Abstract
Protoapigenone (1), isolated from Thelypteris torresiana, previously showed significant cytotoxic activity against five human cancer cell lines. In a continued structure-activity relationship study, the first total synthesis and modification of 1 were achieved. All synthesized compounds and related intermediates were evaluated for cytotoxic activity against five human cancer cell lines, HepG2, Hep3B, MDA-MB-231, MCF-7 and A549. Among them, 24 showed 2.2-14.2 fold greater cytotoxicity than 1 and naphthyl A-ring analogs remarkably enhanced the activity.
Introduction
Flavonoids are plant pigments that generally display marvelous colors and are ubiquitous to green plants. Their multiple bioactivities (leading to the term bioflavonoid) and medicinal significance have been summarized recently.1,2 In our previous study, we isolated the unique flavonoid protoapigenone (1) from Thelypteris torresiana (Gaud.) Alston, which showed potent cytotoxic activity against HepG2 and Hep3B (liver), MDA-MB-231 and MCF-7 (breast), and A549 (lung) human cancer cell lines with IC50 values of 0.94-5.59 μM.3 In our subsequent study, this compound showed 7.5- and 4.6-fold greater cytotoxic activity against two human breast cancer cell lines MCF-7 and MDA-MB-231 compared with the MCF-10A normal human breast epithelial cell line.4
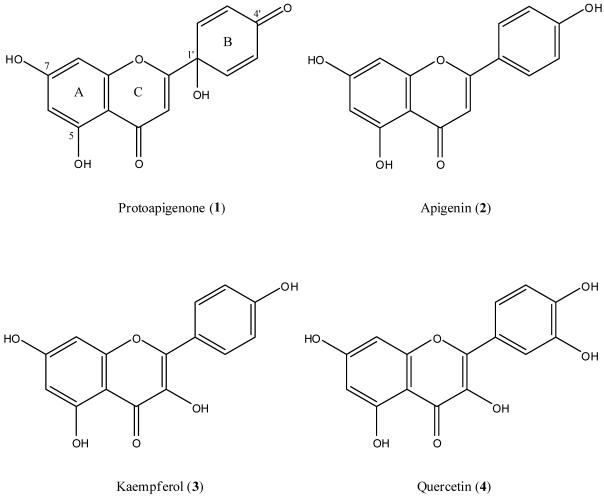
Flavonoid 1 has an unusual non-aromatic B-ring with a hydroxy group on the C-1′ position, which might arise from oxidation of a 4′-hydroxyphenyl. Apigenin (2), which is the conceivable biosynthetic precursor of 1, kaempferol (3), and quercetin (4) are well-known flavonoids bearing a 4′-hydroxyphenyl B-ring (Figure 1). These three compounds showed no antiproliferative activity (IC50 > 20 μg/mL) against six human cancer cell lines,5 while 1 displayed significant cytotoxic activity. This unique difference spurred our interest in the characteristics of the 1′-hydroxycyclohexa-2′,5′-dien-4′-one B-ring in this flavonoid skeleton. Although the antitumor mechanism of this functional group is still not clear, recent studies on some quinol derivatives showed that the oxidized species are more active than their reduced counterparts.6-9
Figure 1.
Chemical structures of 1 and related flavonoids.
To investigate a new class of anticancer agents based on this novel plant-derived natural flavonoid, the first total synthesis of 1 and the preparation of some analogs were accomplished. All newly synthesized compounds including structurally related intermediates were assayed for in vitro cytotoxic activity against five human cancer cell lines, e.g., HepG2, Hep3B, MDA-MB-231, MCF-7, and A549. In this paper, we describe the synthesis, bioactivity data, and preliminary structure-activity relationship (SAR) studies related to 1.
Chemistry
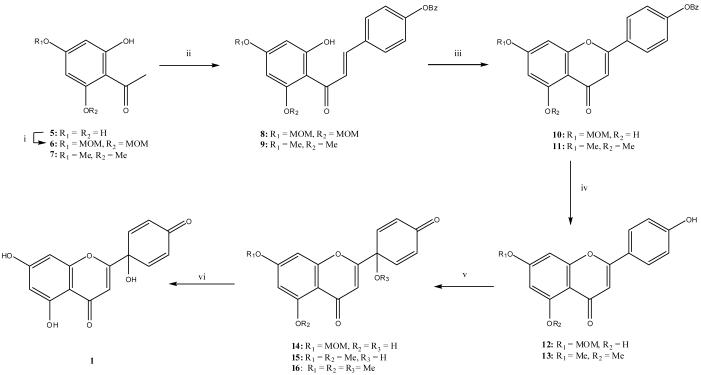
Our initial attempt to obtain 1 from 2 by oxidation failed because of oxidation of the 5- and 7- hydroxy groups. Due to the difficulty of selectively protecting these two groups on 2 in the presence of the 4′-hydroxyl, diprotected trihydroxyacetophenones (6 and 7)10 (commercially available) were selected as starting materials.
Our successful flavonoid synthesis started with a Claisen-Schmidt condensation carried out individually on 6 and 7 with 4-benzyloxybenzaldehyde in the presence of aqueous potassium hydroxide to provide 8 (87%) and 9 (79%).11 Chalcone 9 was further cyclized with a catalytic amount of iodine in DMSO to give the corresponding 4′-benzyloxyflavonoid 11 (74%).11 Similar cyclization of 8 gave only a low yield of the expected product. Instead, the use of pyridine as solvent caused an unexpected cleavage of the MOM (methoxymethyl) ether from the 5-position, along with cyclization, to produce 10 (86%). The benzyl groups of 10 and 11 were removed by treatment with catalytic 10% palladium carbon under H2 to afford 4′-hydroxyflavonoids 12 (86%) and 13 (83%).12 Oxidation of 12 and 13 with [bis(trifluoroacetoxy)iodo]benzene (TAIB) in acetonitrile/H2O at room temperature gave the novel flavonoids 14 (22%) and 15 (33%).13,14 The 5-hydroxy group was unaffected under these conditions because of strong intermolecular hydrogen bonding with the carbonyl group on the 4-position. When MeOH rather than acetonitrile/H2O was used as solvent, trimethoxyprotoapigenone (16) (33%) was produced from 13. Finally, 1 was obtained by cleavage of the MOM group on 14 using 15% HCl in i-PrOH (yield 47%),10,12 while the methoxy groups on 15 were not cleaved using 47% HBr/HOAc at reflux as the demethylation condition (Scheme 1).15-16
Scheme 1.
Total Synthesis of 1 and its Analogs
Reagents: (i) K2CO3, MOMCl, acetone, (ii) EtOH, 50% KOH / H2O, 4-benzyloxybenzaldehyde; (iii) I2, DMSO or pyridine; (iv) 10% Pd-C / H2; (v) TAIB (1 equiv), CH3CN/H2O 9/1 (12→14, 13→15) or MeOH (13→16), 25 °C; (vi) 15% cHCl / iPrOH (14→1).
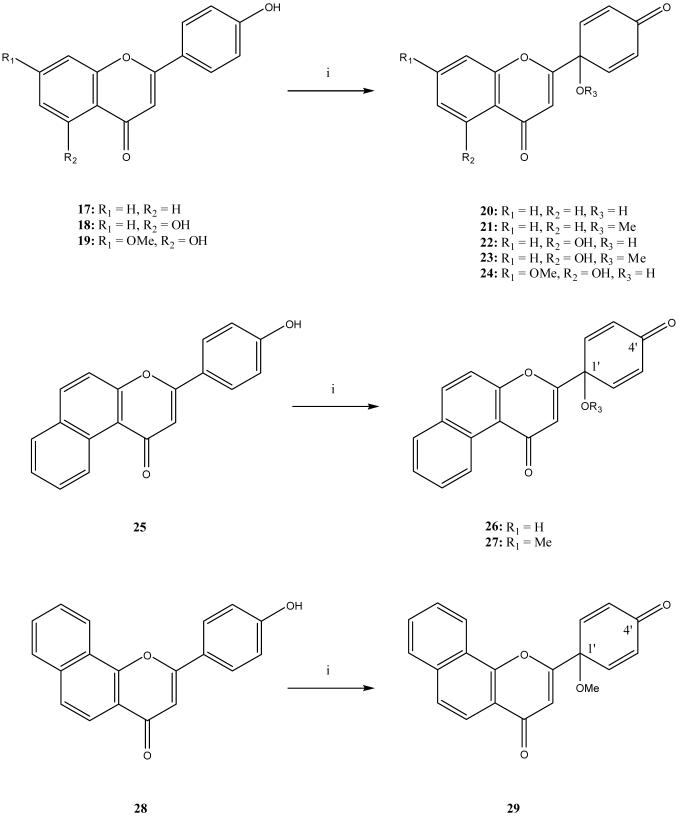
In addition, five commercially available 4′-hydroxyflavonoids (17, 18, 19, 25, and 28) were oxidized with TAIB using acetonitrile/H2O or methanol as the solvent to obtain the related quinol derivatives, 20 (36%) and 21 (28%) from 17, 22 (16%) and 23 (15%) from 18, 24 (20%) from 19, 26 (16%) and 27 (15%) from 25, and 29 (23%) from 28 (Scheme 2).13,14 Accordingly, the first total synthesis of 1 as well as the synthesis of twelve A-ring and C-1′ analogs, 14-16, 20-24, 26, 27, and 29 were accomplished.
Scheme 2.
Synthesis of Flavonoid and Naphthoflavone Analogs with Quinol B-ring
Reagent: (i) TAIB (1 equiv), CH3CN/H2O 9/1 or MeOH, 25 °C.
Results and Discussion
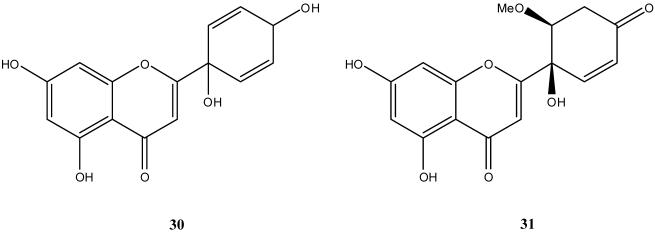
Together with 1, newly synthesized flavonoids 14-16, 20-24, 26, 27, and 29 were examined for in vitro cytotoxic activities against several human cancer lines, including HepG2, Hep3B, MDA-MB-231, MCF-7, and A549. Table 1 lists the IC50 values obtained with these compounds as well as the related flavonoids protoapigenin (30) and 5′,6′-dihydro-6′-methoxyprotoapigenone (31) (Figure 2), as well as doxorubicin, included as a positive control.
Table 1.
Cytotoxic Effects of Synthesized 1 and Analogs
| IC50(μM)a |
|||||
|---|---|---|---|---|---|
| Compound | HepG2 | Hep3B | MDA-MB-231 | MCF-7 | A549 |
| 1 | 8.11±0.01 | 2.27±0.01 | 1.43±0.00 | 3.74±0.02 | 13.85±0.01 |
| 14 | 15.58±0.08 | 2.39±0.00 | 1.73±0.00 | 5.24±0.01 | 24.61±0.02 |
| 15 | 5.03±0.01 | 0.67±0.01 | 0.54±0.01 | 1.21±0.01 | 1.27±0.03 |
| 16 | 27.01±0.01 | 3.78±0.00 | 4.70±0.01 | 4.21±0.04 | >60.98 |
| 20 | 6.73±0.01 | 1.26±0.02 | 0.71±0.00 | 1.73±0.01 | 5.24±0.05 |
| 21 | 40.86±0.08 | 5.41±0.00 | 5.49±0.01 | 7.99±0.03 | >74.63 |
| 22 | 4.96±0.02 | 1.30±0.01 | 0.67±0.00 | 3.44±0.04 | 5.07±0.03 |
| 23 | 26.90±0.01 | 2.32±0.04 | 1.90±0.02 | 5.46±0.06 | 57.61±0.31 |
| 24 | 0.57±0.02 | 0.67±0.00 | 0.43±0.00 | 1.70±0.06 | 3.10±0.08 |
| 26 | 3.55±0.04 | 0.30±0.00 | 0.39±0.00 | 0.66±0.00 | 1.81±0.00 |
| 27 | 9.94±0.10 | 1.10±0.01 | 1.16±0.01 | 2.70±0.01 | 25.79±0.05 |
| 29 | 14.37±0.03 | 0.60±0.01 | 1.07±0.01 | 2.96±0.08 | 28.33±0.07 |
| 30a | 5.56±0.33 | 69.44±0.58 | >69.44 | >69.44 | 65.42±0.60 |
| 31a | 18.49±0.47 | 5.47±0.08 | 4.09±0.10 | 18.62±0.29 | 41.82±0.23 |
| Doxorubicinb | 0.50±0.04 | 0.62±0.03 | 0.14±0.01 | 0.74±0.00 | 0.36±0.02 |
Data are expressed as mean ± SD ( mean = 2).
Data from our previous study.
Positive control.
Figure 2.
Structures of 1-related cytotoxic flavonoids.
The parent compound 1 showed potent cytotoxic activity against the tested human cancer cell lines. Comparison of 1 to its synthetic analogs 14-16 showed that 14, with a MOM ether at C-7, had comparable or reduced activity compared with 1, and trimethoxy 16 was uniformly less active. However, 5,7-dimethoxy compound 15 showed enhanced cytotoxic activity with notable IC50 values of 0.54-1.27 μM against four cell lines (Hep3B, MDA-MB-231, MCF-7 and A549). Remarkably, 1′-hydroxy analogs 15, 20, 22, and 26 were 1.6 to >48.0 times more active than the related 1′-methoxy analogs, 16, 21, 23, and 27. These findings indicate the importance of a non-substituted OH at the C-1′ position.
Among the quinol derivatives, changing the C-5 substituent from hydrogen to methoxy (20 vs 22, 21 vs 23) did not affect potency, while variation in the C-7 functional group did exert an influence. For example, compound 24 with a C-7 methoxy group showed the highest potency against all five cancer cell lines with IC50 values of 0.43-3.10 μM. Amazingly, this compound also exhibited significantly enhanced activity against the HepG2 human liver cancer cell line with an IC50 value of 0.57 μM, and consequently was 14.2 times more active than 1, while most analogs showed comparatively weak activity against this cell line. Compound 22, which lacks a C-7 methoxy group, was generally less active than 24, but was about two-fold more active than 1 against all cell lines. In particular, growth inhibition of Hep3B (IC50 = 1.30 μM) and MDA-MB-231 (IC50 = 0.67 μM) was significantly increased.
Naphthoflavones 26, 27, and 29, which have an additional conjugated aromatic ring attached to the A-ring, showed better activity compared with 1 against the three cell lines Hep3B, MDA-MB-231, and MCF-7. Particularly, analog 26 was more potent than doxorubicin against the Hep3B cell line with an IC50 value of 0.30 μM. In addition, this compound was 7.7 times more potent than 1 against A549 cell growth with an IC50 value of 1.81 μM.
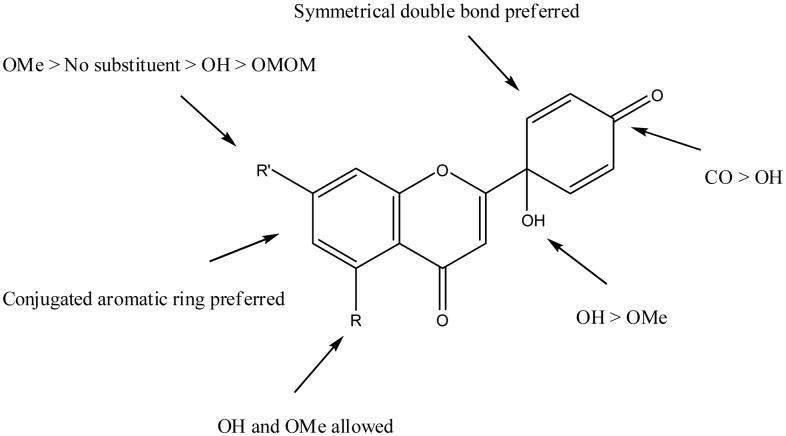
Our prior report stated that the 4′-oxocyclohexa-2′,5′-dienyl moiety as found in 1 is critically important for biological activity in flavonoids.3 Accordingly, we found in this study that the natural product 30, which instead contains a 4′-hydroxycyclohexa-2′,5′-dienyl moiety, was active against only the HepG2 cell line. However, corresponding synthetic 1′-hydroxyquinol analogs 20, 22, and 24 with different combinations of hydrogen, hydroxy, and methoxy substituents rather than two hydroxy groups on the A-ring, showed significant cytotoxic activity. In addition, we previously found that 31, which has only a single double bond in the B-ring, exhibited lower cytotoxic activity; thus, a symmetrical double bond structure in the B-ring might also be important.3 The summary of this preliminary SAR study is shown in Figure 3.
Figure 3.
SAR in newly synthesized cytotoxic flavonoids.
In conclusion, among all screened compounds, the 1′-hydroxycyclohexa-2′,5′-dien-4′-one B-ring is critically important in the bioflavonoid skeleton, but substituent changes on the A-ring can affect selectivity or potency against different kinds of cancer cell lines. A methoxy group on the C-7 position can enhance cytotoxic selectivity toward the human HepG2 liver cancer cell line, and an additional conjugated aromatic ring on the A-ring increases cytotoxicity against all five tested human cancer cell lines. These prototype analogs of 1 have demonstrated valuable growth inhibition of selected human cancer cell lines. In addition, our preliminary data (unpublished) indicate that treatment of MDA-MB-231 with 1 leads to cell cycle arrest at the G2/M phase and apoptosis; the precise mechanisms responsible for these effects are currently under investigation. We are synthesizing additional candidate structures that will address specific cancer cell lines.
Experimental Section
Materials and Methods
Melting points were measured with a Fisher John melting apparatus without correction. 1H NMR spectra were measured on a 300 MHz Varian Gemini 2000 spectrometer. The solvent used were CDCl3 or pyridine-d5. Mass spectra were measured on a PECIEX API 3000 instrument with turbo ion spray source, Agilent-1100, LC/MSD-Trap, or Shimadzu LCMS-IT-TOF with ESI interface. Thin-layer chromatography (TLC) and preparative TLC were performed on precoated silica GF plates purchased from Merck, Inc. Biotage Flash+ or Isco Companion systems were used for flash chromatography. Silica gel (200-400 mesh) from Aldrich, Inc. was used for column chromatography. Flavonoids were obtained from Indofine Chemical Company, Inc. All other chemicals were obtained from Aldrich, Inc.
Cell Viability Assays
Human breast (MCF-7 and MDA-MB-231), liver (HepG2 and Hep3B), and lung (A549) cancer cell lines were obtained from American Type Culture Collection. All cell lines were propagated in RPMI-1640 medium supplement with 10% (v/v) FBS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. Cell viability was measured by the MTT colorimetric method. Cells were seeded at densities of 5,000-10,000 cells/well in 96-well tissue culture plates. On day two, cells were treated with test compounds for another 72 h. After drug treatment, attached cells were incubated with MTT (0.5 mg/mL, 1 h) and subsequently solubilized in DMSO. The absorbency at 550 nm was then measured using a microplate reader. The IC50 is the concentration of agent that reduced the cell viability by 50% under the experimental conditions.3,17
Protoapigenone (1)
Compound 14 (30 mg, 0.09 mmol) was dissolved in 15% HCl / iPrOH (1:5, 5 mL), and the reaction mixture was stirred at the room temperature for 140 min. The solvent was evaporated and the residue chromatographed using silica gel (isocratic elution, 33% MeOH / 67% CHCl3) to give 1 (12.0 mg, 47%) as a yellow solid; ESI-MS (m/z, %): 285 (M--1, 100); 1H NMR (pyridine d5) δ 13.38 (1H, br s, OH-5), 7.24 (2H, d, J = 10.2 Hz, H-2′, H-6′), 7.07 (1H, s, H-3), 6.72 (1H, d, J = 2.4 Hz, H-8), 6.60 (1H, d, J = 2.4 Hz, H-6), 6.54 (2H, d, J = 10.2 Hz, H-3′, H-5′); 13C NMR (pyridine d5) δ 185.2 (C-4′), 182.9 (C-4), 167.8 (C-2), 166.3 (C-7), 163.1 (C-5), 158.7 (C-9), 148.4 (C-2′, C-6′), 129.5 (C-3′, C-5′), 107.4 (C-3), 105.1 (C-10), 100.2 (C-6), 95.0 (C-8), 69.7 (C-1′).
1-[2-Hydroxy-4,6-bis(methoxymethoxy)phenyl]ethanone (6)
2′,4′,6′-Trihydroxyacetophenone monohydrate (5) (2.0 g, 11.9 mmol) and K2CO3 (11.5 g) were dissolved in anhydrous acetone (80 mL). Chloromethyl methyl ether (MOMCl, 2.4 g) was added dropwise to the stirring solution, and then the suspension was refluxed for 90 min. After cooling, the solid was filtered off, and the crude product was evaporated and purified by column chromatography on silica gel (isocratic elution, 90% n-hexane / 10% EtOAc) to afford 6 (1.5 g, 50%) as a white solid; 1H NMR (CDCl3) δ 13.72 (1H, br s, OH-2), 6.25 (1H, d, J = 2.4 Hz, H-5), 6.23 (1H, d, J = 2.4 Hz, H-3), 5.24, 5.16 (2H each, s, CH2-MOM), 3.51, 3.46 (3H each, s, OMe-MOM), 2.64 (1H, s, COCH3); 13C NMR (pyridine d5) δ 203.2 (CO), 166.8 (C-2), 163.4 (C-4), 160.3(C-6), 106.9 (C-1), 97.1 (C-3), 94.4 (C-5), 93.9 (CH2-MOM, overlapping), 56.6, 56.4 (OMe-MOM), 33.0 (COCH3).
3-[4-(Benzyloxy)phenyl]-1-[2-hydroxy-4,6-bis(methoxymethoxy)phenyl]prop-2-en-1-one (8)
Compound 6 (1.8 g, 7.1 mmol) and 4-benzyloxybenzaldehyde (3.0 g, 14.3 mmol) were dissolved in 50% EtOH, KOH / H2O solution (20 mL). The reaction was stirred at rt for 30 h, and then the solvent was evaporated under reduced pressure. The mixture was chromatographed on silica gel (isocratic elution, n-hexane/EtOAc, 6:1) to afford 8 (2.8 g, 87%) as a white solid; 1H NMR (CDCl3) δ 13.95 (1H, br s, OH-2′), 7.84 (1H, d, J = 15.6 Hz, H-β), 7.78 (1H, d, J = 15.6 Hz, H-α), 7.56 (2H, d, J = 8.7 Hz, , H-2, H-6), 7.32-7.46 (5H, m, H-OBz), 7.01 (2H, d, J = 8.7 Hz, H-3, H-5), 6.32 (1H, d, J = 2.4 Hz, H-5′), 6.25 (1H, d, J = 2.4 Hz, H-3′), 5.29, 5.19 (2H each, s, CH2-MOM), 5.11 (2H, s, CH2-OBz), 3.54, 3.49 (3H each, s, OMe-MOM); 13C NMR (CDCl3) δ 192.8 (CO), 167.3 (C-4′), 163.3 (C-2′), 160.6 (C-6′), 159.8 (C-4), 142.6 (C-β), 136.4 (C-1-OBz), 130.1 (C-2, C-6), 128.6 (C-3-OBz, C-5-OBz), 128.4 (C-1), 128.1 (C-α), 127.4 (C-2-OBz, C-6-OBz), 125.0 (C-4-OBz), 115.3 (C-3, C-5), 107.5 (C-1′), 97.5 (C-5′), 95.1 (C-3′), 94.7, 94.0 (CH2-MOM), 70.1 (CH2-OBz), 56.8, 56.4 (OMe-MOM).
3-[4-(Benzyloxy)phenyl]-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one (9)
Compound 7 (1.0 g, 5.1 mmol) was reacted with 4-benzyloxybenzaldehyde (2.2 g, 10.2 mmol) as described above for 6 to afford 9 (1.6 g, 79%) as a white solid; 1H NMR (CDCl3) δ 13.87 (1H, br s, OH-2′), 7.82 (1H, d, J = 15.6 Hz, H-β), 7.77 (1H, d, J = 15.6 Hz, H-α), 7.56 (2H, d, J = 9.0 Hz, H-2, H-6), 7.32-7.46 (5H, m, H-OBz), 7.00 (2H, d, J = 9.0 Hz, H-3, H-5), 6.11 (1H, d, J = 2.4 Hz, H-5′), 5.96 (1H, d, J = 2.4 Hz, H-3′), 5.11 (2H, s, CH2-OBz), 3.92, 3.83 (3H each, s, OMe); 13C NMR (CDCl3) δ 192.6 (CO), 168.4 (C-4′), 166.0 (C-2′), 162.4 (C-6′), 160.5 (C-4), 142.4 (C-β), 136.5 (C-1-OBz), 130.1 (C-2, C-6), 128.7 (C-3-OBz, C-5-OBz), 128.5 (C-1), 128.1 (C-α), 127.5 (C-2-OBz, C-6-OBz), 125.2 (C-4-OBz), 115.2 (C-3, C-5), 106.3 (C-1′), 93.8 (C-5′), 91.2 (C-3′), 70.1 (CH2-OBz), 55.8, 55.6 (OMe).
2-[4-(Benzyloxy)phenyl]-5-hydroxy-7-(methoxymethoxy)chromen-4-one (10)
Compound 8 (500 mg, 1.1 mmol) was dissolved anhydrous pyridine (5 mL), and a catalytic amount of iodine (28.4 mg, 0.1 mmol) was added. The solution mixture was stirred and refluxed. After 3 h, 10% Na2S2O3 (30 mL) was added to remove iodine. The mixture was extracted with EtOAc (50 mL) and then purified by column chromatography on silica gel (isocratic elution, EtOAc only) to give 10 (380 mg, 86%) as a yellow solid; 1H NMR (CDCl3) δ 12.77 (1H, br s, OH-5), 7.81 (2H, d, J = 8.7 Hz, H-2′, H-6′), 7.32-7.46 (5H, m, H-OBz), 7.06 (2H, d, J = 8.7 Hz, H-3′, H-5′), 6.64 (1H, d, J = 2.1 Hz, H-8), 6.55 (1H, s, H-3), 6.46 (1H, d, J = 2.1 Hz, H-6), 5.23 (2H, s, CH2-OMOM), 5.13 (2H, s, CH2-OBz), 3.50 (3H, s, OMe); 13C NMR (CDCl3) δ 182.4 (C-4), 163.9 (C-7), 162.8 (C-2), 161.9 (C-5), 161.7 (C-9), 157.4 (C-4′), 136.1 (C-1-OBz), 128.7 (C-2, C-6), 128.2 (C-4-OBz), 128.0 (C-2-OBz, C-6-OBz), 127.4 (C-3-OBz, C-5-OBz), 123.6 (C-1′), 115.3 (C-3, C-5), 106.1 (C-10), 104.3 (C-3), 100.0 (C-6), 94.2 (C-8), 94.2 (CH2-MOM), 70.1 (CH2-OBz), 56.3 (OMe).
2-[4-(Benzyloxy)phenyl]-5,7-dimethoxychromen-4-one (11)
Compound 9 (1.6 g, 4.0 mmol) was treated analogously as 8 to give 11 (1.1 g, 74%) as a yellow solid; 1H NMR (pyridine-d5) δ 7.95 (2H, d, J = 9.0 Hz, H-2′, H-6′), 7.32-7.58 (5H, m, H-OBz), 7.23 (2H, d, J = 9.0 Hz, H-3′, H-5′), 6.96 (1H, s, H-3), 6.83 (1H, d, J = 2.4 Hz, H-8), 6.57 (1H, d, J = 2.4 Hz, H-6), 5.19 (2H, s, CH2-OBz), 3.85 (6H, s, OMe); 13C NMR (CDCl3) δ 176.6 (C-4), 164.3 (C-7), 161.6 (C-2), 161.3 (C-5), 160.5 (C-4′), 160.2 (C-9), 137.2 (C-1-OBz), 129.0 (C-3-OBz, C-5-OBz), 128.5 (C-4-OBz), 128.1 (C-2-OBz, C-6-OBz), 128.1 (C-2, C-6), 124.5 (C-1′), 115.6 (C-3, C-5), 108.2 (C-10), 109.8 (C-3), 96.7 (C-6), 93.7 (C-8), 70.3 (CH2-OBz), 56.2, 55.9 (OMe).
General Procedure for Benzyl Group Removal. Synthesis of Compounds 12 and 13
A mixture of 10 (200 mg, 0.5 mmol), dry Pd/C (10%, 106 mg) and EtOAc/MeOH (1:1, 15 mL) was stirred at 25°C under an atmosphere of hydrogen for 3 h. The mixture was filtered through a layer of silica gel on cotton, washed with EtOAc, concentrated under vacuum, and purified by column chromatography on silica gel (isocratic elution, EtOAc only) to give 12 (134 mg) as a yellow solid.
5-Hydroxy-2-(4-hydroxyphenyl)-7-(methoxymethoxy)chromen-4-one (12)
86% yield; 1H NMR (pyridine-d5) 7.93 (2H, d, J = 8.7 Hz, H-2′, H-6′), 7.27 (2H, d, J = 8.7 Hz, H-3′, H-5′), 6.96 (1H, s, H-3), 6.91 (1H, d, J = 2.1 Hz, H-8), 6.78 (1H, d, J = 2.1 Hz, H-6), 5.35 (2H, s, CH2-OMOM), 3.45 (3H, s, OMe); 13C NMR (CDCl3) δ 182.4 (C-4), 164.4 (C-7), 162.8 (C-2), 163.4 (C-5), 162.1 (C-9), 157.4 (C-4′), 128.5 (C-2, C-6), 121.5 (C-1′), 116.4 (C-3, C-5), 106.0 (C-10), 103.6 (C-3), 99.7 (C-6), 94.3 (C-8), 94.2 (CH2-MOM), 55.8 (OMe).
2-(4-Hydroxyphenyl)-5,7-dimethoxychromen-4-one (13)
83% yield; 1H NMR (pyridine-d5) δ 7.93 (2H, d, J = 9.0 Hz, H-2′, H-6′), 7.27 (2H, d, J = 9.0 Hz, H-3′, H-5′), 6.95 (1H, s, H-3), 6.82 (1H, d, J = 2.4 Hz, H-8), 6.56 (1H, d, J = 2.4 Hz, H-6), 3.84 (6H, s, OMe); 13C NMR (pyridine-d5) δ 176.7 (C-4), 164.2 (C-7), 162.1 (C-2), 161.3 (C-5), 161.1 (C-4′), 160.2 (C-9), 128.4 (C-2′, C-6′), 122.6 (C-1′), 116.8 (C-3′, C-5′), 109.7 (C-10), 107.5 (C-3), 96.7 (C-6), 93.7 (C-8), 56.2, 55.9 (OMe).
General Procedure for Oxidation Reaction with TAIB. Synthesis of Compounds 14-16, 20-24, 26, 27 and 29
The precusor flavonoid [for example, 12 (90 mg)] was dissolved in acetonitrile/H2O (9:1) or MeOH (5 mL) individually, and a catalytic amount of TEMPO (0.2 eq) was added to the flask, followed by 2 eq of TAIB. The reaction mixture was stirred vigorously at 25 °C for 90 min. The reaction mixture was then evaporated to dryness under reduced pressure, and the residue purified on a silica gel column (isocratic elution, 2.5% MeOH / 97.5 % CH2Cl2) to give 14 in 22% yield. Corresponding oxidations of the remaining flavonoids (13, 17-19, 25 and 28) gave 15/16, 20/21, 22/23, 24, 26/27, and 29, respectively, with different solvents.
7-Methoxymethoxyprotoapigenone (14)
22% yield from 12; 1H NMR (CDCl3) δ 12.34 (1H, s, OH-5), 6.87 (2H, d, J = 9.9 Hz, H-2′, H-6′), 6.66 (1H, s, H-3), 6.46 (2H, s, H-6, H-8), 6.41 (2H, d, J = 9.9 Hz, H-3′, H-5′), 5.20 (2H, s, CH2-OMOM), 3.46 (6H, s, OMe); 13C NMR (CDCl3) δ 184.5 (C-4′), 182.4 (C-4), 165.6 (C-7), 163.4 (C-2), 162.0 (C-5), 157.6 (C-9), 145.5 (C-2′, C-6′), 130.2 (C-3′, C-5′), 107.5 (C-3, C-10), 100.6 (C-6), 94.4 (C-8), 94.2 (CH2-MOM), 69.5 (C-1′), 56.5 (OMe).
5,7-Dimethoxyprotoapigenone (15)
33% yield from 13; ESI-MS (m/z, %): 313 (M--H, 100); 1H NMR (pyridine-d5) δ 7.26 (2H, d, J = 10.2 Hz, H-2′, H-6′), 7.10 (1H, s, H-3), 6.56 (2H, d, J = 10.2 Hz, H-3′, H-5′), 6.55 (2H, s, H-6, H-8), 3.82, 3.68 (3H each, s, OMe); 13C NMR (pyridine-d5) δ 185.3 (C-4′), 176.4 (C-4), 164.5 (C-7), 164.3 (C-2), 161.3 (C-5), 160.3 (C-9), 148.8 (C-2′, C-6′), 129.4 (C-3′, C-5′), 110.9 (C-3), 109.8 (C-10), 96.9 (C-6), 93.4 (C-8), 69.4 (C-1′), 56.2, 55.8 (OMe).
5,7,1′-Trimethoxyprotoapigenone (16)
33% yield from 13; ESI+MS (m/z, %): 328 (M++H, 100); 1H NMR (pyridine-d5) δ 6.95 (2H, d, J = 10.2 Hz, H-2′, H-6′), 6.76 (1H, s, H-3), 6.67 (2H, d, J = 10.2 Hz, H-3′, H-5′), 6.55 (1H, s, H-8), 6.52 (1H, s, H-6), 3.81, 3.68, 3.25 (3H each, s, OMe); 13C NMR (pyridine-d5) δ 184.6 (C-4′), 176.0 (C-4), 164.6 (C-7), 161.4 (C-2), 161.3 (C-5), 160.1 (C-9), 146.0 (C-2′, C-6′), 133.2 (C-3′, C-5′), 111.5 (C-3, C-10), 97.0 (C-6), 93.4 (C-8), 74.7 (C-1′), 56.2, 55.8, 52.4 (OMe).
2-(1-Hydroxy-4-oxocyclohexa-2,5-dienyl)-4H-chromen-4-one (20)
36% yield from 17; ESI-MS (m/z, %): 254 (M--H, 100); 1H NMR (CDCl3) δ 8.14 (1H, dd, J = 7.8, 1.5 Hz, H-5), 7.66 (1H, ddd, J = 8.4, 7.2, 1.5 Hz, H-7), 7.40 (1H, ddd, J = 7.8, 7.2, 0.9 Hz, H-6), 7.36 (1H, dd, J = 8.4, 0.9 Hz, H-8), 6.95 (2H, d, J = 10.2 Hz, H-2′, H-6′), 6.88 (1H, s, H-3), 6.41 (2H, d, J = 10.2 Hz, H-3′, H-5′); 13C NMR (CDCl3) δ 185.0 (C-4′), 178.9 (C-4), 166.3 (C-2), 156.2 (C-9), 146.2 (C-2′, C-6′), 134.3 (C-7), 129.9 (C-3′, C-5′), 125.7 (C-5, C-6), 123.5 (C-7), 118.1 (C-8), 108.8 (C-3), 69.8 (C-1′).
2-(1-Methoxy-4-oxocyclohexa-2,5-dienyl)-4H-chromen-4-one (21)
28% yield from 17; ESI+MS (m/z, %): 268 (M+, 100); 1H NMR (CDCl3) δ 8.17 (1H, dd, J = 7.8, 1.5 Hz, H-5), 7.65 (1H, ddd, J = 8.4, 7.2, 1.5 Hz, H-7), 7.40 (1H, ddd, J = 7.8, 7.2, 0.9 Hz, H-6), 7.35 (1H, dd, J = 8.4, 0.9 Hz, H-8), 6.82 (2H, d, J = 10.2 Hz, H-2′, H-6′), 6.76 (1H, s, H-3), 6.58 (2H, d, J = 10.2 Hz, H-3′, H-5′); 13C NMR (CDCl3) δ 185.5 (C-4′), 178.0 (C-4), 163.8 (C-2), 156.0 (C-9), 145.5 (C-2′, C-6′), 134.0 (C-7), 133.1 (C-3′, C-5′), 125.7, 125.5 (C-5, C-6), 123.9 (C-7), 118.0 (C-8), 109.5 (C-8), 74.8 (C-1′), 52.7 (OMe).
5-Hydroxy-2-(1-hydroxy-4-oxocyclohexa-2,5-dienyl)-4H-chromen-4-one (22)
16% yield from ESI-MS 18; (m/z, %): 270 (M--H, 100); 1H NMR (CDCl3) δ 12.24 (1H, s, OH-5), 7.52 (1H, dd, J = 8.4, 8.4 Hz, H-7), 6.99 (2H, d, J = 9.9 Hz, H-2′, H-6′), 6.81 (1H, d, J = 8.4 Hz, H-8), 6.80 (1H, d, J = 8.4 Hz, H-6), 6.73 (1H, s, H-3), 6.42 (2H, d, J = 9.9 Hz, H-3′, H-5′); 13C NMR (CDCl3) δ 184.4 (C-4′), 183.5 (C-4), 166.4 (C-2), 160.8 (C-5), 156.3 (C-9), 145.3 (C-2′, C-6′), 135.9 (C-7), 130.4 (C-3′, C-5′), 112.0 (C-6), 110.7 (C-10), 107.7 (C-3), 107.0 (C-8), 69.6 (C-1′).
5-Hydroxy-2-(1-methoxy-4-oxocyclohexa-2,5-dienyl)-4H-chromen-4-one (23)
15% yield from 18; ESI-MS (m/z, %): 283 (M--H, 25), 268 (M--CH , 8), 253 (M--CH3-OH, 100); 1H NMR (CDCl3) δ 12.29 (1H, s, OH-5), 7.50 (1H, dd, J = 8.4, 8.4 Hz, H-7), 6.79 (2H, m, H-6, H-8), 6.78 (2H, d, J = 9.9 Hz, H-2′, H-6′), 6.70 (1H, s, H-3), 6.58 (2H, d, J = 9.9 Hz, H-3′, H-5′), 3.41 (3H, s, OMe); 13C NMR (CDCl3) δ 184.3 (C-4′), 183.5 (C-4), 165.1 (C-2), 160.7 (C-5), 156.2 (C-9), 145.1 (C-2′, C-6′), 135.7 (C-7), 133.4 (C-3′, C-5′), 111.8 (C-6), 110.8 (C-10), 108.2(C-3), 107.0 (C-8), 74.8 (C-1′), 52.8 (OMe).
5-Hydroxy-2-(1-hydroxy-4-oxocyclohexa-2,5-dienyl)-7-methoxy-4H-chromen-4-one (24)
20% yield from 19; ESI-MS (m/z, %): 299 (M--H, 100); 1H-NMR (pyridine-d5) δ 13.22 (1H, s, OH-5), 7.27 (2H, d, J = 10.2 Hz, H-2′, H-6′), 7.11 (1H, s, H-3), 6.61 (1H, d, J = 2.7 Hz, H-8), 6.59 (2H, d, J = 10.2 Hz, H-3′, H-5′), 6.49 (1H, d, J = 2.7 Hz, H-6), 3.67 (3H , s, OMe); 13C NMR (pyridine-d5) δ 185.2 (C-4′), 183.0 (C-4), 168.3 (C-7), 166.2 (C-2), 162.6 (C-5), 158.3 (C-9), 148.4 (C-2′, C-6′), 129.6 (C-3′, C-5′), 107.7 (C-3), 106.1 (C-10), 99.0 (C-6), 92.9 (C-8), 69.7 (C-1′), 56.0 (OMe).
3-(1-Hydroxy-4-oxocyclohexa-2,5-dienyl)-1H-benzo[f]chromen-1-one (26)
16% yield from 25; ESI-MS (m/z, %): 303 (M--H, 100); 1H-NMR (CDCl3) δ 9.92 (1H, dd, J = 9.0, 1.0 Hz, H-5), 8.04 (1H, d, J = 9.0 Hz, H-9), 7.88 (1H, dd, J = 8.4, 1.2 Hz, H-8), 7.76 (1H, ddd, J = 8.4, 8.4, 1.0 Hz, H-7), 7.63 (1H, ddd, J = 9.0, 8.4, 1.2 Hz, H-6), 7.36 (1H, d, J = 9.0 Hz, H-10), 7.02 (1H, s, H-3), 7.00 (2H, d, J = 10.2 Hz, H-2′, H-6′), 6.43 (2H, d, J = 10.2 Hz, H-3′, H-5′); 13C NMR (CDCl3) δ 184.9 (C-4′), 180.4 (C-4), 163.3 (C-2), 157.6 (C-10a), 146.1 (C-2′, C-6′), 130.0 (C-3′, C-5′), 136.1, 130.7, 130.1, 129.6, 128.3, 127.0, 126.9 (C-4b, C-5, C-6, C-7, C-8, C-8a, C-9), 117.2 (C-10), 117.1 (C-4a), 111.8 (C-3), 69.6 (C-1′).
3-(1-Methoxy-4-oxocyclohexa-2,5-dienyl)-1H-benzo[f]chromen-1-one (27)
15% yield from 25; ESI-MS (m/z, %): 288 (M++H-OCH3, 100), 319 (M++H, 95); 1H-NMR (CDCl3) δ 9.98 (1H, dd, J = 9.0, 1.2 Hz, H-5), 8.07 (1H, d, J = 9.0 Hz, H-9), 7.94 (1H, dd, J = 8.4, 1.5 Hz, H-8), 7.76 (1H, ddd, J = 8.4, 8.4, 1.2 Hz, H-7), 7.62 (1H, ddd, J = 9.0, 8.4, 1.2 Hz, H-6), 7.40 (1H, d, J = 9.0 Hz, H-10), 6.88 (1H, s, H-3), 6.88 (2H, d, J = 9.9 Hz, H-2′, H-6′), 6.60 (2H, d, J = 9.9 Hz, H-3′, H-5′); 13C NMR (CDCl3) δ 184.5 (C-4′), 179.8 (C-4), 161.1 (C-2), 157.4 (C-10a), 145.9 (C-2′, C-6′), 133.1 (C-3′, C-5′), 135.8, 130.6, 130.3, 129.4, 128.2, 127.0, 126.8 (C-4b, C-5, C-6, C-7, C-8, C-8a, C-9), 117.3 (C-10), 117.3 (C-4a), 112.6 (C-3), 74.7 (C-1′), 52.8 (OMe).
2-(1-Methoxy-4-oxocyclohexa-2,5-dienyl)-4H-benzo[h]chromen-4-one (29)
23% yield from 28; ESI-MS (m/z, %): 319 (M++H, 100), 288 (M++H-OCH3, 17); 1H-NMR (CDCl3) δ 8.12 (1H, dd, J = 7.8, 1.2 Hz, H-10), 8.10 (1H, d, J = 8.7 Hz, H-5), 7.91 (1H, dd, J = 7.2, 1.5 Hz, H-7), 7.76 (1H, d J = 8.7 Hz, H-6), 7.69 (1H, ddd, J = 8.4, 7.8, 1.5 Hz, H-9), 7.62 (1H, ddd, J = 8.4, 7.2, 1.2 Hz, H-8), 6.94 (1H, s, H-3), 6.93 (2H, d, J = 10.2 Hz, H-2′, H-6′), 6.67 (2H, d, J = 10.2 Hz, H-3′, H-5′); 13C NMR (CDCl3)δ 184.6 (C-4′), 177.9 (C-4), 162.9 (C-2), 160.6 (C-10b), 1462. (C-2′, C-6′), 133.1 (C-3′, C-5′), 136.0, 129.5, 128.1, 127.5, 125.7, 123.7, 122.0, 120.5, 120.3 (C-4a, C-5, C-6, C-6a, C-7, C-8, C-9, C-10, C-10a), 110.8 (C-3), 75.1 (C-1′), 52.9 (OMe).
Supplementary Material
Acknowledgment
This investigation was support by Grants CA 17625 from National Cancer Institute (K. H. Lee), National Science Council, Taiwan, and National SunYat-Sen University-Kaohsiung Medical University Joint Research Center (Y.-C. Wu).
Abbreviations
- (TAIB)
[bis(trifluoroacetoxy)iodo]benzene
- (MOM)
methoxymethyl
References
- (1).Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- (2).Middleton E, Jr., Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- (3).Lin A-S, Chang F-R, Wu C-C, Liaw C-C, Wu Y-C. New cytotoxic flavonoids from Thelypteris torresiana. Planta Med. 2005;71:867–870. doi: 10.1055/s-2005-871292. [DOI] [PubMed] [Google Scholar]
- (4).Cytotoxicity was measured with the same MTT colorimetric method as in the Experimental Section. IC50 is defined as the concentration of agent that reduced the cell viability by 50% under the experimental conditions. The IC50 values of protoapigenone toward human breast normal (MCF-10A) and cancer (MCF-7 and MDA-MB-231) cell lines were 8.2 ± 1.0, 1.8 ± 0.1 and 1.1 ± 0.4 μM, respectively.
- (5).Manthey JA, Guthrie N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002;50:5837–5843. doi: 10.1021/jf020121d. [DOI] [PubMed] [Google Scholar]
- (6).Wells G, Berry JM, Bradshaw TD, Burger AM, Seaton A, Wang B, Westwell AD, Stevens MFG. 4-Substituted 4-hydroxycyclohexa-2,5-dien-1-one with selective activities against colon and renal cancer cell lines. J. Med, Chem. 2003;46:532–541. doi: 10.1021/jm020984y. [DOI] [PubMed] [Google Scholar]
- (7).Lion CJ, Vasselin DA, Schwalbe CH, Matthews CS, Stevens MFG, Westwell AD. Novel reaction products from the hypervalent iodine oxidation of hydroxy stilbenes and isoflavones. Org, Biomol. Chem. 2005;3:3996–4001. doi: 10.1039/b510240e. [DOI] [PubMed] [Google Scholar]
- (8).Berry JM, Bradshaw TD, Fichtner I, Ren R, Schwalbe CH, Wells G, Chew E-H, Stevens MFG, Westwell AD. Quinols as novel Therapeutic agents. 2. 4-(1-arylsulfonylindol-2-yl)-4-hydroxycyclohexa-2,5-diene-1-ones and related agents as potent and selective antitumor agents. J. Med, Chem. 2005;48:639–644. doi: 10.1021/jm040859h. [DOI] [PubMed] [Google Scholar]
- (9).Bradshaw TD, Matthews CS, Cookson J, Chew E-H, Shah M, Bailey K, Monks A, Harris E, Westwell AD, Wells G, Laughton CA, Stevens MFG. Elucidation of thioredoxin as a molecular target for antitumor quinols. Cancer Res. 2005;65:3911–3919. doi: 10.1158/0008-5472.CAN-04-4141. [DOI] [PubMed] [Google Scholar]
- (10).Zhao L-M, Jin H-S, Sun L-P, Piao H-R, Quan Z-S. Synthesis and evaluation of antiplatelet activity of trihydroxychalcone derivatives. Bioorg. Med. Chem. 2005;15:5027–5029. doi: 10.1016/j.bmcl.2005.08.039. [DOI] [PubMed] [Google Scholar]
- (11).Dao TT, Chi YS, Kim J, Kim HP, Kim S, Park H. Synthesis and inhibitory activity against COX-2 catalyzed prostaglandin production of chrysin derivatives. Bioorg. Med. Chem. 2004;14:1165–1167. doi: 10.1016/j.bmcl.2003.12.087. [DOI] [PubMed] [Google Scholar]
- (12).Kumazawa T, Minatogawa T, Matsuba S, Sato S, Onodera J. An effective synthesis of isoorientin: the regioselective synthesis of a 6-C-glucosylflavone. Carbohydr. Res. 2000;329:507–513. doi: 10.1016/s0008-6215(00)00226-3. [DOI] [PubMed] [Google Scholar]
- (13).McKillop A, McLaren L, Taylor RJK. A simple and efficient procedure for the preparation of p-quinols by hypervalent iodine oxidation of phenols and phenol tripropylsilyl ethers. J. Chem. Soc. Perkin Trans. 1. 1994:2047–2048. [Google Scholar]
- (14).Wipf P, Kim Y. π-Facial selectivity in nucleophilic additions to 4,4-disubstituted dienones: experimental support for electrostatic control. J. Am. Chem. Soc. 1994;116:11678–11688. [Google Scholar]
- (15).Shaw J, Lee A-R, Huang W-H. Methods of synthesizing flavonoids and chalcones. no.20040242907. US patent application series. 2004 May 28;
- (16).Kim SK, Sohn DW, Kim YC, Kim S-A, Lee SK, Kim HS. Fine tuning of a reported synthetic route for biologically active flavonoid, baicalein. Arch. Pharm. Res. 2007;30:18–21. doi: 10.1007/BF02977773. [DOI] [PubMed] [Google Scholar]
- (17).Elliott WM, Auersperg N. Comparison of the neutral red and methylene blue assays to study cell growth in culture. Biotech. Hitochem. 1993;68:29–35. doi: 10.3109/10520299309105573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.