rat brains and cultured for 7 days as described above, were exposed to either 1 μM U69593, or 1 μM U69593 and 1 μM norbinaltorphimine, for the final 48 h of culture and to [3H]thymidine (total and specific activity, as described above) for the last 23 h. In control experiments, U69593 and norbinaltorphimine were omitted. Cell culture medium was removed by centrifugation, then aggregates were resuspended in 0.2% agarose and centrifuged at 8,000 g for 2 min. The pellet, containing aggregates embedded in agarose solution, was frozen on dry ice and stored at −20°C. Sections (10 μm) were cut from pellets using a cryostat and thaw-mounted onto microscopic slides covered with gelatin. Specimens were covered with liquid autoradiographic emulsion (Amersham, LM1) according to the manufacturer's instructions and exposed for 4 days at room temperature. The emulsion was developed in Kodak D19 developer for 4 min at 20°C and fixed in Kodak Rapid Fixer for 10 min at 20°C. The section through the center of an aggregate was selected and the number of labeled nuclei, as well as unlabeled nuclei, was recorded over the whole section. The resulting labeling index represents a percentage of labeled cells among the total number scored. The accuracy of the index was calculated as a standard error of the mean for a binomial distribution (Sokal and Rohlf, 1981).
Inhibition or activation of thymidine incorporation
Cultures were treated with pertussis toxin (List Biological Laboratories, Campbell, CA, U.S.A.), the selective PKC inhibitor chelerythrine (Herbert et al., 1990; Vipont Laboratories, Fort Collins, CO, U.S.A.), atropine, or LiCl in the presence or absence of various concentrations of U50488 or U69593 for 48 h prior to harvesting and with [3H]thymidine (0.1 μCi/ml) for the final 23 h.
PtdIns turnover measurements
myo[3H]Inositol (2.5 μCi/ml, 15.5 Ci/mmol; NEN–Du Pont, Boston, MA, U.S.A.) was added to cultures 18 h prior to harvesting. [3H]Inositol trisphosphate ([3H]IP3) analysis was performed following the experimental procedure described by Xu and Chuang (1987).
Statistical analysis
Means of data from three or more experiments were compared using the Student's t test.
Results
The effect of the κ-selective agonist U69593 on [3H]thymidine incorporation into DNA of fetal rat brain cell aggregates grown for 7, 14, or 21 days is shown in Fig. 1. U69593 decreased [3H]thymidine incorporation by 35% in 7-day cultures. Although U69593 had no effect on thymidine incorporation in 14-day aggregates, a 3.5-fold increase was evident by day 21. Note the lower rate of thymidine incorporation into DNA in 21-day cultures (Fig. 1). Dose dependence studies performed on 7-day cultures indicated that at levels ≥0.5 μM U69593, [3H]thymidine incorporation was inhibited (Fig. 2). Attenuation of thymidine incorporation was reversed by the selective κ antagonist norbinaltorphimine (Fig. 2). Under conditions comparable to those of κ-agonist inhibition in 7-day cultures, opioid agonists etorphine (1 μM), DAMGE (1 μM), and β-endorphin (0.1 μM) diminished [3H]thymidine incorporation by 35, 28, and 52%, respectively. The opioid peptide analog DADLE (1 μM), which binds to both δ and μ sites, had an insignificant effect on [3H]thymidine incorporation into DNA (Fig. 3).
FIG. 1.
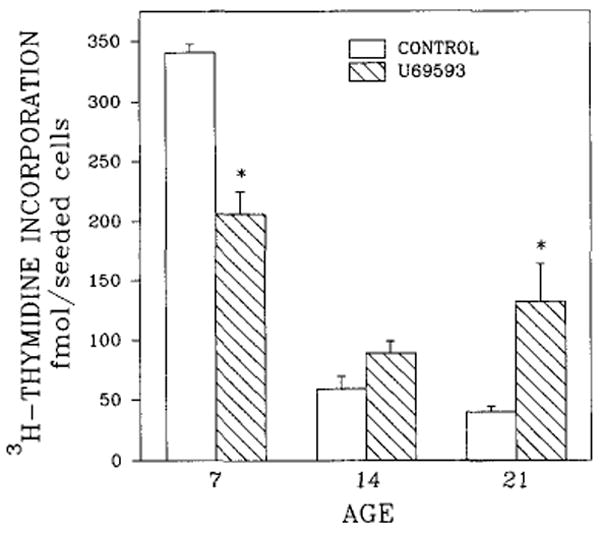
Effects of U69593 on [3H]thymidine incorporation into DNA of rat brain cell aggregates as a function of age (days in culture). Cultures were treated with 1 μM U69593 for the final 48 h, and [3H]thymidine (0.1 μCi/ml) was included in the last 23 h. Data are the means ± SEM of three to five experiments. *p < 0.05, significantly different from untreated controls.
FIG. 2.
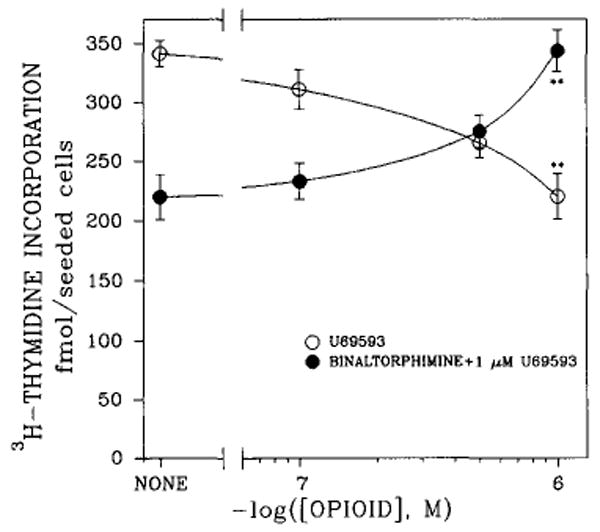
Dose-dependent effects of κ-opioid agonist U69593 and antagonist norbinaltorphimine on [3H]thymidine incorporation into DNA of rat brain cell aggregates. Cultures were grown for 5 days, then treated with various concentrations of U69593 or norbinaltorphimine in the presence of 1 μM U69593 for the final 48 h. Data are the means ± SEM of three to five experiments. **p < 0.01, significant difference between U69593 and norbinaltorphimine.
FIG. 3.
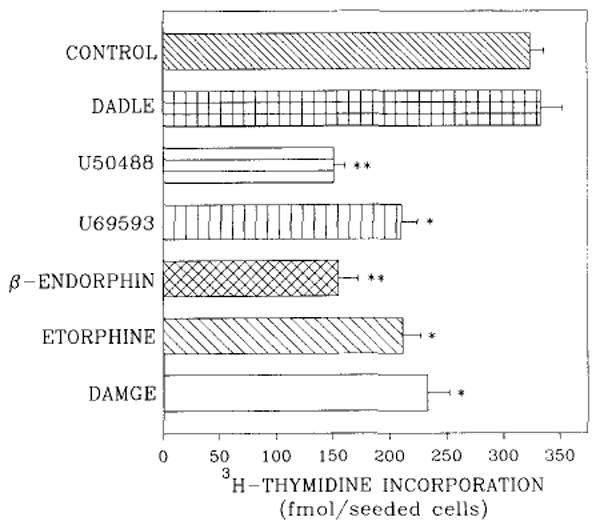
Opioid modulation of [3H]thymidine incorporation into DNA of 7-day rat brain cell aggregates. Cultures were treated with 1 μM DAMGE, 1 μM etorphine, 1 μM U69593, 1 μM U50488, 0.1 μM β-endorphin, or 1 μM DADLE for the final 48 h, and [3H]thymidine (0.1 μCi/ml) was included for the last 23 h. Data are the means ± SEM of three to five experiments. *p < 0.05 and **p < 0.01, significantly different from untreated controls.
Autoradiographic experiments revealed that 25.3 ± 1.2% of cells in 7-day brain aggregates were labeled with [3H]thymidine after 23 h of exposure to the labeled nucleoside. The labeling index decreased to 6.6 ± 0.7% in the same culture upon treatment with 1 μM U69593. Addition of both κ agonist (U69593) and antagonist (norbinaltorphimine) to the culture medium resulted in reversal of the U69593 effect (labeling index of 24.2 ± 1.0%).
The question of whether κ agonists exert their action through the cholinergic receptor system was addressed by treating brain cell aggregates with atropine and U50488. Atropine (10−7M) decreased [3H]thymidine incorporation 43%, and inclusion of 1 μM U50488 had no additional effect. Norbinaltorphimine (1 μM) did not prevent inhibition of thymidine incorporation induced by atropine.
Because many opioid receptors are coupled to pertussis toxin-sensitive G proteins, we attempted to determine whether the inhibitory action of U69593 on DNA synthesis was abolished by pertussis toxin (Fig. 4). Relatively low doses of this toxin blocked the inhibitory effect of U69593.
FIG. 4.
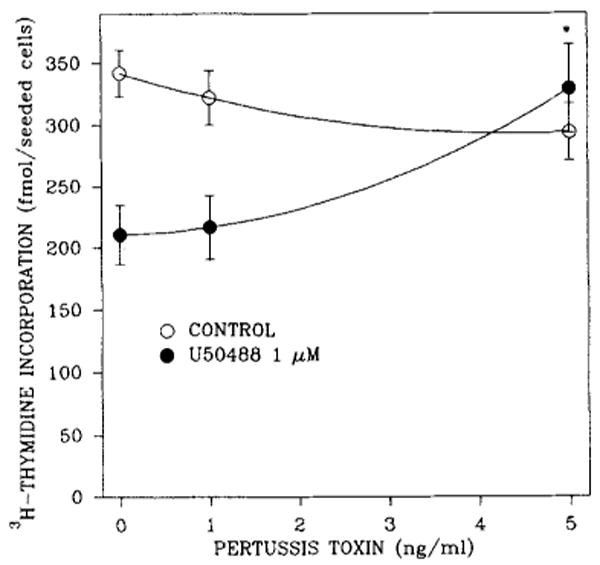
Reversal of the U50488 blockade of [3H]thymidine incorporation into DNA of 7-day rat brain cell aggregates by pertussis toxin. Cultures were exposed to 1 μM κ agonist and/or toxin 48 h prior to being harvested and to [3H]thymidine (0.1 μCi/ml) for the final 23 h. Data are the means ± SEM of three to seven experiments. *p < 0.05, significantly different from untreated controls.
The possibility that κ-opioid receptor agonist inhibition of thymidine incorporation may operate through the PtdIns turnover system was also addressed. U50488 modulation of thymidine incorporation was assessed in the absence or presence of 0.5 mM LiCl (Fig. 5), a concentration demonstrated to be less than the IC50 value (10 mM) obtained from studies of dose dependence (Barg et al., 1992). The effect of LiCl on κ-opioid receptor inhibition appeared to be biphasic. At low concentrations of U50488 (10−7–10−8M), LiCl enhanced the U50488 inhibitory effect. At high concentrations, it did not confer additional inhibition.
FIG. 5.
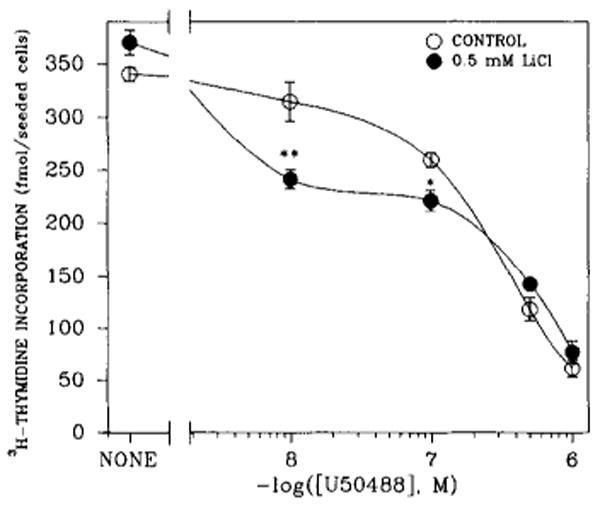
Concentration-dependent effects of U50488 on LiCl inhibition of [3H]thymidine incorporation into DNA of 7-day rat brain cell aggregates. Cultures were exposed to various concentrations of U50488 and/or to 0.5 mM LiCl 48 h prior to being harvested and to [3H]thymidine (0.1 μCi/ml) for the final 23 h. Data are the means ± SEM of three to seven experiments. *p < 0.05 and **p < 0.01, significantly different from their respective controls (cultures not treated with LiCl).
To implicate the PtdIns signal transduction system further, the effect of U69593 on IP turnover was studied in 7-, 14-, and 21-day brain cell aggregates (Fig. 6). U69593 decreased the formation of [3H]IP3 in 7-day brain cell aggregates by 79% (Fig. 6A). The decline in [3H]IP3 formation was reversed by norbinaltorphimine. In 14-day cultures, U69593 had no significant effect (Fig. 6B), whereas in cultures maintained for 21 days, U69593 stimulated formation of [3H]IP3 (Fig. 6C). The U69593 effect exhibited in 21-day cultures was also reversed by the antagonist norbinaltorphimine.
FIG. 6.
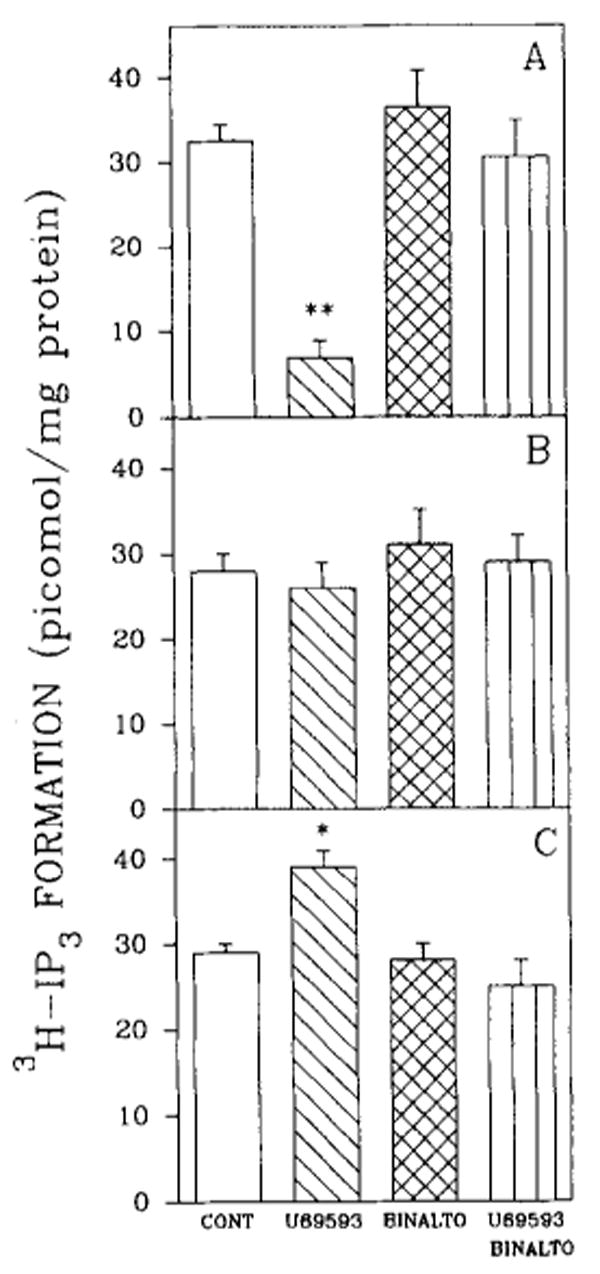
Effects of κ-opioid agonist and antagonist on [3H]IP3 accumulation in rat brain cell aggregates. The effect of U69593 and/or norbinaltorphimine (BINALTO; 1 μM) on [3H]IP3 accumulation in 7-day (A), 14-day (B), and 21-day cultures (C). Brain cell aggregates were exposed to opioids 30 min prior to being harvested and to myo[3H]inositol for the final 18 h. Data are the means ± SEM of three to five experiments. *p < 0.05 and **p < 0.01, significantly different from untreated controls (CONT).
Involvement of PKC in opioid agonist-mediated inhibition of thymidine incorporation was tested by adding a PKC inhibitor to the cells along with the κ agonist (Fig. 7). Chelerythrine, a selective PKC inhibitor, decreased thymidine incorporation in both 7- and 21-day brain cell aggregates in a dose-dependent manner. It is interesting that the U69593 effect was attenuated when the opioid was combined with chelerythrine, and a net inhibition of 55% of thymidine incorporation was evident (Fig. 7A). In the absence of chelerythrine, U69593 caused a net loss of 122 fmol of thymidine, whereas in the presence of 10−5M PKC inhibitor, the reduction elicited by the opioid was 36 fmol. Additive effects were not seen. In 21-day cultures, chelerythrine partially blocked the stimulatory effect of U69593 on thymidine incorporation (Fig. 7B). In contrast to 7-day brain cells, additive effects were evident.
FIG. 7.
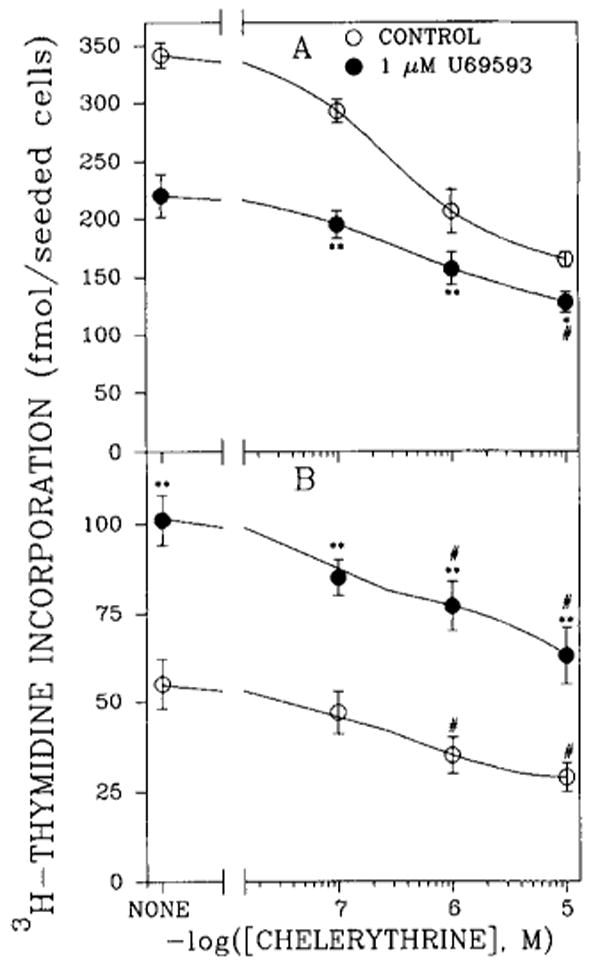
Effects of the PKC inhibitor chelerythrine and U69593 on [3H]thymidine incorporation into DNA of rat brain cell aggregates in 7-day (A) and 21-day (B) cultures. Aggregates were exposed to chelerythrine and/or opioid 48 h prior to being harvested and to [3H]thymidine for the final 23 h. Data are the means ± SEM of three to six experiments. *p < 0.05 and **p < 0.01, significantly different from cultures treated only with chelerythrine (CONTROL); *p < 0.05, significantly different from untreated controls.
Treatment of 21-day rat brain mixed glial cell aggregates with 1 μM U50488 significantly increased thymidine incorporation (Fig. 8). These results suggest that in cultures maintained for 21 days, κ-opioid stimulation of [3H]thymidine incorporation occurs primarily in mixed glial cells.
FIG. 8.
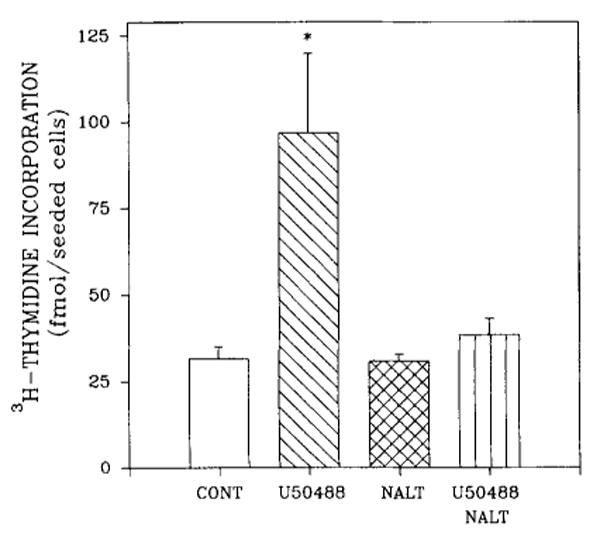
Stimulation of [3H]thymidine incorporation into DNA of rat brain mixed glial cell aggregates by U50488. Cultures were exposed to U50488 and/or naltrexone (NALT) 48 h prior to being harvested and to [3H]thymidine (0.1 μCi/ml) for the final 23 h. Incorporation of radioactivity was measured after 7 days of culture. Data are the means ± SEM of three experiments. *p < 0.05, significantly different from all the other treatments, including untreated controls (CONT).
Discussion
Similar to the μ-selective agonist DAMGE (Barg et al., 1990, 1992; Coscia et al., 1991), κ-opioid receptor agonists attenuated thymidine incorporation in 7-day cultures. This action proved to depend on agonist concentration and the time brain cell aggregates were maintained in culture. Moreover, the U69593 effect was reversed by the highly selective κ-opioid receptor antagonist, norbinaltorphimine. In contrast to DAMGE (Barg et al., 1990), U69593 induced an increase of thymidine incorporation in brain cell aggregates maintained for 21 days. Also unlike the DAMGE inhibition of DNA synthesis (Barg et al., 1992), the action of U50488 was sensitive to pertussis toxin. Taken together, these results suggest that the κ opioids utilize a different mechanism of signal transduction than μ agonists.
Opioid signaling has been shown to be transduced by pertussis toxin-sensitive G proteins (Gi and Go) that act on both adenylyl cyclase and ion channels (Burns et al., 1983; Kurose et al., 1983; Hsia et al., 1984). However, κ-mediated inhibition of dopamine-stimulated cyclic AMP production was not detected at any stage of rat brain development (De Vries et al., 1990), whereas it has been demonstrated in fetal spinal cord–dorsal root ganglion cultures (Attali et al., 1991). κ-Regulated Ca2+ ion channels have also been characterized in the same cocultures. It is also plausible that the κ-opioid receptor may interact with a second receptor that transmits the signal to the PtdIns system (Ross et al., 1990). For example, although direct inhibition of phosphatidylinositol was not demonstrable in cultured neurons from chick embryo cerebral hemispheres, both carbachol- and bradykinin-induced stimulation of IP release was blocked by chronic exposure to opioids (Mangoura and Dawson, 1991). Moreover, cholinergic receptor agonists also transiently modulate DNA synthesis in primary glial cultures of embryonic rat brain (Ashkenazi et al., 1989). The finding that U50488 together with the cholinergic antagonist atropine did not have an additive effect on the inhibition of thymidine incorporation raises several possibilities. The two compounds may compete at the same cholinergic site (Periyasamy and Hoss, 1991) or at two different binding sites. Receptor communication could occur by “cross-talk” (Hansson et al., 1990). Because norbinaltorphimine did not reverse the inhibition of thymidine incorporation caused by atropine, it is unlikely that the two drugs bind to the same site.
U69593 modulated basal rates of [3H]IP3 formation by a process that is not only reversed by norbinaltorphimine but is also temporally correlated with the inhibitory and stimulatory opioid effects on DNA synthesis. These results suggest that IP may have a role in κ-opioid receptor-mediated inhibition of thymidine incorporation. The absence of an additive effect of LiCl at either lower or higher levels of U50488 is consistent with this interpretation. The observation that IP3 formation is enhanced in postnatal day 21 brain is in agreement with the finding that κ agonists stimulated IP turnover in adult rat brain (Periyasamy and Hoss, 1990). At earlier times (7-day cultures) the κ-agonist effect entails inhibition of IP formation. As κ-subtype multiplicity is well documented (Attali et al., 1982; Zukin et al., 1988; Clark et al., 1989), different κ-receptor isoforms may be involved at the two stages of development.
Evidence that attenuation of thymidine incorporation entails inhibition of PKC was gained by combining opioids with a PKC inhibitor. Chelerythrine, a recently discovered, selective PKC inhibitor (Herbert et al., 1990), suppressed thymidine incorporation in both 7- and 21-day cultures in a dose-dependent manner. This PKC inhibitor also attenuated both the inhibitory and stimulatory effects of U69593. If chelerythrine and U69593 were acting totally independently, additive effects of the opioid would have been expected. If U69593 were acting exclusively via PKC, opioid inhibition in 7-day cultures or stimulation in 21-day cultures would not be predicted under conditions of maximal chelerythrine blockade. The results observed here suggest only a partial mediation of the opioid action by PKC. Other more complex interpretations are possible, e.g., cell types undergoing division may change with time, down-regulation of opioid receptors with subsequent changes in G protein levels may occur as a result of long-term agonist treatment (Vogel et al., 1990), or different isozymes of PKC may be involved at different developmental stages (Huang et al., 1987).
[3H]Thymidine incorporation into DNA is a function of several variables: the number of DNA-synthesizing cells, the rate of DNA synthesis in individual cells, and the specific activity of the labeled thymidine in the intracellular pool of DNA precursors (Cleaver, 1967). Thus, observed changes in [3H]thymidine incorporation may be related to the regulation of one or more of the above factors, and may or may not entail genomic events. However, the autoradiographic experiment, in which the percentage of labeled cells was measured, affords additional evidence to suggest that cell proliferation is involved.
Data demonstrating κ-agonist stimulation of [3H]thymidine incorporation into DNA in 7-day mixed glial cell aggregates are consistent with the observation that approximately 50% of the opioid receptors are of the κ type in rat glial cultures (Eriksson et al., 1990; Barg et al., 1991). This is also in accord with other reports that demonstrate the presence of κ sites in glial cells from rat brain and pituicytes in vivo (Lightman et al., 1983; Bunn et al., 1985; Eriksson et al., 1990) and U69593 modulation of DNA synthesis in mixed glial cultures (Stiene-Martin and Houser, 1991). Moreover, the extent of κ-agonist stimulation of [3H]thymidine incorporation in 21-day brain neural cell aggregates (3.5-fold, Fig. 1) was comparable to that observed for mixed glial aggregates (3.1-fold, Fig. 8). Finally, 21-day fetal rat brain cell aggregates (Fig. 1) are developmentally comparable to 14-day-old postnatal brain (Barg et al., 1989b), a time at which only glial cell proliferation occurs (Hatten, 1987). Viewed collectively, these and other observations support the theory of a glial site of neurotrophic action for κ agonists.
Acknowledgments
This work was supported by grant DA05412 from the National Institute of Drug Abuse.
Abbreviations
- DADLE
[d-Ala2,d-Leu5]enkephalin
- DAMGE
[d-Ala2,Me-Phe4,Gly-ol5]enkephalin
- G protein
GTP-binding regulatory protein
- IP
inositol phosphate
- IP3
inositol trisphosphate
- PKC
protein kinase C
- PtdIns
phosphoinositide
References
- Ashkenazi A, Ramachandran J, Capon DJ. Acetylcholine analogue stimulates DNA synthesis in brain-derived cells via specific muscarinic receptor subtypes. Nature. 1989;340:146–150. doi: 10.1038/340146a0. [DOI] [PubMed] [Google Scholar]
- Attali B, Gouarderes V, Mazarguil H, Cros J. Evidence for multiple kappa binding sites by use of opioid peptides in the guinea pig lumbosacral spinal cord. Neuropeptides. 1982;3:53–64. doi: 10.1016/0143-4179(82)90065-8. [DOI] [PubMed] [Google Scholar]
- Attali B, Nah SY, Vogel Z. Phorbol ester pretreatment desensitizes the inhibition of Ca2+ channels induced by κ-opiate, α2-adrenergic, and muscarinic receptor agonists. J Neurochem. 1991;57:1803–1806. doi: 10.1111/j.1471-4159.1991.tb06384.x. [DOI] [PubMed] [Google Scholar]
- Barg J, Levy R, Simantov RJ. Paradoxical and subtype-specific effects of opiate antagonists on the expression of opioid receptors in rat brain cultures. J Neurosci Res. 1989a;22:322–330. doi: 10.1002/jnr.490220312. [DOI] [PubMed] [Google Scholar]
- Barg J, Levy R, Simantov R. Expression of the three opioid receptor subtypes μ, δ, and κ in guinea pig and rat brain cell cultures and in vivo. Int J Dev Neurosci. 1989b;7:173–180. doi: 10.1016/0736-5748(89)90067-1. [DOI] [PubMed] [Google Scholar]
- Barg J, Belcheva M, McHale RJ, Coscia CJ. Opioid inhibition of [3H]thymidine incorporation into DNA of fetal rat brain aggregates. In: Van Ree JM, Mulder TW, Wiegant VM, Wimersma Greidanus TV, editors. New Leads in Opioid Research. Excerpta Medica; Amsterdam: 1990. pp. 252–253. [Google Scholar]
- Barg J, Belcheva M, Bem WT, Lambourne B, McLachlan JA, Tolman KC, Johnson FE, Coscia CJ. Desipramine modulation of sigma and opioid peptide receptor expression in glial cells. Peptides. 1991;12:845–849. doi: 10.1016/0196-9781(91)90144-e. [DOI] [PubMed] [Google Scholar]
- Barg J, Belcheva M, Coscia CJ. Evidence for the implication of phosphoinositol signal transduction in μ-opioid inhibition of DNA synthesis. J Neurochem. 1992;59:1145–1152. doi: 10.1111/j.1471-4159.1992.tb08357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolome JV, Bartolome MB, Daltner LA, Evans CJ, Barchas JD, Kuhn CM, Schanberg SM. Effects of β-endorphin on ornithine decarboxylase in tissues of developing rats: a potential role for this endogenous neuropeptide in the modulation of tissue growth. Life Sci. 1986;38:2355–2362. doi: 10.1016/0024-3205(86)90643-0. [DOI] [PubMed] [Google Scholar]
- Bartolome JV, Bartolome MB, Lorber BA, Dileo SJ, Schanberg SM. Effects of central administration of beta-endorphin on brain and liver DNA synthesis in preweanling rats. Neuroscience. 1991;40:289–294. doi: 10.1016/0306-4522(91)90191-p. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol lipids and DNA replication. Philos Trans R Soc Lond (Biol) 1987;317:525–536. doi: 10.1098/rstb.1987.0079. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Downes CP, Hanley MR. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982;206:587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn SJ, Hanley MR, Wilkin GP. Evidence for a kappa-opioid receptor on pituitary astrocytes: an autoradiographic study. Neurosci Lett. 1985;55:317–323. doi: 10.1016/0304-3940(85)90455-0. [DOI] [PubMed] [Google Scholar]
- Burns DL, Hewlett EL, Moss J, Vaughan M. Pertussis toxin inhibits enkephalin stimulation of GTPase of NG108-15 cells. J Biol Chem. 1983;258:1435–1438. [PubMed] [Google Scholar]
- Chuang DM. Neurotransmitter receptors and phosphoinositide turnover. Annu Rev Pharmacol Toxicol. 1989;29:71–110. doi: 10.1146/annurev.pa.29.040189.000443. [DOI] [PubMed] [Google Scholar]
- Clark JA, Liu L, Price M, Hersh B, Edelson M, Pasternak GW. Kappa opiate receptor multiplicity: evidence for two U50,488-sensitive κ1 subtypes and a novel κ3 subtype. J Pharmacol Exp Ther. 1989;251:461–468. [PubMed] [Google Scholar]
- Cleaver JE. In: Thymidine Metabolism and Cell Kinetics. Neuberger A, Tatum EL, editors. John Wiley & Sons, Inc.; New York: 1967. pp. 70–100. [Google Scholar]
- Coscia CJ, Szücs M, Barg J, Belcheva MM, Bem WT, Khoobehi K, Donnigan TA, Juszczak R, McHale RJ, Hanley MR, Barnard EA. A monoclonal anti-idiotypic antibody to μ and δ opioid receptors. Mol Brain Res. 1991;9:299–306. doi: 10.1016/0169-328x(91)90076-a. [DOI] [PubMed] [Google Scholar]
- Davila-Garcia MI, Azmitia EC. Effects of acute and chronic administration of Leu-enkephalin on cultured serotonergic neurons: evidence for opioids as inhibitory neuronal growth factors. Dev Brain Res. 1989;49:97–103. doi: 10.1016/0165-3806(89)90062-x. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Hogenboom F, Mulder AH, Schoffelmeer NM. Ontogeny of μ-, δ-, and κ-opioid receptors mediating inhibition of neurotransmitter release and adenylate cyclase activity in rat brain. Dev Brain Res. 1990;54:63–69. doi: 10.1016/0165-3806(90)90065-7. [DOI] [PubMed] [Google Scholar]
- Eccleston PA, Bannerman PGC, Pleasure DE, Winter J, Mirsky R, Jessen KR. Control of peripheral glial cell proliferation: enteric neurons exert an inhibitory influence on Schwann cell and enteric glial cell DNA synthesis in culture. Development. 1989;107:107–112. doi: 10.1242/dev.107.1.107. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Hansson E, Ronnback L. δ and κ opiate receptors in primary astroglial cultures from rat cerebral cortex. Neurochem Res. 1990;15:1123–1126. doi: 10.1007/BF01101714. [DOI] [PubMed] [Google Scholar]
- Farrar HC, Blumer JL. Fetal effects of maternal drug exposure. Annu Rev Pharmacol Toxicol. 1991;31:525–547. doi: 10.1146/annurev.pa.31.040191.002521. [DOI] [PubMed] [Google Scholar]
- Gilmore W, Weiner LP. The opioid specificity of beta-endorphin enhancement of murine lymphocyte proliferation. Immunopharmacology. 1989;17:19–30. doi: 10.1016/0162-3109(89)90004-0. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Kittstein W, Marks F. Protein kinase C activation by phorbol esters: do cysteine-rich regions and pseudosubstrate motifs play a role? Trends Biochem Sci. 1991;16:167–169. doi: 10.1016/0968-0004(91)90064-3. [DOI] [PubMed] [Google Scholar]
- Guentert-Lauber B, Monnet-Tschudi F, Omlin FX, Favrod P, Honegger P. Serum-free aggregate cultures of rat CNS glial cells: biochemical, immunocytochemical, and morphological characterization. Dev Neurosci. 1985;7:33–44. doi: 10.1159/000112274. [DOI] [PubMed] [Google Scholar]
- Hansson E, Simonsson P, Ailing C. Interactions between cyclic AMP and inositol phosphate transduction systems in astrocytes in primary culture. Neuropharmacology. 1990;29:591–598. doi: 10.1016/0028-3908(90)90072-y. [DOI] [PubMed] [Google Scholar]
- Hatten ME. Neuronal inhibition of astroglial cell proliferation is membrane mediated. J Cell Biol. 1987;104:1353–1360. doi: 10.1083/jcb.104.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayford SM, Epps RP, Dahl-Regis M. Behavior and development patterns in children born to heroin-addicted and methadone-addicted mothers. J Natl Med Assoc. 1988;80:1197–1200. [PMC free article] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hsia JA, Moss J, Hewlett EL, Vaughan M. ADP-ribosylation of adenylate cyclase by pertussis toxin: effects on inhibitory agonist binding. J Biol Chem. 1984;259:1086–1090. [PubMed] [Google Scholar]
- Huang FL, Yoshida Y, Nakabayashi H, Huang KP. Differential distribution of protein kinase C isozymes in the various regions of brain. J Biol Chem. 1987;262:15714–15720. [PubMed] [Google Scholar]
- Huang KP. Role of protein kinase C in cellular regulation. Biofactors. 1990;2:171–178. [PubMed] [Google Scholar]
- Kikkawa U, Kishimoto A, Nishizuka Y. The protein kinase C family: heterogeneity and its implications. Annu Rev Biochem. 1989;58:31–44. doi: 10.1146/annurev.bi.58.070189.000335. [DOI] [PubMed] [Google Scholar]
- Kornblum HI, Loughlin SE, Leslie FM. Effects of morphine on DNA synthesis in neonatal rat brain. Dev Brain Res. 1987;31:45–52. doi: 10.1016/0165-3806(87)90081-2. [DOI] [PubMed] [Google Scholar]
- Kurose H, Katada T, Amano T, Ui M. Specific uncoupling by islet-activating protein, pertussis toxin, of negative signal transduction via α-adrenergic, cholinergic, and opiate receptors in neuroblastoma × glioma hybrid cells. J Biol Chem. 1983;258:4870–4875. [PubMed] [Google Scholar]
- Kusnecov AW, Husband AJ, King MG, Smith R. Modulation of mitogen-induced spleen cell proliferation and the antibody-forming cell response by beta-endorphin in vivo. Peptides. 1989;10:473–479. doi: 10.1016/0196-9781(89)90061-2. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Ninkovic M, Hunt SP, Iversen LL. Evidence for opiate receptors on pituicytes. Nature. 1983;305:235–237. doi: 10.1038/305235a0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maneckjee R, Minna JD. Opioid and nicotine receptors affect growth regulation of human lung cancer cell lines. Proc Natl Acad Sci USA. 1990;87:3294–3298. doi: 10.1073/pnas.87.9.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneckjee R, Biswas R, Vonderhaar BK. Binding of opioids to human MCF-7 breast cancer cells and their effects on growth. Cancer Res. 1990;50:2234–2238. [PubMed] [Google Scholar]
- Mangoura D, Dawson G. Chronic opioid treatment attenuates carbachol-mediated polyphosphoinositide hydrolysis in chick embryo neuronal cultures. Brain Res. 1991;548:273–278. doi: 10.1016/0006-8993(91)91132-k. [DOI] [PubMed] [Google Scholar]
- McDowell J, Kitchen I. Development of opioid systems: peptides, receptors, and pharmacology. Brain Res Rev. 1987;12:397–421. doi: 10.1016/0165-0173(87)90006-3. [DOI] [PubMed] [Google Scholar]
- Periyasamy S, Hoss W. Kappa opioid receptors stimulate phosphoinositide turnover in rat brain. Life Sci. 1990;47:219–225. doi: 10.1016/0024-3205(90)90323-j. [DOI] [PubMed] [Google Scholar]
- Periyasamy S, Hoss W. Inhibition of carbachol-stimulated phosphoinositide turnover by U-50,488H in rat hippocampus: involvement of GTP-binding protein. Eur J Pharmacol. 1991;207:101–109. doi: 10.1016/0922-4106(91)90084-u. [DOI] [PubMed] [Google Scholar]
- Ross CA, Bredt D, Snyder SH. Messenger molecules in the cerebellum. Trends Neurosci. 1990;13:216–222. doi: 10.1016/0166-2236(90)90163-5. [DOI] [PubMed] [Google Scholar]
- Schmahl W, Funk R, Miaskowski U, Plendl J. Long-lasting effects of naltrexone, an opioid receptor antagonist, on cell proliferation in developing rat forebrain. Brain Res. 1989;486:297–300. doi: 10.1016/0006-8993(89)90515-5. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. In: Biometry. 2nd. Wilson J, Cotter S, editors. W. H. Freeman and Company; New York: 1981. p. 77. [Google Scholar]
- Stiene-Martin A, Houser KF. Glial growth is regulated by agonists selective for multiple opioid receptor types in vitro. J Neurosci Res. 1991;29:538–548. doi: 10.1002/jnr.490290415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub DD, Eisenstein TK, Geller EB, Adler MW, Rogers TJ. Immunomodulatory activity of μ- and κ-selective opioid agonists. Proc Natl Acad Sci USA. 1991;88:360–364. doi: 10.1073/pnas.88.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempel A, Habas J, Paredes W, Barr GA. Morphine-induced down regulation of μ-opioid receptors in neonatal rat brain. Dev Brain Res. 1988;41:129–133. doi: 10.1016/0165-3806(88)90176-9. [DOI] [PubMed] [Google Scholar]
- Vertes Z, Melegh G, Vertes M, Kovacs S. Effect of naloxone and d-Met2-Pro5-enkephalinamide treatment on the DNA synthesis in the developing rat brain. Life Sci. 1982;31:119–126. doi: 10.1016/0024-3205(82)90423-4. [DOI] [PubMed] [Google Scholar]
- Vogel Z, Barg J, Attali B, Simantov R. Differential effect of μ, δ, and κ ligands on G protein α subunits in cultured brain cells. J Neurosci Res. 1990;27:106–111. doi: 10.1002/jnr.490270116. [DOI] [PubMed] [Google Scholar]
- Xu J, Chuang DM. Muscarinic acetylcholine receptor-mediated phosphoinositide turnover in cultured cerebellar granule cells: desensitization by receptor agonists. J Pharmacol Exp Ther. 1987;242:238–244. [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Endogenous opioid systems regulate cell proliferation in the developing rat brain. Brain Res. 1987;412:68–72. doi: 10.1016/0006-8993(87)91440-5. [DOI] [PubMed] [Google Scholar]
- Zukin RS, Eghbali M, Olive D, Unterwald EM, Tempel A. Characterization and visualization of rat and guinea pig brain κ opioid receptors: evidence for κ1, and κ2 opioid receptors. Proc Natl Acad Sci USA. 1988;85:4061–4065. doi: 10.1073/pnas.85.11.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]


