Abstract
Following brief stimulation, macroscopic NMDA receptor currents decay with biphasic kinetics that is believed to reflect glutamate dissociation and receptor desensitization. We found that the fast and slow decay components arise from the simultaneous deactivation of receptor populations that gate with short and long openings, respectively. Because individual receptors switched infrequently between gating modes, the relaxation time course was largely determined by the proportion of channels in each gating mode at the time of stimulation.
NMDA receptors mediate the slow component of excitatory postsynaptic currents in the CNS and participate in synaptic integration and the coincidence detection that underlies certain forms of synaptic plasticity1. NMDA receptor currents decay with biphasic kinetics2, and the fast and slow components of decay are thought to reflect agonist dissociation and receptor desensitization3. We have shown that individual NMDA receptors show distinct types of gating patterns (modes), which arise from differences in the kinetics of transitions between fully occupied closed states4,5. Here, we investigated whether modal gating contributes to the biphasic decay of NMDA receptor currents.
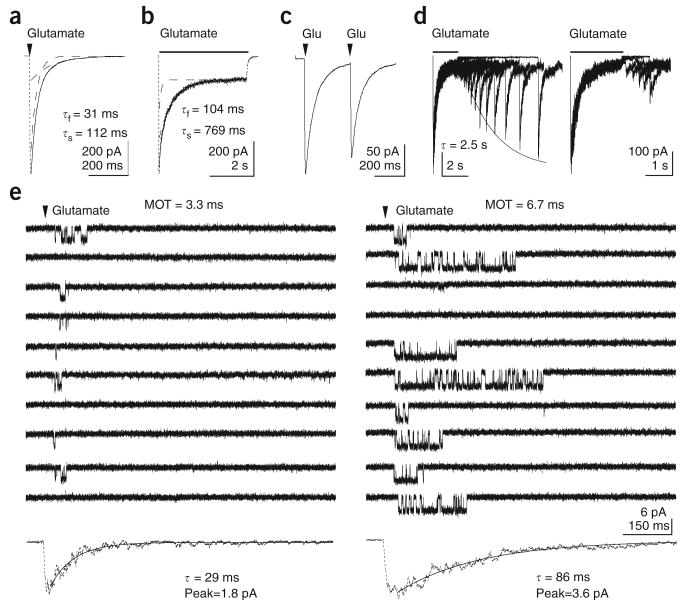
First, we characterized NMDA receptor responses in a preparation of known molecular identity: outside-out patches from tsA201 cells expressing rat recombinant NR1/NR2A receptors (Supplementary Methods online). The decay of ensemble currents evoked by 1-ms pulses of glutamate (1 mM) was consistently bi-exponential (time constants: τf = 31 ms, τs = 110 ms, n = 21; Fig. 1a). To study desensitization in the same patches, we applied 4-s pulses of 1 mM glutamate. The ensemble currents decayed more slowly (τf = 129 ms, τs = 514 ms, n = 5; Fig. 1b) and varied substantially from patch to patch, with some responses following single exponentials (τ = 617 ms, n = 6). To test directly the fraction of receptors that desensitize during a brief pulse, we applied pairs of 1-ms pulses at inter-pulse intervals of 300 ms, at which point the response to the first application had decayed nearly completely. The peak amplitude of the second pulse decreased by only 12% (n = 8; Fig. 1c). Furthermore, recovery followed a single exponential time-course and was only 10–15% complete at 300 ms (τ = 2.2 s, n = 5; Fig. 1d). We conclude that the fraction of desensitizing receptors is too small following a brief pulse of glutamate and the time course of recovery from desensitization is too slow for desensitization to represent one of the kinetic components seen in the decay of responses to synaptic-like pulses of glutamate (Supplementary Table 1 online).
Figure 1.
Deactivation and desensitization of NR1/NR2A channels. (a) Current elicited with a 1-ms pulse of 1 mM glutamate (arrowhead) from an excised patch. The decay was fitted with two exponential components (dashed lines). (b) Current evoked by a 4-s application of 1 mM glutamate (bar), on same patch as in a. (c) Responses to two 1-ms pulses of 1 mM glutamate (arrowheads) applied with a 300-ms interval. (d) Left, results from a paired-pulse protocol using 2-s applications of 1 mM glutamate. Recovery was mono-exponential (solid line). Right, early phase of recovery on an expanded time scale. (e) Two groups of consecutive traces recorded from a patch containing one NR1/NR2A channel in response to 1-ms applications of 1 mM glutamate (arrowheads). The different MOTs during each set of traces are indicative of low- and medium-mode gating that result in ensemble averages that decay with different mono-exponential time courses.
NMDA receptor mechanisms that are derived from analysis of steady-state, single-channel activity contain several states, and in theory it is expected that agonist dissociation, receptor desensitization and gating transitions between open and closed states will collectively contribute more than ten components to the relaxation of the response to a brief concentration jump4–10. In practice, however, only two exponential components can be distinguished experimentally, as shown here and in previous studies3,11. This consistent experimental observation implies that in response to a brief pulse of glutamate individual channels generate activations that can be roughly categorized as short or long.
To investigate the causes of activations having distinct durations, we recorded current responses to brief pulses from outside-out patches that contained only one active receptor. Most patches (5 of 7, > 3,000 sweeps, 20,900 events) showed average current traces that decayed with biphasic kinetics (τf = 32 ± 2 ms (62 ± 4%) and τs = 90 ± 6 ms), which is consistent with the macroscopic measurements made here (Fig. 1a) and in previous reports10–12. In four of the five patches where the ensemble current average decayed bi-exponentially, we could sort individual activations into two groups according to their mean open times (MOTs). The MOTs for the two groups appeared to correspond to the low (3.0 ± 0.2 ms) and medium (6.5 ± 0.3 ms) gating modes described previously in prolonged cell-attached recordings4. Notably, in the two patches where the ensemble current decayed mono-exponentially (τ = 62 ms and 105 ms), all activations had similar open durations (4.2 ms or 7 ms, respectively).
In the records where we detected activations with both short and long open durations, activations with similar kinetics tended to cluster in a record, consistent with the long lifetimes of individual modes4 (Fig. 1e). When all activations in the patch illustrated (157 traces with at least one opening) were sorted by their opening mode (low = 2.6 ± 1.4 ms versus medium = 6.3 ± 1.3 ms, n = 93 and 64, respectively), we found that low-mode activations were shorter than medium-mode activations (low = 65 versus medium = 101 ms) and that the respective ensemble averages decayed mono-exponentially with substantially different time constants (low = 29 versus medium = 86 ms) and peak amplitudes (low = 1.8 versus medium = 3.6 pA) (Fig. 1e). These results show that modal gating behavior influences both the time course and the peak amplitude of NMDA receptor responses.
To examine whether modal kinetics also occurs in native receptors, we recorded on-cell equilibrium single-channel currents from rat cortical neurons (Fig. 2). As in the intact animal, NR2B and NR2A expression is developmentally regulated in this preparation13–15. Consistent with this observation, the activity that we recorded by 19 d in vitro (DIV) in three separate neuronal preparations had similar kinetic characteristics to those previously reported for recombinant NR1/NR2B receptors, whereas cultures older than 25 DIV had similar kinetics to recombinant NR1/NR2A receptors (Fig. 2)6,10. Specifically, in early cultures (5–19 DIV), we measured an open probability, Po, and MOT (mean ± s.d.) of 0.12 ± 0.06 and 3.2 ± 0.5 ms (n = 8, 783,147 events), whereas in older cultures (21–35 DIV), these measurements were 0.33 ± 0.10 and 5.6 ± 0.9 ms (n = 7, 890,060 events), respectively. Notably, in both early and late preparations, we routinely identified clusters of activity that were characterized by three distinct open durations. On the basis of these observations, we conclude that, as with recombinant receptors, native NMDA receptors switch between gating behaviors that differ in the mean duration of openings and can last for minutes. This suggests that the biphasic decay of ensemble currents reflects kinetic heterogeneity that arises from modal gating.
Figure 2.
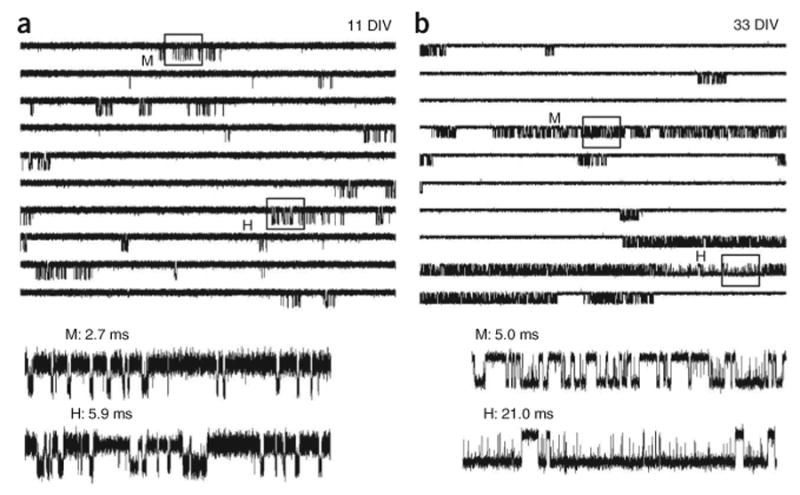
Neuronal NMDA receptors display modal gating. (a,b) Equilibrium single-channel currents elicited with saturating concentrations of glutamate (1 mM) and glycine (0.1 mM) were recorded from on-cell patches of dissociated rat cortical neurons maintained in culture for 11 (a) or 33 d (b). In each panel, the upper ten traces illustrate an uninterrupted 50-s fragment from each recording shown filtered at 2 kHz (openings are downward). Portions (500 ms each) of two clusters that differ in MOT (M, medium; L, low) are boxed and shown at higher resolution (5 kHz) below. Channel kinetics were distinct in the two records illustrated. The first recording (a) had a low Po (0.05) and a short MOT (3.4 ms, 41,726 events), whereas the second recording (b) had a higher Po (0.2) and a longer MOT (4.1 ms, 263,512 events). Consistently, recordings were noisier (mean ± s.d.) in early cultures (5–19 DIV, n = 8, 2.3 ± 0.5 pA) than those obtained from late cultures (25–23 DIV, n = 7, 1.4 + 0.3 pA).
To evaluate this hypothesis quantitatively, we sought to compare the measured macroscopic kinetics with predictions from single-channel experiments. To do this, we needed to evaluate receptor behaviors in terms of a reaction mechanism. This has been done previously for several NMDA receptor preparations4,6,8,9. However, because quantitative differences in kinetics exist between expression systems, as well as attached versus excised patches, we first determined the gating rate constants of NMDA receptors in the preparation used above (Fig. 1). During minutes-long exposure to high concentrations of agonists, the activity recorded from excised patches containing only one NR1/NR2A receptor occurred in long clusters that were separated by desensitization gaps whose durations were distributed mono-exponentially (1,580 ± 180 ms, 2.6 ± 0.4%, n = 5). We used these data to derive rate constants separately for portions of records consisting exclusively of low- and medium-mode gating (Supplementary Results and Supplementary Fig. 1 online). To these equilibrium mechanisms, we appended transitions for glutamate binding with previously reported rate constant values (KD = 3 μM)5 (Fig. 3a).
Figure 3.
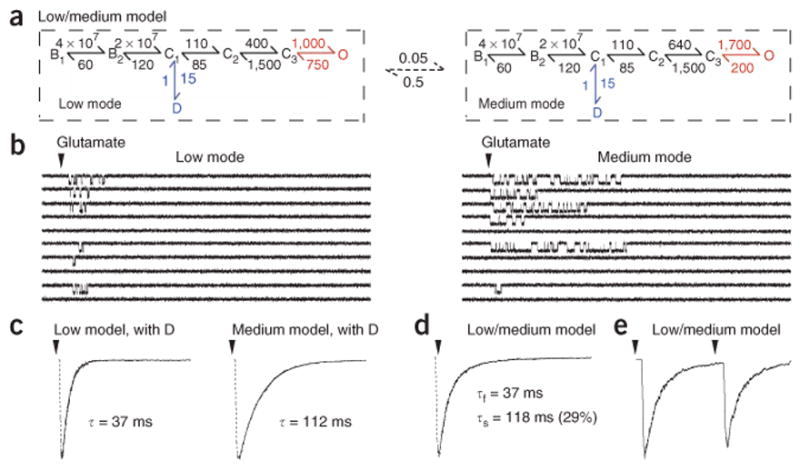
Modal gating accounts for biphasic decay. (a) Tiered model assuming slow switch between two gating modes that differ primarily in open durations. Rate constant values are averages (rounded to two significant figures) obtained by fittings to low- and medium-mode periods selected from five single-channel patches. Values were set to be identical when they did not differ > 10% for the two modes. (b) Single-channel responses simulated with the mechanism in a were similar to those seen experimentally (Fig. 1e). (c–e) Simulations with 1,000 channels gave deactivation decays (c,d) and two-pulse results (e) similar to those recorded experimentally (Fig. 1a,c). D, desensitized state.
Finally, we used each mechanism to simulate single-channel and large ensemble currents. As expected, the two mechanisms reproduced all of the unitary characteristics of low- and medium-mode gating (Fig. 3b), and the decays of the macroscopic responses to 1-ms pulses obtained with either the low- and medium-mode reaction mechanisms were each mono-exponential, regardless of whether a desensitized state was included (Fig. 3c). However, when we mixed the ensemble currents that were generated independently for each reaction mechanism (assuming the low:medium ratio to be 10:1; Supplementary Results), the response decayed bi-exponentially with time constants and relative amplitudes for the two deactivation components that were very close to our experimental values (Figs. 1a and 3d,e).
From these results, we propose that the biphasic decay of NMDA receptor responses evoked by short applications of glutamate reflects largely modal gating and that desensitization has only a minor role. Thus, shifting the equilibrium fraction of channels in each mode may represent a previously unknown mechanism for altering both the amplitude and the time course of NMDA receptor synaptic currents.
Supplementary Material
Note: Supplementary information is available on the Nature Neuroscience website.
Acknowledgments
This work was supported in part by grants from the US National Institutes of Health (to J.R.H. and G.K.P.), the American Heart Association (to G.K.P.) and a travel grant from the Department of Anesthesiology (to G.K.P.).
References
- 1.McBain CJ, Mayer ML. Physiol Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- 2.Lester RA, Clements JD, Westbrook GL, Jahr CE. Nature. 1990;346:565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- 3.Lester RA, Jahr CE. J Neurosci. 1992;12:635–643. doi: 10.1523/JNEUROSCI.12-02-00635.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popescu G, Auerbach A. Nat Neurosci. 2003;6:476–483. doi: 10.1038/nn1044. [DOI] [PubMed] [Google Scholar]
- 5.Popescu G, Robert A, Howe JR, Auerbach A. Nature. 2004;430:790–793. doi: 10.1038/nature02775. [DOI] [PubMed] [Google Scholar]
- 6.Banke TG, Traynelis SF. Nat Neurosci. 2003;6:144–152. doi: 10.1038/nn1000. [DOI] [PubMed] [Google Scholar]
- 7.Colquhoun D, Hawkes AG. Proc R Soc Lond B. 1981;211:205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- 8.Schorge S, Elenes S, Colquhoun D. J Physiol. 2005;569:395–418. doi: 10.1113/jphysiol.2005.095349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auerbach A, Zhou Y. J Neurosci. 2005;25:7914–7923. doi: 10.1523/JNEUROSCI.1471-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erreger K, Dravid SM, Banke TG, Wyllie DJA, Traynelis SF. J Physiol (Lond) 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vicini S, et al. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- 12.Wyllie DJ, Behe P, Colquhoun D. J Physiol (Lond) 1998;510:1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stocca G, Vicini S. J Physiol (Lond) 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Priestley T, Ochu E, Kemp JA. Neuroreport. 1994;5:1763–1765. doi: 10.1097/00001756-199409080-00019. [DOI] [PubMed] [Google Scholar]
- 15.Zhong J, Russell SL, Pritchett DB, Molinoff PB, Williams K. Mol Pharmacol. 1994;45:846–853. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: Supplementary information is available on the Nature Neuroscience website.