Abstract
Objective
Abnormalities in the anterior interhemispheric connections provided by the corpus callosum (CC) have long been implicated in bipolar disorder (BD). In this study, we used complementary diffusion tensor imaging (DTI) methods to study the structural integrity of the CC and localization of potential abnormalities in BD.
Methods
Subjects included 33 participants with BD and 40 healthy comparison participants. Fractional anisotropy (FA) measures were compared between groups using region of interest (ROI) methods to investigate the anterior, middle and posterior CC and voxel-based methods to further localize abnormalities.
Results
In ROI-based analyses, FA was significantly decreased in the anterior and middle CC in the BD group (P<0.05). Voxel-based analyses similarly localized group differences to the genu, rostral body and anterior midbody of CC (P<0.05, corrected).
Conclusion
The findings demonstrate abnormalities in the structural integrity of the anterior CC in BD which may contribute to altered inter-hemispheric connectivity in this disorder.
The anterior corpus callosum (CC) has been implicated in bipolar disorder (BD) since at least 1903 when Starr described symptoms detected after anterior CC lesions similar to those of BD, such as “undue excitement, causeless laughter, unusual crying, great depression and a lack of harmony between the association of ideas and the state of feeling which they should awaken” (1). An early magnetic resonance imaging (MRI) study of cerebral morphology in BD detected mid-sagittal CC area decreases (2). Subsequent structural MRI studies provided further evidence for CC white matter abnormalities in BD including in volume, signal intensity and structural integrity (3–5), although reports varied in the CC subregions studied and regional differences detected. In this study, complementary diffusion tensor imaging (DTI) region of interest (ROI) and voxel-based methods were used to study the structural integrity of CC white matter in BD and assess the regional localization of differences. Anterior CC reductions in FA in BD were anticipated.
Methods
Participants
The Structured Clinical Interview for DSM-IV Axis I Disorders Version 2.0 (SCID) (6) confirmed the presence or absence of Axis I Disorders and mood state at scanning for the 33 BD and 40 healthy comparison (HC) participants studied. Symptoms were assessed using the Hamilton Depression Rating Scale (HAMD) (7) and Clinician-Administered Rating Scale for Mania (CARS-M) (8). No subject had a history of neurological illness, head trauma with loss of consciousness over 5min. or major medical disorder, except 4 female BD participants with treated hypothyroidism. HCs did not have a history of Axis I disorder themselves or in their first-degree relatives. Table 1 and Table 2 provide sample details, including socioeconomic status (SES) (9), and clinical features of BD participants. After a complete description of the study, written informed consent was obtained from all participants in accordance with the human investigation committees of the Yale School of Medicine and the Department of Veterans Affairs.
Table 1.
Sample demographics
| HC | BD | |
|---|---|---|
| Number | 40 | 33 |
| Age (mean±SD) | 29.2±9.2 | 32±10.1 |
| Sex (number female, percent) | 27, 68% | 24, 73% |
| SES (mean±SD) | 3.6±1.43 | 4.1±1.18 |
| Ethnicity (EA:AA:A:others) | 21:8:7:4 | 26:6:0:1 |
| HAMD (mean±SD) | 0.7±1.3 | 12.0±10.3 |
| CARS-M (mean±SD) | 0.2±0.5 | 5.6±6.4 |
Notes: SD, standard deviation; SES, socioeconomic status; EA, European American; AA, African American; A, Asian; HAMD, Hamilton Depression Rating Scale; CARS-M, Clinician-administered rating scale for mania.
Table 2.
Clinical factors for participants with bipolar disorder
| number (percent) | |
|---|---|
| Rapid cycling | 19 (58%) |
| Mood states | |
| manic/mixed or hypomanic | 7 (21%) |
| depressive | 7 (21%) |
| euthymic | 19 (58%) |
| Medication | |
| unmedicated | 6 (18%) |
| lithium carbonate | 8 (24%) |
| anticonvulsants | 17 (52%) |
| atypical antipsychotics | 16 (48%) |
| benzodiazepines | 8 (24%) |
| History of substance related disorders* | 14 (42%) |
| Other comorbidity** | 3 (9%) |
This included 11 (33%) with a history of alcohol abuse or dependence, 6 (18%) of whom also had a history of other substance abuse or dependence, and an additional 3 (9%) BD participants had a history of other substance abuse or dependence. All of 14 subjects were at full remission for at least one year.
This included panic disorder (1, 3%) and post-traumatic stress disorder (2, 6%).
MRI acquisition
Diffusion-weighted images were acquired on a 3T Trio MR scanner (Siemens, Erlangen, Germany) with a single-shot echo planar imaging sequence in alignment with the anterior commissure-posterior commissure (AC-PC) plane. Diffusion sensitizing gradients were applied along 32 non-colinear directions uniformly distributed on a unit sphere: b-value=1000s/mm2, together with a non-diffusion weighted acquisition (b-value=0) (TR=7400ms, TE=115ms, FOV=256×256mm2, matrix=128×128, slice thickness=3mm without gap,40 slices, 1 average). High resolution structural images were acquired with a three-dimensional Magnetization Prepared Rapid Acquisition Gradient Echo T1-weighted sequence for anatomical determinations (TR=1500ms, TE=2.83ms, FOV=256×256mm2, matrix=256×256, slice thickness=1.0 mm without gap,160 slices,2 averages).
ROI-based DTI processing
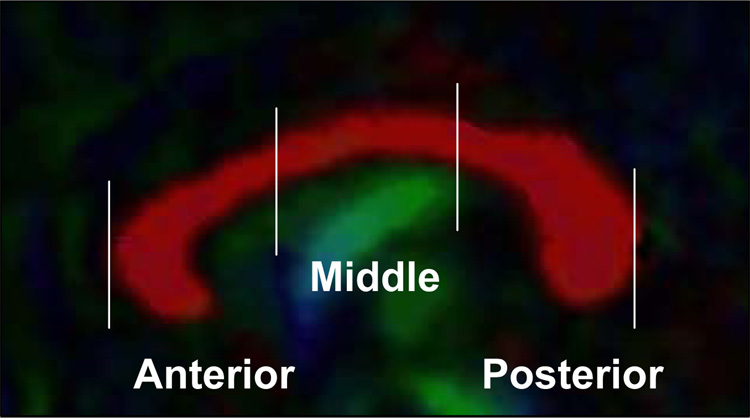
DTI data were processed with BioImage Suite (www.bioimagesuite.org). Diffusion-weighted data were interpolated to 2mm thickness along the coronal-oblique direction with in-plane resolution of 1mm×1mm, and denoised by a three-dimensional isotropic Gaussian kernel with sigma 1mm full-width-at-half-maximum (FWHM). Diffusion tensor matrices and regional fractional anisotropy (FA) were calculated (10). The absolute red-green-blue color-encoding scheme defined the directionality of the principal eigenvector: left-right fibers in red, anterior-posterior in green and superior-inferior in blue (Figure 1). The middle sagittal slice was determined on the AC-PC aligned T1-images (11) to which DTI data were coregistered with Bioimage Suite software. The CC was delineated on the mid-sagittal slice and subdivided into three subregions of equal length (Figure 1). Mean FA was calculated separately for these anterior, middle and posterior subregions. Interrater reliability coefficients for the subregion delineations were 0.95–0.97.
Figure 1.
Sagittal image from the tensor color map displays the left-right coursing fibers of the corpus callosum in red.
Voxel-based DTI processing
DTI data reslicing and smoothing were performed with BioImage Suite software. After diffusion tensor matrices and FA were calculated, FA maps were normalized with SPM5 (http://www.fil.ion.ucl.ac.uk/spm) to the Montreal Neurological Institute space using a white matter tissue probability map as a template and spatially smoothed by an 8 mm FWHM Gaussian kernel. The CC was defined by WFU Pick Atlas Tool (http://www.fmri.wfubmc.edu/download.htm).
Statistical analysis for ROI-based DTI
Statistical analyses were conducted using SAS software, version 9.1 (SAS Institute, Cary, NC). FA values were tested for normality using Kolmogorov-Smirnov test statistics and normal probability plots. A linear mixed model was used in which diagnostic group (HC vs. BD) represented a between-subjects factor and CC subregion (anterior, middle, posterior) was a within-subjects factor. The interaction between diagnostic group and subregion was modeled. Age and sex were considered as covariates but were not significant and therefore dropped for parsimony. Least square means and standard errors were computed from the model and plotted to interpret diagnosis effects.
Post-hoc exploratory analyses were performed for potential main effects of clinical factors on FA among BD participants. Clinical factors examined included rapid cycling, mood state, psychotropic medication at scanning, and history of substance related disorders overall and alcohol related disorders. Spearman Correlation analyses were performed to test for relationships between FA and HAMD or CARS-M scores.
Statistical analysis for voxel-based DTI
Differences between HC and BD groups were assessed using two-sample (HC and BD) t-tests in SPM with FA values as the dependent variables. Findings in CC were considered significant for P<0.005 (uncorrected) and clusters >100 voxels. Furthermore, to minimize the chance of type I error, small volume correction (SVC) was used to correct multiple comparisons within the CC with false discovery rate of P<0.05.
Results
The BD and HC groups did not differ significantly in age, sex or SES scores (P’s >0.1). Data adhered to a normal distribution.
ROI-based DTI
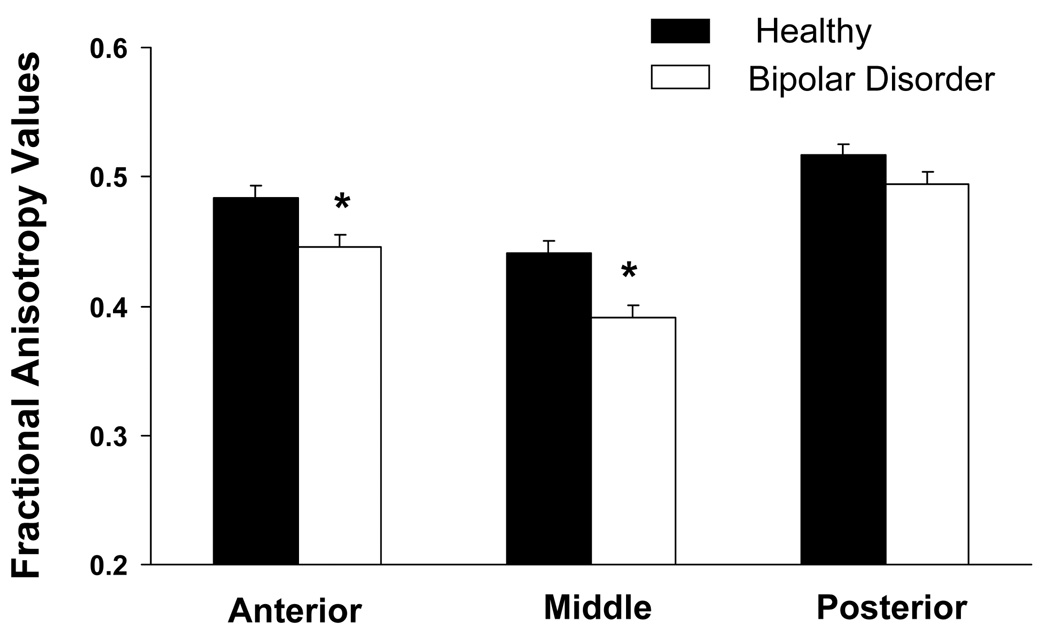
The main effect of diagnosis was statistically significant [F(1,71)=11.2,P=0.001]; FA was significantly lower in the BD than the HC group. Subregional analyses, Bonferroni-corrected for 3 subregions, confirmed the presence of significant FA decreases in BD in the anterior (Pbonf=0.01) and middle CC (Pbonf<0.001) (Figure 2). Exploratory analyses did not reveal significant main or interactive effects of ethnicity overall (P’s>0.7), clinical factors within the BD group (P’s>0.5) on CC FA, or correlations between CARS-M or HAMD scores and FA values (P’s>0.2).
Figure 2.
Least square (ls) mean anisotropy values in the anterior, middle and posterior corpus callosum and standard errors for the bipolar disorder (BD) group (n = 33) and the healthy comparison (n = 40) group. The difference of ls means indicated that the contribution to group differences was derived mainly from significant decreases in FA in the anterior CC [F(1,142)=8.43, PBonf=0.01, Bonferroni corrected for the 3 subregions] and middle CC [F(1,142)=14.6, PBonf <0.001] in the BD compared to the HC group; posterior CC FA decreases were not significant [F(1,142)=2.71,PBonf=0.3]. Results remained significant when excluding the 2 BD subjects with posttraumatic stress disorder and the subject with panic disorder (effect of diagnosis P<0.001 and in anterior and middle subregions PBonf=0.003 and PBonf <0.001, respectively).
*denotes significant decreases
Voxel-based DTI
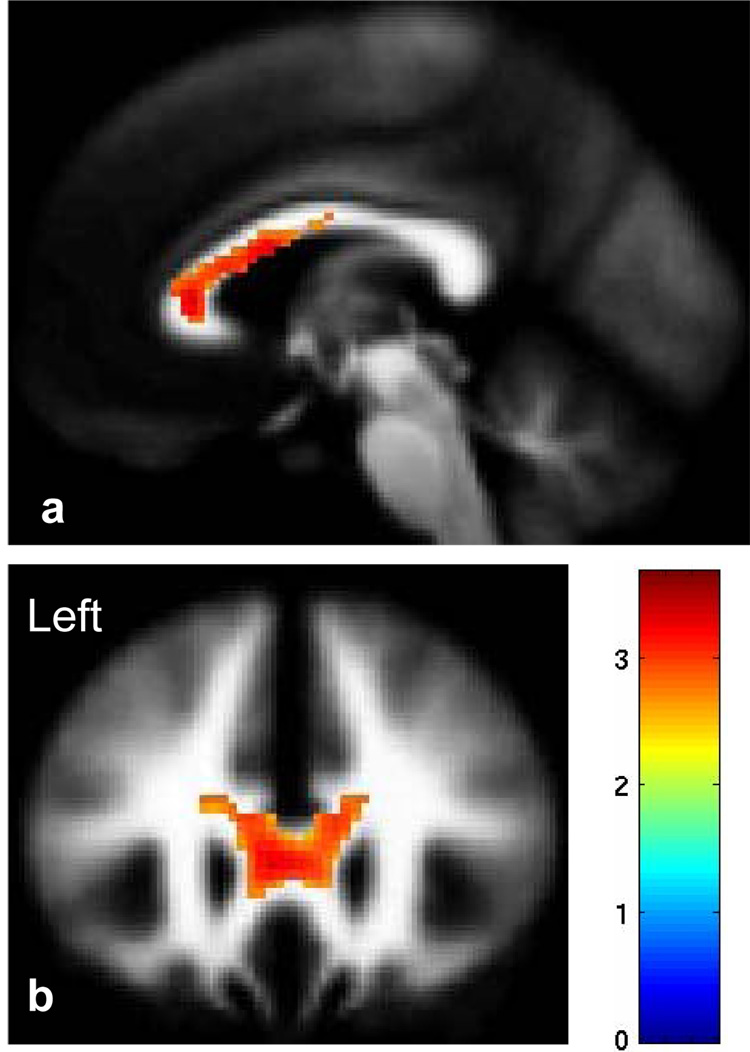
FA was significantly reduced in the genu, rostral body and anterior midbody of CC (P<0.005, uncorrected, Figure 3). The difference remained significant after SVC was performed (P=0.02, corrected).
Figure 3.
The sagittal (a, MNI plane: x=−2mm) and coronal (b, MNI plane: y=26mm) images display the genu, rostral body and anterior midbody of the corpus callosum where fractional anisotropy was reduced significantly in participants with bipolar disorder as compared to healthy comparison participants (P<0.005). The color bar represents the range of T values. The findings are displayed in a tissue probability map of white matter.
Discussion
Using complementary ROI-and voxel-based DTI methods, we found decreased FA values in the participants with BD compared to HC participants in anterior and middle CC subregions encompassing the genu, rostral body and the anterior portion of the midbody. The interhemispheric white matter connections of the CC are critical to interhemispheric communication and to the integration of emotional, cognitive, motor and sensory information. Abnormalities in interhemispheric functioning in BD have been theorized previously (12, 13). Specifically, CC fibers coursing through the anterior CC regions in which differences were detected provide connectivity between right and left prefrontal cortices as well as anterior cingulate and insula (14). These CC regions are implicated in emotional dysregulation (1) and in the hemispheric lateralization of prefrontal abnormalities associated with acute mood states of BD (15). Significant associations between CC FA and clinical features of BD were not detected; however, our ability to detect effects could have been limited by inadequate statistical power.
In a recent DTI study of 11 BD and 10 HC adults that sampled two focal ROIs in the CC genu and splenium, increased FA was detected only in the genu (4). The different direction of the findings could relate to differences between subject samples or imaging methods. The methods used herein permitted examination of the full CC length. We found differences that covered a larger genu region and that extended to the anterior midbody, implicating a larger section of the anterior CC in BD. The complementary ROI- and voxel-based DTI methods used yielded consistent results and drew upon the relative strengths of each method (16). ROI-based DTI permitted testing in specific subregions of the CC and minimized the risk of a type I error, whereas voxel-based DTI permitted further localization within the CC. Neither study detected significant group differences in subregional diffusivity (Supplementary Materials). Furthermore, we did not detect differences in CC cross-sectional areas (Supplementary Materials). Taken together, the studies suggest that DTI FA measures may be relatively sensitive to the CC white matter abnormalities of BD.
Bipolar disorder frequently co-occurs with substance related disorders (17). To attempt to balance generalizability of the findings, and potential effects of substances on white matter, we included only BD participants who were in full remission from substance related disorders for at least 1 year. Medication exposure of the BD participants is another potential confound of this study. Although we did not detect significant effects of these factors, and evidence suggests that effects of chronic alcohol and other substances of abuse on the CC may be at least partially reversible with remission (18, 19), power may have been limited and these analyses did not take into account previous exposures. Further work is necessary to definitively differentiate white matter integrity alterations related to BD from those resulting from medication or substance exposure.
The majority of our BD participants reported symptom onset in adolescence. Interestingly, the CC reaches its maximal myelination in late adolescence/early adulthood (20), a time period coinciding with this peak in the onset of BD, suggesting that further study of CC development in adolescence/early adulthood in BD may help to elucidate a neurodevelopmental mechanism contributing to the disorder.
Supplementary Material
Acknowledgments
The authors were supported by grants from the National Institute of Mental Health R01MH69747 (HPB), R01MH070902 (HPB) and T32MH14276 (LGC, JHK), K23MH077914 (ZB), the Department of Veterans Affairs Career Development (HPB), Merit Review (HPB) and Research Enhancement Award Program (REAP) (HPB, LGC) programs , the National Alliance for Research in Schizophrenia and Depression (Great Neck, NY) (HPB, JHK, ZB), The Ethel F. Donaghue Women’s Investigator Program at Yale (New Haven, CT) (HPB), The Attias Family Foundation (HPB), Marcia Simon Kaplan (JHK) and the Klingenstein Foundation (JHK), and NIH/NCRR/CTSA UL1RR024139-01 (ZB). BioImage Suite was supported in part by the National Institutes of Health /National Institute of Biomedical Imaging and Bioengineering under the grant R01 EB006494 (XP).
This article is dedicated to Ms. Kathleen Colonese who was devoted to helping those suffering from psychiatric illnesses and to advancing the field of bipolar disorder research. The authors thank Cheryl Lacadie, B.S., Karen Martin, R.T.R.M.R., Terry Hickey, R.T.R.M.R. and Hedy Sarofin, R.T.R.M.R. for their technical expertise and the research subjects for their participation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures:
Dr. Blumberg reports having received lecture honoraria from Eli Lilly and Abbott Laboratories and consultant fees from Pfizer. Dr Bhagwagar is on the speaker’s panel for Astra Zeneca and BMS. Drs. Wang, Kalmar, Chepenik, Jackowski, Papademetris, Constable, Mr. Pittman, Ms. Edmiston and Ms. Spencer reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Starr MA. Organic Nervous Disease. New York and Philadelphia: Lea Brothers & CO.; 1903. [Google Scholar]
- 2.Coffman JA, Bornstein RA, Olson SC, Schwarzkopf SB, Nasrallah HA. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biol Psychiatry. 1990;27:1188–1196. doi: 10.1016/0006-3223(90)90416-y. [DOI] [PubMed] [Google Scholar]
- 3.Atmaca M, Ozdemir H, Yildirim H. Corpus callosum areas in first-episode patients with bipolar disorder. Psychol Med. 2007;37:699–704. doi: 10.1017/S0033291706009743. [DOI] [PubMed] [Google Scholar]
- 4.Yurgelun-Todd DA, Silveri MM, Gruber SA, Rohan ML, Pimentel PJ. White matter abnormalities observed in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2007;9:504–512. doi: 10.1111/j.1399-5618.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- 5.Brambilla P, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Keshavan MS, et al. Corpus callosum signal intensity in patients with bipolar and unipolar disorder. J Neurol Neurosurg Psychiatry. 2004;75:221–225. [PMC free article] [PubMed] [Google Scholar]
- 6.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I & II Disorders (Version 2.0) New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- 7.Williams JBW, Link MJ, Rosenthal NE, Terman M. Structured interview guide for the Hamilton depression rating scale: seasonal affective disorder version (SIGH-SAD) New York, NY: New York State Psychiatric Institute; 1994. [Google Scholar]
- 8.Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM. The Clinician-Administered Rating Scale for Mania (CARS-M): development, reliability, and validity. Biol Psychiatry. 1994;36:124–134. doi: 10.1016/0006-3223(94)91193-2. [DOI] [PubMed] [Google Scholar]
- 9.Hollingshead AB. Two factor index of social position. New Haven, CT: 1957. [Google Scholar]
- 10.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 11.Meisenzahl EM, Frodl T, Greiner J, Leinsinger G, Maag KP, Heiss D, et al. Corpus callosum size in schizophrenia--a magnetic resonance imaging analysis. Eur Arch Psychiatry Clin Neurosci. 1999;249:305–312. doi: 10.1007/s004060050104. [DOI] [PubMed] [Google Scholar]
- 12.Bruder GE, Schnur DB, Fergeson P, Mukherjee S, Leite P, Sackeim HA. Dichotic-listening measures of brain laterality in mania. J Abnorm Psychol. 1994;103:758–766. doi: 10.1037//0021-843x.103.4.758. [DOI] [PubMed] [Google Scholar]
- 13.Pettigrew JD, Miller SM. A 'sticky' interhemispheric switch in bipolar disorder? Proc Biol Sci. 1998;265:2141–2148. doi: 10.1098/rspb.1998.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- 15.Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, et al. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 16.Snook L, Plewes C, Beaulieu C. Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. Neuroimage. 2007;34:243–252. doi: 10.1016/j.neuroimage.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Brown ES. Management of comorbid bipolar disorder and substance abuse. J Clin Psychiatry. 2006;67:e05. doi: 10.4088/jcp.0806e05. [DOI] [PubMed] [Google Scholar]
- 18.DeLisi LE, Bertisch HC, Szulc K, Majcher M, Brown K, Bappal A, et al. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduct J. 2006;3:7. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res. 2000;24:1214–1221. [PubMed] [Google Scholar]
- 20.Keshavan MS, Diwadkar VA, DeBellis M, Dick E, Kotwal R, Rosenberg DR, et al. Development of the corpus callosum in childhood, adolescence and early adulthood. Life Sci. 2002;70:1909–1922. doi: 10.1016/s0024-3205(02)01492-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.