Abstract
1,25D3 exhibits anti-tumor activity in a variety of cancers including squamous cell carcinoma (SCC). Intrinsic resistance of SCC cells to cisplatin was observed and led to the investigation into whether 1,25D3 sensitizes SCC cells to cisplatin. Pretreatment with 1,25D3 followed by cisplatin enhanced growth inhibition in SCC cells compared with 1,25D3 alone, as assessed by cytotoxicity and in vitro clonogenic assays. In addition, 1,25D3 sensitized SCC cells to cisplatin-mediated apoptosis. Treatment of tumor-bearing C3H mice with 1,25D3 prior to cisplatin reduced clonogenic survival using in vivo excision clonogenic assay. These results were not observed in a 1,25D3-resistant SCC variant, indicating the critical role of 1,25D3 in sensitizing SCC cells to cisplatin. Further, a marked decrease in fractional tumor volume was observed when SCC tumor-bearing mice were treated with 1,25D3 prior to cisplatin as compared to either agent administered alone. Cisplatin has been shown to modulate p73 protein level in certain cancer cells. Our data showed that p73 level was not affected by cisplatin, but increased by 1,25D3 in SCC cells. Knocking down p73 by siRNA protected SCC cells against 1,25D3 and cisplatin-mediated clonogenic cell kill and apoptosis. Increasing p73 protein level by knocking down UFD2a, which mediates p73 degradation, promoted 1,25D3 and cisplatin-mediated clonogenic cell kill. These results suggest that 1,25D3 potentiates cisplatin anti-tumor activity in vitro and in vivo in a SCC model system, possibly through p73 induction and apoptosis. The combination treatment may provide a more effective therapeutic regimen in cancer treatment.
Keywords: 1,25D3; p73; cisplatin
Introduction
Vitamin D regulates diverse physiological functions including calcium homeostasis, bone metabolism, cell differentiation and immunity (1, 2). 1α,25-dihydroxyvitamin D3 (1,25D3), the most active metabolite of vitamin D, inhibits the growth of a number of cancer types such as prostate, breast, colorectal, ovarian, and skin cancers (1, 2). 1,25D3 is currently being evaluated, alone or in combination with other chemotherapeutic agents, in clinical trials for the treatment of several solid tumors (1, 3).
Cisplatin (cis-diammine-dichloro-platinum (II), cDDP) is a potent chemotherapeutic agent widely used for the treatment of a variety of cancers, including testicular, ovarian, cervical, lung cancer and head and neck squamous cell carcinoma (SCC) (4, 5). However, its effectiveness as an anti-cancer agent is limited by drug resistance and side effects including nephrotoxicity, emetogeneiss and neurotoxicity (6). Tumor resistance to cisplatin may be caused by insufficient DNA binding, increased DNA repair ability, bypass of DNA adducts, or impaired apoptosis (7). Hence, it will be beneficial if tumor cells can be sensitized to cisplatin treatment with a combination therapy.
DNA damage caused by cisplatin may induce the activation of tumor suppressor p53 (6, 8), which inhibits cell proliferation by promoting cell cycle arrest or apoptosis. The presence of wild type p53 correlates to the sensitivity to cisplatin (6). Since p53 is frequently mutated or functionally impaired in human cancers, the status of a p53 related protein, p73, is considered to be an important determinant of cellular sensitivity to chemotherapeutic drugs.
p73 has significant homology to p53. p73 gene encodes multiple isoforms due to the usage of alternative promoters and the alternative splicing (9). Transcription of p73 gene from promoter P1 results in the isoforms containing an N-terminal transactivation (TA) domain (TAp73), whereas the isoforms transcribed from promoter P2 are N-terminal truncated and lack the TA domain (ΔNp73) (9). TAp73 is a transcription factor and regulates genes involved in cell cycle arrest and apoptosis and other cellular functions. Some genes are common targets of p53, such as Bax, Puma, and Noxa, while others are not regulated by p53. In contrast, ΔNp73 may serve as dominant negative inhibitors of p53 family (10).
Unlike p53, p73 mutation is rare in human cancers (10). Loss of heterozygosity and methylation-mediated gene silencing are observed in many cancer types (10). In addition, p73 gene polymorphism is implicated in tumorigenesis (11). p73 protein expression is deregulated in many cancers (10, 11). Loss of p73 has been reported to associate with tumor progression and poor prognosis in several cancers (12–15). p73 loss triggers the conversion of kerotinocytes to SCC (16). Although p73 knockout mice do not develop spontaneous tumors in the initial studies, mice heterozygous for p73 (p73+/−) or p63 (p63+/−) develop malignant tumors at high frequency (17). Moreover, higher tumor burden and metastasis are observed in p53+/−; p73+/− and p53+/−; p63+/− mice compared to p53+/− mice (17). These observations indicate p73 plays a role in tumor development.
We previously demonstrated that 1,25D3 exerts anti-proliferative effects in murine SCC cell line SCCVII/SF (18–20). These effects are mediated by the induction of cell cycle arrest and apoptosis (20, 21). We also demonstrated that pretreatment with 1,25D3 enhances paclitaxel, cisplatin, or carboplatin-mediated antitumor activities (22–24). However, the mechanisms for 1,25D3-enhanced cisplatin antitumor effects remain unclear.
In the current study, we established a variant of SCC cell line, SCC-DR, which is resistant to 1,25D3 and thereby serves as a control to study the effects of 1,25D3. Further, we investigate the mechanisms of 1,25D3 and cisplatin-mediated growth inhibition, especially the role of p73 and apoptosis in SCC cells.
Materials and Methods
Materials
1,25D3 was a generous gift from Hoffmann-LaRoche (Nutley, NJ). Cisplatin (Platinol-AQ) was obtained from Bristo-Myers Squibb Company (Princeton, NJ). Anti-VDR (sc-1008) and anti-phosphorylated ERK1/2 (sc-7383) were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-caspase 3 (9662), anti-caspase 8 (4927), anti-caspase 9 (9504), anti-caspase 10 (9752), anti-phosphorylated Akt (Ser473; 9271), anti-Akt (9272), anti-ERK1/2 (9102) and anti-p53 (2524) were from Cell Signaling Technology (Beverly, MA). Anti-p73 (IMG-246, clone 5B429) was from Imgenex (San Diago, CA). Anti-PARP (556362) and anti-p63 (550025) was from BD Pharmingen. Anti-actin (CP-01) was from Calbiochem (San Diego, CA).
Cell culture and tumor model systems
Murine SCC (SCCVII-SF) is a moderately well-differentiated SCC derived from a spontaneously arising tumor of the C3H mouse (25). SCC cells were maintained in 6–10 weeks old female C3H/HeJ mice from Jackson Laboratory (Bar Harbor, ME). SCC cells were cultured in RPMI 1640 media supplemented with 12% FBS and 1% penicillin/streptomycin sulfate. The mice protocols used for in vivo excision clonogenic assays were approved by the Roswell Park Cancer Institutional Animal Care and Use Committee. The mice protocols used for tumor regrowth delay were approved by University of Pittsburgh animal care committee according to USPHS guidelines.
Generation of SCC-DR cells
SCC cells were continuously cultured in RPMI 1640/FBS media containing 10 nM of 1,25D3 for over 10 months until no cytotoxicity was observed on a light microscope. The resulting stable SCC-DR cell line was maintained in RPMI 1640/FBS containing 10 nM of 1,25D3. For experiments, SCC-DR cells were plated in RPMI 1640/FBS without 1,25D3 over night and subjected to further treatment.
Trypan blue exclusion assay
Cell viability was quantitatively assessed by Trypan blue exclusion assay using Vi-CELL™ Series Cell Viability Analyzers (Beckman-Coulter, Fullerton, CA).
Cytotoxicity assay
Cytotoxicity was quantified by the released lactate dehydrogenase (LDH) from the cytosol of damaged cells using Cytotoxicity Detection KitPLUS (LDH) kit following the manufacturer’s protocol (Roche Applied Science, Indianapolis, IN).
In vitro Clonogenic assay
SCC or SCC-DR cells were pretreated with ethanol (ETOH) or 10 nM 1,25D3 for 24 h, and then treated with 0.5 μg/ml cisplatin for 2 h or left untreated. Cisplatin was then washed away and 1,25D3 was replaced in the groups treated with 1,25D3. The in vitro clonogenic assays were performed as described (23, 26).
In Vivo Clonogenic Assay
The in vivo effects of 1,25D3 and cisplatin on clonogenic SCC cells were determined by in vivo excision clonogenic assay as described (22, 26–28). Briefly, C3H mice bearing 9-day SCC or SCC-DR tumors were treated in 4 groups (3 to 5 per group): saline, 1,25D3, cisplatin, or 1,25D3 and cisplatin combination. Mice were treated for 3 d with daily i.p. injection of saline or 0.625 μg/mouse of 1,25D3. On day 3, mice also received i.p. injection of 3 mg/kg of cisplatin. Twenty-four h after the last injection, mice were sacrificed, and their tumors were excised. Clonogenic assays were performed as described (26).
Tumor regrowth delay
SCC cells (4.5×105) were inoculated s.c. into the flank of the C3H mice. Studies were initiated when the tumors were palpable. Mice were treated in 4 groups (10 per group): saline, 1,25D3, cisplatin, or 1,25D3 and cisplatin combination. Mice were treated for 3 d with single, daily i.p. injections of saline or 0.25 μg/mouse of i.p. injection of 6 mg/kg of cisplatin. 1,25D3. On day 3, mice also received a singleTumor measurements were done as described (22).
Immunoblot analysis
Cell lysates were prepared and immunoblot analysis was performed as described previously (19, 21).
Apoptosis assay - DNA fragmentation ELISA
SCC cells were harvested, lysed, and DNA fragmentation was quantitatively evaluated by Cell Death Detection ELISAPLUS according to the manufacturer’s instructions as described (19, 21).
Real-time quantitative RT-PCR
Total RNA from SCC cells was isolated using the RNeasy Mini kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 1 μg total RNA using oligo(dT) primers (iScript cDNA synthesis kit, Bio-Rad, Hercules, CA). Gene quantification was performed on an Applied Biosystems 7300 real-time system (Applied Biosystems, Foster City, CA) with standard thermal cycler conditions. TaqMan primers and probes for p73 and GAPDH were purchased from Applied Biosystems. Relative gene expression was determined by the ΔΔ-CT method.
siRNA transfection
Synthetic small interfering RNA (siRNA) siGENOME SMARTpool siRNAs (4 individual siRNA pooled together) specific for p73, UFD2a, siCONTROL non-specific siRNA (siRNA-NS), and DharmaFECT1 transfection reagent were from Dharmacon (Lafayette, CO). SCC cells were transfected with 50 nM siRNA-NS or siRNA against p73 or UFD2a for 24 h using DharmaFECT1 transfection reagent following the manufacturer’s protocol.
Statistics
Statistical significances between groups were determined by two-tailed student’s t-test.
Results
Generation of 1,25D3 resistant SCC cells
We previously demonstrated that 1,25D3 has antitumor effects in SCC cells (18–20). Cisplatin is widely used to treat patients with head and neck SCC with moderate success (29). Therefore, SCC cell line serves as an ideal model to study the effects of combination treatment with 1,25D3 and cisplatin.
We previously reported that 1,25D3 induces cell cycle arrest and apoptosis in SCC cells (19, 20, 30). However, only a small percentage of the cells responded to 1,25D3. To have a better understanding on the effects of 1,25D3 in SCC cells, we established a 1,25D3-resistant variant of SCC, SCC-DR cell line, by continuously culturing SCC cells in media containing 10 nM of 1,25D3, the dose that has anti-proliferative effects in SCC cells and is clinically achievable in man (19, 20, 30). To examine whether SCC-DR cells are resistant to the growth inhibitory effects of 1,25D3, SCC-DR cells or control parental SCC cells were treated with 10 or 500 nM of 1,25D3 and subjected to in vitro clonogenic assay. The colony-forming capacity of SCC cells was greatly inhibited by 1,25D3 (Fig. 1A). In contrast, the colony-forming capacity of 1,25D3-treated SCC-DR cells was mostly intact (Fig. 1B).
Figure 1.
SCC-DR cells are resistant to 1,25D3 treatment. A, SCC or SCC-DR cells were treated with 0, 10, or 500 nM of 1,25D3 and subjected to in vitro clonogenic assay. After staining, colonies were viewed and counted on a light microscope and photographed. Surviving fraction was calculated by dividing the cloning capacity of treated cells to that of ETOH control. Results of the surviving fraction are the mean ± SD of triplicate experiments and are representative of two independent experiments. B, SCC and SCC-DR cells were treated with 0 to 1000 nM of 1,25D3 for 48 h, and the levels of VDR and caspase 3 were assessed by immunoblot analysis. Actin was the loading control. Results are representative of two independent experiments. (c) SCC-DR cells were treated with 10 nM of 1,25D3 for 5 to 240 min, and the levels of phosphorylated Akt and ERK1/2 were evaluated by immunoblot analysis. Total Akt or ERK1/2 level was assessed as the loading control.
To further characterize the cellular functions of SCC-DR cells, 1,25D3-mediated transcriptional activity and apoptosis were examined. No induction of vitamin D receptor (VDR) was observed in SCC-DR cells until 1000 nM of 1,25D3 was used, while VDR was induced in SCC cells upon 10 nM 1,25D3 treatment (Fig. 1B), suggesting that SCC-DR cells have compromised transcriptional activity of VDR. Additionally, pro-caspase 3 was readily cleaved in SCC cells with 10 nM 1,25D3 treatment while it remained intact in SCC-DR cells (Fig. 1B); suggesting that SCC-DR cells are resistant to 1,25D3-induced apoptosis. We previously demonstrated that 1,25D3 induces nongenomic activation (occurs within 5 min) of Akt and ERK1/2 in SCC cells (21). 1,25D3 did not induce rapid activation of Akt in SCC-DR cells (Fig. 1C). Interestingly, 1,25D3 activated ERK1/2 in SCC-DR cells at ~ 30 min (Fig. 1C), indicating the nongenomic signaling of 1,25D3 is partially affected. Together, these results show that SCC-DR cells are resistant to 1,25D3 treatment at several aspects, therefore, it may serve as a control model to study the effects of 1,25D3 in sensitive cell lines.
SCC and SCC-DR cells are resistant to cisplatin
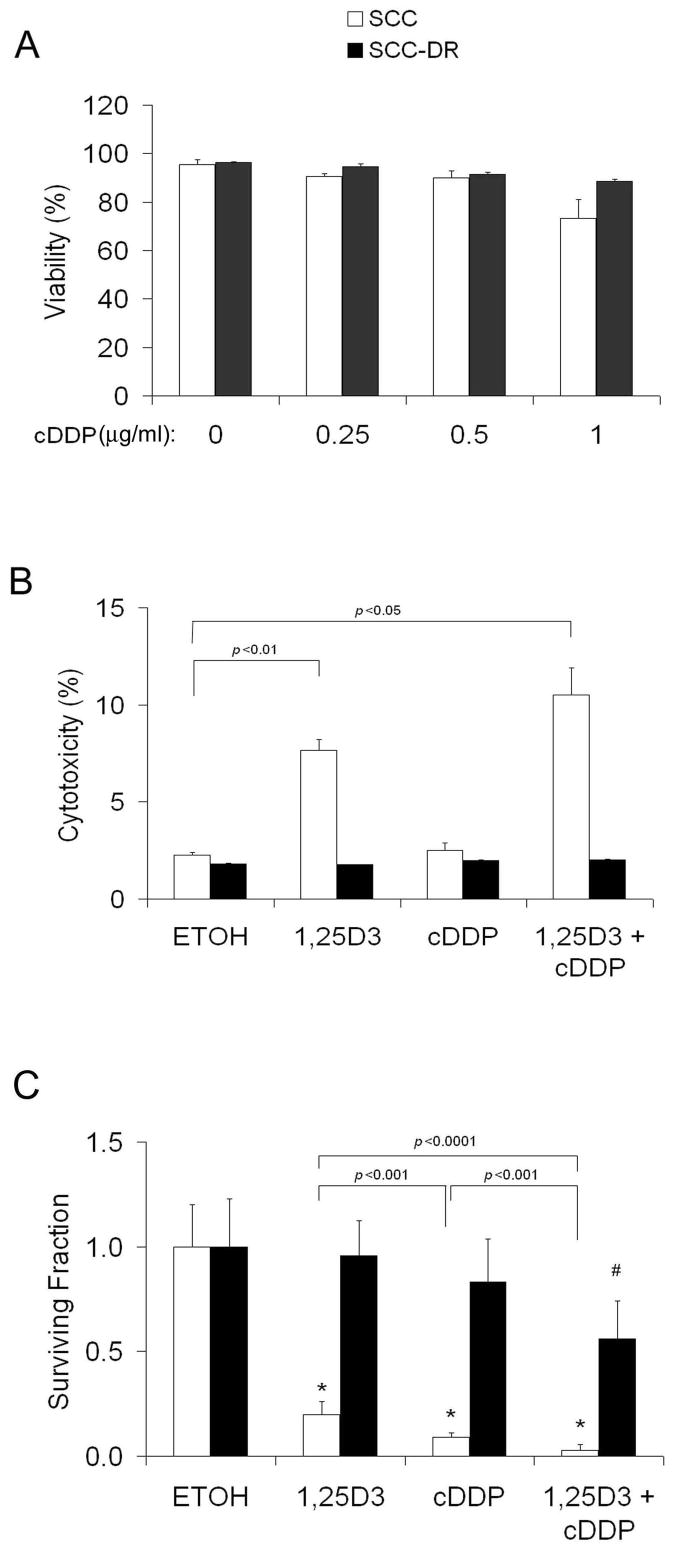
To examine whether cisplatin has cytotoxic effects in SCC or SCC-DR cells, the cells were treated with various doses (0 to 1 μg/ml) of cisplatin for 48 h and cell viability was assessed by Trypan blue exclusion assay. Surprisingly, cisplatin had no cytotoxic effects in SCC and SCC-DR cells even at 1 μg/ml, suggesting they are resistant to cisplatin over the range of concentration tested (Fig. 2A).
Figure 2.
1,25D3 sensitizes SCC cells to cisplatin treatment. A, SCC or SCC-DR cells were treated with 0 to 1 μg/ml of cisplatin for 48 h, and cell viability was assessed by Trypan blue exclusion assay. Results are the mean ± SD of triplicate experiments and are representative of two independent experiments. B, SCC or SCC-DR cells were pretreated with vehicle control ETOH or 10 nM 1,25D3 for 24 h followed by 0.5 μg/ml of cisplatin for 2 h. Cells were harvested after an additional 48 h of incubation. Cytotoxicity was examined by LDH Cytotoxicity Detection Kit. C, Various dilutions of SCC or SCC-DR cells were plated in six-well tissue culture plates over night. They were pretreated with ETOH or 10 nM 1,25D3 for 24 h, and then incubated without further treatment or 0.5 μg/ml cDDP for 2 h and subjected to in vitro clonogenic assay. Results are representative of two to three independent experiments. cDDP, cisplatin. *, P < 0.00001, vs. ETOH; #, P < 0.01, vs. cDDP.
1,25D3 sensitizes SCC cells to cisplatin treatment in vitro
We previously demonstrated that 1,25D3 and cisplatin have synergistic grow inhibition in SCC cells indicated by MTT assays (24). To further determine whether 1,25D3 can sensitize SCC cells to cisplatin, two other methods were employed, cytotoxicity assay by measuring the released lactate dehydrogenase from damaged cells and the in vitro clonogenic assay. SCC or SCC-DR cells were pretreated with 10 nM 1,25D3 or vehicle control ETOH for 24 h followed by 0.5 μg/ml cisplatin or control media for 2 h. Cytotoxicity was assessed after an additional 48 h of incubation. 1,25D3 induced significant (P < 0.01) cytotoxicity in SCC cells (Fig. 2B). Cisplatin did not induce cytotoxicity, however, pretreatment of 1,25D3 for 24 h followed by cisplatin resulted in enhanced cytotoxicity compared with 1,25D3 alone, suggesting that 1,25D3 sensitized SCC cells to cisplatin-induced cell killing (Fig. 2B). In contrast, cytotoxicity was not observed upon any treatment in SCC-DR cells (Fig. 2B). The more sensitive clonogenic assay revealed that 1,25D3 or cisplatin alone markedly inhibited the clonogenic capacity of SCC cells. The combination treatment had more profound effect than 1,25D3 or cisplatin alone (Fig. 2C). 1,25D3 alone did not alter the clonogenic capacity of SCC-DR cells (Fig. 2C), while cisplatin or the combination modestly suppressed the clonogenic ability of SCC-DR cells (Fig. 2C). These results suggest that 1,25D3 potentiates cisplatin anti-proliferative effects in SCC cells.
1,25D3 promotes cisplatin anti-tumor activity in vivo
To evaluate whether 1,25D3 also enhances the anti-proliferative effects of cisplatin in vivo, the in vivo excision clonogenic assay was used. We previously showed that this assay is an indication of in vivo anti-tumor activity (23, 27, 28, 31, 32). SCC or SCC-DR tumor-bearing mice were treated with saline, 0.625 μg 1,25D3 daily for 3 d, 3 mg/kg cisplatin on day 3, or the combination of 0.625 μg 1,25D3 daily for 3 d and 3 mg/kg cisplatin on day 3. The combination of 1,25D3 and cisplatin resulted in a significantly greater decrease in surviving fraction as compared with 1,25D3 (P < 0.01) or cisplatin (P < 0.0001) alone compared with 1,25D3 (Fig. 3A). In contrast, 1,25D3 or cisplatin alone had no significant activity in SCC-DR cells (Fig. 3A), and the combination treatment resulted in a slight decrease in surviving fraction (Fig. 3A), suggesting critical role of 1,25D3 in clonogenic cell kill.
Figure 3.
1,25D3 promotes cisplatin anti-tumor activity in SCC in vivo. A, SCC tumor- bearing mice (3 to 5 per group) were treated with saline or 0.625 μg of 1,25D3 daily for 3 d. On day 3, mice also received 3 mg/kg of cisplatin. Both agents were administered i.p. The in vivo excision clonogenic assay was performed 24 h after the last treatment. Each point represents the mean surviving fraction for total clonogenic cells per gram of tumor. *, P < 0.001, **, P < 0.0001, vs. saline; #, P < 0.01, vs. saline. B, C3H mice (10 per group) bearing palpable subcutaneous SCC tumors were treated with either saline, 0.25 μg of 1,25D3 daily for 3 d, 6 mg/kg of cisplatin on day 3, or the combination of 0.25 μg of 1,25D3 daily for 3 d and 6 mg/kg of cisplatin on day 3. Both agents were administered i.p. Tumor measurements were obtained on the days indicated, and fractional tumor volumes were calculated as described in Methods. Data points represent the mean ± SD fractional tumor volume for 10 mice/group. *, P < 0.05. cDDP, cisplatin.
To determine the effects of 1,25D3 and cisplatin on tumor growth in vivo, SCC tumor-bearing daily for 3 d, 6 mg/kg cisplatin on mice were treated with saline, 0.25 μg 1,25D3day 3, or the combination of 0.25 μg 1,25D3 daily for 3 d with 6 mg/kg cisplatin on day dosing regimen was reported previously to maximize anti-tumor efficacy 3. This 1,25D3 while minimizing toxicity or hypercalcemia (23). 1,25D3 or cisplatin alone exhibited tumor-inhibitory effects in SCC as compared with the saline control (Fig. 3B). The combination of 1,25D3 and cisplatin resulted in enhanced tumor regression compared to single agent (Fig. 3B). Mice in saline, 1,25D3, and cisplatin treatment groups had to be enhances sacrificed early as a result of tumor burden. These results indicate that 1,25D3in vivo antitumor activity of cisplatin in the SCC model.
1,25D3 promotes cisplatin to induce apoptosis
1,25D3 induces apoptosis in SCC cells (19, 21, 30). 1,25D3 and cisplatin treatment led to increased caspase 3 cleavage compared to single agent treatment (24). To further characterize 1,25D3 and cisplatin-induced apoptosis, DNA fragmentation was evaluated by Cell Death Detection ELISA. 1,25D3 enhanced DNA fragmentation in SCC cells compared with controls, while cisplatin did not induce apoptosis (Fig. 4A). The combination treatment resulted in a significantly (P < 0.01) higher level of apoptosis compared to 1,25D3 alone (Fig. 4A). Immunoblot analysis showed that 1,25D3 induced the cleavage of pro-caspases 8, 10, 3 and Poly (ADP-ribose) polymerase (PARP) in SCC cells, while cisplatin did not (Fig. 4B). The combination treatment resulted in enhanced cleavage of pro-caspases 8, 10 and PARP (Fig. 4B). None of these were observed in SCC-DR cells (Fig. 4B). Pro-caspase 9 was not cleaved by any treatment (Fig. 4B). These results suggest that 1,25D3 promotes cisplatin to induce apoptosis through a caspase 8/10 – caspase 3 pathway in SCC cells.
Figure 4.
1,25D3 promotes cisplatin to induce apoptosis. A, SCC cells were pretreated with ETOH or 10 nM 1,25D3 for 24 h followed by 0.5 μg/ml of cisplatin for 2 h and an additional 48 h of incubation. Cells were harvested, lysed, and DNA fragmentation was evaluated by Cell Death Detection ELISAPLUS according to the manufacturer’s protocol. The enrichment factor was used as a parameter of apoptosis and shown on the y axis as mean ± SD of triplicate experiments. B, SCC or SCC-DR cells were pretreated with vehicle control ETOH or 10 nM 1,25D3 for 24 h followed by 0.5 μg/ml of cisplatin for 2 h. After an additional 48 h of incubation, cells were harvested and pro-caspases 8, 9, 10, 3 and PARP levels were evaluated by immunoblot analysis. Actin was the loading control. Results are representative of three independent experiments. cDDP, cisplatin.
1,25D3-augmented p73 level contributes to cisplatin-induced growth inhibition
p73 is one of the p53 family members and may regulate apoptosis (10). Cisplatin has been reported to promote 73 protein accumulation in HCT116 cells (33). Therefore, we next examined whether 1,25D3 and cisplatin alter the protein levels of p73 and other p53 family members in SCC or SCC-DR cells. 1,25D3 alone or in combination with cisplatin enhanced full length TAp73 (p73) levels in SCC cells as assessed by immunoblot analysis using a monoclonal antibody recognizing TAp73 but not reacting to Np73 nor p53 (Fig. 5A). In contrast, 1,25D3 resulted in reduced p53 protein level and the combination treatment also reduced p53 level (Fig. 5A). 1,25D3 alone or in combination with cisplatin also reduced p63 levels (Fig. 5A). Cisplatin did not affect the levels of p53, p63 or p73 in SCC cells (Fig. 5A). p53, p63, and p73 levels were not affected by any of the treatment in SCC-DR cells (Fig. 5A). To determine whether p73 accumulation contributes to 1,25D3 and cisplatin-induced growth inhibition, p73 was knocked down by siRNA. Since the endogenous p73 level was low, the efficiency of the p73 gene silencing was assessed by quantitative Real-time PCR (Fig. 5B). Following siRNA-transfection, SCC cells were further treated with ETOH or 10 nM 1,25D3 for 24 h followed by 0.5 μg/ml of cisplatin for 2 h and additional 6-day incubation for the in vitro clonogenic assay. siRNA-p73 significantly (P<0.001) promoted the surviving fraction following the treatment of 1,25D3, cisplatin, or the combination (Fig. 5B). To determine whether augmenting p73 protein level further promotes the anti-proliferative effects of 1,25D3 and cisplatin, we targeted a U-box-type E3/E4 ubiquitin ligase UFD2a which promotes the degradation of p73 (34). Knocking down endogenous UFD2a by siRNA resulted in p73 accumulation in SCC cells (Fig. 5C). With siRNA-UFD2a transfection, 1,25D3 or combination treatment resulted in significantly reduced surviving fraction when compared to the non-specific siRNA transfection (Fig. 5C). These data indicate that p73 contributes to the anti-proliferative effects of 1,25D3 and cisplatin.
Figure 5.
1,25D3-incrased p73 protein level contributes to cisplatin-induced growth inhibition. A, SCC or SCC-DR cells were pretreated with ETOH or 10 nM 1,25D3 for 24 h followed by 0.5 μg/ml of cisplatin for 2 h and an additional 48 h of incubation. Cells were harvested and p53, p63 and p73 levels were evaluated by immunoblot analysis. Actin was the loading control. B, SCC cells were transfected with siRNA-NS, siRNA- p73, or left untransfected for 24 h, followed by the treatment with 1,25D3 for 48 h. p73 mRNA level was evaluated by Real-time quantitative PCR. Results are the mean ± SD of the relative expression level to GAPDH. NS, non-specific; *, undetectable. Following siRNA-transfection, SCC cells were pretreated with ETOH or 10 nM 1,25D3 for 24 h followed by 0.5 μg/ml of cisplatin for 2 h. Anti-proliferative effect was assessed by in vitro clonogenic assay. Results are representative of two independent experiments. C, SCC cells were transfected with siRNA-NS, siRNA-UFD2a, or left untransfected for 24 h, followed by the treatment with 1,25D3 for 48 h. p73 protein level was evaluated by immunoblot analysis. Actin was the loading control. UFD, UFD2a. Following siRNA- transfection, SCC cells were pretreated with ETOH or 10 nM 1,25D3 for 24 h followed by 0.5 μg/ml of cisplatin for 2 h. Anti-proliferative effect was assessed by in vitro clonogenic assay. Results are representative of two independent experiments. cDDP, cisplatin.
p73 contributes to 1,25D3 and cisplatin-induced apoptosis
To further elucidate the mechanisms for 1,25D3 and cisplatin-induced growth inhibition, whether p73 plays a role in apoptosis was examined. Following siRNA transfection, SCC cells were treated with 1,25D3 and/or cisplatin and DNA fragmentation was evaluated after an additional 48 h of incubation. siRNA-p73 transfection resulted in reduced DNA fragmentation induced by 1,25D3 alone or 1,25D3 and cisplatin compared to controls (Fig. 6A). These results indicate that p73 contributes to 1,25D3 and cisplatin-mediated apoptosis.
Figure 6.
A, p73 contributes to 1,25D3 and cisplatin-induced apoptosis. SCC cells were transfected with siRNA-NS, siRNA-p73, or left untransfected for 24 h. Cells were then pretreated with ETOH or 10 nM 1,25D3 for 24 h followed by 0.5 μg/ml of cisplatin for 2 h and an additional 48 h of incubation. Cells were harvested and DNA fragmentation was evaluated by Cell Death Detection ELISAPLUS kit. The enrichment factor was used as a parameter of apoptosis and shown on the y axis as mean ± SD of triplicate experiments and are representative of three independent experiments. cDDP, cisplatin. B, A schematic presentation of the 1,25D3 potentiation of cisplatin anti-tumor activity. SCC cells are resistant to cisplatin treatment. 1,25D3 induces p73 accumulation and sensitizes SCC cells to cisplatin-mediated growth inhibition through caspase 8/10-caspase 3-dependent apoptotic pathway.
Altogether, our data showed that 1,25D3 sensitizes SCC cells to cisplatin-induced growth inhibition by the induction of p73 which promotes apoptosis through a caspase 8/10-caspase 3 dependent pathway (Fig. 6B).
Discussion
1,25D3 exerts anti-tumor effects in vitro and in vivo through inhibition of proliferation, induction of differentiation and apoptosis, and suppression of invasiveness of cancer cells (1). 1,25D3 has also been shown to synergistically or additively enhance the antitumor activities of a number of chemotherapeutic agents including carboplatin, cisplatin, docetaxel and paclitaxel in prostate cancer, breast cancer, and SCC models (22, 23, 35, 36). The mechanisms for the enhanced anti-tumor effects are not well understood. 1,25D3 promoted caspase 3 cleavage when used in combination with cisplatin in SCC cells (24). 1,25D3 potentiates antitumor activity of paclitaxel by reducing p21 in PC3 cells (22). In addition, 1,25D3 promotes docetaxel-induced growth inhibition by reducing multidrug resistance-associated protein 1 (36). 1,25D3 has been shown to enhance the cytotoxicity of carboplatin when used in clinical trials in patients with prostate cancer and advanced cancer (3).
To better understand the role of 1,25D3 in SCC cells, 1,25D3-resistant SCC variant was generated. SCC-DR cells showed resistance to 1,25D3-mediated growth inhibition and apoptosis, and compromised VDR transcription activity and nongenomic signaling.
Cisplatin is a widely used chemotherapeutic agent. Unfortunately, drug resistance and toxic side effects limit its usage. Therefore, if tumor cells can be sensitized to cisplatin treatment, lower and thus more tolerated dose can be used in the treatment. Potential mechanisms for acquired resistance to cisplatin include drug inactivation by glutathione and metallothionein, enhanced DNA repair, decreased cisplatin accumulation, increased cisplatin adducts tolerance and impaired apoptotic pathway (6). We previously showed that cellular concentration of cisplatin and cisplatin-DNA adducts did not change in response to 1,25D3 and cisplatin combination treatment compared to cisplatin alone (24).
Although we previously demonstrated that 1,25D3 enhanced cisplatin antiproliferative effects and caspase 3 cleavage in SCC cells (24), the mechanisms for these effects are largely unknown. Our current study identified p73 as a target of 1,25D3, the level of which is increased upon 1,25D3 treatment. We further demonstrate that p73 contributes to 1,25D3 and cisplatin-mediated growth inhibition.
We show that SCC and SCC-DR cells are resistant to cisplatin, and 1,25D3 sensitizes SCC cells to cisplatin-induced growth inhibition. Pretreatment with 1,25D3 followed by cisplatin resulted in enhanced clonogenic cell kill in SCC, but not SCC-DR, cells in vitro and in vivo. 1,25D3 in combination with cisplatin suppressed SCC tumor growth compared with either agent administered alone. This is in line with our previous data showing that a vitamin D analog, Ro23-7553, increased tumor regrowth delay in a combination therapy with cisplatin when compared to either agent administered alone (23). Cisplatin alone does not induce apoptosis in SCC cells, while pretreatment with 1,25D3 followed by cisplatin greatly enhances apoptosis compared to 1,25D3 alone. Others have shown that damaged apoptotic pathway is one of the mechanisms for cisplatin resistance (6, 37). Impaired apoptosis may involve dysregulation and mutations of apoptosis-mediating molecules which result in the inability of cells to detect DNA damage or to induce apoptosis (38–40). Therefore, SCC cells may be resistant to cisplatin treatment because cisplatin alone fails to induce apoptosis. When SCC cells are pretreated with 1,25D3, the apoptotic pathway is restored and cisplatin is able to further promote apoptosis, which is indicated by enhanced cleavage of pro-caspase 10 and PARP and increased DNA fragmentation.
Cisplatin may induce apoptosis through the regulation of p53 family member p73, which is regulated by DNA damage, oncogenes, and viral proteins (10). Cisplatin enhances p73 level in HCT116 cells by stabilizing p73 protein (33). In addition, cisplatin-mediated p73 accumulation contributes to cisplatin-induced apoptosis in Hep3B cells (41). When overexpressed, p73 promotes cisplatin-induced apoptosis in HeLa cells (42). Surprisingly, cisplatin did not induce p73 in SCC cells in this study, which may be one of the reasons why cisplatin alone failed to induce apoptosis in SCC cells. In contrast, 1,25D3 alone or in combination with cisplatin enhanced p73 protein level in SCC cells, most likely through increasing the stability of p73, since 1,25D3 did not alter the mRNA level of p73 as shown by quantitative Real-time RT-PCR. 1,25D3 did not sensitize SCC-DR cells to cisplatin treatment. Further, p73, p53, and p63 levels were not affected by 1,25D3 in SCC-DR cells. These results indicate that 1,25D3 signaling plays a critical role in potentiating the growth inhibitory effects of cisplatin. When p73 is knocked down by siRNA approach, 1,25D3 and cisplatin-induced growth inhibition and apoptosis were suppressed. The endogenous protein level of p73 is very low in SCC cell cultures. The stability of p73 is regulated by the proteasome through ubiquitin-dependent and ubiquitin-independent pathways (43). UFD2a, a U-box-type ubiquintin protein ligase, has recently been reported to interact with and promote the degradation of p73 in a ubiquitin-independent manner (34). It does not affect the half life of p53 (34). We took advantage of this phenomenon and augmented p73 protein level by siRNA-UFD2a. Increased p73 level promoted 1,25D3 and cisplatin-induced growth inhibition in SCC cells. These results suggest that p73 contributes to the anti-proliferative and pro-apoptotic effects of 1,25D3 and cisplatin. In line with this concept, two recent studies show that p73 induction sensitizes tumor cells to therapies through enhanced apoptosis. CD154 sensitizes leukemia cells to fludarabine treatment via the activation of p73 and the consequent overcoming of the resistance to apoptosis (44). Endogenous expression of p73 was observed only in the radiosensitive cervical cancer cells, and p73 transfection in the radioresistant cells resulted in enhanced cellular sensitivity to radiation by increase of apoptosis (45).
The mechanisms for p73-induced apoptosis remain to be fully understood. p73 may induce apoptosis through the mitochondrial pathway by inducing Puma which causes Bax mitochondrial translocation and cytochrome c release in Saos-2 cells (46). This apoptosis can be inhibited by the ΔNp73 isoform (46). The induction of cyclin-dependent kinase inhibitor p57kip2 is required for p73-mediated apoptosis in H1299 cells (47). Another study shows that p73 transcriptionally promotes the expression of death receptor Fas and sensitizes cells to apoptosis via a caspase-dependent pathway (48). Further studies are required to elucidate the mechanisms for p73-mediated apoptosis in SCC cells.
In summary, the current study demonstrates for the first time that 1,25D3 increased p73 protein level in SCC cells which sensitized SCC cells to cisplatin-mediated growth inhibition and apoptosis. We propose that the combination of 1,25D3 and cisplatin as a strategy to overcome cisplatin resistance and dose limitation.
Acknowledgments
Financial support: This study was supported by NIH/NCI grants CA67267 and CA85142 to Dr. Candace S. Johnson, and CA95045 to Dr. Donald L. Trump.
Abbreviations list
- 1,25D3
1α,25-dihydroxyvitamin D3
- cDDP
cisplatin, cis-diammine-dichloro-platinum (II)
- ETOH
ethanol
- LDH
lactate dehydrogenase
- PARP
poly (ADP-ribose) polymerase
- SCC
squamous cell carcinoma
- TA
transactivation
- VDR
vitamin D receptor
References
- 1.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 2.Brown AJ, Dusso A, Slatopolsky E. Vitamin D. Am J Physiol. 1999;277:F157–75. doi: 10.1152/ajprenal.1999.277.2.F157. [DOI] [PubMed] [Google Scholar]
- 3.Trump DL, Hershberger PA, Bernardi RJ, et al. Anti-tumor activity of calcitriol: pre-clinical and clinical studies. J Steroid Biochem Mol Biol. 2004;89–90:519–26. doi: 10.1016/j.jsbmb.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 4.Zamble DB, Lippard SJ. Cisplatin and DNA repair in cancer chemotherapy. Trends Biochem Sci. 1995;20:435–9. doi: 10.1016/s0968-0004(00)89095-7. [DOI] [PubMed] [Google Scholar]
- 5.Cohen SM, Lippard SJ. Cisplatin: from DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol. 2001;67:93–130. doi: 10.1016/s0079-6603(01)67026-0. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–20. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 7.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 8.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 9.Murray-Zmijewski F, Lane DP, Bourdon JC. p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 2006;13:962–72. doi: 10.1038/sj.cdd.4401914. [DOI] [PubMed] [Google Scholar]
- 10.Melino G, De Laurenzi V, Vousden KH. p73: Friend or foe in tumorigenesis. Nat Rev Cancer. 2002;2:605–15. doi: 10.1038/nrc861. [DOI] [PubMed] [Google Scholar]
- 11.Zaika AI, El-Rifai W. The role of p53 protein family in gastrointestinal malignancies. Cell Death Differ. 2006;13:935–40. doi: 10.1038/sj.cdd.4401897. [DOI] [PubMed] [Google Scholar]
- 12.Araki D, Uzawa K, Watanabe T, et al. Frequent allelic losses on the short arm of chromosome 1 and decreased expression of the p73 gene at 1p36.3 in squamous cell carcinoma of the oral cavity. Int J Oncol. 2002;20:355–60. [PubMed] [Google Scholar]
- 13.Ahomadegbe JC, Tourpin S, Kaghad M, et al. Loss of heterozygosity, allele silencing and decreased expression of p73 gene in breast cancers: prevalence of alterations in inflammatory breast cancers. Oncogene. 2000;19:5413–8. doi: 10.1038/sj.onc.1203914. [DOI] [PubMed] [Google Scholar]
- 14.Puig P, Capodieci P, Drobnjak M, et al. p73 Expression in human normal and tumor tissues: loss of p73alpha expression is associated with tumor progression in bladder cancer. Clin Cancer Res. 2003;9:5642–51. [PubMed] [Google Scholar]
- 15.Matsumoto H, Matsuyama H, Fukunaga K, Yoshihiro S, Wada T, Naito K. Allelic imbalance at 1p36 may predict prognosis of chemoradiation therapy for bladder preservation in patients with invasive bladder cancer. Br J Cancer. 2004;91:1025–31. doi: 10.1038/sj.bjc.6602073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson J, Lagowski J, Sundberg A, Lawson S, Liu Y, Kulesz-Martin M. p73 Loss Triggers Conversion to Squamous Cell Carcinoma Reversible upon Reconstitution with TAp73{alpha} Cancer Res. 2007;67:7723–30. doi: 10.1158/0008-5472.CAN-07-1195. [DOI] [PubMed] [Google Scholar]
- 17.Flores ER, Sengupta S, Miller JB, et al. Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell. 2005;7:363–73. doi: 10.1016/j.ccr.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 18.McElwain MC, Modzelewski RA, Yu WD, Russell DM, Johnson CS. Vitamin D: an antiproliferative agent with potential for therapy of squamous cell carcinoma. Am J Otolaryngol. 1997;18:293–8. doi: 10.1016/s0196-0709(97)90022-3. [DOI] [PubMed] [Google Scholar]
- 19.McGuire TF, Trump DL, Johnson CS. Vitamin D(3)-induced apoptosis of murine squamous cell carcinoma cells. Selective induction of caspase-dependent MEK cleavage and up-regulation of MEKK-1. J Biol Chem. 2001;276:26365–73. doi: 10.1074/jbc.M010101200. [DOI] [PubMed] [Google Scholar]
- 20.Bernardi RJ, Trump DL, Yu WD, McGuire TF, Hershberger PA, Johnson CS. Combination of 1alpha,25-dihydroxyvitamin D(3) with dexamethasone enhances cell cycle arrest and apoptosis: role of nuclear receptor cross-talk and Erk/Akt signaling. Clin Cancer Res. 2001;7:4164–73. [PubMed] [Google Scholar]
- 21.Ma Y, Yu WD, Kong RX, Trump DL, Johnson CS. Role of Nongenomic Activation of Phosphatidylinositol 3-Kinase/Akt and Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase Kinase/Extracellular Signal-Regulated Kinase 1/2 Pathways in 1,25D3-Mediated Apoptosis in Squamous Cell Carcinoma Cells. Cancer Res. 2006;66:8131–8. doi: 10.1158/0008-5472.CAN-06-1333. [DOI] [PubMed] [Google Scholar]
- 22.Hershberger PA, Yu WD, Modzelewski RA, Rueger RM, Johnson CS, Trump DL. Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin Cancer Res. 2001;7:1043–51. [PubMed] [Google Scholar]
- 23.Light BW, Yu WD, McElwain MC, Russell DM, Trump DL, Johnson CS. Potentiation of cisplatin antitumor activity using a vitamin D analogue in a murine squamous cell carcinoma model system. Cancer Res. 1997;57:3759–64. [PubMed] [Google Scholar]
- 24.Hershberger PA, McGuire TF, Yu WD, et al. Cisplatin potentiates 1,25-dihydroxyvitamin D3-induced apoptosis in association with increased mitogen-activated protein kinase kinase kinase 1 (MEKK-1) expression. Mol Cancer Ther. 2002;1:821–9. [PubMed] [Google Scholar]
- 25.Suit HD, Sedlacek RS, Silver G, Dosoretz D. Pentobarbital anesthesia and the response of tumor and normal tissue in the C3Hf/sed mouse to radiation. Radiat Res. 1985;104:47–65. [PubMed] [Google Scholar]
- 26.Yu WD, McElwain MC, Modzelewski RA, et al. Enhancement of 1,25-dihydroxyvitamin D3-mediated antitumor activity with dexamethasone. J Natl Cancer Inst. 1998;90:134–41. doi: 10.1093/jnci/90.2.134. [DOI] [PubMed] [Google Scholar]
- 27.Chang MJ, Yu WD, Reyno LM, et al. Potentiation by interleukin 1 alpha of cisplatin and carboplatin antitumor activity: schedule-dependent and pharmacokinetic effects in the RIF-1 tumor model. Cancer Res. 1994;54:5380–6. [PubMed] [Google Scholar]
- 28.Johnson CS, Chang MJ, Yu WD, et al. Synergistic enhancement by interleukin-1 alpha of cisplatin-mediated antitumor activity in RIF-1 tumor-bearing C3H/HeJ mice. Cancer Chemother Pharmacol. 1993;32:339–46. doi: 10.1007/BF00735916. [DOI] [PubMed] [Google Scholar]
- 29.Murdoch D. Standard, and novel cytotoxic and molecular-targeted, therapies for HNSCC: an evidence-based review. Curr Opin Oncol. 2007;19:216–21. doi: 10.1097/01.cco.0000264952.98166.99. [DOI] [PubMed] [Google Scholar]
- 30.Hershberger PA, Modzelewski RA, Shurin ZR, Rueger RM, Trump DL, Johnson CS. 1,25-Dihydroxycholecalciferol (1,25-D3) inhibits the growth of squamous cell carcinoma and down-modulates p21 (Waf1/Cip1) in vitro and in vivo. Cancer Res. 1999;59:2644–9. [PubMed] [Google Scholar]
- 31.Braunschweiger PG, Johnson CS, Kumar N, Ord V, Furmanski P. Antitumor effects of recombinant human interleukin 1 alpha in RIF-1 and Panc02 solid tumors. Cancer Res. 1988;48:6011–6. [PubMed] [Google Scholar]
- 32.Braunschweiger PG, Jones SA, Johnson CS, Furmanski P. Potentiation of mitomycin C and porfiromycin antitumor activity in solid tumor models by recombinant human interleukin 1 alpha. Cancer Res. 1991;51:5454–60. [PubMed] [Google Scholar]
- 33.Gong JG, Costanzo A, Yang HQ, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–9. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 34.Hosoda M, Ozaki T, Miyazaki K, et al. UFD2a mediates the proteasomal turnover of p73 without promoting p73 ubiquitination. Oncogene. 2005;24:7156–69. doi: 10.1038/sj.onc.1208872. [DOI] [PubMed] [Google Scholar]
- 35.Cho YL, Christensen C, Saunders DE, et al. Combined effects of 1,25-dihydroxyvitamin D3 and platinum drugs on the growth of MCF-7 cells. Cancer Res. 1991;51:2848–53. [PubMed] [Google Scholar]
- 36.Ting HJ, Hsu J, Bao BY, Lee YF. Docetaxel-induced growth inhibition and apoptosis in androgen independent prostate cancer cells are enhanced by 1alpha,25-dihydroxyvitamin D3. Cancer Lett. 2007;247:122–9. doi: 10.1016/j.canlet.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Eliopoulos AG, Kerr DJ, Herod J, et al. The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and Bcl-2. Oncogene. 1995;11:1217–28. [PubMed] [Google Scholar]
- 38.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–22. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 39.Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–14. doi: 10.1182/blood.v98.9.2603. [DOI] [PubMed] [Google Scholar]
- 40.Niedner H, Christen R, Lin X, Kondo A, Howell SB. Identification of genes that mediate sensitivity to cisplatin. Mol Pharmacol. 2001;60:1153–60. [PubMed] [Google Scholar]
- 41.Kim JS, Lee JM, Chwae YJ, et al. Cisplatin-induced apoptosis in Hep3B cells: mitochondria-dependent and -independent pathways. Biochem Pharmacol. 2004;67:1459–68. doi: 10.1016/j.bcp.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Kim KC, Jung CS, Choi KH. Overexpression of p73 enhances cisplatin-induced apoptosis in HeLa cells. Arch Pharm Res. 2006;29:152–8. doi: 10.1007/BF02974277. [DOI] [PubMed] [Google Scholar]
- 43.Watson IR, Irwin MS. Ubiquitin and ubiquitin-like modifications of the p53 family. Neoplasia. 2006;8:655–66. doi: 10.1593/neo.06439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dicker F, Kater AP, Prada CE, et al. CD154 induces p73 to overcome the resistance to apoptosis of chronic lymphocytic leukemia cells lacking functional p53. Blood. 2006;108:3450–7. doi: 10.1182/blood-2006-04-017749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu SS, Chan KY, Leung RC, Law HK, Leung TW, Ngan HY. Enhancement of the radiosensitivity of cervical cancer cells by overexpressing p73alpha. Mol Cancer Ther. 2006;5:1209–15. doi: 10.1158/1535-7163.MCT-05-0451. [DOI] [PubMed] [Google Scholar]
- 46.Melino G, Bernassola F, Ranalli M, et al. p73 Induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J Biol Chem. 2004;279:8076–83. doi: 10.1074/jbc.M307469200. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez S, Perez-Perez MM, Hernando E, Serrano M, Cordon-Cardo C. p73beta-Mediated apoptosis requires p57kip2 induction and IEX-1 inhibition. Cancer Res. 2005;65:2186–92. doi: 10.1158/0008-5472.CAN-04-3047. [DOI] [PubMed] [Google Scholar]
- 48.Terrasson J, Allart S, Martin H, et al. p73-dependent apoptosis through death receptor: impairment by human cytomegalovirus infection. Cancer Res. 2005;65:2787–94. doi: 10.1158/0008-5472.CAN-04-2019. [DOI] [PubMed] [Google Scholar]