Abstract
Two factors, the ETS transcription factor ER81 and skeletal muscle-derived neurotrophin-3 (NT3), are essential for the formation of muscle spindles and the function of spindle afferent–motoneuron synapses in the spinal cord. Spindles either degenerate completely or are abnormal, and spindle afferents fail to project to spinal motoneurons in Er81 null mice; however, the interactions between ER81 and NT3 during the processes of afferent neuron and muscle spindle development are poorly understood. To examine if overexpression of NT3 in muscle rescues spindles and afferent–motoneuron connectivity in the absence of ER81, we generated myoNT3;Er81−/− double-mutant mice that selectively overexpress NT3 in muscle in the absence of ER81. Spindle reflex arcs in myoNT3;Er81−/− mutants differed greatly from Er81 null mice. Muscle spindle densities were greater and more afferents projected into the ventral spinal cord in myoNT3;Er81−/− mice. Spindles of myoNT3;Er81−/− muscles responded normally to repetitive muscle taps, and the monosynaptic inputs from Ia afferents to motoneurons, grossly reduced in Er81−/− mutants, were restored to wild-type levels in myoNT3;Er81−/− mice. Thus, an excess of muscle-derived NT3 reverses deficits in spindle numbers and afferent function induced by the absence of ER81. We conclude that muscle-derived NT3 can modulate spindle density and afferent–motoneuron connectivity independently of ER81.
Keywords: muscle spindles, sensory neurons, motor neurons, neurotrophins, NT3, ETS transcription factors, ER81, mutant mice
INTRODUCTION
Group Ia neurons located in the dorsal root ganglia (DRGs) form the afferent arm of the stretch reflex, the principal segmental motor circuit of the spinal cord. The peripheral projections of Ia neurons innervate muscle spindles, whereas their central projections synapse with α-motoneurons (MNs) of the ventral spinal cord. The ETS (for E26 Transformation Specific) transcription factor, ER81, and the neurotrophic factor, neurotrophin-3 (NT3), play major roles in the assembly of the stretch reflex circuitry (Ernfors et al., 1994; Chen and Frank, 1999; Arber et al., 2000; Chen et al., 2002).
ER81 is required for the development of muscle spindles and their associated Ia afferent projections to the spinal cord in mice. Spindles either degenerate completely or are abnormal and spindle afferents fail to project to spinal motoneurons in Er81 null mice (Arber et al., 2000; Kucera et al., 2002). As a result, Er81−/− mutants exhibit uncoordinated movements and abnormal gait (Arber et al., 2000). NT3 is also required for development of the stretch reflex. NT3 regulates the survival of Ia afferent neurons, formation of muscle spindles, and function of afferent–MN synapses. NT3 null mice are devoid of group Ia neurons and spindles in limb muscles (Ernfors et al., 1994; Fariñas et al., 1994, 1996). Afferent–MN synaptic function is also abnormal in the absence of muscle-derived NT3 (Chen et al., 2002).
ER81 and NT3 interact during development of the stretch reflex, but the nature of this interaction in mediating the development of the stretch reflex is poorly delineated. For example, DRG neurons do not express ER81 in the absence of NT3, such as in NT3−/−;Bax−/− mice, and administration of NT3 induces expression of ER81 in a subset of wild-type neurons in DRG explants in vitro (Patel et al., 2003). The ability of NT3 to induce ER81 expression in DRG neurons raises the possibility that at least some of the effects of NT3 on sensory neurons, Ia afferent function, and muscle spindle development could be mediated through ER81 (Patel et al., 2003).
This report describes an in vivo mouse model designed to determine whether NT3 regulates the development of stretch reflex circuitry independently of ER81. We generated myoNT3; Er81−/− double mutants that selectively overexpress NT3 in muscle (Wright et al., 1997) in the absence of ER81. We observed that muscle-specific overexpression of NT3 completely reversed the loss of muscle spindles observed in Er81 null mutants and largely restored Ia afferent–motoneuron synaptic function. Thus, NT3 can affect spindle density and afferent–motoneuron connectivity independently of ER81. Preliminary reports of these data have been presented in abstract form (Li et al., 2004).
RESULTS
MyoNT3;Er81−/− compound mutants, mice that selectively overexpress NT3 in skeletal muscle in the absence of ER81, died during the fourth postnatal week (P21–P25) due to a failure to thrive, similar to Er81 null mice (Arber et al., 2000). Deficits in motor coordination such as ataxia and abnormal flexor–extensor posturing of limbs, characteristic of Er81−/− mutants (Arber et al., 2000), were less pronounced in the compound mutants. Thus, overexpression of NT3 in muscle improved gross motor performance in the absence of ER81, although it had no discernible impact on morbidity.
MyoNT3 Transgene Reverses Spindle Deficits Caused by Er81 Deletion
Muscle spindles form in all muscles of Er81 null embryos, but their postnatal fate differs according to the muscle position along the proximal–distal axis of the hindlimb. In proximal muscles such as the gluteus (GL), nearly all spindles degenerate by birth (Arber et al., 2000). In contrast, normal or increased numbers of spindles are present in distal limb muscles, such as the medial gastrocnemius (MG) of Er81−/− mutants (Kucera et al., 2002). Introduction of the myoNT3 transgene into muscle, which in turn increases intramuscular levels of NT3 protein, results in an increased number of spindles in GL and MG muscles of myoNT3 transgenic mice (Wright et al., 1997). Similarly, spindle densities were increased in compound myoNT3; Er81−/− mutants relative to Er81−/− mutants or wild-type mice when examined in neonatal (P5–P7) or young adolescent (P21) mice. Spindles typically occur singly in wild-type mice; however, clusters of adjoining spindles were often observed in muscles of myoNT3 and myoNT3;Er81−/− mice (Fig. 1). Thus, muscle-specific overexpression of NT3 (Wright et al., 1997) enhanced generation and/or survival of spindles and altered their spatial distribution in both proximal and distal muscles of hindlimbs in ER81-deficient mice (Table 1).
Fig. 1.
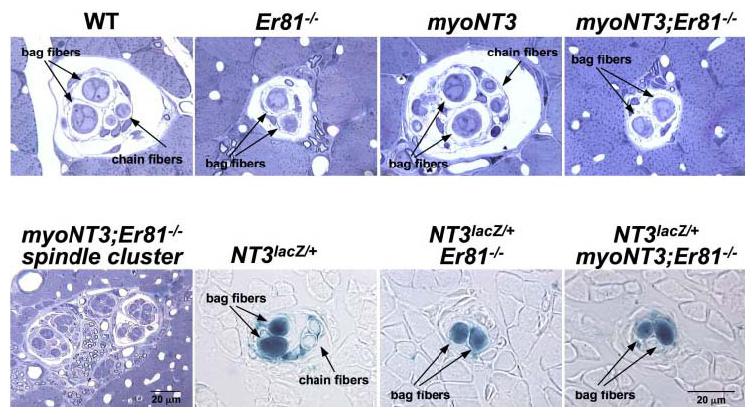
Morphological features of muscle spindles in wild-type (WT) and mutant mice. The 1-μm transverse sections of the MG muscles stained with toluidine blue (WT, Er81−/−, myoNT3;Er81−/−, and myoNT3) or 10-μm sections were processed for β-galactosidase activity (NT3lacZ/+, NT3lacZ/; Er81−/−, and NT3lacZ/+;myoNT3;Er81−/−). Note that WT and myoNT3 spindles contain the normal complement of two nuclear bag fibers and two nuclear chain fibers (arrows), whereas spindles in all mutant muscles lacking ER81 contain two nuclear bag fibers only (top row). Spindles in NT3-overexpressing muscles are often clustered (bottom row, left panel). Also note that spindles in ER81-deficient muscles express NT3 similar to WT spindles, as revealed by expression of the lacZ reporter gene (bottom row, right three panels). Magnification is identical for all panels except the one (bottom row, left panel) of which is indicated separately. NT3, neurotrophin-3.
TABLE 1.
Counts of Muscle Spindles in a Proximal (GL) and a Distal (MG) Muscle of Mutant and WT Mice at P7–P21a
| Spindle counts | % of WT | % myoNT3 | |
|---|---|---|---|
| A. GL muscle | |||
| WT | 35.5 ± 0.3 (4) | — | — |
| myoNT3 | 47.3 ± 0.9 (3)* | 133% | — |
| Er81−/− | 3.5 ± 0.4 (8)* | 9.8% | — |
| myoNT3;Er81−/− | 86.7 ± 2.8 (10)*,b,c | 252% | 189% |
| B.MG muscle | |||
| WT | 10.2 ± 0.3 (6) | — | — |
| myoNT3 | 30.3 ± 0.7 (8)* | 297% | — |
| Er81−/− | 18.5 ± 0.6 (6)* | 181% | — |
| myoNT3;Er81−/− | 50.5 ± 2.9 (8)*,b,c | 495% | 167% |
Note that the introduction of the myoNT3 transgene results in the formation of supernumerary spindles and that this effect is augmented by the absence of ER81 (myoNT3;Er81−/−double mutants). Spindle counts are expressed as mean ± SEM (N). Groups denoted an asterisk are significantly different from wild-type at P < 0.05.
A significant difference from myoNT3 at P < 0.05.
A significant difference from Er81−/− at P < 0.05.
GL, gluteus; MG, medial gastrocnemius; WT, wild-type; P, postnatal day; NT3, neurotrophin-3.
ER81 Absence Enhances the Effect of NT3 on Muscle Spindle Formation
Densities of spindles in limb muscles correlate with levels of muscle NT3. Spindles are absent in NT3 null mice and are present in excess in myoNT3 mice overexpressing NT3 in muscles (Ernfors et al., 1994; Wright et al., 1997). Unexpectedly, spindle densities in the GL and MG muscles of myoNT3;Er81−/− mutants exceeded by a considerable margin (200 –500%) those observed in wild-type, Er81−/− mutant, and myoNT3 transgenic mice. Thus, the effect of increased levels of muscle NT3 on the formation and/or survival of spindles in hindlimb muscles was enhanced by the deletion of Er81 (Table 1).
Based on the unexpected findings regarding spindle densities in myoNT3;Er81−/− mutants, we addressed whether elements responsible for spindle formation might also be more responsive to a decreased level of NT3 if ER81 were absent. To address this hypothesis, we crossed the NT3 and Er81 mutant lines to generate NT3lacZ/+;Er81−/− double mutants. Mice with the NT3lacZ/+ genotype lack 50% of the wild-type complement of spindles, presumably due to a 50% decrease in tissue levels of NT3 (Ernfors et al., 1994). In contrast to Er81−/− mice, the haploid NT3 gene condition of NT3lacZ/+;Er81−/− mutants entirely precluded the formation of supernumerary spindles characteristically seen in the distal (such as MG) muscles of Er81−/− mutants. Furthermore, no spindles at all formed in the GL, a muscle that typically has at least a few spindles in Er81−/− mutants. Thus, the reduction in spindle densities in hindlimb muscles observed in NT3 heterozygous mutant mice is enhanced, and formation of supernumerary spindles is inhibited, in the absence of ER81 (Table 2).
TABLE 2.
Counts of Spindles in the GL and MG Muscles of Mutants Deficient in ER81, NT3, or Both Factorsa
| Genotype | MG muscle | GL muscle |
|---|---|---|
| WT | 10.2 ± 0.3 (6) | 35.5 ± 0.3 (4) |
| Er81−/− | 18.5 ± 0.6 (6)* | 3.5 ± 0.4 (8)* |
| NT3lacZ/+ | 5.0 ± 0.7 (4)* | 15.3 ± 0.3 (3)* |
| NT3lacZ/+;Er81−/− | 4.7 ± 0.3 (3)* | 0.0 ± 0 (3) |
| NT3lacZ/lacZ;Er81−/− | 0 ± 0 (2) | 0 ± 0 (2) |
Note that the absence of one copy of the NT3 gene in NT3lacZ/+;Er81−/− mutants precludes the overproduction of spindles in the MG muscle typically observed in the absence of ER81, and results in spindle-less GL muscles. Muscles of mutants lacking both copies of NT3 were devoid of any spindles regardless of the presence or absence of ER81. Spindle counts are expressed as means ± SEM (N muscles examined). Groups denoted by an asterisk are significantly different from wild-type values at P < 0.05. NT3lacZ/+;Er81−/− and NT3lacZ/lacZ;Er81−/− groups had means of zero and were not be compared statistically to wild-type mice.
GL, gluteus; MG, medial gastrocnemius; WT, wild-type; P, postnatal day; NT3, neurotrophin-3.
MyoNT3 Transgene Does Not Rescue Intrafusal Fibers in ER81-Deficient Spindles
Muscle spindles in wild-type mice typically contain four intrafusal muscles fibers: one nuclear bag1, one nuclear bag2, and two nuclear chain fibers (Kozeka and Ontell, 1981). Spindles of Er81−/− mutants have fewer intrafusal fibers than wild-type spindles (Kucera et al., 2002). Similar to Er81−/− mutants, more than 70% of the GL and 65% of MG spindles in myoNT3;Er81−/− mutant mice lacked a full complement of four intrafusal fibers. Most ER81-deficient spindles contained two or three intrafusal fibers at P18–P22. Thus, overexpression of NT3 in muscles did not result in generation of a full complement of intrafusal fibers in spindles of ER81-deficient mice (Figs. 1, 2).
Fig. 2.
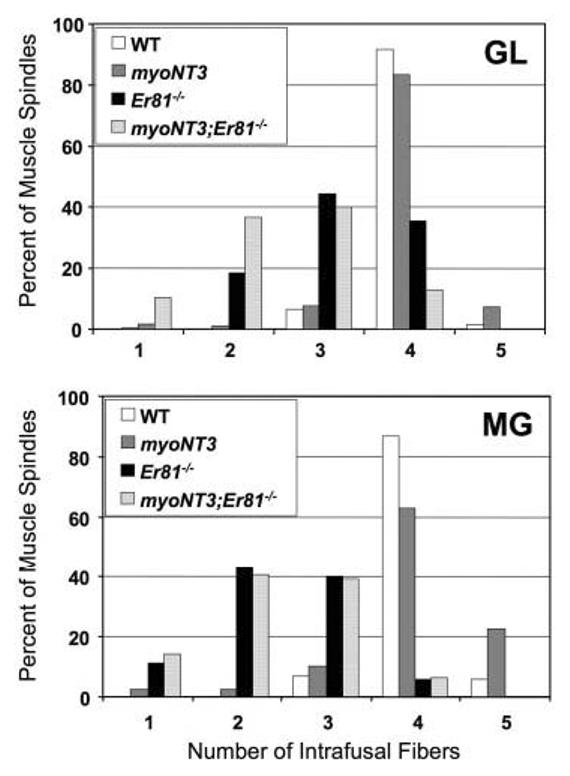
Histogram of complements of intrafusal muscle fibers in spindles of the gluteus (GL) and medial gastrocnemius (MG) muscle of mutant and wild-type (WT) mice. A,B: Note that most WT spindles contain four intrafusal fibers, whereas most Er81−/− spindles contain two or three intrafusal fibers in both the GL and MG muscles. Also note that overexpression of NT3 in muscle has little or no effect on numbers of intrafusal fibers in spindles (myoNT3;Er81−/− compared with Er81−/−).
Intrafusal fibers form in the sequence—nuclear bag2, nuclear bag1, and nuclear chain (Milburn, 1973). The type of intrafusal fiber absent from spindles of Er81−/− mutants was typically the last fiber to form, a nuclear chain fiber. In a combined sample of 20 ER81-deficient MG and GL spindles examined at P18–P22, 13 spindles lacked at least one nuclear chain fiber. Of these, four spindles lacked a nuclear bag1 fiber in addition to a chain fiber(s) and one spindle lacked a bag1 fiber only. A nuclear bag2 fiber was present in each spindle. Intrafusal fiber complements in myoNT3;Er81−/− mutants paralleled those of ER81-deficient spindles. In a sample of 20 MG and GL spindles of myoNT3;Er81−/− mutants, 12 spindles lacked one or two nuclear chain fibers and 2 spindles lacked a nuclear bag1 fiber. Each spindle contained at least one nuclear bag2 fiber. Thus, increasing the level of muscle NT3 produced no significant restoration of fiber type composition of intrafusal bundles in the absence of ER81. Moreover, the presence of the nuclear bag2 fiber, the first fiber to form, and frequent absence of one or two chain fibers, the last fibers to form, in myoNT3;Er81−/− mutant mice suggested that an excess of muscle NT3 has little or no effect on the incomplete assembly of the intrafusal bundle associated with the absence of ER81.
Greater Density of Afferents in myoNT3;Er81−/− Mutants
We compared the numbers of neurons in the L4 DRG, afferents in the L4 dorsal spinal root (DR), and myelinated nerve fibers in the MG muscle nerve of mutant and wild-type mice (Table 3). The presence of the myoNT3 transgene resulted in more DRG neurons as well as more DR afferents and MG muscle nerve fibers in myoNT3;Er81−/− than Er81−/− mutants. Thus, the myoNT3 transgene augmented numbers of sensory neurons in DRGs, which in turn resulted in increased numbers of afferent projections from the DRGs centrally to the spinal cord and peripherally into muscles. We assume that most of the supernumerary MG muscle nerve fibers were afferents, because we did not observe increased numbers of large-caliber efferent nerve fibers in the ventral roots of myoNT3;Er81−/− mutants (data not shown). These observations suggest that the increased numbers of spindles in myoNT3;Er81−/− muscles may result, at least in part, from a greater number of Ia afferents innervating the ER81-deficient muscles in the presence of the myoNT3 transgene. However, numbers of DRG neurons, DR afferents, and MG muscle nerve fibers in myoNT3;Er81−/− mutants, although increased relative to Er81−/− mutants, were less than those observed in myoNT3 mutants (Table 3).
TABLE 3.
Counts of the L4 DRG Neurons and Myelinated Nerve Fibers in the L4 DRs and Medial Gastrocnemius Muscle Nerve in WT and Mutant Mice at P21a
| Genotype | L4 DRG neurons | L4 DR nerve fibers | Muscle nerve fibers |
|---|---|---|---|
| WT | 9,255 ± 192 (8) | 2,495 ± 74; (7) | 138 ± 2 (11) |
| myoNT3 | 10,050 ± 325 (8)* | 2,914 ± 156 (7)* | 210 ± 6 (8)** |
| Er81−/− | 7,585 ± 189 (7)** | 1,776 ± 39 (7)** | 121 ± 3 (15)* |
| myoNT3;Er81−/− | 8,871 ± 197 (13)*,b,c | 2,451 ± 60 (16)*,b,c | 178 ± 2 (8)*,b,c |
Note the deficits in numbers of sensory neurons and nerve fibers in Er81−/− mutants and excess neurons and nerve fibers in myoNT3 mutants relative to wild-type mice. Overexpression of NT3 in muscle augmented the number of neurons and nerve fibers in myoNT3;Er81−/− double relative to Er81−/− single mutants. Means ± SEM (N) of counts are shown. Values denoted by an a single or double asterisk are significantly different from wild-type values at P < 0.05 or P < 0.001, respectively.
A significant difference from myoNT3 at P < 0.05.
A significant difference from Er81−/− at P < 0.05.
L4, lumbar 4; DRG, dorsal root ganglia; DR, dorsal spinal root; WT, wild-type; P21, postnatal day 21; NT3, neurotrophin-3.
Anatomical Rescue of the Central Afferent Projections in myoNT3;Er81−/− Mutants
Afferents projected from the DRGs into the ventral spinal cord and overlapped with the dendrites of motoneurons both at the rostral (L2) and caudal (L4/L5) cord segmental levels in DiI (1,1′, di-octadecyl-3,3,3′,3′,-tetramethylindocarbocyanine perchlorate) -labeled preparations of wild-type mice at P0 –P1. In contrast, we observed a rostral– caudal difference in the number of afferent projections in Er81−/− mutants (Fig. 3A). Fewer afferents projected toward MNs in the more rostral lumbar spinal cord than in the more caudal segments. In DiI-labeled preparations, no afferents were observed projecting from DRGs into the motoneuron region at the L2 cord level in Er81−/− mutants (N = 4), whereas DiI-labeled afferents (estimated at 10–20% of the normal complement) entered the ventral motoneuron zone at the L4/L5 levels in the mutants (N = 3).
Fig. 3.
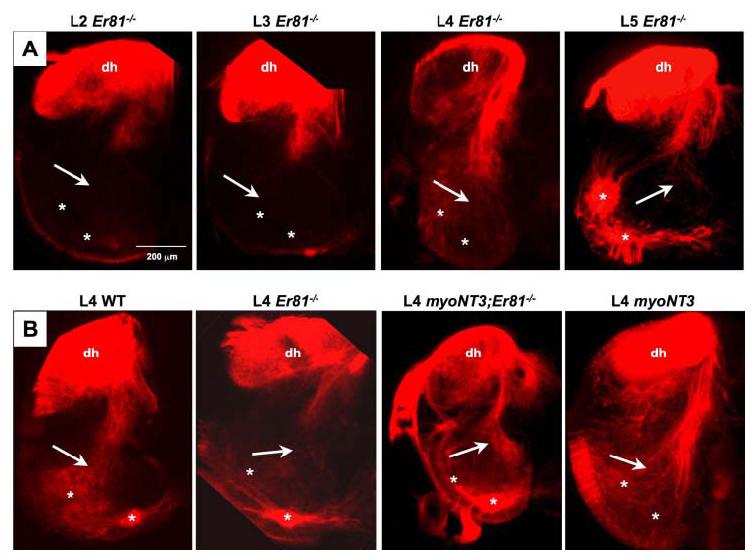
DiI (1,1′, di-octadecyl-3,3,3′,3′,-tetramethylindocarbocyanine perchlorate) labeling of spinal projections of afferents in control and mutant mice at postnatal day (P) 0–P1. A: Extent of the central projections of sensory axons from dorsal spinal roots (DRs) into the spinal cord at different lumbar (L2–L5) segmental levels in Er81−/− mice. B: Spinal projections of afferents at the L4 level in wild-type (WT), Er81−/−, myoNT3;Er81−/−, and myoNT3 mice. Arrows in each panel indicate the normal location of Ia afferents in WT mice, although these projections are largely absent in Er81−/− mice. Locations of α-motoneurons (MNs) are indicated with asterisks. Some MNs are also DiI (1,1′-dioctadecyl-3,3,3′,3′,-tetramethylindocarbocyanine perchlorate) -labeled in several panels. Note that more afferent projections are present at the L5 than L2 level in Er81−/− mice and that the density of the projections is higher in myoNT3;Er81−/− than in Er81−/− mice. dh, dorsal horn.
Next, we compared the apparent density of central afferent projections among Er81−/−, myoNT3;Er81−/−, myoNT3, and wild-type mice at P0 –P1 (Fig. 3B). More DiI-labeled afferents projected from the L4 DRGs to the ventral spinal cord region in myoNT3 mice than in wild-type mice. In addition, more labeled afferents projected into the ventral cord in myoNT3;Er81−/− than in Er81−/− mutants, indicative of a rescue of at least some Ia afferent projections by increased levels of muscle NT3. However, the degree of the afferent rescue varied among individual mutants. Typically, the density of labeled afferent projections into the ventral cord of myoNT3;Er81−/− specimens was less than in wild-type mice, but greater than in Er81−/− mutants.
In older neonates (P5–P8), we used dextran fills to trace afferent projections from the lumbar DRs to the spinal cord (Fig. 4). Dextran labeling showed a paucity of projections in Er81−/− mutants, consistent with the results obtained using DiI and with the observations of Arber et al. (2000). A partial rescue (afferents were more numerous and penetrated deeper into the ventral cord) was observed in the myoNT3;Er81−/− mice (N = 3). However, afferent projections were clearly less dense in myoNT3;Er81−/− than in wild-type mice (N = 7).
Fig. 4.
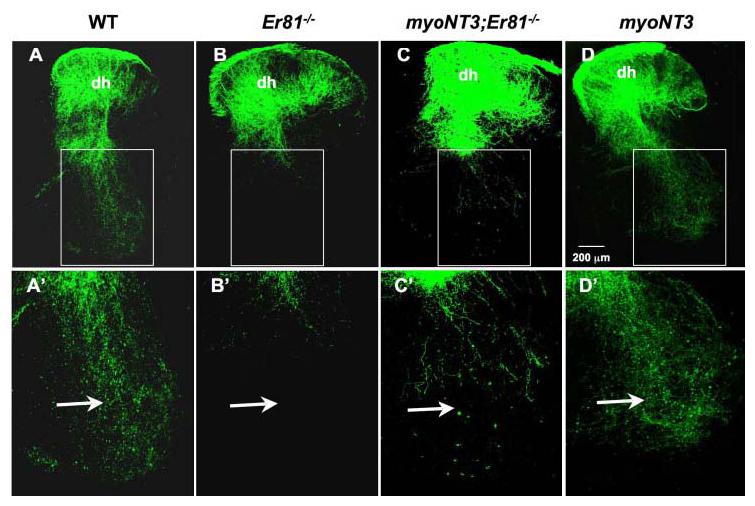
Dextran labeling of spinal projections of afferents in wild-type (WT), Er81−/−, myoNT3;Er81−/−, and myoNT3 mice at postnatal day (P) 7. A–D: Extent of the central projections of sensory axons from the lumbar (L4) dorsal spinal root (DR) into the spinal cord in control and mutant mice. A′–D′: Higher magnification of the areas shown above in white rectangles. Arrows indicate the normal ventral projections of Ia sensory afferent collaterals, which are absent in Er81−/− mice but partially rescued by overexpression of neurotrophin-3 (NT3) in myoNT3;Er81−/− mice.
Functional Rescue of Ia Afferent–Motoneuron Connectivity in myoNT3;Er81−/− Mutants
To assess functional connectivity between afferents and spinal MNs, we stimulated lumbar spinal DRs and recorded synaptic responses in MNs from the corresponding ventral spinal roots (VRs). In wild-type mice (N = 12), DR stimulation elicited compound synaptic responses consisting of a short latency monosynaptic component arising from the direct connections of Ia afferents onto motoneurons, and longer latency polysynaptic responses arising from a variety of afferent classes. In Er81−/− mutants (N = 10), the early monosynaptic response was either grossly diminished or entirely absent and only the later polysynaptic responses persisted (Fig. 5A), similar to results obtained by Arber et al. (2000). The amplitude of the residual monosynaptic component varied at different lumbar segmental levels; synaptic potentials were typically larger at the L5 than at L2 level in Er81−/− mutants (Fig. 5B). Thus, the reduction in excitatory postsynaptic potentials (EPSP) amplitude was more pronounced at rostral compared with caudal segmental levels in Er81−/− mice (Fig. 5B). These data are consistent with the data obtained from DiI labeling, demonstrating a greater density of afferent projections at more caudal levels of the ventral spinal cord in mice lacking ER81.
Fig. 5.
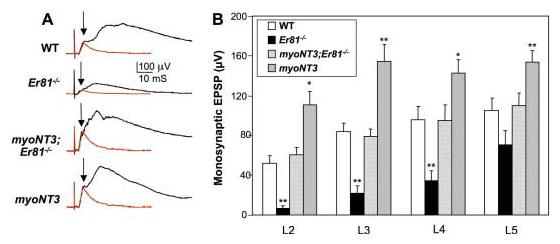
Monosynaptic connections between afferent axons and α-motoneurons (MNs) in wild-type (WT), Er81−/−, myoNT3;Er81−/−, and myoNT3 mice. A: Excitatory postsynaptic potentials (EPSPs) were recorded from the lumbar region (L2–L5) ventral spinal roots (VRs) in response to stimulation of the dorsal spinal roots (DRs) of the corresponding segments. Representative traces of EPSPs recorded from the L4 VR are shown. The superimposed red traces show the model EPSPs used to measure monosynaptic amplitudes (see Experimental Procedures section), which are indicated by arrows. B: Average amplitudes of monosynaptic EPSPs in segments L2 to L5 in mutant and control mice. Statistical significance of differences at each segment from WT mice is denoted by asterisks (*P < 0.05, **P < 0.01).
The magnitude of monosynaptic responses was restored to approximately wild-type levels in myoNT3; Er81−/− mutants (N = 13); the values were intermediate between those in Er81−/− (N = 12) and myoNT3 (N = 14) mutants (Fig. 5B). Although the monosynaptic VR response to DR stimulation was insufficient to evoke orthodromic action potentials in any of the Er81−/− mutants examined (N = 10), orthodromic action potentials were evoked in 12 of the 13 myoNT3;Er81−/− mutants. Stimulation of DRs in myoNT3 mutants elicited monosynaptic responses that were approximately 1.6-fold larger than in wild-type mice, indicative of enhanced Ia afferent–motoneuron function in mice carrying the myoNT3 transgene. Thus, overexpression of NT3 in muscle resulted in an enhancement of Ia afferent–motoneuron synaptic function, both in wild-type mice and in Er81−/− mutants.
Ia Afferents in Er81−/− Mice Retain the Ability to Form Selective Synapses With MNs
To explore further the segmental regulation by ER81 of Ia-MN synapses, we used intracellular recording to compare Ia afferent inputs from specific limb muscles onto identified MNs (Fig. 6). Activity in quadriceps (Q) and adductor (AD) Ia afferents elicited no detectable synaptic potentials in Q or AD MNs (0.1 ± 0.1 mV, N = 10 MNs in four Er81−/− mice), whereas these connections were strong in wild-type mice (10.7 ± 0.5 mV, N = 28 MNs in 8 wild-type [WT] mice). At more caudal levels, however, some Ia-MN connectivity remained. Stimulation of afferents in the lateral or medial gastrocnemius (LG or MG) muscle nerves elicited an average response of 2.6 ± 0.7 mV (N = 18) in LG and MG MNs, approximately one fourth of the amplitude of these inputs in wild-type mice (Fig. 6). These results confirm those obtained earlier with extracellular recordings from VRs (see above and Arber et al., 2000).
Fig. 6.
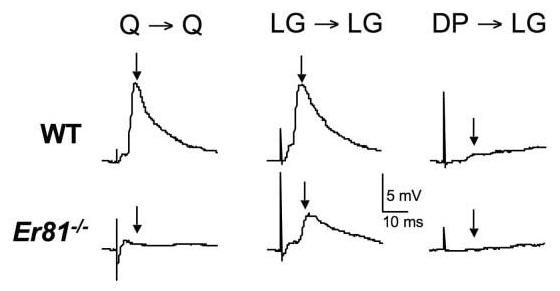
Intracellular recordings of synaptic potentials evoked by stimulation of Ia muscle afferents and recorded in the corresponding homonymous (projecting to the same muscle) α-motoneurons (MNs). The amplitude of the monosynaptic component of each excitatory postsynaptic potential (EPSP) is indicated with an arrow, as in Figure 5. Note that homonymous Ia–MN EPSPs in rostral lumbar segments (Q MNs at L2) are more severely attenuated in Er81−/− mice than those in more caudal segments (LG MNs at L4). The remaining connections in caudal segments of Er81−/− mice are selective; homonymous (LG → LG) Ia EPSPs are larger than those from other muscles (DP → LG), just as in wild-type (WT) mice. Q, quadriceps; LG, lateral gastrocnemius; DP, deep peroneal; L, lumbar.
The presence of detectable inputs to caudal MNs made it possible to assess whether ER81 was required for the formation of selective connections between Ia fibers and MNs. Normally, Ia afferents make their strongest projections to MNs supplying the same (homonymous) MNs, whereas inputs to MNs supplying antagonist muscles are weak or nonexistent. Homonymous Ia–MN connections within the group of ankle extensors (MG and LG muscles), for example, are strong (9.5 ± 0.5 mV, N = 18), whereas input from Ia afferents supplying the deep peroneal (DP) muscle to these MNs is weak (0.03 ± 0.03 mV, N = 10). This same selectivity of Ia–MN connections was present in mice that lacked Er81 (Fig. 6). Homonymous Ia–MN connections within the ankle extensor group were 2.6 ± 0.7 mV (N = 18), whereas there was no measurable input from the DP nerve to this same MN group (0.00 ± 0.03 mV; N = 18). These results show that subsets of Ia-MN connections form selectively even in the absence of ER81.
The increased synaptic connectivity in myoNT3;Er81−/− mutants led us to check if these additional connections were also specific. Surprisingly, we found that Ia–MN connections were not specific in myoNT3 transgenic mice even when expression of ER81 was normal (myoNT3 transgenic mice, data not shown). These results suggest that overexpression of NT3 during embryonic development augments Ia–MN synapses indiscriminately, increasing the strength of both correct and incorrect connections. The reasons that the formation of selective Ia–MN connections is disrupted in myoNT3 transgenic mice are unclear and warrant further study. Because overexpression of NT3 by itself disrupted the normal pattern of connections, however, it was not possible to test for an additional effect of the absence of ER81.
Rescue of Muscle Spindle Function in myoNT3;Er81−/− Mutants
To assess the impact of the myoNT3 transgene on the peripheral projections of Ia afferents, we tested muscle spindle function by recording from the Q muscle nerve while stretching the Q muscle to elicit spindle discharges in myoNT3;Er81−/− mutants (Fig. 7). Mechanical muscle stimulation in Er81−/− mutants (N = 6) elicited compound action potentials of lower amplitude than in wild-type mice (N = 10), suggesting fewer functional spindle afferents (Fig. 7). In contrast, spindle responses to muscle stimulation in myoNT3;Er81−/− mutants (N = 4) were indistinguishable from those in wild-type mice and were occasionally larger than in wild-type mice (although the difference was not statistically significant). In three of four mice, bursts of action potentials were elicited in myoNT3;Er81−/− mutants compared with the single compound action potentials elicited in wild-type mice, suggesting that the number of functional spindles was larger than in wild-type mice. Moreover, spindles in myoNT3; Er81−/− mutants followed rapid repetitive muscle taps at frequencies up to approximately 10 Hz, similar to the response characteristics of normal spindles. Thus, the extra spindles induced by overexpression of NT3 in myoNT3; Er81−/− muscles functioned normally.
Fig. 7.
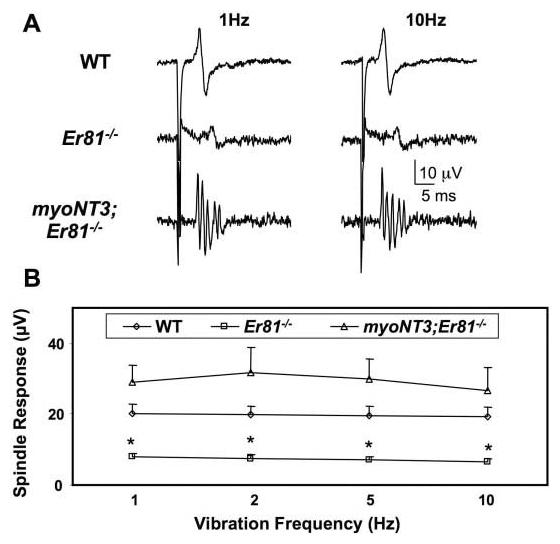
Responses of spindles to muscle stretch in wild-type (WT), Er81−/−, and myoNT3;Er81−/− mice. A: Representative records of Ia afferent action potentials recorded from the quadriceps (Q) muscle nerve in response to small stretches of the Q muscle at stretch frequencies of 1 Hz and 10 Hz. B: Average responses of spindles in mutant and control mice to varying stretch frequencies. The amplitude of the extracellularly recorded action potential reflects the number of active spindles. The more complex waveforms in myoNT3;Er81−/− mice indicate a burst of action potentials in response to single stretches. Statistical significance of reductions in amplitude of Er81−/− compared with WT spindles is denoted by asterisks (**P < 0.01).
Expression of NT3 in Muscle Spindles Is Independent of ER81 Expression
Finally, we examined whether differences in the expression of NT3 by muscle spindles might account for the observed differences in central Ia afferent connectivity between Er81−/− and myoNT3;Er81−/− mice. Intrafusal fibers express NT3 in their equatorial region, subjacent to the terminals of Ia afferents (Copray and Brouwer, 1994), and spindle-derived NT3 is required for the function of central Ia afferent–motoneuron synapses (Chen et al., 2002). Spindles of Egr3 null mice do not express NT3, and the central Ia sensory-motor connections, although present anatomically, are grossly dysfunctional in these mutants (Chen et al., 2002). If spindles in Er81−/− mice did not express NT3, a similar loss of functionality would be predicted. We examined NT3 expression in spindles using a heterozygous lacZ reporter substitution for the NT3 gene. Spindles of both NT3lacZ/+;Er81−/− and myoNT3; NT3lacZ/+;Er81−/− compound mutants expressed NT3 in the equatorial region of nuclear bag fibers at P7–P10 in a pattern similar to that of wild-type spindles (Copray and Bouwer, 1994), as shown by the presence of blue reaction product deposits in β-galactosidase staining (Fig. 1). Thus, NT3 expression in spindles is independent of ER81 expression, and the rescue of central Ia afferent–motoneuron connectivity in myoNT3;Er81−/− mutants is not due to reintroduction of NT3 into ER81-deficient spindles by the myoNT3 transgene.
DISCUSSION
In wild-type mice, muscle-derived NT3 up-regulates ER81 expression in group Ia sensory neurons that mediate the stretch reflex (Patel et al., 2003). In turn, ER81 is required for the establishment of appropriate anatomical projections of Ia afferents into the ventral horn and development of muscle spindles, the mechanoreceptors of the reflex (Arber et al., 2000). The present study shows that selectively overexpressing NT3 in muscle ameliorates the abnormalities of Ia afferent–MNs connectivity and spindle development in Er81−/− mutants. Thus, muscle-derived NT3 influences the structural and functional development of the stretch reflex through a pathway independent of tissue expression of ER81 or by acting on factors downstream of ER81.
Rescue of the Peripheral Arm of the Stretch Reflex
Muscle-derived NT3 was particularly effective in ameliorating defects in the peripheral arm of the stretch reflex in ER81-deficient mice. myoNT3;Er81−/− mutants had more DRG neurons, a greater density of muscle innervation and more muscle spindles, than Er81−/− mice. A single group Ia afferent induces the formation of a muscle spindle; hence, spindles and afferents exist in a 1:1 ratio in wild-type mice (Zelená, 1957; Hippenmeyer et al., 2002). This ratio is maintained in NT3 mutant mice. Ia neurons do not survive in NT3 null mice; consequently, no spindles are present in limb muscles (Ernfors et al., 1994; Klein et al., 1994). In contrast, more Ia neurons survive and more spindles form in mice that overexpress NT3 in muscle than in wild-type mice (Wright et al., 1997). Thus, any excess or deficiency of muscle spindles correlates with an excess or deficiency of Ia sensory neurons in DRGs of mutant mice.
We observed that the increase in number of spindles correlated with an increase in the number of DRG neurons as well as an increased number of nerve fibers in the MG muscle nerve of myoNT3;Er81−/− relative to Er81−/− mice. These observations suggest that the overproduction of spindles in myoNT3;Er81−/− mutants relative to Er81−/− mice may result partially from a greater survival of Ia neurons in the presence of excess muscle NT3. Target-derived NT3 is known to modulate naturally occurring cell death, and more Ia neurons survive in DRGs of myoNT3 mice (Davies, 1996; Fariñas et al., 1996; Wright et al., 1997).
Numbers of muscle spindles in hindlimbs of myoNT3;Er81−/− mutants exceeded not only those of Er81−/− or wild-type mice, but also myoNT3 mice. In contrast, fewer spindles were present in NT3lacZ/+;Er81−/− than in NT3lacZ/+ or Er81−/− mutants. No spindles were present in the GL muscle of NT3lacZ/+;Er81−/− mice, even though NT3lacZ/+ mice have 50% of the wild-type spindle complements (Ernfors et al., 1994). These observations suggest that more Ia neurons survive in ER81-deficient mice when levels of NT3 in tissue are increased, and fewer Ia neurons survive in ER81-deficient mice when levels of NT3 in tissue are decreased, than might be expected based solely on NT3 levels. The relative underproduction of spindles in NT3lacZ/+;Er81−/− mice and overproduction of spindles in myoNT3;Er81−/− mice suggest that ER81, which is normally expressed in Ia neurons (Arber et al., 2000), may act to dampen the Ia neuron survival response to reduced or elevated levels of NT3.
Factors other than increased survival of Ia neurons may contribute to the overproduction of muscle spindles in the myoNT3;Er81−/− mice. The excess of spindles exceeded the excess of DRG neurons in myoNT3;Er81−/− relative to Er81−/− mice; hence, the gross overproduction of spindles could not be attributed solely to more Ia afferents innervating muscles in myoNT3;Er81−/− mice. In some experimental situations, single Ia afferents may branch to innervate more than one spindle, resulting in a cluster of spindles (Kucera et al., 1993; Wright et al., 1997). The clustering of spindles observed in both myoNT3 and myoNT3;Er81−/− muscles is consistent with abnormal intramuscular branching of Ia afferents. Excess muscle NT3 may maintain supernumerary Ia afferent branches that are normally eliminated coincident with down-regulation of NT3 in late embryonic wild-type muscles (Kucera et al., 1988; Fariñas et al., 1996). This phenomenon may be enhanced by the absence of ER81 because counts of spindles in myoNT3;Er81−/− mutants exceeded those observed in myoNT3 mice.
Excess muscle NT3 may also enhance the postnatal stability of ER81-deficient muscle spindles. Spindles are scarce or absent in GL muscles of Er81−/− mutants due to a defect in spindle stability rather than spindle formation (Arber et al., 2000). In contrast to Er81−/− mutants, myoNT3;Er81−/− mice showed an excess of spindles in the GL muscle relative to wild-type mice. Thus, an excess muscle NT3 may also prevent degeneration of ER81-deficient spindles.
The dependence of developing muscle spindles on ER81 is more pronounced in proximal than distal limb segments. Few muscle spindles are present in proximal muscles (GL), but distal muscles (MG) contain numerous spindles in Er81−/− mutants (Arber et al., 2000; Kucera et al., 2002). myoNT3;Er81−/− compound mutants had supernumerary spindles in both the proximal and distal muscles compared with the corresponding muscles of wild-type mice. Thus, excess muscle NT3 abolishes the differences in spindle densities observed in different hindlimb compartments in the absence of ER81.
In contrast to spindle numbers, complements of intrafusal fibers in individual spindles were approximately equivalent between Er81−/− and myoNT3;Er81−/− mutants, independent of the limb segment. Similar to Er81−/− spindles, many spindles of myoNT3;Er81−/− muscles were deficient in nuclear chain fibers, the last intrafusal fiber type to form during ontogeny. Developing intrafusal fibers normally express ER81, and ER81 may represent one of the target factors that are regulated by sensory innervation during intrafusal fiber morphogenesis (Albert et al., 2005). Thus, the aborted assembly of intrafusal bundles characteristic of ER81-deficient spindles may reflect a critical requirement for ER81 during spindle development that is not ameliorated by excess NT3. Peripheral assembly of other mechanoreceptors such as Pacinian corpuscles is also dependent on ER81 but not NT3 (Šedý et al., 2006).
Rescue of the Central Arm of the Stretch Reflex Circuit
ER81 expression in Ia neurons and NT3 expression in muscle spindles are both required for development of proper monosynaptic afferent–MN function in wild-type mice. Group I afferents fail to project to the ventral spinal cord and monosynaptic sensory–motor function is impaired in Er81−/− mutants (Arber et al., 2000). Ia afferent–MN synaptic transmission is also defective in Egr3 mutants, which do not express NT3 in muscle spindles (Chen et al., 2002). Disruption of NT3 expression in spindles of Er81 mutants might explain the deficit in Ia–MN synaptic function. However, NT3 expression in Er81−/− spindles, as revealed by the lacZ reporter in NT3lacZ/+;Er81−/− double mutants, is normal in terms of location and apparent level. Thus, the alteration in Ia–MN connectivity in Er81−/− mutants is not due to the failure of ER81-deficient spindles to provide retrograde NT3 trophic support to Ia afferents. Instead, the loss of connectivity is probably a direct consequence of the greatly reduced anatomical projection of Ia afferents into the ventral spinal cord in Er81−/− mutants.
Overexpression of NT3 in muscle partially rescued the anatomical defects of afferent–motor connectivity resulting from ER81 absence. DiI and dextran labeling showed that more afferent branches penetrated into the ventral spinal cord of myoNT3; Er81−/− than Er81−/− neonates. Several mechanisms may account for these increased anatomical projections. Counts of neurons and DR fibers suggest that more DRG neurons survive, thereby providing more afferents to project to the spinal cord in myoNT3;Er81−/− than Er81−/− mice. Increased intraspinal branching of the afferents may also be a contributing factor to functional rescue. NT3 is known to promote DRG neuronal arborization in vitro (Lentz et al., 1999) and in vivo (Genc et al., 2004) as well as a layer-specific axonal branching during the development of the mammalian cerebral cortex (Castellani and Bolz, 1999). Excess NT3 has been shown to promote growth of lesioned dorsal column axons with an abundance of fiber sprouting at the lesion site (Bradbury et al., 1999; Ramer et al., 2002). Thus, both an increased number of incoming afferents and increased intraspinal branching of afferents may underlie the increased projections of Ia afferents in myoNT3;Er81−/− mice.
Increased anatomical projections are unlikely to be the complete explanation of the functional rescue of Ia–MN connectivity in myoNT3;Er81−/− mutants, however. These projections were never as robust as in WT mice, yet the monosynaptic component of the EPSP in MNs evoked by DR stimulation was as large as normal (Fig. 5B). Overexpression of NT3 must, therefore, increase synaptic connectivity by means of other mechanisms. The rescue of connectivity in Egr3−/− mutant mice by exogenous NT3 provides an example of such an effect. In these mice, Ia–MN connectivity is reduced as dramatically as in Er81−/− mutants, even though anatomical projections of Ia afferents into the ventral horn are maintained (Chen et al., 2002). Retrograde transport of NT3 to the spinal cord by Ia afferents is compromised because muscle spindles do not express NT3 postnatally in Egr3−/− mice; however, exogenous NT3 injections can restore normal levels of connectivity by potentiating Ia afferent–synaptic function (Chen et al., 2002). In the present experiments, it is possible that motor neurons retrogradely transport NT3 from NT3-overexpressing muscles to the spinal cord, resulting in Ia afferent–MN synaptic function potentiation. Alternatively, an NT3 leakage from muscles carrying the myoNT3 transgene may increase systemic levels of NT3. In either case, even the reduced anatomical projections of Ia afferents may be able to generate a normal level of Ia–MN connectivity in myoNT3;Er81−/− mutants.
CONCLUSIONS
In Er81−/− mutant mice, previous investigations revealed that group Ia afferents do not project appropriately into the ventral spinal horn and muscle spindles either degenerate or are abnormal. In this study, we found these deficits to be more pronounced at caudal than rostral lumbar levels, suggesting a segmental difference in dependence on ER81. An excess of muscle-derived NT3 reversed the deficits in spindle density and afferent connectivity induced by the absence of ER81. These data indicate that NT3 promotes muscle spindle formation and afferent–MN connectivity through pathways independently of ER81. NT3 and ER81, therefore, play related but distinct roles in the development of the stretch reflex circuit.
EXPERIMENTAL PROCEDURES
Mutant Animals
Generation of mice carrying mutated Er81ETS/ETS (Er81−/−; 129/Sv genetic background) or NT3lac/Z (C57bl/6 background) alleles has been described previously (Fariñas et al., 1994, 1996; Arber et al., 2000). We bred the myoNT3 transgene (Wright et al., 1997) into the Er81 mutant line to generate myoNT3;Er81−/− compound mutants. We also crossed the Er81 and NT3 mutant lines to generate NT3lacZ/+;Er81−/− double mutants and used β-galactosidase histochemistry to determine whether mutant spindles expressed NT3. Mixing the two genetic backgrounds did not alter the abnormal motor pheno-type resulting from the absence of ER81 (Arber et al., 2000). Genotyping was performed by polymerase chain reaction (PCR) amplification of DNA extracted from tails of neonatal mice (0–10 days after birth). Primer sets and PCR protocols were as described in Arber et al. (2000) and (Fariñas et al. 1994, 1996).
Counts of Neurons, Nerve Fibers, and Muscle Spindles
Mutant mice and their wild-type lit-termates were perfused with 2.5% paraformaldehyde–2% glutaraldehyde fixative after sodium pentobarbital anesthesia (50 mg/kg bbody weight, intraperitoneally) at birth (P0) or at weaning (P20–P22). Lumbar DRGs, together with their associated VR and DR, and hindlimb muscles were dissected, embedded in resin, cut in serial 1-μm-thick sections, and stained with toluidine blue. Myelinated VR and DR nerve fibers were counted using a ×100 oil-immersion objective and the Neurolucida image analysis system. Counts of DRG neurons were obtained using a modification of the stereotaxic method of Coggeshall (1992) as described previously in Wright et al. (1997). Counts of spindles were obtained from semi-serial 1-μm-thick cross-sections of the gluteus (GL) and medial gastrocnemius (MG) muscles. Every 10th or 20th section was stained with toluidine blue and examined using light microscopy. Spindles were identified as encapsulated bundles of small-diameter muscle fibers that exhibited accumulations of central nuclei at their equator. Spindle equators were counted to ensure that each spindle was counted only once. Counts performed in neonatal mice are reflective of spindle densities in adults (Zelená, 1957).
Immunocytochemistry of Muscles
We used S46, an antibody specific for slow developmental myosin heavy chain, as a marker for spindles and to differentiate nuclear bag from nuclear chain intrafusal fibers (Kucera and Walro, 1995). Nuclear bag2 and nuclear bag1 intrafusal fibers bind S46, whereas extrafusal fibers and nuclear chain fibers do not (Kucera and Walro, 1995). We used MF30, an antibody specific for neonatal/fast MyHC (Bader et al., 1982) to discriminate between the bag2 and bag1 fibers. Bag2 fibers react to MF30, whereas bag1 fibers do not (Kucera and Walro, 1995). Muscles of mutant and wild-type lit-termates were excised at P0–P22, frozen in liquid nitrogen, cut transversely into serial 8-μm-thick sections and reacted with either S46 (diluted 1:200) or MF 30 (diluted 1:50). Binding of S46 and MF30 in the muscles was visualized using an indirect peroxidase technique (Vectastain ABC kit, Vector Laboratories).
Electrophysiological Recordings of Synaptic Responses From Spinal Motoneurons
Lumbar spinal cords with attached DRs, VRs, and peripheral nerves were dissected from mutant and wild-type mouse pups at P5–P10 and maintained in an oxygenated Krebs solution (Mears and Frank, 1997; Arber et al., 2000). Extracellular recordings of synaptic potentials in motoneurons were made by means of tight-fitting suction electrodes from the proximal ends of cut VRs, with the recording electrodes positioned immediately adjacent to the spinal cord to minimize electrotonic decrement of the synaptic potentials. For intracellular recordings, MNs were impaled with glass micropipettes filled with 2 M potassium methylsulfate, including 500 mM QX-314 [Alamone Labs, Jerusalem, Israel] to delete antidromic action potentials and 0.5% Fast Green [Sigma] to increase the visibility. Motoneurons were identified by antidromic activation by means of one of the muscle nerves. Only those MNs with a resting potential more negative than −50 mV are included in this report. Sensory axons were stimulated with a suction electrode placed either on the cut proximal end of a DR or on the individual nerves to the Q, AD, LG and MG, soleus, or DP muscles. Electrical recordings of the responses in MNs to sensory stimulation were amplified, filtered (DC to 3 kHz), and recorded digitally at 10 kHz using custom software (LabVIEW). Ten to twenty individual responses generated at 0.5 Hz were averaged on-line and stored digitally for off-line analysis.
The amplitude of the monosynaptic (group Ia) component of the synaptic responses, which represents the direct input from Ia afferents that innervate muscle spindles, was estimated by fitting the synaptic response with a “model” trace from an age-matched wild-type neonate obtained using the same stimulating and recording protocol as described previously (Sah and Frank, 1984; Mears and Frank, 1997; Arber et al., 2000). The model trace was elicited using a stimulus slightly greater than the threshold for any synaptic response. Because Ia fibers have a low threshold for electrical stimulation, the response was, therefore, composed almost entirely of monosynaptic input from a few Ia afferents. Scaling this model response to fit the early (monosynaptic) portion of the input to be measured in a mutant, therefore, provided a good estimate of the monosynaptic component.
Analyses of Muscle Spindle Afferent Function
To study the response properties of muscle spindles, we activated Ia afferents by brief muscle stretches and recorded Ia afferent responses from the distal ends of cut DRs with a suction electrode at P5–P10. The Q muscle was dissected in continuity with its muscle nerve and the corresponding L3 DR and maintained in an oxygenated bath. Spindle Ia afferents were selectively activated by vibrating the distal tendon of the Q muscle with a piezoelectric bimorph, using 1- to 2-msec pulses that produced ∼50 μm displacements of the tendon (Lichtman and Frank, 1984; Arber et al., 2000). The amplitude of the sensory response in the DR gave an approximate measure of the number of Ia afferents activated with each tap, because the amplitude of the action potentials in all Ia afferents are similar (Lichtman and Frank, 1984; Arber et al., 2000). In some muscles, a single tap elicited a burst of action potentials (see Fig. 7). These bursts represent activity in multiple Ia axons, because single Ia axons cannot respond repetitively at such high frequencies. As a measure of the response properties of the spindle–Ia system, we also measured how faithfully the Ia afferents could follow repetitive taps at increasing frequencies, from 1 to 10 Hz.
Retrograde Labeling of Central Projections of Sensory Neurons
To examine the central projections of sensory neurons, we labeled DR afferents with the lipophilic dye, DiI, in situ or fluorescent dextran in vivo (Molecular Probes, Eugene, OR). We used the lipophilic dye DiI at P0, before sensory axons are heavily myelinated. Newborn mice were fixed in 4% paraformaldehyde under sodium pentobarbital anesthesia (50 mg/kg body weight, intraperitoneally) at P0, DRGs were exposed, and injected with 0.25% DiI in dimethyl formamide, and the preparation was incubated for 7 days at 37°C. The spinal cord with attached DRGs was embedded in 2% agar at 50°C, then cooled and sectioned transversely at 50 μm thickness on a Vibratome. Labeled afferents projecting into the ventral spinal horns were identified as Ia afferents (Ernfors et al., 1994).
In older neonates (P5–P8), we used fluorescent dextran to label afferents. DRs were drawn into tight-fitting glass capillaries, which were then back-filled with an aqueous solution of 100 mg/ml fluorescein dextran, lysine-fixable, 3,000 molecular weight (Molecular Probes) with 1% lysolecithin (Sigma). Labeling was allowed to proceed overnight at 25°C and was followed by overnight fixation in 4% paraformaldehyde in 0.1 M phosphate buffer at 4°C. Transverse sections of the spinal cord were cut at 50 μm thickness on a cryostat, and images were captured using a Leica confocal microscope (TCS SP2). Images were processed in Photoshop 7.0.
Statistical Analyses
Counts of spindles, L4 DRG neurons, L4 DR nerve fibers, and muscle nerve fibers were grouped according to strain/genotype of mouse. Counts obtained from mutant strains/genotypes of mice were compared with wild-type mice by a two-sided t -test to test the hypotheses that mutations in myoNT3 or Er81 altered elements of the stretch reflex. Similarly, myoNT3;Er81−/− compound mutants were compared with myoNT3 and Er81−/− mice to test the hypothesis that over-expression of myoNT3 rescued elements of the spindle reflex pathway when ER81 was absent. Data are reported as mean ± SEM (N). Statistical significance was set at P < 0.05.
ACKNOWLEDGMENTS
We thank Drs. S. Arber, T.M. Jessell, and I. Fariñas for providing Er81 and NT3 mutant mice. MyoNT3 mice were kindly provided by Dr. W.D. Snider. Ms. S. Tseng and M. Campo provided excellent technical assistance. J.S. was supported by Ministry of Education of the Czech Republic, J.K. received funding from the Veterans Administration, and E.F., L.Y.L., and Z.W. received funding from the National Institutes of Health.
Grant sponsor: Ministry of Education of the Czech Republic; Grant number: VZ 0021620806; Grant number: 1M0021620803; Grant number: AVOZ 50390512; Grant number: EC FP6 project RESCUE (LSHB-CT-2005-518233); Grant sponsor: the Veterans Administration; Grant sponsor: the National Institutes of Health.
REFERENCES
- Albert Y, Whitehead J, Eldredge L, Carter J, Gao X, Tourtellotte WG. Transcriptional regulation of myotube fate specification and intrafusal muscle fiber morphogenesis. J Cell Biol. 2005;169:257–268. doi: 10.1083/jcb.200501156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- Bader D, Masaki T, Fischmann DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E, Khemani S, King VR, Priestley JV, McMahon SB. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur J Neuro-sci. 1999;11:3873–3883. doi: 10.1046/j.1460-9568.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- Castellani V, Bolz J. Opposing roles for neurotrophin-3 in targeting and collateral formation of distinct sets of developing cortical neurons. Development. 1999;126:3335–3345. doi: 10.1242/dev.126.15.3335. [DOI] [PubMed] [Google Scholar]
- Chen HH, Frank E. Development and specification of muscle sensory neurons. Curr Opin Neurobiol. 1999;9:405–409. doi: 10.1016/S0959-4388(99)80061-0. [DOI] [PubMed] [Google Scholar]
- Chen HH, Tourtellotte WG, Frank E. Muscle spindle-derived neurotrophin-3 regulates synaptic connectivity between muscle sensory and motor neurons. J Neurosci. 2002;22:2512–2519. doi: 10.1523/JNEUROSCI.22-09-03512.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Copray JC, Brouwer N. Selective expression of neurotrophin-3 messenger RNA in muscle spindles of the rat. Neuroscience. 1994;63:1125–1135. doi: 10.1016/0306-4522(94)90578-9. [DOI] [PubMed] [Google Scholar]
- Davies AM. The neurotrophic hypothesis: where does it stand? Phils Trans Biol Sci. 1996;351:389–394. doi: 10.1098/rstb.1996.0033. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Fariñas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Fariñas I, Yoshida CK, Backus C, Reichardt LF. Lack of neurotrophin-3 results in death of spinal sensory neurons and premature differentiation of their precursors. Neuron. 1996;17:1065–1078. doi: 10.1016/s0896-6273(00)80240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc BP, Ozdinler P, Mendoza AE, Erzurumlu RS. A chemoattractant role for NT-3 in proprioceptive axon guidance. Public Library of Science. 2004;2:e403–e418. doi: 10.1371/journal.pbio.0020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S, Shneider NA, Birchmeier C, Burden S, Jessell TM, Arber S. A role for neuregulin-1 signaling in muscle spindle differentiation. Neuron. 2002;36:1035–1049. doi: 10.1016/s0896-6273(02)01101-7. [DOI] [PubMed] [Google Scholar]
- Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD, Barbacid M. Disruption of the neurotrophin-3 receptor gene trkC eliminates Ia muscle afferents and results in abnormal movements. Nature. 1994;368:249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- Kozeka K, Ontell M. The three-dimensional cytoarchitecture of developing murinemuscle spindles. Dev Biol. 1981;87:133–147. doi: 10.1016/0012-1606(81)90067-1. [DOI] [PubMed] [Google Scholar]
- Kucera J, Walro JM. An immunocy-tochemical marker for early type I muscle fibers in the developing rat hindlimb. Anat Embryol (Berl) 1995;192:137–147. doi: 10.1007/BF00186002. [DOI] [PubMed] [Google Scholar]
- Kucera J, Walro JM, Reichler J. Innervation of developing intrafusal muscle fibers in the rat. Am J Anat. 1988;183:344–358. doi: 10.1002/aja.1001830408. [DOI] [PubMed] [Google Scholar]
- Kucera J, Walro JM, Gao Y. Fusimotor-free spindles in reinnervated muscles of neonatal rats treated with nerve growth factor. Neuroscience. 1993;52:219–228. doi: 10.1016/0306-4522(93)90194-k. [DOI] [PubMed] [Google Scholar]
- Kucera J, Cooney W, Que A, Szeder V, Stancz-Szeder H, Walro J. Formation of supernumerary muscle spindles at the expense of Golgi tendon organs in ER81-deficient mice. Dev Dyn. 2002;223:389–401. doi: 10.1002/dvdy.10066. [DOI] [PubMed] [Google Scholar]
- Lentz S, Knudson M, Korsmeyer S, Snider W. Neurotrophins support the development of diverse sensory axon morphologies. J Neurosci. 1999;19:1038–1048. doi: 10.1523/JNEUROSCI.19-03-01038.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang Z, Šedý J, Quazi R, Frank E, Kucera J. Partial rescue of propri-oceptive deficits in mice lacking the ETS factor ER81 by neurotrophin-3. SFN. 2004 Abstracts 615.1. [Google Scholar]
- Lichtman JW, Frank E. Physiological evidence for specificity of synaptic connections between individual sensory and motor neurons in the brachial spinal cord of the bullfrog. J Neurosci. 1984;4:1745–1753. doi: 10.1523/JNEUROSCI.04-07-01745.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears SC, Frank E. Formation of specific monosynaptic connections between muscle spindle afferents and motoneurons in the mouse. J Neurosci. 1997;17:3128–3135. doi: 10.1523/JNEUROSCI.17-09-03128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn A. The early development of muscle spindles in the rat. J Cell Sci. 1973;12:175–195. doi: 10.1242/jcs.12.1.175. [DOI] [PubMed] [Google Scholar]
- Patel TD, Kramer I, Kucera J, Niederkofler V, Jessell TM, Arber S, Snider WD. Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron. 2003;38:403–416. doi: 10.1016/s0896-6273(03)00261-7. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Bishop T, Dockery P, Mobarak MS, O'Leary D, Fraher JP, Priestley JV, McMahon SB. Neurotrophin-3-mediated regeneration and recovery of proprioception following dorsal rhizotomy. Mol Cell Neurosci. 2002;19:239–249. doi: 10.1006/mcne.2001.1067. [DOI] [PubMed] [Google Scholar]
- Sah DW, Frank E. Regeneration of sensory-motor synapses in the spinal cord of the bullfrog. J Neurosci. 1984;4:2784–2791. doi: 10.1523/JNEUROSCI.04-11-02784.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šedý J, Tseng S, Walro JM, Grim M, Kucera J. ETS transcription factor ER81 is required for the Pacinian corpuscle development. Dev Dyn. 2006;235:1081–1089. doi: 10.1002/dvdy.20710. [DOI] [PubMed] [Google Scholar]
- Wright DE, Zhou D, Kucera J, Snider WD. Introduction of a neurotrophin-3 transgene into muscle selectively rescues proprioceptive neurons in mice lacking endogenous neurotrophin-3. Neuron. 1997;19:503–517. doi: 10.1016/s0896-6273(00)80367-0. [DOI] [PubMed] [Google Scholar]
- Zelená J. The morphogenetic influence of innervation on the ontogenetic development of muscle spindles. J Embryol Exp Morphol. 1957;5:283–292. [Google Scholar]


