Abstract
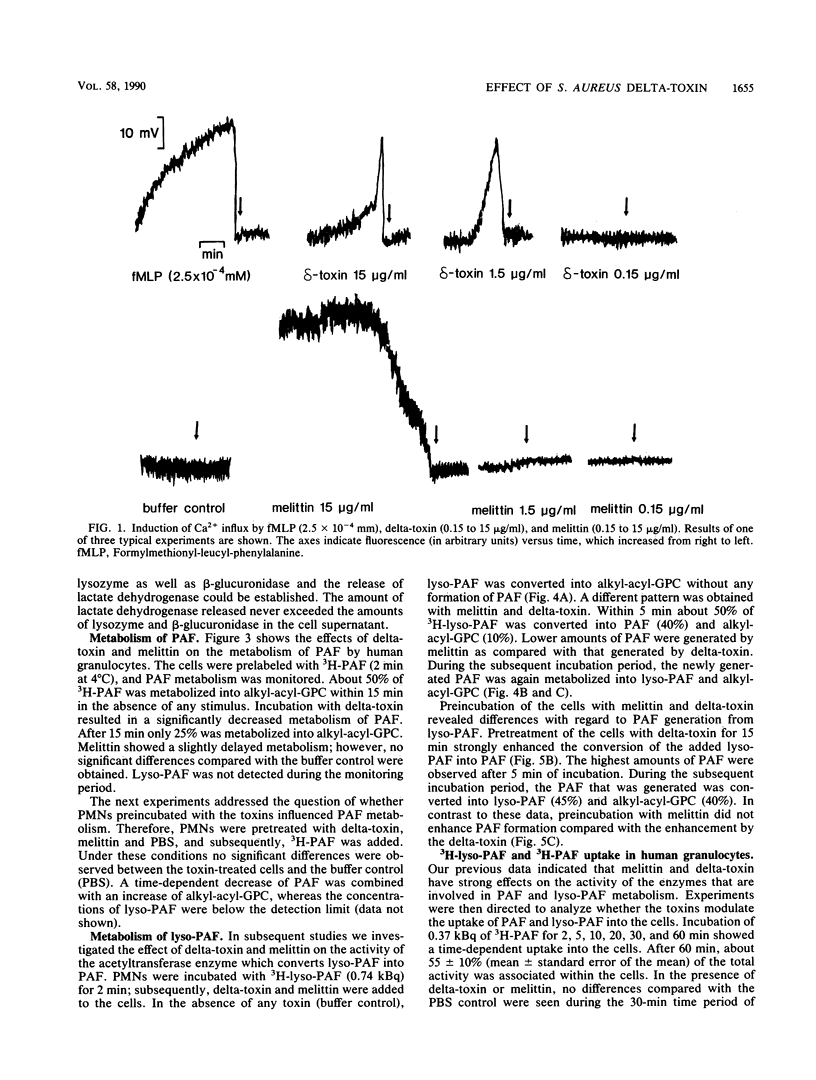
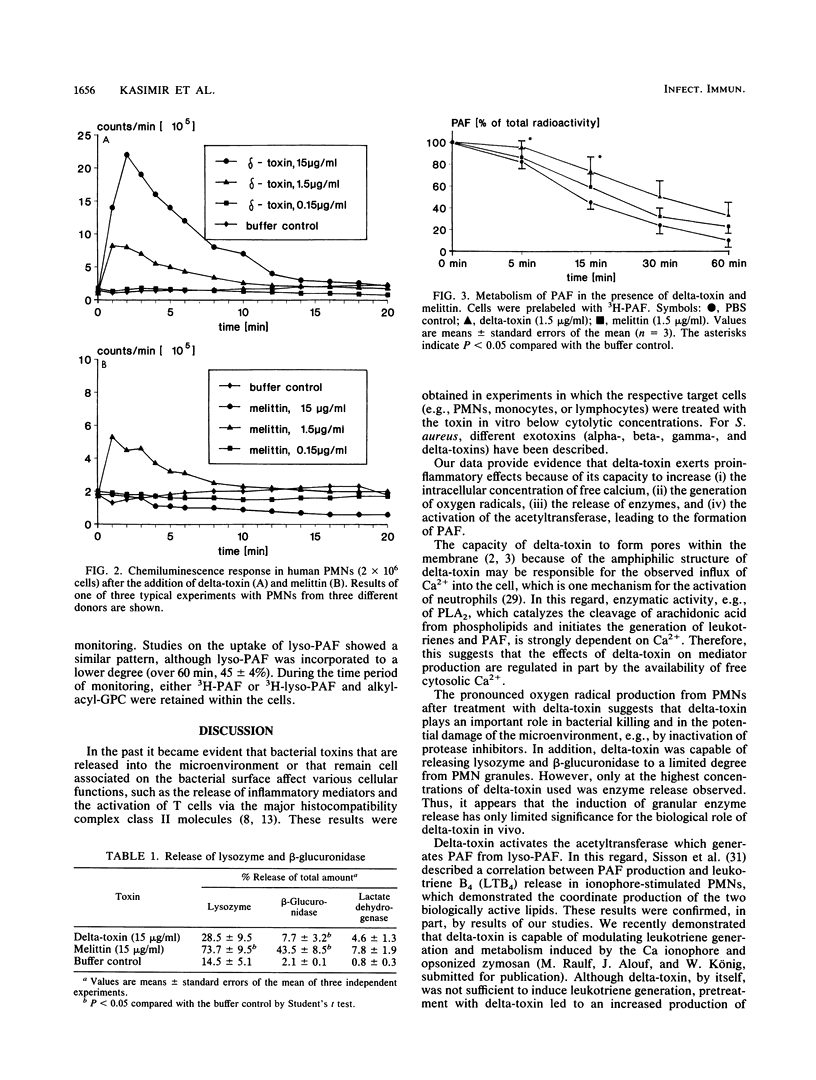
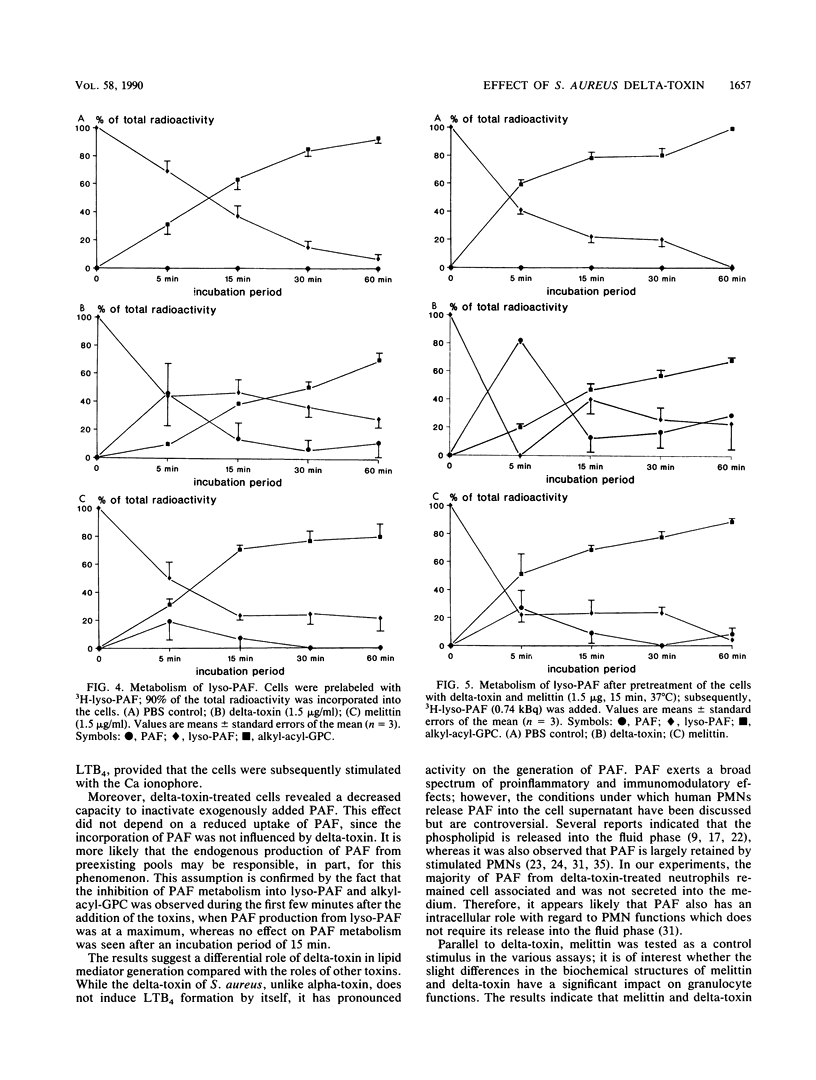
The production of delta-toxin is supposed to be responsible for various pathophysiological effects during infection with Staphylococcus aureus. We compared the effects of delta-toxin with the structurally related bee venom toxin melittin on granulocyte functions and inflammatory mediator release. Delta-toxin and melittin induced a rapid Ca2+ influx, as was shown by fluorescence detection. Furthermore, oxygen radical production, as determined by luminol-enhanced chemiluminescence, was triggered by delta-toxin (0.15 to 15 micrograms/ml), whereas melittin showed only marginal effects. Release of lysozyme and beta-glucuronidase was observed only at high concentrations of 15 micrograms of melittin and delta-toxin per ml. Preincubation (15 min) of neutrophils with both toxins resulted in the formation of 3H-platelet-activating factor (3H-PAF) from 3H-lyso-PAF. After 5 min of incubation, the exogenously added lyso-PAF was converted to PAF (delta-toxin, 80 +/- 2%; melittin, 27 +/- 12% of total radioactivity; n = 3, mean +/- standard error of the mean) and 1-O-alkyl-2-acyl-glycerophosphorylcholine (alkyl-acyl-GPC) (corresponding values, 20 +/- 3% and 51 +/- 14% of total radioactivity). The newly generated PAF was rapidly metabolized to lyso-PAF and alkyl-acyl-GPC during the subsequent incubation period of 60 min. In the absence of any toxin, no formation of PAF from lyso-PAF was observed. Further studies indicated that the metabolism of PAF into lyso-PAF and alkyl-acyl-GPC was inhibited in the presence of delta-toxin. Melittin had no significant effects on PAF metabolism. Neither delta-toxin nor melittin modulated the uptake of PAF and lyso-PAF significantly. Our data provide evidence that delta-toxin has an effect on the activity of neutrophil granulocytes with regard to its proinflammatory capacity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernheimer A. W., Rudy B. Interactions between membranes and cytolytic peptides. Biochim Biophys Acta. 1986 Jun 12;864(1):123–141. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Bhakoo M., Birkbeck T. H., Freer J. H. Interaction of Staphylococcus aureus delta-lysin with phospholipid monolayers. Biochemistry. 1982 Dec 21;21(26):6879–6883. doi: 10.1021/bi00269a039. [DOI] [PubMed] [Google Scholar]
- Bremm K. D., König W., Pfeiffer P., Rauschen I., Theobald K., Thelestam M., Alouf J. E. Effect of thiol-activated toxins (streptolysin O, alveolysin, and theta toxin) on the generation of leukotrienes and leukotriene-inducing and -metabolizing enzymes from human polymorphonuclear granulocytes. Infect Immun. 1985 Dec;50(3):844–851. doi: 10.1128/iai.50.3.844-851.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremm K. D., König W., Thelestam M., Alouf J. E. Modulation of granulocyte functions by bacterial exotoxin and endotoxins. Immunology. 1987 Nov;62(3):363–371. [PMC free article] [PubMed] [Google Scholar]
- Chilton F. H., Connell T. R. 1-ether-linked phosphoglycerides. Major endogenous sources of arachidonate in the human neutrophil. J Biol Chem. 1988 Apr 15;263(11):5260–5265. [PubMed] [Google Scholar]
- Chilton F. H., Ellis J. M., Olson S. C., Wykle R. L. 1-O-alkyl-2-arachidonoyl-sn-glycero-3-phosphocholine. A common source of platelet-activating factor and arachidonate in human polymorphonuclear leukocytes. J Biol Chem. 1984 Oct 10;259(19):12014–12019. [PubMed] [Google Scholar]
- Elstad M. R., Prescott S. M., McIntyre T. M., Zimmerman G. A. Synthesis and release of platelet-activating factor by stimulated human mononuclear phagocytes. J Immunol. 1988 Mar 1;140(5):1618–1624. [PubMed] [Google Scholar]
- Fitton J. E., Dell A., Shaw W. V. The amino acid sequence of the delta haemolysin of Staphylococcus aureus. FEBS Lett. 1980 Jun 30;115(2):209–212. doi: 10.1016/0014-5793(80)81170-7. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988 May 1;167(5):1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P. Toxins of Staphylococcus aureus. Pharmacol Ther. 1982;19(1):55–106. doi: 10.1016/0163-7258(82)90042-0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Jouvin-Marche E., Ninio E., Beaurain G., Tence M., Niaudet P., Benveniste J. Biosynthesis of Paf-acether (platelet-activating factor). VII. Precursors of Paf-acether and acetyl-transferase activity in human leukocytes. J Immunol. 1984 Aug;133(2):892–898. [PubMed] [Google Scholar]
- Kroegel C., König W., Mollay C., Kreil G. Generation of the eosinophil chemotactic factor (ECF) from various cell types by melittin. Mol Immunol. 1981 Mar;18(3):227–236. doi: 10.1016/0161-5890(81)90089-4. [DOI] [PubMed] [Google Scholar]
- Mellor I. R., Thomas D. H., Sansom M. S. Properties of ion channels formed by Staphylococcus aureus delta-toxin. Biochim Biophys Acta. 1988 Jul 21;942(2):280–294. doi: 10.1016/0005-2736(88)90030-2. [DOI] [PubMed] [Google Scholar]
- Michel L., Denizot Y., Thomas Y., Jean-Louis F., Pitton C., Benveniste J., Dubertret L. Biosynthesis of paf-acether factor-acether by human skin fibroblasts in vitro. J Immunol. 1988 Aug 1;141(3):948–953. [PubMed] [Google Scholar]
- Mollinedo F., Gómez-Cambronero J., Cano E., Sánchez-Crespo M. Intracellular localization of platelet-activating factor synthesis in human neutrophils. Biochem Biophys Res Commun. 1988 Aug 15;154(3):1232–1239. doi: 10.1016/0006-291x(88)90271-9. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Surles J. R., Redman J., Jacobson D., Piantadosi C., Wykle R. L. Binding and metabolism of platelet-activating factor by human neutrophils. J Clin Invest. 1986 Aug;78(2):381–388. doi: 10.1172/JCI112588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Wykle R. L. Biology and biochemistry of platelet-activating factor. Clin Rev Allergy. 1983 Sep;1(3):353–367. doi: 10.1007/BF02991226. [DOI] [PubMed] [Google Scholar]
- Oda M., Satouchi K., Yasunaga K., Saito K. Molecular species of platelet-activating factor generated by human neutrophils challenged with ionophore A23187. J Immunol. 1985 Feb;134(2):1090–1093. [PubMed] [Google Scholar]
- Raulf M., Stüning M., König W. Metabolism of leukotrienes by L-gamma-glutamyl-transpeptidase and dipeptidase from human polymorphonuclear granulocytes. Immunology. 1985 May;55(1):135–147. [PMC free article] [PubMed] [Google Scholar]
- Rola-Pleszczynski M., Pouliot C., Turcotte S., Pignol B., Braquet P., Bouvrette L. Immune regulation by platelet-activating factor. I. Induction of suppressor cell activity in human monocytes and CD8+ T cells and of helper cell activity in CD4+ T cells. J Immunol. 1988 May 15;140(10):3547–3552. [PubMed] [Google Scholar]
- Rossi F., Della Bianca V., Grzeskowiak M., Bazzoni F. Studies on molecular regulation of phagocytosis in neutrophils. Con A-mediated ingestion and associated respiratory burst independent of phosphoinositide turnover, rise in [Ca2+]i, and arachidonic acid release. J Immunol. 1989 Mar 1;142(5):1652–1660. [PubMed] [Google Scholar]
- Scheffer J., König W., Braun V., Goebel W. Comparison of four hemolysin-producing organisms (Escherichia coli, Serratia marcescens, Aeromonas hydrophila, and Listeria monocytogenes) for release of inflammatory mediators from various cells. J Clin Microbiol. 1988 Mar;26(3):544–551. doi: 10.1128/jcm.26.3.544-551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson J. H., Prescott S. M., McIntyre T. M., Zimmerman G. A. Production of platelet-activating factor by stimulated human polymorphonuclear leukocytes. Correlation of synthesis with release, functional events, and leukotriene B4 metabolism. J Immunol. 1987 Jun 1;138(11):3918–3926. [PubMed] [Google Scholar]
- Somerfield S. D., Stach J. L., Mraz C., Gervais F., Skamene E. Bee venom melittin blocks neutrophil O2- production. Inflammation. 1986 Jun;10(2):175–182. doi: 10.1007/BF00915999. [DOI] [PubMed] [Google Scholar]
- Tomita T., Momoi K., Kanegasaki S. Staphylococcal delta toxin-induced generation of chemiluminescence by human polymorphonuclear leukocytes. Toxicon. 1984;22(6):957–965. doi: 10.1016/0041-0101(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Wardlaw A. J., Moqbel R., Cromwell O., Kay A. B. Platelet-activating factor. A potent chemotactic and chemokinetic factor for human eosinophils. J Clin Invest. 1986 Dec;78(6):1701–1706. doi: 10.1172/JCI112765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthen G. S., Seccombe J. F., Clay K. L., Guthrie L. A., Johnston R. B., Jr The priming of neutrophils by lipopolysaccharide for production of intracellular platelet-activating factor. Potential role in mediation of enhanced superoxide secretion. J Immunol. 1988 May 15;140(10):3553–3559. [PubMed] [Google Scholar]



