Abstract
The repertoire of T cell receptor (TCR) specificities is established by a selection process in the thymus during which precursor survival and maturation is dictated by the nature of the TCR signals. The differences in signals that determine whether precursors will survive and mature or be induced to die remain poorly understood. Among the molecular effectors involved in executing the differentiation process initiated by TCR-ligand interactions is a family of Zn-finger transcription factors termed early growth response genes (Egr). Indeed, ablation of the Egr1 gene impairs ligand-induced maturation (positive selection) but not ligand-induced deletion (negative selection). The partial impairment of positive selection by Egr1-deficiency is not enhanced by simultaneous deletion of another Egr family member, Egr3. Accordingly, we asked whether this results from compensation by another family member, Egr2. In this manuscript, we demonstrate that deletion of Egr2 impairs positive selection of both CD4 and CD8 SP thymocytes. Interestingly, many of the genes involved in positive selection and T cell differentiation are upregulated normally in the Egr2-deficient thymocytes. However, Bcl-2 upregulation is not sustained during late stages of positive selection. This defect is at least partially responsible for the developmental blockade in Egr2-deficient thymocytes, as enforced expression of Bcl-2 rescues T cell development in Egr2−/− thymocytes. Taken together, these data suggest that Egr2 plays a central role in the upregulation of the survival molecule Bcl-2 during positive selection.
Keywords: Thymus, Transcription factors, Gene regulation, Differentiation
INTRODUCTION
During T cell development, the organism generates a T cell population with an extended repertoire of antigen specificities. This repertoire is molded in the thymus through signals derived from the interaction of T cell receptors (TCRs) on thymocytes with their ligands, major histocompatibility complex (MHC) molecules with bound peptides. The majority of thymocytes bear TCRs that do not recognize the MHC molecules present in the thymus, and these cells die relatively rapidly (3–4 days). Those cells bearing a TCR able to interact with self-MHC can receive signals that induce either their differentiation into mature T cells (positive selection) or apoptosis (negative selection). Furthermore, those cells that are positively selected develop into two different lineages, CD4 or CD8, depending on the ability of their TCRs to bind MHC class II or I, respectively. Most of what we understand about these processes has been learnt using genetically modified mice, especially mice engineered to express a rearranged transgenic TCR. The majority of DP thymocytes in these mice express the same TCR and therefore behave as a relatively homogeneous population. Breeding TCR transgenic mice to mice with alterations in different signaling molecules has provided most of our current knowledge regarding the role of different signal transduction pathways in the regulation of the different cell fate decisions during T cell development.
One of the first pathways identified as important for positive selection during T cell development was the Ras/Erk cascade (1). The immediate/early gene Egr1 has been proposed as one downstream effector of Erk during positive selection. Egr-1 is one of the earliest transcription factors expressed after TCR stimulation on DP thymocytes, and its induction is Erk dependent (2). Furthermore, mice overexpressing Egr1 have more efficient positive selection of mature CD4 and CD8 thymocytes even in genetic backgrounds that are very weakly selecting (3). Although the initial analyses of Egr-1 null mice did not reveal apparent defects in T cell development (4), a more recent study examined thymocyte development in Egr1-deficient mice in more detail using three different transgenic TCR backgrounds (5). This study suggests that Egr-1 is important for positive selection of both CD4 and CD8 cells. The lack of an observable effect on positive selection in the original Egr1−/− report may have been due to partial compensation by other members of the Egr family.
There are four Egr genes (Egr1– 4) expressed during thymocyte differentiation (2) (DLW, unpublished observations). Egr proteins contain highly conserved zinc-finger DNA-binding domains that can bind a number of common target gene promoters and potentially cooperate in regulating their expression (6). In thymocytes, Egr1, Egr2, and Egr3 are induced by pre-TCR signaling and overexpression of these proteins in pre-TCR signaling-deficient thymocytes can partially facilitate progression through the β-selection checkpoint (7) Conversely, dominant-negative Egr proteins inhibit progression from DN3 to DN4 in vitro (7). Similarly, Egr1, Egr2 and Egr3 can be induced by TcR signals in a DP thymoma-derived cell line (2). In vivo, Egr1-deficient thymocytes have a small but significant defect in positive selection but have no apparent defect in negative selection (5). In contrast, Egr3-deficient thymocytes have a partial block at the DN3 stage that is largely explained by reduced proliferation of DN4 thymocytes (8, 9). Simultaneous abrogation of Egr1 and Egr3 function in developing thymocytes results in a severe thymic atrophy not seen in mice lacking only Egr1 or Egr3. In contrast, the defects in positive selection were not more pronounced in Egr1/3 double knockout mice than in Egr1 single knockouts (9). This suggested to us that Egr2 could be more involved at this stage of T cell development.
We have explored this possibility using conditional Egr2 KO, and our results indicate that deletion of Egr2 in DP thymocytes results in a significant reduction in the generation of both CD4 and CD8 SP thymocytes, without affecting negative selection. Interestingly many of the genes known to be involved in positive selection and T cell differentiation are upregulated normally in the Egr2 null thymocytes. However, Bcl-2 upregulation is not sustained late in this process, and using rescue experiments we show that forced expression of Bcl-2 can rescue the defect in positive selection in Egr-2 thymocytes, suggesting that a central role of this transcription factor during positive selection is the upregulation of the survival molecule Bcl-2.
MATERIAL AND METHODS
Mice
All mice were maintained in the AALAC-accredited animal colonies of the Fox Chase Cancer Center and OMRF, and were handled in compliance with guidelines established by the Institutional Animal Care and Use Committees. Both conventional Egr2-deficient (Krox20-deficient; Krox20−/−) mice (10) (provided by T. Gridley, Jackson Labs, Bar Harbor, ME) and floxed conditional Egr2-deficient (Egr2f/f) mice (11) (a gift of Patrick Charnay, Institut National de la Santé et de la Recherche Médicale, Paris, France) were imported into our laboratory animal facility by embryo rederivation. Deletion of floxed Egr2 alleles was accomplished through crossing to lck-Cre transgenic mice (purchased from Jackson labs, Bar Harbor, ME) either alone or in conjunction with AND (12) or H–Y TCR transgenic mice (13) (provided by Dietmar Kappes, FCCC). B6-LY5.2/Cr congenic mice were obtained from the NCI Frederick animal production program.
Flow Cytometry
Thymic lobes were gently ground with a syringe plunger in PBA (PBS, 1% BSA, 0.02% NaN3) to produce a single cell suspension which was filtered through mesh, dispensed into a round-bottomed microtiter plate (1×105–1×106 cells per well), and stained and analyzed as described previously (14). Flurochrome coupled Ab were purchased from BD PharMingen (San Diego, CA) and eBiosciences (San Diego, CA). Cells were stained with the indicated commercially-prepared Ab and samples were analyzed using a BD LSR cytometer (BD PharMingen) and FlowJo Software (Treestar, Inc Ashland, OR).
Bcl-2 upregulation
For Bcl-2 staining thymocytes were isolated from Egr2f/f-Cre or Egr2f/f mice and cultured for 20 h in plates coated with or without 1 μg/ml anti-CD3 (145.2C11). Cells were harvested and surface stained for CD69 expression followed by intracellular Bcl-2 staining using the BD Cytofix/Cytoperm kit. Subsequently, the cells were analyzed for CD69 and Bcl-2 expression by flow cytometry, as described above.
Fetal thymic organ culture
At 13.5dpc, fetuses were harvested from pregnant Krox20+/− females and cultured on top of filter discs on gelfoam in a conventional fetal thymic organ culture (FTOC) system described previously (15) and cultured for the indicated time before analysis by flow cytometry as described above.
In vitro antibody stimulation
For negative selection studies, CD4+8+ DP thymocytes from AND transgenic Egr2f/f and Egr2f/f-Cre mice were purified by cell sorting and plated at 1×106/ml on control coated plates or plates coated with anti-CD3 (10□g/ml) + anti-CD28 (50□g/ml). After incubation for 20 hours at 37°C the fraction of cells surviving was determined by flow cytometry and staining with annexin. Survival cells were defined as those which were negative for both annexin and propidium iodide staining.
Sorting
The cell populations used in Figure 1A were isolated from the indicated mice after staining with fluorescently-labelled antibodies against CD4, CD8, CD69 and TCR (Pharmingen, Becton-Dickinson, e-Biosciences). Thymocytes were stained with antibodies against CD69 (fluorescein isothiocyanate, FITC), TCR beta (phycoerythrin, PE), CD4 (PE-Cy5, CyC) and CD8 (allophycocyanine, APC) and sorted for the populations indicated in the figure. Three- and four-color sorts were performed on a FACS Diva (Becton-Dickinson). The purity of all sorted populations was above 95%, except that the CD69-PE antibody became photobleached during the sort giving an apparent CD69 downregulation on reanalysis.
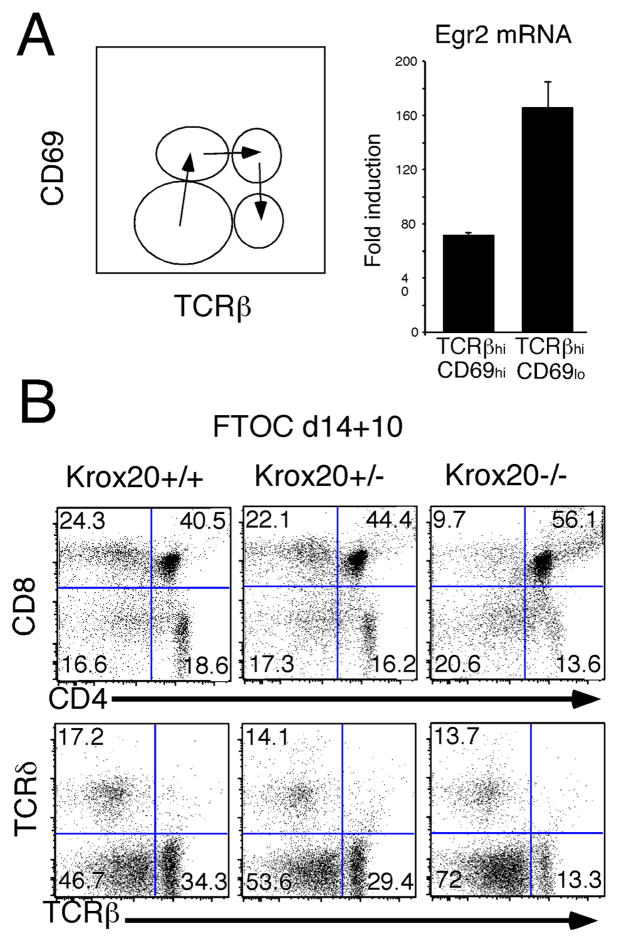
Figure 1. Egr2-deficiency impairs development of fetal TCRhi SP thymocytes.
(A) A schematic of the intermediates of positive selection defined by changes in expression of TCR and CD69 is depicted on the left. The expression level of Egr2 mRNA in TCRhiCD69hi and TCRhiCD69lo intermediates from C57BL/6 and in TCRintCD69− cells from MHC-I/II double-deficient mice was measured in triplicate by real time PCR and normalized to β-actin. The fold induction in C57BL/6 subsets was calculated relative to that in MHC-I/II double-deficient thymocytes whose TCR complexes have not been engaged by intrathymic ligand. The mean and standard deviation of fold induction of Egr2 mRNA expression in C57BL/6 intermediates is depicted graphically on the right side of the panel.
(B). Fetal thymic lobes explanted at day14 of gestation were cultured on filters at the air:medium interface for 14 days following which thymocyte development was assessed by flow cytometry using the indicated antibodies. Analysis was conducted on a minimum of 3 lobes per genotype. Gate frequencies are listed on the histograms. Results are representative of 2 experiments performed.
Isolation of RNA
RNA was isolated from cells using Qiagen RNeasy mini kit (cat #74104) according to the manufacturer’s protocol with minor modifications. Briefly, the cells were homogenized and lysed using QIAshredder columns (Qiagen cat #79654) in 1ml/1×106 cells of buffer RLT lysis buffer provided with the RNeasy mini kit and stored at −80°C until ready for RNA isolation. Samples were analyzed for quantity and quality by spectrophotometry (A260/280 ratio), agarose gel electrophoresis, and RNA bioanalysis (Agilent technologies 2100 Bioanalyzer). RNA samples exhibiting very high purity with no detected degradation of 18S or 28S rRNAs were used for preparation of cDNA for real time PCR.
Relative quantitation of gene expression by real-time PCR
cDNA was synthesized from 2μg total RNA purified as described above using Taqman reverse transcription reagents (Applied Biosystems, Foster City, CA, cat #n808-0234). Gene expression was analyzed by TaqMan real-time PCR using commercially prepared primers and probes (Applied Biosystems). Reactions were performed in triplicate for each gene, and expression was normalized to β-actin expression. Quantitation was done using the comparative CT method as described by ABI 7500 system SDS software.
Retroviral production and hematopoietic cell infection
Retroviral constructs in the MIG vector (16) were co-transfected along with the pCL/Eco plasmid into 293 cells as described (17). For viral transduction, 1 to 2 × 106 cells were mixed with 2 ml of retroviral supernatant plus 20μg/ml Polybrene per well in 24-well plates and centrifuged for 1 hr, at 210 × g, RT, as described (18). Supernatant was replaced with fresh media. Cells were cultured overnight before using them for adoptive transfer experiments
Bone marrow chimeras
Hematopoietic stem cells (HSC) were enriched from bone marrows using the mouse hematopoietic progenitor cell enrichment kit (BD biosciences, San Diego, CA). The cells thus isolated were rested overnight in the presence of SCF, IL-3 and IL-6 (10ng/ml) in 24 well culture plates. Next day the HSC-enriched cells were infected with MIG or MIG-Bcl2 virus as described above, and incubated at 37°C overnight. Infection efficiency was monitored by flow cytometry. The HSC were suspended at 2×106/ml and 100μl of the suspension was retro-orbitally injected into lethally radiated B6-Ly5.2 mice (NCI, Frederick). The mice were placed on Baytril and Doxycyclin treated antibiotic water for 6 weeks. Analysis was performed by flow cytometry, as described above.
RESULTS
Egr-2 is upregulated during positive selection
The process of thymocyte positive selection can be minimally subdivided into stages based on changes in expression of the CD69 activation marker and TCRβ. They are: 1) TCRβintCD69hi; 2) TCRβhiCD69hi; and 3) TCRβhiCD69lo (Fig. 1A). To determine if Egr2 mRNA levels are increased during positive selection, TCRβhiCD69hi and TCRβhiCD69lo intermediates were isolated by flow cytometry. Real time PCR analysis on mRNA samples from these populations revealed that Egr2 was substantially induced in both the TCRβhiCD69hi and TCRβhiCD69lo subpopulations (Fig. 1A), suggesting that Egr2 may play a role in positive selection.
Egr2-deficiency disrupts the development of αβ but not γδ thymocytes in FTOC
To assess the role of Egr2 in positive selection, we analyzed thymocyte development in Egr2-deficient (Krox20−/−) mice. Since Egr2-deficiency results in perinatal lethality due to defective myelination (10), we analyzed explanted d14 fetal lobes cultured for 10 days in FTOC (15). Flow cytometric analysis revealed no significant differences in the number of γδlineage cells, DN subsets, or the number of DP thymocytes in Krox20−/− thymocytes (Fig. 1B and data not shown); however, there was a reduction in both the CD4SP and CD8SP subsets as well as in TCRβhi cells (Fig. 1B). Development of Krox20+/− thymocytes was not impaired relative to that of littermate controls. Taken together, these data demonstrate that Egr2 plays an important role in the late stages of T cell development.
Conditional deletion of Egr-2 in T lineage precursors partially blocks development of mature αβ T cells
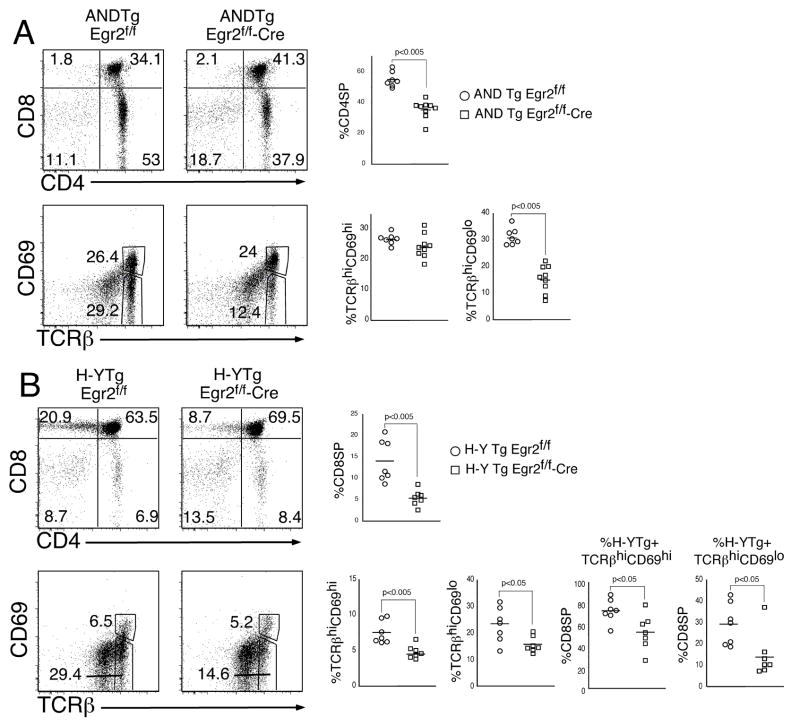
To address the role of Egr-2 in T cell development during adult hematopoiesis we employed floxed-Egr2 (Egr2f/f) mice in which Egr2 was conditionally ablated in T cells mice by Cre recombinase expressed under the control of the lck proximal promoter (lck-Cre) (19). Deletion of Egr2 in DN thymocytes results in thymii with slightly decreased cellularity (Fig 2A). The percentage and absolute numbers of mature CD4 and CD8 SP thymocytes are also decreased, indicating that the lack of Egr-2 impairs maturation f αβthymocytes. This block is already evident in early stages of the positive selection process as the TCRβhiCD69hi and TCRβhiCD69lo subpopulations are also somewhat reduced (Fig 2B). Interestingly, Egr2-deficiency appears to skew the CD4:8 ratio among the TCRβhiCD69hi early intermediates, increasing CD4SP and decreasing CD8SP; however, this differential effect on the CD4 and CD8 lineages is minimized upon differentiation to the TCRβhiCD69lo stage (Fig. 2B). Consequently, Egr2-deficiency impairs development of both fetal and adult mature thymocytes, with the inhibition being slightly more pronounced for CD8 lineage cells. It should be noted that the number of CD4 T cells is preferentially decreased in the spleen raising the possibility that Egr2-deficiency may impair growth or survival of CD4 T cells after exit from the thymus (Fig. 2C).
Figure 2. Egr2-deficiency impairs development of adult mature SP thymocytes with heterogenous TCR complexes.
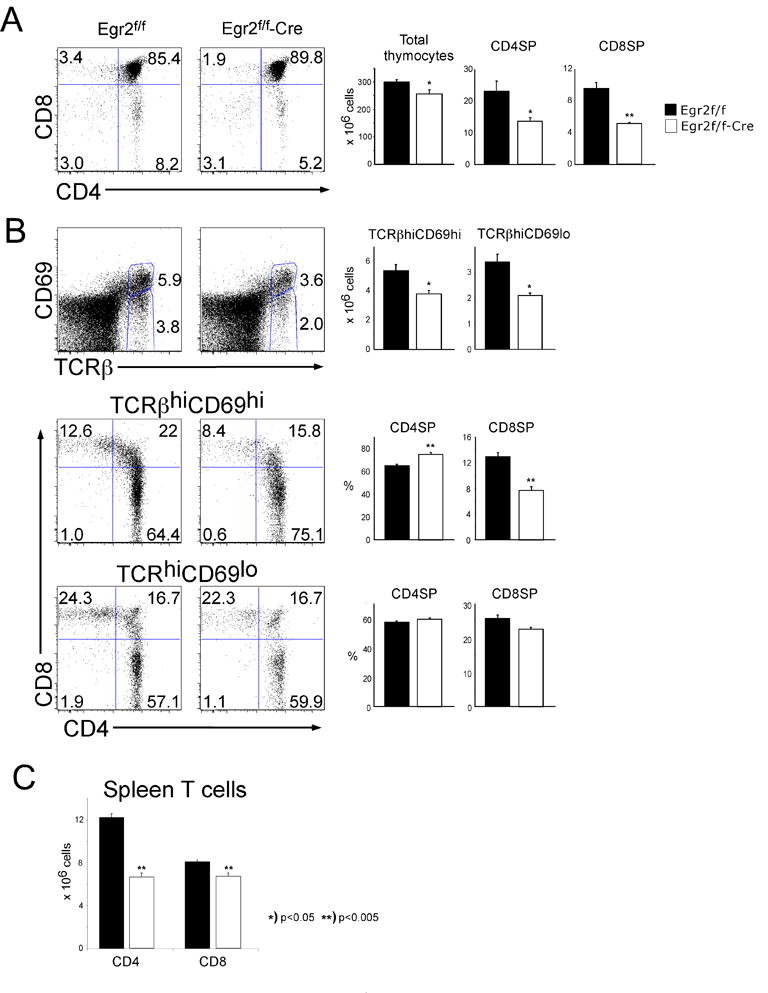
(A–B) Single cell suspensions of thymocytes from adult Egr2f/f or Egr2f/f-Cre mice were stained with mAb reactive to CD4, CD8, TCRβand CD69. Gate frequencies of the indicated populations were used to calculate the absolute number of mature CD4 and CD8 SP thymocytes or positive selection intermediate. The mean and standard deviations are depicted graphically to the right. The depicted analysis was performed on a minimum of 5 mice per group and is representative of 3 experiments performed. (C) Number of CD4 and CD8 T cells in Egr2f/f or Egr2f/f-Cre. Data from two experiments, n=3 for each group in each experiment.
As observed in the FTOC with conventional Krox20−/− mice, no effect on γδT cell development was observed in conditional Egr2-deficient mice; however, the number of αβTCRhi CD4−CD8− cells was diminished somewhat, suggesting that development of NKT cells might also be impaired by Egr2-deficiency (data not shown).
Egr-2 deficiency interferes with positive selection
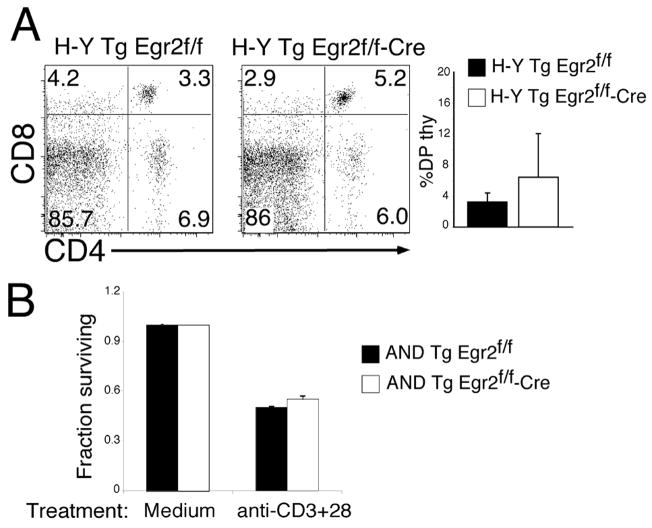
The effects of a gene product during positive selection can sometimes be obscured by a heterogeneous TCR repertoire, where TCR specificities of differing affinity may compensate for the absence of an important molecular effector. To assess the effect of Egr2-deficiency on development of thymocytes with a monoclonal TCR repertoire, we crossed Egr2f/f/lck-cre mice with mice bearing the transgenic AND TCR (12). AND transgenic thymocytes are positively selected on I-Ab and I-Ek molecules but not I-Ad (20) and this model has previously used to reveal the importance of many signal transduction pathways in positive selection and CD4/CD8 lineage commitment (21–23). As shown in Fig. 3A, Egr-2 deficiency clearly decreased both the percentage and absolute number of CD4SP thymocytes. Indeed, Egr-2 deficiency reduced the absolute number of CD4 SP from 27.9(±3.5) × 106 to 10.8(±3.2) × 106 cells. This decrease is even more evident when the thymocytes were resolved into positive selection intermediates based on expression of TCR and CD69. As discussed above (Fig. 2), changes in expression of TCR and CD69 distinguish cells early in the process of positive selection (TCRhiCD69hi) from those in the more advanced phases (TCRhiCD69lo). Egr2 deficiency caused a substantial reduction of the TCRhiCD69lo subset, indicating that Egr-2 function may be important primarily during the later stages of positive selection. This reduction was still evident in the periphery, where the numbers of splenic AND CD4 cells was 24.22(±6.7) × 106 in Egr2f/f mice versus 13.5(±1.4) × 106 cells in Egr2f/f -cre mice. Since Egr2 deficiency interfered with selection of CD4SP thymocytes we asked whether selection of CD8SP was similarly impaired. For this we employed the H-Y TCR transgenic model (Fig 3B). The H-Y TCR is reactive with the male antigen presented by H-2k/Db and thymocytes bearing this TCR are positively selected and commit to the CD8 lineage in female mice (13). In female H-Y TCR transgenic mice, Egr2 deficiency impaired the generation of mature CD8 cells with the defect manifesting primarily in the TCRhiCD69lo population, as was observed for CD4SP. Similarly, the T3.70 + CD8 population in the spleen was reduced from 1.4(±0.3) × 106 in Egr2f/f mice to 0.5(±0.2) × 106 cells in Egr2f/f -cre mice. Taken together, these data indicate that Egr2 is required for generation of both CD4SP and CD8SP thymocytes with no obvious differential effects on the CD4 and CD8 lineages. In contrast with the effect on positive selection, Egr2-deficiency did not interfere with thymocyte deletion. Neither the male-antigen induced deletion of H-Y TCR Tg thymocytes in vivo, nor the anti-CD3+CD28-induced killing of AND Tg DP thymocytes cells in vitrowere impaired by Egr2-deficiency (Fig. 4A,B).
Figure 3. Egr2-deficiency impairs development of transgenic TCR CD4 and CD8 SP thymocytes.
(A–B) Single cell suspensions of thymocytes from either TCR Tg mice that were either Egr2f/f (control) or Egr2f/f-Cre were analyzed by flow cytometry as in Figure 2. Frequencies of SP populations or positive selection intermediates were calculated for both the MHC-II restricted CD4-selecting AND TCR Tg (A) and the CD8-selecting MHC-I restricted H-Y Tg (B) models. Scatter plots in which each mouse is represented as an individually graphed symbol are depicted to the right.
Figure 4. Egr2-deficiency does not affect thymocyte deletion.
(A) Thymocytes from HY/Egr2f/f and HY/Egr2f/f-Cre males were stained with CD4 and CD8 and analyzed by flow cytometry. The percentage of DP thymocytes, shown on the graph is not significantly different. (n=6).
(B) Histogram showing survival of DP thymocytes from ANDTg Egr2f/f and ANDTg Egr2f/f-Cre mice. Purified DP thymocytes were treated with anti-CD3+CD28 antibody and subsequently analyzed by flow cytometry using annexin-V and PI. Survival was calculated from triplicate samples as the percentage of annexin/PI negative cells remaining after stimulation.
Egr-2 selectively controls Bcl-2 upregulation during positive selection
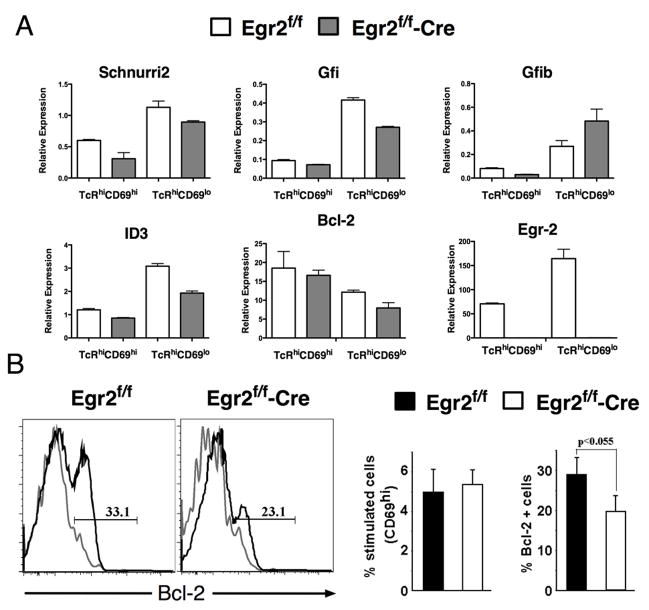
To elucidate the mechanism responsible for the blockade in positive selection in Egr2-deficient thymocytes, we sorted TCRhiCD69hi and TCRhiCD69lo intermediates from normal littermates (Egr2f/f) and Egr2f/f-Cre thymocytes, and analyzed the expression of a panel of genes that play an important role during positive selection and T cell differentiation, including Schnurri2 (24)), Id3 (25), Tox (26, 27), Gfi1 and Gfi1b (28, 29), GATA-3 (18, 30), runx3 (31, 32) and TH-POK (33, 34). Surprisingly, the upregulation of most of these genes was not altered in Egr2f/f-Cre thymocytes (Fig 5A and data not shown) with the exception of Id3, which seems partially inhibited. Id3 up-regulation during positive selection is mediated by the Ras/MAPK pathway (35), which also contributes to the regulation of Egr2. Id3-deficiency has been reported to impair positive selection (25); however, the phenotype of Id3−/− mice is less profound than that of Egr2-deficiency, specially in regards to CD8 development (25), suggesting that it is probably not solely responsible for impairing positive selection in Egr2−/− mice.
Figure 5. Egr-2-deficient thymocytes have a defective Bcl-2 upregulation after positive selection.
(A) TcRβhiCD69hi and TcRβhiCD69lo populations from Egr2f/f or Egr2f/f-Cre mice were sorted, and the expression of different genes analyzed by real-time PCR, as described in Materials and Methods. (B) Histograms showing Bcl-2 expression in CD69high cells from un-stimulated (gray) or anti-CD3 stimulated (black) Egr2f/f or Egr2f/f-Cre thymocytes. The percentage of stimulated CD69high and Bcl-2 expressing CD69high thymocytes is shown in the graph on the right. Egr2f/f or Egr2f/f-Cre cells are shown in black and white bars, respectively. Statistical differences between Egr2f/f or Egr2f/f-Cre cells were determined using Student’s t-test.
Given that previous reports suggested that Egr1-deficient thymocytes had defects in Bcl-2 upregulation after TcR activation (5), we tested whether Bcl-2 induction was impaired by Egr2-deficiency by measuring mRNA levels in sorted intermediates and measuring protein levels by intracellular staining after anti-CD3 crosslinking. As shown in Fig 5B, Egr2-deficiency blunts the induction of Bcl-2 in response to TCR signals, although the blockade is not complete. mRNA encoding Bcl-2 is not significantly altered early during selection (i.e. in CD69hi cells), but is reduced in the later stage CD69lo cells (Fig. 5A), suggesting that in the absence of Egr2, positively selected thymocytes start upregulating Bcl-2 normally, but fail to sustain its expression. Since Bcl-2 is important for survival, this defect could be responsible for the defect in mature T cell development.
Overexpression of Bcl-2 rescues the development of Egr2−/− thymocytes
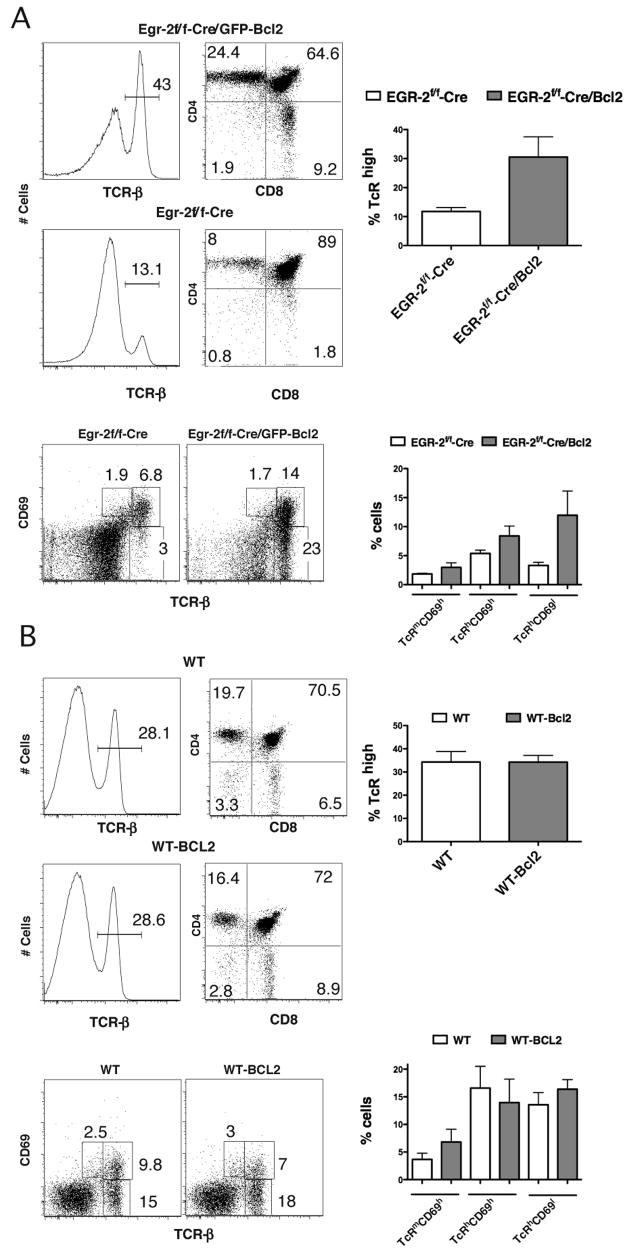
To test this possibility, we infected bone marrow Lin− Sca+c-Kit+ Egr2f/f-cre cells with a retrovirus encoding for a Bcl2-GFP fusion protein (36), and adoptively transferred the infected cells into lethally irradiated Ly5.1 recipients. The mice were analyzed after 6 weeks, and the effect of Bcl-2 expression on the development of T cells was assessed by comparing the percentages of TcRhigh thymocytes in GFP+ Egr2f/f-Cre vs. GFP− Egr2f/f cells. As shown in Fig 6A, overexpression of Bcl-2 dramatically increased the percentage of TcRhi single positive cells in the thymus of the recipient animals, suggesting that the defect in Bcl-2 maintenance is responsible for the blockade in positive selection caused by Egr2-deficiency. Consistent with our previous results the effect is more marked in the TcRhiCD69lo population, suggesting that the role of Egr2 is more important during late stages of positive selection. In contrast, overexpression of Bcl-2 in Egr2-sufficient thymocytes did not result in an increase in positive selection (Fig 6B).
Figure 6. Forced expression of Bcl-2 rescues the generation of TcRhi cells in Egr-2 deficient mice.
Lin− bone marrow cells from Egr2f/f-cre mice were infected with MIG-Bcl-2 and injected i.v. into lethally irradiated Ly5.2 recipients. After six weeks the mice were euthanized, and T cell development of the transferred cells was assessed by gating on Ly5.1+ cells. The effect of Bcl-2 is determined by comparing development in Ly5.1+/GFP− vs. Ly5.1+/GFP+ cells. (A) We show a representative histogram of thymocytes stained with TCR or CD4/CD8 comparing GFP+ cells (top) to GFP− (bottom), and a quantification of the TcRhigh cells (n=8). Expression of Bcl-2 rescues the development of mature T cells in an Egr2f/f-Cre background. The same thymocytes were stained with TcR and CD69 to determine the effect of Bcl-2 at the different stages of T cell differentiation after positive selection. We show a representative histogram, and the quantification (n=8). As can be clearly observed the effect of Bcl-2 is more prominent at the latest stages of T cell differentiation. (B) Similar experiments were performed on Lin− bone marrow cells from wild-type mice (n=7).
DISCUSSION
Egr proteins play a role during positive selection. Overexpression of Egr-1 improved the efficiency of positive selection (3), while its deletion resulted in a partial blockade in positive selection, both in class I- and class II-restricted T cells (5). The partial phenotype suggests that other Egr family members may also play a role in this process, and expression of a dominant negative pan-Egr construct in rFTOCs results in a much more dramatic block in mature T cell development ((7) and JAI, unpublished results). Since the defects in positive selection were not more pronounced in Egr1/3 double knockout mice than in Egr1 single knockouts (9), we suspected that Egr-2 could be the family member involved in this process. In this manuscript we provide evidence that demonstrates a role for Egr-2 in positive selection. As with Egr1-deficient thymocytes, the block in positive selection is partial, and seems equally profound for class I- and class II-restricted T cells. The prediction from our experiments is that a double Egr1-Egr2 knockout would result in a much more profound blockade in positive selection, and the experiments to test this hypothesis are currently in progress.
Besides positive selection, interactions of the TcR with MHC+peptide in immature DP thymocytes may result in deletion. Thymocytes defective in Egr-2 seem to undergo normal deletion, as do Egr-1 deficient thymocytes, both using an in vitro model with α-CD3+α-CD28 antibodies, and in vivo, using the HY transgenic model (13). Neither of these approaches perfectly mimics the physiological process of negative selection (i.e., a caveat of the in vivo results is that HY deletion could occur before Cre-mediated deletion of the Egr-2 gene), so further experiments will be required to assess the contribution of Egr-2 to negative selection. However, these results are consistent with the lack of effect on negative selection of many alterations in the Ras/MAPK pathway, one of the principal regulators of Egr expression (1). Likewise, Egr2 induction is also dependent upon Ca++ signaling and perturbations in Ca++ signaling caused by ablation of the CnB1 (calcineurin B1) blocks positive, but not negative selection (37).
One interesting aspect of the phenotype we observed in Egr2-deficient thymocytes is that the defect is more pronounced in late stages of positive selection, as defined by the expression of TCR and CD69. This is intriguing because Egr-2 is induced very early during positive selection, and suggests that Egr-2 controls the expression of other genes that are necessary during late stages in the process of positive selection. Surprisingly, its effects are very selective, because among the genes required for positive selection and whose expression is altered during this process, (Schnurri2, Id3, Tox, Gfi1 and Gfi1b, GATA-3, runx3, TH-POK and Bcl-2 (18, 24–34, 38), only Id3 and Bcl-2 expression were significantly altered in Egr2-deficient intermediates.
Id3 is a negative regulator of E-box transcription factors, and in its absence positive selection is partially blocked. Interestingly, it has been reported that Id3 upregulation in DP thymocytes is regulated by the Ras/MAPK pathway and Egr1 (35), although these results have been controversial (39). However, the phenotype of Id3−/− mice is less profound than that of Egr2-deficient mice, especially in regards to CD8 development (25), suggesting that it is probably not the primary Egr2-modulated gene responsible for the defects in Egr2-deficient mice. It is however possible that it may contribute to the phenotype, and genetic rescue experiments will be necessary to address this possibility.
Upregulation of Bcl-2 is important for the survival of positively selected thymocytes (38), but not much is known about the factors that regulate its expression during this process. Because Egr2-deficiency both attenuated induction of Bcl-2 after in vitro stimulation and lowered its expression in purified intermediate populations, diminished Bcl-2 was very attractive as an explanation for the positive selection defect. It must be pointed out that the Bcl-2 expression defect is not very profound. This is probably due in part to Egr-1 compensation. This is likely to be compounded by selective pressure which enables only those Egr2-deficient precursors whose Bcl-2 induction exceeds a minimum threshold to mature to the TCRhiCD69lo stage. Accordingly, the requirement of Egr transcription factors in controlling Bcl-2 expression during positive selection is probably more profound than the subtle defect noted here suggests. Analysis of double Egr1/Egr2-deficient thymocytes will probably make this more evident.
Our genetic rescue experiments of Egr2-deficient thymocytes with a Bcl2-encoding retrovirus clearly demonstrate that positive selection is enhanced in the presence of Bcl-2, both for CD4 and CD8 single positive thymocytes. Therefore, while quantitatively modest, the defect in Bcl-2 induction during positive selection in Egr2-deficient thymocytes is biologically significant and plays a predominant role in impairing positive selection in Egr2-deficient mice. It remains unclear at present whether Bcl-2 is a direct target of Egr2. In that regard, Egr2 induction is most pronounced late in positive selection (TCRhiCD69lo) where Bcl-2 levels are most adversely affected by Egr2-deficiency. Nevertheless, it remains possible that Egr2 influences Bcl-2 expression through an intermediate(s), which in turn contribute to Bcl-2 induction or maintenance at late stages of the positive selection process. A possible mediator of this effect could be an alteration in IL7Ra expression in the absence of Egr2, and we have observed a small reduction in the percentage of IL7Ra positive cells in post-selected populations from Egr2f/f-cre thymocytes (DW, unpublished observations). Current experiments are focused on the identification of this mediator.
In conclusion, we demonstrate here that Egr2 plays an important role during positive selection of αβT cells, and identify the regulation of Bcl-2 expression as the likely mechanism that underlies this effect. These results are in contrast with a recent published observation (40) where no alterations in peripheral T cell populations were observed in Egr2 deficient bone marrow chimeras. However, in this report only peripheral populations were analyzed, and the defect in positive selection in the mice may be masked by homeostatic expansion of the selected populations in the periphery.
Our findings also raise the interesting possibility of differential involvement of particular Egr family members in positive selection. Indeed, while Egr1-deficiency and Egr2-deficiency partially impair positive selection, mice doubly deficient for both Egr1 and Egr3 do not exhibit a more profound defect than do Egr1-deficient mice (9). This suggests that among Egr family members, the function of Egr1 and 2 is most important during positive selection whereas the function of Egr1 and 3 are most important during early thymocyte development and β-selection. Analysis of multiply-deficient mice lacking combinations of Egr1, Egr2, and Egr3 will be necessary to address this possibility and establish its molecular basis.
Footnotes
This work was supported by NIH grants CA73656, CA87407, CA100144, NIH core grant P01CA06927, Center grant #P30-DK-50306, and an appropriation from the Commonwealth of Pennsylvania to DLW and NIH grant AI059302 to JAI
Bibliography
- 1.Alberola-Ila J, Hernandez-Hoyos G. The Ras/MAPK cascade and the control of positive selection. Immunol Rev. 2003;191:79–96. doi: 10.1034/j.1600-065x.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 2.Shao H, Kono DH, Chen LY, Rubin EM, Kaye J. Induction of the early growth response (Egr) family of transcription factors during thymic selection. J Exp Med. 1997;185:731–744. doi: 10.1084/jem.185.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyazaki T, Lemonnier FA. Modulation of thymic selection by expression of an immediate-early gene, early growth response 1 (Egr-1) J Exp Med. 1998;188:715–723. doi: 10.1084/jem.188.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SL, Tourtellotte LC, Wesselschmidt RL, Milbrandt J. Growth and differentiation proceeds normally in cells deficient in the immediate early gene NGFI-A. J Biol Chem. 1995;270:9971–9977. doi: 10.1074/jbc.270.17.9971. [DOI] [PubMed] [Google Scholar]
- 5.Bettini M, Xi H, Milbrandt J, Kersh GJ. Thymocyte development in early growth response gene 1-deficient mice. J Immunol. 2002;169:1713–1720. doi: 10.4049/jimmunol.169.4.1713. [DOI] [PubMed] [Google Scholar]
- 6.Swirnoff AH, Milbrandt J. DNA-binding specificity of NGFI-A and related zinc finger transcription factors. Mol Cell Biol. 1995;15:2275–2287. doi: 10.1128/mcb.15.4.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carleton M, Haks MC, Smeele SA, Jones A, Belkowski SM, Berger MA, Linsley P, Kruisbeek AM, Wiest DL. Early growth response transcription factors are required for development of CD4(−)CD8(−) thymocytes to the CD4(+)CD8(+) stage. J Immunol. 2002;168:1649–1658. doi: 10.4049/jimmunol.168.4.1649. [DOI] [PubMed] [Google Scholar]
- 8.Xi H, Kersh GJ. Early growth response gene 3 regulates thymocyte proliferation during the transition from CD4-CD8- to CD4+CD8+1. J Immunol. 2004;172:964–971. doi: 10.4049/jimmunol.172.2.964. [DOI] [PubMed] [Google Scholar]
- 9.Carter JH, Lefebvre JM, Wiest DL, Tourtellotte WG. Redundant role for early growth response transcriptional regulators in thymocyte differentiation and survival. J Immunol. 2007;178:6796–6805. doi: 10.4049/jimmunol.178.11.6796. [DOI] [PubMed] [Google Scholar]
- 10.Swiatek PJ, Gridley T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev. 1993;7:2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- 11.Taillebourg E, Buart S, Charnay P. Conditional, floxed allele of the Krox20 gene. Genesis. 2002;32:112–113. doi: 10.1002/gene.10062. [DOI] [PubMed] [Google Scholar]
- 12.Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 13.Kisielow P, Teh HS, Bluthmann H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 14.Haks MC, Belkowski SM, Ciofani M, Rhodes M, Lefebvre JM, Trop S, Hugo P, Zuniga-Pflucker JC, Wiest DL. Low activation threshold as a mechanism for ligand-independent signaling in pre-T cells. J Immunol. 2003;170:2853–2861. doi: 10.4049/jimmunol.170.6.2853. [DOI] [PubMed] [Google Scholar]
- 15.Hare KJ, Jenkinson EJ, Anderson G. In vitro models of T cell development. Semin Immunol. 1999;11:3–12. doi: 10.1006/smim.1998.0151. [DOI] [PubMed] [Google Scholar]
- 16.Van Parijs L, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- 17.Naviaux RK, Costanzi E, Haas M, Verma IM. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Hoyos G, Anderson MK, Wang C, Rothenberg EV, Alberola-Ila J. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity. 2003;19:83–94. doi: 10.1016/s1074-7613(03)00176-6. [DOI] [PubMed] [Google Scholar]
- 19.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 20.Vasquez NJ, Kaye J, Hedrick SM. In vivo and in vitro clonal deletion of double-positive thymocytes. J Exp Med. 1992;175:1307–1316. doi: 10.1084/jem.175.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharp LL, Schwarz DA, Bott CM, Marshall CJ, Hedrick SM. The influence of the MAPK pathway on T cell lineage commitment. Immunity. 1997;7:609–618. doi: 10.1016/s1074-7613(00)80382-9. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez-Hoyos G, Sohn SJ, Rothenberg EV, Alberola-Ila J. Lck activity controls CD4/CD8 T cell lineage commitment. Immunity. 2000;12:313–322. doi: 10.1016/s1074-7613(00)80184-3. [DOI] [PubMed] [Google Scholar]
- 23.Barbee SD, Alberola-Ila J. Phosphatidylinositol 3-kinase improves the efficiency of positive selection. Int Immunol. 2006;18:921–930. doi: 10.1093/intimm/dxl027. [DOI] [PubMed] [Google Scholar]
- 24.Takagi T, Harada J, Ishii S. Murine Schnurri-2 is required for positive selection of thymocytes. Nat Immunol. 2001;2:1048–1053. doi: 10.1038/ni728. [DOI] [PubMed] [Google Scholar]
- 25.Rivera RR, Johns CP, Quan J, Johnson RS, Murre C. Thymocyte selection is regulated by the helix-loop-helix inhibitor protein, Id3. Immunity. 2000;12:17–26. doi: 10.1016/s1074-7613(00)80155-7. [DOI] [PubMed] [Google Scholar]
- 26.Aliahmad P, Kaye J. Development of all CD4 T lineages requires nuclear factor TOX. J Exp Med. 2008;205:245–256. doi: 10.1084/jem.20071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkinson B, Chen JY, Han P, Rufner KM, Goularte OD, Kaye J. TOX: an HMG box protein implicated in the regulation of thymocyte selection. Nat Immunol. 2002;3:272–280. doi: 10.1038/ni767. [DOI] [PubMed] [Google Scholar]
- 28.Yucel R, Karsunky H, Klein-Hitpass L, Moroy T. The transcriptional repressor Gfi1 affects development of early, uncommitted c-Kit+ T cell progenitors and CD4/CD8 lineage decision in the thymus. J Exp Med. 2003;197:831–844. doi: 10.1084/jem.20021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doan LL, Kitay MK, Yu Q, Singer A, Herblot S, Hoang T, Bear SE, Morse HCR, Tsichlis PN, Grimes HL. Growth factor independence-1B expression leads to defects in T cell activation, IL-7 receptor alpha expression, and T cell lineage commitment. J Immunol. 2003;170:2356–2366. doi: 10.4049/jimmunol.170.5.2356. [DOI] [PubMed] [Google Scholar]
- 30.Pai SY, Truitt ML, Ting CN, Leiden JM, Glimcher LH, Ho IC. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity. 2003;19:863–875. doi: 10.1016/s1074-7613(03)00328-5. [DOI] [PubMed] [Google Scholar]
- 31.Taniuchi I, Osato M, Egawa T, Sunshine MJ, Bae SC, Komori T, Ito Y, Littman DR. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111:621–633. doi: 10.1016/s0092-8674(02)01111-x. [DOI] [PubMed] [Google Scholar]
- 32.Sato T, Ohno S, Hayashi T, Sato C, Kohu K, Satake M, Habu S. Dual functions of Runx proteins for reactivating CD8 and silencing CD4 at the commitment process into CD8 thymocytes. Immunity. 2005;22:317–328. doi: 10.1016/j.immuni.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 33.He X, He X, Dave VP, Zhang Y, Hua X, Nicolas E, Xu W, Roe BA, Kappes DJ. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433:826–833. doi: 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- 34.Keefe R, Dave V, Allman D, Wiest D, Kappes DJ. Regulation of lineage commitment distinct from positive selection. Science. 1999;286:1149–1153. doi: 10.1126/science.286.5442.1149. [DOI] [PubMed] [Google Scholar]
- 35.Bain G, Cravatt CB, Loomans C, Alberola-Ila J, Hedrick SM, Murre C. Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat Immunol. 2001;2:165–171. doi: 10.1038/84273. [DOI] [PubMed] [Google Scholar]
- 36.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 38.Linette GP, Grusby MJ, Hedrick SM, Hansen TH, Glimcher LH, Korsmeyer SJ. Bcl-2 is upregulated at the CD4+ CD8+ stage during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1994;1:197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 39.McNeil LK, Starr TK, Hogquist KA. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci U S A. 2005;102:13574–13579. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins S, Lutz MA, Zarek PE, Anders RA, Kersh GJ, Powell JD. Opposing regulation of T cell function by Egr-1/NAB2 and Egr-2/Egr-3. Eur J Immunol. 2008;38:528–536. doi: 10.1002/eji.200737157. [DOI] [PMC free article] [PubMed] [Google Scholar]