Abstract
Purpose
Intrasynovial tendon injuries are at greatest risk of failure in the first three weeks after surgical repair. Attempts to improve the strength of repair by modifying rehabilitation variables have not always been successful. Manipulation of the biological environment of the sutured tendon holds great promise for accelerating the repair process. The goals of this study were to examine: 1) the range of conditions (e.g., dosage, delivery system formulation, presence of cells) over which delivery of PDGF-BB can be sustained from fibrin matrices using a heparin-binding delivery system (HBDS) and 2) the biological activity of the PDGF-BB released from this system on canine tendon fibroblasts in vitro.
Methods
We examined in vitro release kinetics from cellular and acellular fibrin matrices using Enzyme-Linked ImmunoSorbent Assays (ELISA). We examined the biologic activity of the PDGF-BB in vitro by measuring cell proliferation (i.e., total DNA) and collagen synthesis (i.e., proline incorporation).
Results
The acellular release kinetics of PDGF-BB was modulated by varying the ratio of PDGF-BB to heparin (PDGF-binding sites) or the dose of PDGF-BB in the presence of the delivery system. In the presence of canine tendon fibroblasts, the delivery system prolonged the duration of PDGF-BB release from fibrin matrices, thus demonstrating that cells are able to liberate PDGF-BB retained by the HBDS. Sustained delivery of PDGF-BB promoted increased cell proliferation at a dose of 0.125 and 1.25 μg/mL compared to fibrin without delivery system. Collagen synthesis was enhanced by PDGF-BB at a dose of 0.125 and 1.25 μg/mL, however there was an enhancement over fibrin without the delivery system only at the lower dose.
Conclusions
These results demonstrate that the PDGF-BB released from fibrin matrices containing a HBDS is biologically active and can modulate both cell proliferation and extracellular matrix synthesis, both of which are key factors in the process of tendon repair.
Keywords: drug delivery, fibrin, growth factor, tissue engineering
INTRODUCTION
Intrasynovial tendon injuries are at greatest risk of failure in the first three weeks after surgical repair due to repair-site rupture. Loss of function during flexor tendon healing of intact repairs is typically due to repair-site elongation or adhesion formation between the tendon surface and the digital sheath. Previous work has shown that controlled passive motion after repair is effective in reducing adhesion formation and maintaining digital range of motion (1–3). However, attempts to improve the strength of repair (and hence reduce repair-site elongation) by modifying rehabilitation variables have been unsuccessful, as the immature repair site has failed to respond to increasing levels of applied in vivo load (4–7). Clinical and experimental studies have suggested that future strategies for accelerating the repair process be directed toward altering the biological environment of the sutured tendon (8–20). Growth factors have been shown to be powerful regulators of biological function and their presence in tissues is highly regulated in both time and space. We hypothesized that controlled delivery of growth factors may enhance tendon fibroblast proliferation and extracellular matrix synthesis. This could accelerate tendon healing and reduce the risk of tendon repair-site elongation and rupture in vivo.
Patterns of various growth factors, such as platelet-derived growth factor BB (PDGF-BB), basic fibroblast growth factor (bFGF), transforming growth factor β1, and vascular endothelial growth factor, vary dramatically over the time course of tendon healing (21). However, the endogenous factors released during the process of repair have been insufficient to restore the tendon’s structural characteristics in the first few weeks following injury. Manipulation of the repair environment of dense regular connective tissues through the use of exogenous growth factor delivery from drug delivery systems has shown promise for stimulating collagen synthesis and increasing the stiffness and strength of the repair site. Further, previous authors have noted substantial improvement when growth factor release is maintained for a prolonged period of time during healing (8–12,14,19,22–24). In our previous reports we showed that: 1) PDGF-BB stimulates flexor tendon fibroblast proliferation and collagen synthesis in vitro(24), 2) sustained delivery of PDGF-BB in repaired flexor tendons improves range of motion(25), and 3) sustained delivery of PDGF-BB in repaired flexor tendons promotes collagen synthesis and cell proliferation(26). However, we did not demonstrate an increase in repair site strength due to PDGF-BB. The lack of improvement in repair site strength with PDGF-BB treatment may have been due to sub-optimal release kinetics and/or growth factor dosage. Based on these studies, we chose to comprehensively characterize the release kinetics and the biologic activity of PDGF-BB in vitro. To manipulate the release characteristics of the growth factor, we chose a fibrin matrix as the scaffold for the delivery system and a heparin-based delivery system (HBDS) to sequester the PDGF-BB and control its release over a two week period. We have previously used this delivery system for controlled delivery of bFGF and neurotrophins to promote neurite extension and nerve regeneration in the rat following sciatic nerve injury or spinal cord injury (27–29). In the current study, we examine both the range of conditions over which delivery of PDGF-BB can be modulated using this HBDS, as well as the biological effect that PDGF-BB, released from this system, has on canine tendon fibroblasts in vitro. Our goal is to identify the concentration of PDGF-BB and heparin that has the greatest ability to promote cell proliferation and collagen synthesis for testing in vivo.
METHODS
Growth factor delivery system
A novel growth factor delivery system was used to provide sustained delivery of PDGF-BB (30,31). Sustained delivery was achieved by immobilizing the high affinity heparin-binding growth factor PDGF-BB in a fibrin matrix, protecting it from degradation prior to release. Release of growth factor from the matrix may occur via three mechanisms: 1) Dissociation of growth factor from matrix-bound heparin and subsequent diffusion of free heparin-binding growth factor; 2) Proteolytic degradation of the fibrin matrix; or 3) Enzymatic degradation of heparin. The first mechanism is passive and occurs in the presence or absence of cells. With the latter two methods, cells infiltrate and remodel the matrix by releasing the enzymes heparinase (32,33) and plasmin (34). Both passive and active (cell-mediated) release was examined in this study.
All reagents and chemicals were purchased from Sigma Aldrich, Saint Louis, MO unless otherwise indicated. In vitro experiments were performed with three different concentrations of PDGF-BB (R&D Systems, Minneapolis, MN) - 0.125 μg/mL, 0.25 μg/mL and 1.25 μg/mL; and varying concentrations of the delivery system component heparin. Fibrin matrices comprising of 10 mg/ml fibrinogen (EMD Chemicals Inc, San Diego, CA), 6.9mM CaCl2, and 12.5units/ml thrombin in Tris-buffered saline (TBS, 137mM NaCl, 2.7mM KCl, 33mM Tris, pH 7.4) were made. In one group, heparin was not added to serve as the “no delivery system” group with a PDGF-BB to heparin ratio of 1:0. In three other groups, concentration of heparin was varied to yield groups with PDGF-BB to heparin molar ratios of 1:0, 1:100, 1:1,000 or 1:1,0000 respectively. ATIII peptide (dLNQEQVSPK(βA)FAKLAARLYRKA-NH2, where dL denotes dansyl leucine) was used at concentrations corresponding to the different amounts of heparin to maintain a constant ratio of 1:10 for heparin to peptide. Passive release was studied for all PDGF-BB to heparin molar ratios. For the cell-mediated release, fibrin degradation, cell proliferation and collagen production studies, the 1:0 and 1:1,000 groups were evaluated. A detailed description of our methodology follows.
Cell isolation
For all cell-based experiments, canine tendon fibroblasts were used. These cells were used to make the results relevant to our canine flexor tendon animal mode (2,4,7,25,26,35,36). Fibroblasts were isolated from canine flexor digitorum profundus (FDP) tendons as described previously (24). The isolated cells were incubated (37°C, 5% CO2, and 100% humidity) in DMEM containing 10% fetal bovine serum with additional antibiotics (5x Pen/Strep, 1x Fungizone). The media was changed three times a week until the cells became confluent. The cells were used between passages 2 and 4 for experiments.
PDGF-BB release kinetics
Passive release kinetics
Fibrinogen solutions were made as described previously (26). Fibrin matrices (20 mg/mL fibrinogen stock solution) were made in 400μL volume in 24-well tissue culture plates pre-coated with bovine serum albumin (BSA) to prevent nonspecific adsorption (N=4 per group in triplicate). The matrices were allowed to polymerize for one hour at 37°C, 100% humidity before a 400μL wash of Tris buffered saline plus 2% BSA (TBS-2% BSA) was added. The entire 400 μL wash volume was collected and replaced five times over the first 24 hours, and then once per day for 10 days. The wash volumes were stored at −80°C. At the end of 10 days, the matrices were cut into fragments and placed in 4 ml of extraction buffer containing 10 mg/ml heparin, 1% BSA, 2M sodium chloride, and 0.01% Triton-X in phosphate buffered saline. The fragmented matrices were incubated in the extraction buffer for 72 h at 4°C with constant shaking. The samples were then centrifuged to pellet the matrix fragments, and the extraction buffer was collected and stored at −80°C. A sandwich ELISA for PDGF-BB (R&D systems Minneapolis, MN) was performed on daily wash samples and endpoint extract volumes to determine passive growth factor release kinetics. The total PDGF-BB was determined to be the sum of the PDGF-BB in the wash solutions and that remaining in the fibrin after 10 days. Percent of cumulative release was calculated as the amount of PDGF-BB in all of the washes up to that time point added together and divided by the total PDGF-BB measured. Changes between groups and over time were tested using a repeated measures analysis of variance (ANOVA) with significance level set at p < 0.05. Pair-wise comparisons for group and time were then made using a Fisher’s Least Squares Difference post-hoc test. In addition to post-hoc tests comparing treatment group and time, post-hoc comparisons of release at days 1 and 10 were run for Figure 1 and Figure 2.
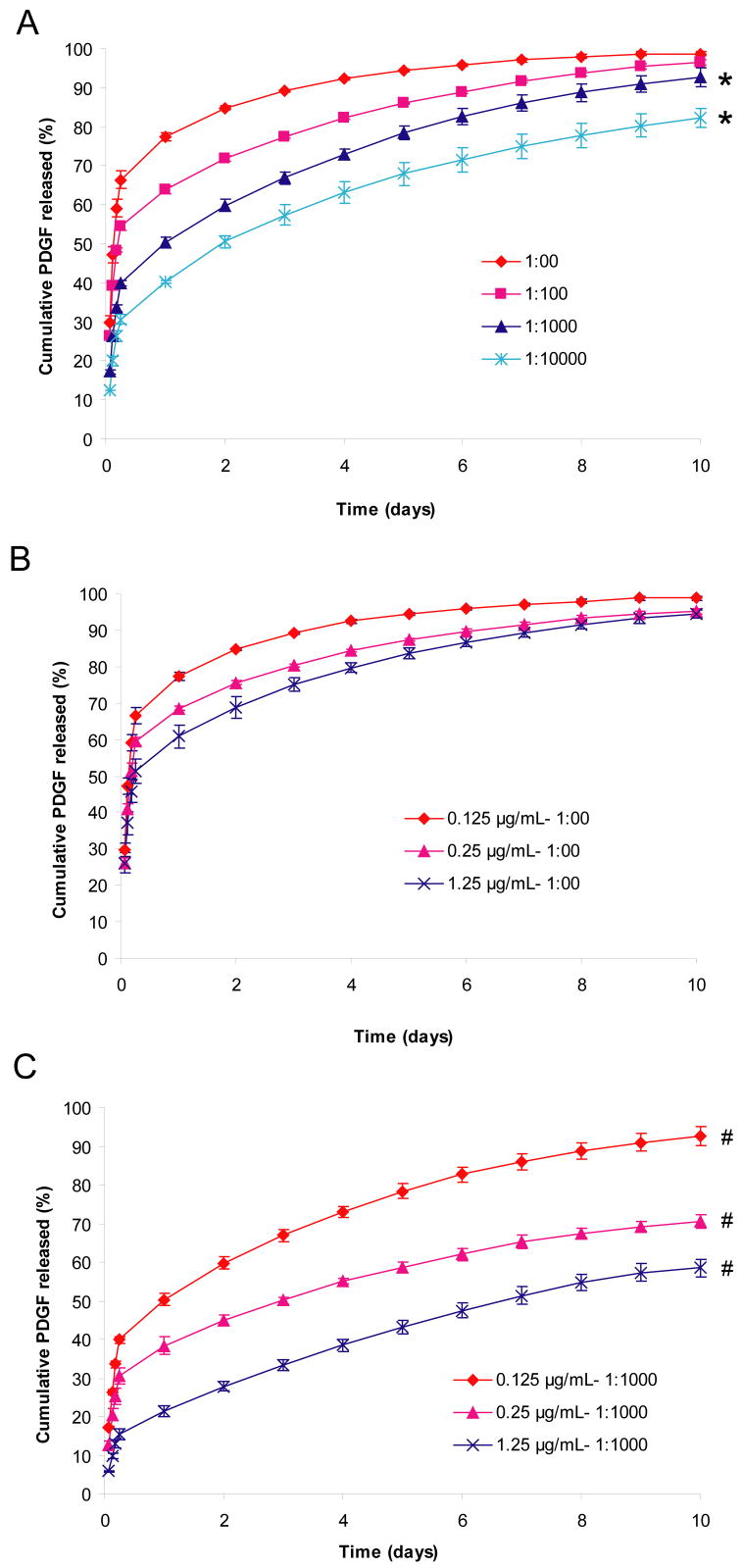
Figure 1. Effect of growth factor to heparin ratio and PDGF-BB concentration on the rate of passive PDGF-BB release.
A) Effect of varying the growth factor to heparin ratio on release at constant PDGF-BB concentration (0.125 μg/μL) in the matrix. B) Effect of varying the growth factor concentration on release from fibrin matrices with no delivery system. C) Effect of varying the growth factor concentration on release from fibrin matrices with delivery system (PDGF:heparin ratio 1:1,000). Fibrin matrices containing HBDS and PDGF-BB were washed with 1 mL of TBS five times for the first 24 h and once every 24 h for the following 9 days. Percentage of cumulative PDGF-BB released was calculated by dividing the amount of PDGF-BB present in the wash cumulative by the total amount of PDGF-BB present cumulatively in both the wash and the matrix at the end of the study. ‘*’ denotes p<0.05 versus no delivery system over time. ‘#’ denotes p<0.001 versus the other two doses of PDGF-BB over time.
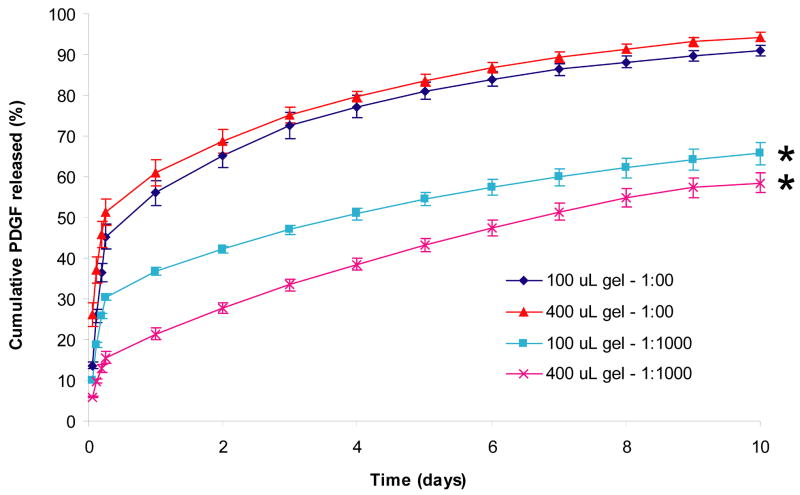
Figure 2. Effect of matrix size on rate of PDGF-BB release.
Fibrin matrices containing HBDS and PDGF-BB were washed with 1 or 0.25 mL of TBS five times for the first 24 h and once every 24 h for the following 9 days. Percentage of cumulative PDGF-BB released was calculated by dividing the amount of PDGF-BB present in the wash cumulative by the total amount of PDGF-BB present cumulatively in both the wash and the matrix at the end of the study. ‘*’ denotes p<0.001 versus no delivery system over time.
Cell mediated release kinetics
Fibrin matrices were made as described above. The matrices were washed five times with TBS-2% BSA in the first 24 h and wash volumes were collected. The next day, canine tendon fibroblasts were seeded on top of the fibrin matrices at a concentration of 50,000 cells/well in 400 μL culture medium containing DMEM, 1% FBS, 1x L-Glutamine, 1x Penicillin/Streptomycin, 1% non-essential amino acids, and 1% Sodium pyruvate. Four experiments were performed with different cell isolations (N=4 per group in triplicate). Media was changed and collected daily for 10 days. The media samples were stored at −80°C. After 10 days, the remaining fibrin matrices were fragmented and treated as described above to collect extract volumes. A sandwich ELISA for PDGF-BB was performed on the TBS-BSA washes, media samples and extract volumes. The percent fraction of PDGF-BB released per day by cell-mediated degradation of the fibrin matrix was calculated from the PDGF-BB measured at each day (from ELISA) normalized to the total amount of PDGF-BB put in (measured by acellular release ELISA) to characterize the cell-mediated release profile of the growth factor from the fibrin matrix. An ANOVA followed by a post-hoc test for each individual time point (day 1–9) was run for Figure 3 to test for significant differences.
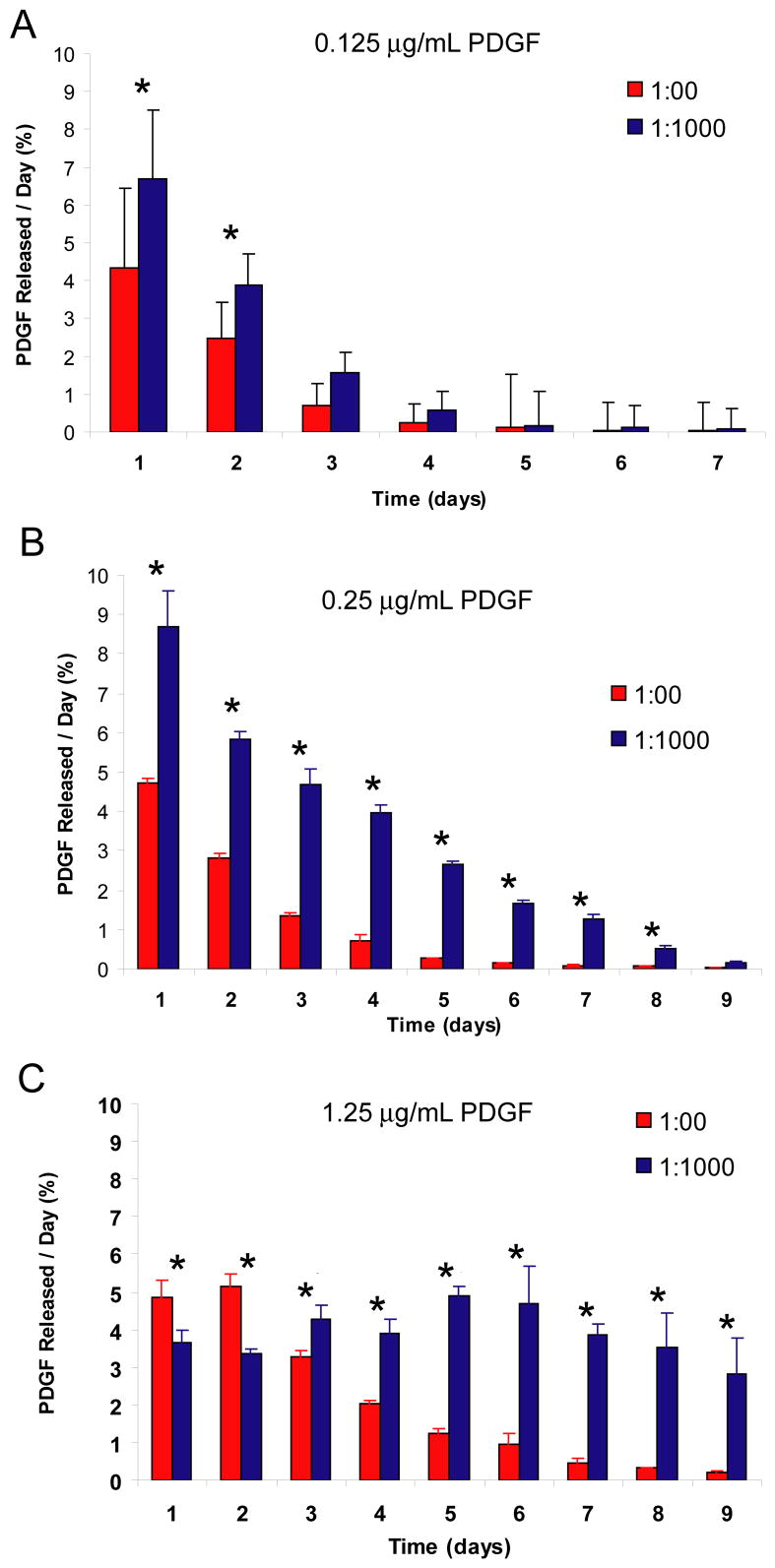
Figure 3. Effect of heparin-based delivery system on cell-mediated release of PDGF-BB.
A) Effect of delivery system on the percentage of PDGF-BB recovered for matrices containing 0.125 μg/mL of PDGF-BB. B) Effect of delivery system on the percentage of PDGF-BB recovered for matrices containing 0.25 μg/mL of PDGF-BB. C) Effect of delivery system on the percentage of PDGF-BB recovered for matrices containing 1.25 μg/mL of PDGF-BB. Fibrin matrices containing HBDS and PDGF-BB were washed with 1 mL of TBS five times for 24 h and then seeded with 50,000 tendon fibroblasts per matrix. The media was collected every 24 h for the following 9 days and the PDGF-BB in the media each day was quantified by ELISA. ‘*’ denotes p<0.05 versus no delivery system at a given time point.
Sandwich ELISA
PDGF-BB release was quantified by using a commercially available Quantikine ELISA kit in a 96-well plate format according to manufacturer’s instructions. Briefly, a seven point standard curve was made ranging from 0 to 2000 ng/mL PDGF-BB. Each sample was read in duplicate and the average of the two readings was used to calculate the concentration of PDGF-BB in the sample. From the concentrations, the amount of PDGF-BB released per day and cumulative release amounts were calculated.
Fibrin matrix degradation
To evaluate the in vitro degradation of the fibrin matrix delivery system, fibrin matrices were made as described above with or without delivery system. The ratio of PDGF:Heparin in the delivery system group was 1:1,000. Four experiments were done with different cell isolations (N=4 per group in triplicate). A small amount of fluorescently tagged fibrinogen (2.5 mg/ml concentration, Alexa-488 conjugated fibrin, Invitrogen Corporation, Carlsbad, CA) was incorporated into the matrices before use. 400 μL matrices were created in triplicate in 24 well tissue culture plates. 50,000 canine tendon fibroblasts were seeded on the top surface of the matrices. A second group of matrices was not seeded with cells to determine baseline fluorescence levels due to unbound fluorescent fibrin. 400 μL of serum containing media (1% FBS) was then added to each well. Media was collected from each well daily and replaced with fresh media. The fluorescence intensity of the sampled media was measured on a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA). The amount of fibrinogen that was released into the media is presented as cumulative fluorescent intensity. An ANOVA followed by a post-hoc test was run to compare doses of PDGF-BB.
Cell proliferation
Fibrin matrices were made in 400 μL volume as described before. The matrices were washed five times with TBS-2% BSA in the first 24 h. The next day, canine tendon fibroblasts were seeded on top of the fibrin matrices at a concentration of 50,000 cells/well. Four experiments were performed with different cell isolations (N=4 per group in triplicate). Media was changed daily and discarded. At the end of 6 days, the fibrin matrix was dissolved with plasminogen (1 U/mL) and the cells were collected. Cell number was quantified in a 96-well plate by a fluorescence-based DNA assay using commercially available CyQUANT kit (Invitrogen Corporation, Carlsbad, CA) according to manufacturer’s instructions. Briefly, the cell pellets were thawed at room temperature and 200 μL of cell lysing buffer containing CyQUANT GR dye was added to each tube. The samples were then transferred to a 96-well plate and fluorescence was measured on a SpectraMax M2 microplate reader at 480 nm excitation and 520 nm emission wavelengths. The cell number was calculated from the fluorescence readings using a standard curve. An ANOVA followed by a post-hoc test was run to compare the delivery system group to the no delivery system group for each dose of PDGF-BB test (treatment versus dose).
Collagen synthesis by fibroblasts
Collagen synthesis by canine tendon fibroblasts in vitro was determined using methods described previously (24). Fibrin matrices were made in 400 μL volume as described before. The matrices were washed five times with TBS-2% BSA in the first 24 h. The next day, canine tendon fibroblasts were seeded on top of the fibrin matrices at a concentration of 50,000 cells/well. Tritiated proline (3H-proline) was added at a concentration of 5μci/mL of media. Three experiments were performed with different cell isolations (N=3 per group in triplicate). Media was changed daily and collected. The media samples were pooled for each matrix at the end of 6 days and dialyzed against 3% acetic acid. The dialysates were freeze-dried and hydrolyzed in 6N hydrochloric acid (HCl) for 24 hours at 108 C. The hydrolysates were then subjected to cation-exchange High Performance Liquid Chromatography (HPLC) to isolate and quantify 3H-hydroxyproline indicative of newly synthesized collagen. An ANOVA followed by a post-hoc test was run to compare the delivery system group to the no delivery system group for each dose of PDGF-BB (treatment versus dose).
RESULTS
Quantifying passive release of PDGF-BB from fibrin matrices containing the HBDS
The effect of varying the heparin concentration on the release of PDGF-BB from fibrin matrices was studied using growth factor to heparin molar ratios that varied from 1:00 to 1:10,000. The cumulative release of PDGF-BB from fibrin matrices was determined over 10 days (Figure 1A). Release of PDGF-BB from control matrices without heparin present (1:00) occurred rapidly, and 89% of the growth factor initially present in the matrix was released within 3 days. With low molar ratios of PDGF-BB to heparin (1:100), the release of PDGF-BB from the matrix was somewhat slower initially (78% released within 3 days) however; similar amounts were released over 10 days (p=0.083 for comparing 1:00 versus 1:100 over all time points). At a PDGF-BB to heparin ratio of 1:1,000 or greater, the PDGF-BB release from the fibrin matrix occurred more slowly (67% and 57% released within 3 days for 1:1,000 and 1:10,000 groups, respectively). The initial burst release from 1:1,000 and 1:10,000 was smaller than for lower ratios and the amount of PDGF-BB remaining bound in the matrices at 10 days was greater (p=0.0002 at day 10 for 1:1,000 and 1:10,000 versus 1:00). This bound portion of PDGF-BB remaining in the matrix may be released by degradation of the matrix either by the addition of plasmin, or by cell-mediated degradation in the presence of cells. These results suggest that PDGF-BB release from the HBDS can be tuned by varying the concentrations of its components to attain the desired release profile over a 10 day period.
The effect of PDGF-BB dose on the rate of release was also evaluated. Three doses of PDGF-BB (0.125, 0.25 and 1.25 μg/mL) were evaluated at a PDGF-BB to heparin ratio of 1:1,000 and 1:00 (no delivery system). In the absence of heparin, there was no difference in the rates of release for the three doses of PDGF-BB tested (p=0.348 comparing dose groups, Figure 1B). However, in the presence of the delivery system, an effect of PDGF-BB concentration was observed. The release rate for each dose of PDGF-BB was significantly different from the other doses (p<0.0001 comparing dose groups) (Figure 1C).
Effect of fibrin matrix size (volume) on the rate of passive release
We examined the effect of fibrin matrix size on the rate of PDGF-BB release. The release of PDGF-BB from the 100 μL fibrin matrices was slightly faster than that for the 400 μL matrices (p=0.004, Figure 2). The release profiles for PDGF-BB were similar for matrices lacking the delivery system over the duration of release (p=0.11). However, in both cases release was significantly faster in matrices without delivery system compared to those with delivery system (p<0.0001). This result suggests that scaling fibrin matrix size will not have major effect on the release rate of PDGF-BB, and that passive release results should correlate between different matrix volumes.
Effect of PDGF-BB to heparin ratio on cell mediated release
Release of PDGF-BB can occur by passive means (dissociation and subsequent diffusion from the matrix) or by active (cell mediated) means. We examined cell mediated PDGF-BB release with and without the delivery system. In these studies, only the available (i.e., extracellular) PDGF-BB could be measured. The PDGF-BB that is internalized by the cells cannot be quantified, as it may be degraded by the cells and/or masked from the antibodies used in the ELISA. This results in some PDGF-BB that cannot be measured. Typically, we were able to recover 60–70% of the PDGF-BB that was originally put in the gels. This yield was similar in all groups. Thus, the data for this study is reported as the percentage of initial PDGF-BB loaded in the fibrin matrix that is measured in the cell culture media each day over a 9 day period (Figure 3A–C). The fraction of PDGF-BB measured in the media in these studies was always higher for matrices containing the delivery system than for those that did not for the two lower dose of PDGF-BB tested (0.125 and 0.25 μg/mL) (p<0.0001). For the highest dose tested (1.25 μg/mL) the PDGF-BB fraction was higher for the first two days in the no delivery system group, but the PDGF-BB fraction for the remaining 7 days was higher in the media from the delivery system matrices than that from the matrices without delivery system (p<0.004 at each time point). At this dose, a significant amount of PDGF-BB was detected in the media for the duration of the cell culture period (9 days). These results demonstrate that fibrin matrices containing delivery system are able to retain PDGF-BB better than matrices lacking delivery system, and the PDGF-BB that is retained can be released by cells in a dose dependent manner.
Effect of PDGF-BB release on fibrin matrix degradation
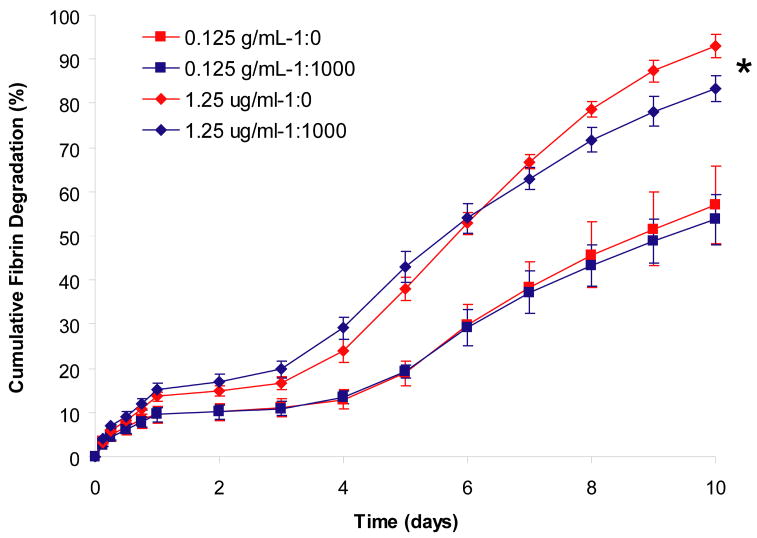
We examined the effect of both the delivery system components (i.e. heparin) and PDGF-BB on the rate of fibrin degradation in the presence of tendon fibroblasts. The presence or absence of delivery system (1:1,000 or 1:00 PDGF-BB to heparin ratio, respectively) did not affect the rate of fibrin matrix degradation (Figure 4), however matrices containing 1.25 μg/mL of PDGF-BB showed a faster rate of degradation than matrices containing 0.125 μg/mL PDGF-BB (p<0.0001). This suggests that the HBDS does not have an effect of the rate of fibrin degradation, which confirms previous studies we have performed (26).
Figure 4. Effect of PDGF-BB concentration and growth factor to heparin ratio on the rate of fibrin matrix degradation by tendon fibroblasts.
Fibrin matrices containing HBDS and PDGF-BB were washed with 1 mL of TBS five times for 24 h and then seeded with 50,000 tendon fibroblasts per matrix. The media was collected every 24 h for the following 9 days and the fibrinogen in the media or TBS was quantified by measuring the fluorescently labeled fibrinogen in solution. ‘*’ denotes p<0.001 versus 0.125 μg/mL of PDGF-BB with or without delivery system over time.
Effect of controlled delivery of PDGF-BB on tendon fibroblast proliferation
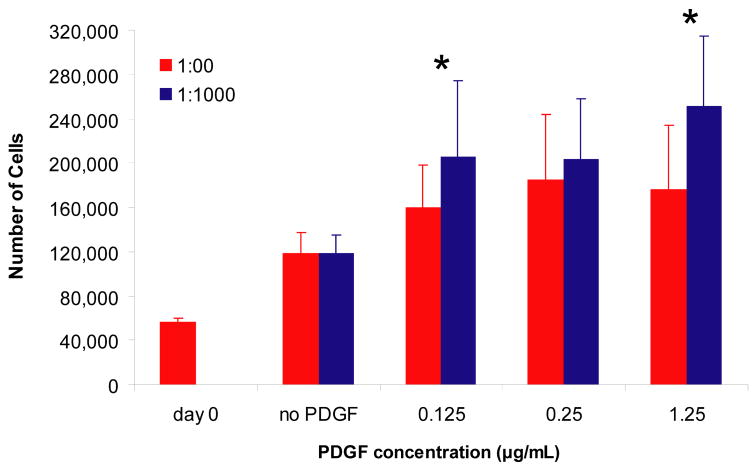
We examined the effect of delivery system on the ability of PDGF-BB to promote cell proliferation. The number of cells present on the matrices was determined after 6 days in culture by measuring the total DNA present (Figure 5). At day 0, after washing the matrices to remove unbound PDGF-BB, the matrices were seeded with approximately 50.000 cells (~57,000 by DNA assay). After 6 days, in the absence of PDGF-BB, the cell number had roughly doubled (~118,000). In the presence of PDGF-BB with delivery system, this number increased to between ~200,000 and ~250,000 depending on the dose of PDGF-BB, whereas without the delivery system this number increased to between ~160,000 and 185,000 cells. At both 0.125 and 1.25 μg/mL of PDGF-BB, these increases in cell number were statistically significant (p=0.036 and p=0.001, respectively). There was also a significant effect of PDGF-BB dose on cell number (p<0.0001). These results demonstrate that controlled delivery PDGF-BB can enhance cell proliferation to a greater extent then PDGF-BB in unmodified fibrin matrices (p=0.003 when comparing all doses tested).
Figure 5. Effect of controlled PDGF-BB delivery on tendon fibroblast proliferation on fibrin matrices.
Fibrin matrices containing HBDS and PDGF-BB were washed with 1 mL of TBS five times for 24 h and then seeded with 50,000 tendon fibroblasts per matrix. After 6 days, the cells were harvested by trypsinization, and the number of cells was quantified by measuring the total DNA present. ‘*’ denotes p<0.05 versus no delivery system at a given PDGF-BB dose.
Effect of controlled delivery on PDGF-BB on collagen production
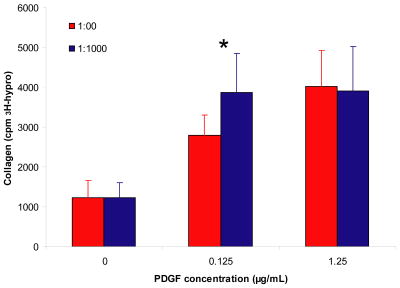
We examined the effect of the delivery system on the ability of PDGF-BB to promote collagen synthesis. In the presence of PDGF-BB with the delivery system, collagen synthesis was significantly increased in the 0.25 μg/mL group (p<0.04) (Figure 6). There was no effect of the delivery system in the absence of PDGF-BB (i.e., the 0 μg/mL group) or in the 1.25μg/mL group. Based on an ANOVA, there was a significant effect of PDGF-BB dose on collagen synthesis (p<0.001 comparing both with and without delivery system). These results demonstrate that controlled delivery PDGF-BB can enhance collagen synthesis to a greater extent then PDGF-BB in unmodified fibrin matrices
Figure 6. Effect of controlled PDGF-BB delivery on tendon fibroblast collagen synthesis.
Fibrin matrices containing HBDS and PDGF-BB were washed with 1 mL of TBS five times for 24 h and then seeded with 50,000 tendon fibroblasts per matrix. The media was changed each day and after 6 days all the media samples were pooled for analysis. 3H-hydroxyproline content was analyzed to determine the relative amount of newly synthesized collagen. ‘*’ denotes p<0.05 versus no delivery system at a given PDGF-BB dose.
DISCUSSION
In this study we examined the release kinetics and the biologic effect of PDGF-BB in vitro. We showed that the release of the growth factor could be controlled by changing the concentration of the heparin in our delivery system. We also showed that sustained release of PDGF-BB resulted in increased cell proliferation and matrix production. The responding cells in our in vitro system were canine fibroblasts isolated from the same region as those used in our previous in vivo flexor tendon studies. As cell proliferation, migration, and matrix synthesis are influenced by a number of growth factors that are released by cellular elements in the initial clot (e.g., PDGF-BB, insulin-like growth factor 1, and transforming growth factor beta 1), the delivery of growth factors from a fibrin matrix may both mimic the initial wound healing environment following tendon repair and allow for control of cell activity. Through the use of growth factors, the rate of cell proliferation, migration and extracellular matrix synthesis could be modulated to enhance and accelerate the healing process.
The sequence and duration of growth factor delivery have been shown to be key variables in stimulating demonstrable biological effects at the time of tendon and ligament repair. As growth factors delivered by bolus injection are likely cleared before local fibroblasts appear at the repair site, we hypothesized that sustained growth factor delivery could result in a more effective repair response. Our data show that we can control the release of PDGF-BB by varying the level of heparin in the delivery system. Increased levels of heparin slowed the release of the PDGF-BB, regardless of growth factor concentration. As a result, the tunability of the release kinetics allows us to target cells during specific phases of healing in vivo.
Previously, we demonstrated the ability of PDGF-BB to promote cell proliferation and improve range of motion in vivo in a canine flexor tendon injury model (25,26). However, the delivery system and dose of PDGF-BB used in those studies was not optimized for the injury model. In an effort to define the appropriate dose and delivery system formulation for use in vivo, we explored the role of growth factor to heparin ratio, PDGF-BB concentration, and fibrin matrix volume on the rate of PDGF-BB delivery from fibrin matrices containing the heparin-based delivery system in vitro. In our previous studies, we tested a PDGF-BB concentration of 0.25 μg/mL in vivo and 0.1 μg/mL in vitro. Initially, there was concern about the formation of deleterious adhesions due to PDGF-BB delivery, so we selected a modest total dose of 100 μg for delivery in vivo. After early studies demonstrated that adhesions did not form between the tendon and sheath (25,26), we selected higher doses for study in both our in vitro and in vivo systems. A further goal was to establish an in vitro model that mimicked the in vivo model as closely as possible (in so far as dose and size of the matrix are concerned).
The results of the current study demonstrate that both the growth factor to heparin ratio and the dosage level of PDGF-BB play a role in the rate of release of PDGF-BB from fibrin matrices containing the HBDS. While the heparin ratio was expected to have an effect because it increases the concentration of free heparin-binding sites that are able to bind and retain PDGF-BB, the effect of dose was not expected. In order to examine the effect of PDGF-BB dose at a constant PDGF-BB to heparin ratio, the concentration of heparin was increased as we increased the dosage level of PDGF. The increased concentration of heparin may have served to shift the initial equilibrium concentration of PDGF-BB to the matrix bound state or to have shifted the fraction of PDGF-BB to the bound state by increasing the concentration of heparin available for binding. The size of the matrix, however, did not appear to have a major influence on the rate of PDGF-BB release. The significance of these findings is twofold: we can now use, with confidence, experimental data obtained with larger matrices, and we can utilize large samples for in vitro cell seeding and release studies.
Additional findings of the current study relate to data on cell-mediated release, a critical factor related to tendon healing in vivo. We demonstrate that the HBDS allows a greater fraction of PDGF-BB to be released in the presence of cells. This finding suggests that cellular activity during tendon healing could lead to a further release of matrix-bound PDGF-BB allowing for an enhanced local effect through increased cell proliferation and matrix production within repair site rather than on the gliding surface. The increased cell numbers demonstrated in Figure 5 support this hypothesis.
There was little effect of PDGF-BB release rate on fibrin degradation. Sustained release of PDGF-BB resulted in a similar rate of fibrin degradation compared to bolus release of PDGF-BB. However, there was a dose effect, with higher doses of PDGF-BB leading to a faster rate of degradation. These results imply that the amount of fibrin gel remaining at the wound site after surgical repair in vivo cannot be used as the sole indicator of PDGF-BB remaining at the wound site because the release rate is also dose dependent. Caution must therefore be taken when estimating the release kinetics of PDGF-BB in vivo. Ideally, ELISA methods could be used to determine in vivo growth factor release, however this can prove to be technically challenging.
The functional cell response assays demonstrated that cell proliferation and collagen synthesis can be enhanced with sustained release of PDGF-BB. However, not all doses led to statistically significant increases when examining the effect of the delivery system. For example, collagen synthesis was increased at the lower dose due to sustained release of PDGF-BB, but was equivalent to bolus release at the higher dose level. It is possible that increased collagen synthesis may result from any dose level above a certain threshold dose. Our high dose may have reached this threshold level, even after washing away the growth factor that was released in the initial bolus without the delivery system present. In addition, we did not look for the expression of cartilage or bone specific markers, so we cannot be certain that the phenotype of the tendon fibroblasts was maintained in our cultures. However, based on the morphology of the cells and the production of collagen, we believe that the main phenotypic features of tendon fibroblasts were maintained.
The localized delivery of growth factors is an important step in achieving flexor tendon repair. Increased matrix production by migrating epitenon cells may aid the process of tendon healing by enhancing the connection between the severed tendon stumps, leading to improved biomechanical properties. However, the delivery must be localized to the interior of the tendon, as cell proliferation and matrix synthesis on the exterior surface of the tendon may lead to adhesion formation between the tendon and the surrounding sheath and a decrease in digital range of motion. For this model, a fibrin matrix containing the heparin-binding delivery system allows localized delivery of growth factor to the interior of the tendon while also serving as a scaffold for cell migration within the repair site. Our initial studies using this delivery system in vivo indicate that the fibrin gel remains localized to the interior of the tendon and does not induce inflammation during the early healing and rehabilitation period (26).
The studies described in this manuscript demonstrate that our initial delivery system and PDGF-BB dose from our pilot study can be further refined to find a PDGF-BB concentration (1.25 μg/mL or 500 μg total dose) and PDGF-BB to heparin ratio (1:1,000) that will promote slower PDGF-BB release and presumable greater cellular response in terms of cell proliferation and matrix synthesis in vivo. This improved system may have the potential to stimulate faster restoration of tendon biomechanical properties. Future studies will focus on testing this improved delivery system and dosage level in an in vivo animal model.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woo SL, Gelberman RH, Cobb NG, Amiel D, Lothringer K, Akeson WH. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study Acta Orthop Scand. 1981;52:615–622. doi: 10.3109/17453678108992156. [DOI] [PubMed] [Google Scholar]
- 2.Gelberman RH, Woo SL, Lothringer K, Akeson WH, Amiel D. Effects of early intermittent passive mobilization on healing canine flexor tendons. Journal of Hand Surgery -American Volume. 1982;7:170–175. doi: 10.1016/s0363-5023(82)80083-x. [DOI] [PubMed] [Google Scholar]
- 3.Takai S, Woo SL, Horibe S, Tung DK, Gelberman RH. The effects of frequency and duration of controlled passive mobilization on tendon healing. Journal of Orthopaedic Research. 1991;9:705–713. doi: 10.1002/jor.1100090510. [DOI] [PubMed] [Google Scholar]
- 4.Boyer MI, Gelberman RH, Burns ME, Dinopoulos H, Hofem R, Silva MJ. Intrasynovial flexor tendon repair. An experimental study comparing low and high levels of in vivo force during rehabilitation in canines. J Bone Joint Surg Am. 2001;83-A:891–899. [PubMed] [Google Scholar]
- 5.Lieber RL, Amiel D, Kaufman KR, Whitney J, Gelberman RH. Relationship between joint motion and flexor tendon force in the canine forelimb. The Journal of hand surgery. 1996;21:957–962. doi: 10.1016/S0363-5023(96)80299-1. [DOI] [PubMed] [Google Scholar]
- 6.Lieber RL, Silva MJ, Amiel D, Gelberman RH. Wrist and digital joint motion produce unique flexor tendon force and excursion in the canine forelimb. Journal of biomechanics. 1999;32:175–181. doi: 10.1016/s0021-9290(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 7.Silva MJ, Brodt MD, Boyer MI, Morris TS, Dinopoulos H, Amiel D, et al. Effects of increased in vivo excursion on digital range of motion and tendon strength following flexor tendon repair. J Orthop Res. 1999;17:777–783. doi: 10.1002/jor.1100170524. [DOI] [PubMed] [Google Scholar]
- 8.Aspenberg P, Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta orthopaedica Scandinavica. 1999;70:51–54. doi: 10.3109/17453679909000958. [DOI] [PubMed] [Google Scholar]
- 9.Chan BP, Chan KM, Maffulli N, Webb S, Lee KK. Effect of basic fibroblast growth factor. An in vitro study of tendon healing. Clinical orthopaedics and related research. 1997:239–247. [PubMed] [Google Scholar]
- 10.Chan BP, Fu S, Qin L, Lee K, Rolf CG, Chan K. Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: a rat patellar tendon model. Acta orthopaedica Scandinavica. 2000;71:513–518. doi: 10.1080/000164700317381234. [DOI] [PubMed] [Google Scholar]
- 11.Chang J, Most D, Stelnicki E, Siebert JW, Longaker MT, Hui K, et al. Gene expression of transforming growth factor beta-1 in rabbit zone II flexor tendon wound healing: evidence for dual mechanisms of repair. Plastic and reconstructive surgery. 1997;100:937–944. doi: 10.1097/00006534-199709001-00016. [DOI] [PubMed] [Google Scholar]
- 12.Chang J, Thunder R, Most D, Longaker MT, Lineaweaver WC. Studies in flexor tendon wound healing: neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plastic and reconstructive surgery. 2000;105:148–155. doi: 10.1097/00006534-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Fukui N, Fukuda A, Kojima K, Nakajima K, Oda H, Nakamura K. Suppression of fibrous adhesion by proteoglycan decorin. J Orthop Res. 2001;19:456–462. doi: 10.1016/S0736-0266(00)90016-0. [DOI] [PubMed] [Google Scholar]
- 14.Klein MB, Yalamanchi N, Pham H, Longaker MT, Chang J. Flexor tendon healing in vitro: effects of TGF-beta on tendon cell collagen production. The Journal of hand surgery. 2002;27:615–620. doi: 10.1053/jhsu.2002.34004. [DOI] [PubMed] [Google Scholar]
- 15.Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res. 2001;19:1199–1202. doi: 10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 16.Rapoff AJ, Heisey DM, Vanderby R., Jr A probabilistic rule of mixtures for elastic moduli. Journal of biomechanics. 1999;32:189–193. doi: 10.1016/s0021-9290(98)00155-9. [DOI] [PubMed] [Google Scholar]
- 17.Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. The American journal of sports medicine. 1999;27:476–488. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]
- 18.Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. The Journal of clinical investigation. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshikawa Y, Abrahamsson SO. Dose-related cellular effects of platelet-derived growth factor-BB differ in various types of rabbit tendons in vitro. Acta orthopaedica Scandinavica. 2001;72:287–292. doi: 10.1080/00016470152846646. [DOI] [PubMed] [Google Scholar]
- 20.Zhao C, Amadio PC, Paillard P, Tanaka T, Zobitz ME, Larson DR, et al. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. The Journal of bone and joint surgery. 2004;86-A:320–327. doi: 10.2106/00004623-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports medicine (Auckland, NZ. 2003;33:381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 22.Rickert M, Jung M, Adiyaman M, Richter W, Simank HG. A growth and differentiation factor-5 (GDF-5)-coated suture stimulates tendon healing in an Achilles tendon model in rats. Growth factors (Chur, Switzerland) 2001;19:115–126. doi: 10.3109/08977190109001080. [DOI] [PubMed] [Google Scholar]
- 23.Rohrich RJ, Trott SA, Love M, Beran SJ, Orenstein HH. Mersilene suture as a vehicle for delivery of growth factors in tendon repair. Plastic and reconstructive surgery. 1999;104:1713–1717. doi: 10.1097/00006534-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. The Journal of hand surgery. 2005;30:441–447. doi: 10.1016/j.jhsa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Gelberman RH, Thomopoulos S, Sakiyama-Elbert SE, Das R, Silva MJ. The early effects of sustained platelet-derived growth factor administration on the functional and structural properties of repaired intrasynovial flexor tendons: an in vivo biomechanic study at 3 weeks in canines. J Hand Surg [Am] 2007;32:373–379. doi: 10.1016/j.jhsa.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Thomopoulos S, Zaegel M, Das R, Harwood FL, Silva MJ, Amiel D, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25:1358–1368. doi: 10.1002/jor.20444. [DOI] [PubMed] [Google Scholar]
- 27.Lee AC, Yu VM, Lowe JB, 3rd, Brenner MJ, Hunter DA, Mackinnon SE, et al. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Experimental neurology. 2003;184:295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 28.Taylor SJ, McDonald JW, 3rd, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J Control Release. 2004;98:281–294. doi: 10.1016/j.jconrel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Taylor SJ, Rosenzweig ES, McDonald JW, 3rd, Sakiyama-Elbert SE. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J Control Release. 2006;113:226–235. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000;69:149–158. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 31.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 32.Presta M, Maier JA, Rusnati M, Ragnotti G. Basic fibroblast growth factor is released from endothelial extracellular matrix in a biologically active form. Journal of cellular physiology. 1989;140:68–74. doi: 10.1002/jcp.1041400109. [DOI] [PubMed] [Google Scholar]
- 33.Vlodavsky I, Fuks Z, Ishai-Michaeli R, Bashkin P, Levi E, Korner G, et al. Extracellular matrix-resident basic fibroblast growth factor: implication for the control of angiogenesis. Journal of cellular biochemistry. 1991;45:167–176. doi: 10.1002/jcb.240450208. [DOI] [PubMed] [Google Scholar]
- 34.Herbert CB, Bittner GD, Hubbell JA. Effects of fibinolysis on neurite growth from dorsal root ganglia cultured in two- and three-dimensional fibrin gels. The Journal of comparative neurology. 1996;365:380–391. doi: 10.1002/(SICI)1096-9861(19960212)365:3<380::AID-CNE4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Gelberman RH, Amiel D, Harwood F. Genetic expression for type I procollagen in the early stages of flexor tendon healing. Journal of Hand Surgery - American Volume. 1992;17:551–558. doi: 10.1016/0363-5023(92)90370-5. [DOI] [PubMed] [Google Scholar]
- 36.Gelberman RH, Boyer MI, Brodt MD, Winters SC, Silva MJ. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg Am. 1999;81:975–982. doi: 10.2106/00004623-199907000-00010. [DOI] [PubMed] [Google Scholar]