Abstract
1,1-Bis(3′-indoly)-1-(p-substituted phenyl)methanes (C-DIMs) exhibit structure-dependent activation of peroxisome-proliferator-activated receptor γ (PPARγ) and nerve growth factor-induced Bα (NGFIBα, Nur77) and induce receptor-dependent and receptor-independent apoptosis in cancer cells and tumors. In this study, we investigated the activation of apoptosis in pancreatic cancer cells by p-bromo (DIM-C-pPhBr) and p-fluoro (DIM-C-pPhF) and structurally-related analogs that do not activate either PPARγ or Nur77. The ortho-, meta- and para-bromo and -fluoro isomers all activated ER stress-dependent apoptosis in pancreatic cancer cells; however, methylation of the indole N group significantly decreased activity, suggesting that a free N was important for activation of ER stress. Both DIM-C-pPhBr and DIM-C-pPhF resembled the classical ER stress inducer thapsigargin in pancreatic cancer cells and activated ER stress markers such as glucose related protein 78 and the c-jun N-terminal kinase (JNK) pathway, resulting in the induction of CCAAT/enhancer-binding protein homologous protein (CHOP), death receptor (DR5) and the extrinsic apoptotic pathway. Moreover, DIM-C-pPhBr also inhibited tumor growth in an orthotopic model for pancreatic cancer demonstrating the clinical potential for this C-DIM compound in pancreatic cancer chemotherapy.
Keywords: C-DIMs, ER stress, apoptosis, pancreatic cancer
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is a major cause of cancer-related deaths in developed countries and it is estimated that in excess of 30,000 new cases will be diagnosed this year in the United States (1). PDAC is a highly aggressive disease that invariably evades early diagnosis (2, 3). The mean survival time for patients with metastatic disease is only 3–6 months, and the 1-year survival time for all pancreatic cancers cases is approximately 20–30%. Several factors are associated with increased risk for pancreatic cancer and these include chronic pancreatitis, prior gastric surgery, smoking, diabetes, exposure to certain classes of organic solvents, and radiation (4–11). Heritable germline mutations in several genes are also associated with increased risk for pancreatic cancer and these include Peutz-Jeghers, hereditary pancreatitis, familial atypical multiple melanoma (FAMM), familial breast cancer 2, and hereditary nonpolyposis colorectal cancer syndromes (3, 12, 13). Several acquired gene mutations have also been identified in sporadic pancreatic tumors (12, 13).
5-Fluorouracil (5-FU) has been extensively used for treatment of advanced pancreatic cancer, and gemcitabine, a deoxycytidine analog (or antimetabolite), has now partially replaced 5-FU for pancreatic cancer chemotherapies. Several other drugs for treatment of pancreatic cancer are also being studied in clinical trials and these include other “antimetabolites”, taxanes, topoisomerase I inhibitors, and various combinations of these drugs as well as other novel mechanism-based agents (14–20). Research in this laboratory has identified a series of 1,1-bis(3-indolyl)-1-(p-substituted phenyl)methanes (C-DIMs) which are being developed for treatment of various cancers including pancreatic cancer (21–28). The activities of these compounds are structure-dependent. For example, the p-trifluromethylphenyl, p-t-butylphenyl and p-biphenyl derivatives activate peroxisome proliferator-activated receptor γ (PPARγ), whereas the unsubstituted p-phenyl and p-methoxyphenyl C-DIMs activate the orphan receptor nerve growth factor-induced Bα (NGF-Bα, Nur77) (21). In addition, C-DIMs also activate receptor-independent proapoptotic/growth inhibitory pathways including endoplasmic reticulum (ER) stress (23, 25).
In this study, we investigated the structure-dependent activation of ER stress in pancreatic cancer cells by two C-DIMs that do not activate either PPARγ or Nur77, namely the p-bromophenyl (DIM-C-pPhBr) and p-fluorophenyl (DIM-C-pPhF) and related isomers and analogs. Both DIM-C-pPhBr and DIM-C-pPhF induce apoptosis in pancreatic cancer cells and this is linked to activation of ER stress and the c-jun N-terminal kinase (JNK) pathway resulting in the induction of CCAAT/enhancer-binding protein homologous protein (CHOP), death receptor 5 (DR5), and the extrinsic apoptotic pathway. These results, coupled with the observed in vivo antitumorigenic activity of DIM-C-pPhBr suggest that C-DIM compounds alone or in combination with other drugs represent a promising new treatment modality for pancreatic cancer.
MATERIALS AND METHODS
Reagents and antibodies
1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes were synthesized in this laboratory from the condensation of indole or substituted indole plus a substituted benzaldehyde derivative and confirmed by gas chromatography-mass spectrometry as previously described (27). Antibodies for PARP (sc-7150), CHOP (sc-793), β-tubulin (sc-33749), and GRP78 (sc-13968) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for cleaved PARP (#9541), cleaved caspase-8 (#9496), cleaved caspase-3 (#9664), phospho-JNK (#9251), JNK (#9252), phospho-c-Jun (#9161), c-Jun (#9162), phosphor-ASK1 (#3765), ASK1(#3762), phospho-MKK4 (#9156), MKK4 (#9152), and DR5 (#3696) were obtained from Cell Signaling Technology (Danvers, MA).
Cell culture and treatment
Panc-1, Panc-28, MiaPaCa-2, and BxPC-3 cells (American Type Culture Collection, Manassas, VA) and L3.6pl cells (kindly provided by Dr. Isaiah Fidler, M. D. Anderson Cancer Center, Houston, TX) were maintained in DMEM/Ham’s F-12 (Sigma) supplemented with 0.22% sodium bicarbonate, 5% fetal bovine serum, and 1X antibiotic/antimycotic solution (Sigma), in a humid atmosphere of 5% CO2. Cells were trypsinized and counted with a Z1 Dual Coulter Particle Counter (Beckman Coulter, Fullerton, CA). Equal number of cells were seeded in 96-well, 6-well, or 12-well plates and allowed to attach overnight. Cells were treated with vehicle (DMSO) or compounds of various concentrations diluted in DMEM/Ham’s F-12 without phenol red and supplemented with 2.5% charcoal-stripped FBS.
WST-1 cell proliferation assay
Panc-1 and Panc-28 cells were seeded in 96-well plates at a density of 2 × 103 per well and then treated with DMSO or various compounds. The WST-1 assay was done according to the manufacturer’s instructions. The absorbance of each sample was analyzed with FLUOstar OPTIMA Elisa reader (Offenburg, Germany) at 450 nm with 620 nm as the reference wavelength. All experiments were determined in triplicate and repeated at least two times and results are expressed as means ± SD for each treatment group.
Western blot analysis
Whole-cell lysates were extracted with high-salt lysis buffer [50 mmol/L HEPES, 0.5 mol/L sodium chloride, 1.5 mmol/L magnesium chloride, 1 mmol/L EGTA, 10% (v/v) glycerol, 1% Triton X-100, and 5 μL/mL of Protease Inhibitor Cocktail (Sigma)] and quantified with Bio-Rad Protein Assay (Hercules, CA). Equal amounts of protein from each treatment group were separated on an SDS-polyacrylamide gel and then transferred to polyvinylidene difluoride membrane (Immobilon-P, Millipore Corp., Bedford, MA). The polyvinylidene difluoride membrane was then blocked with 5% milk in TBS-T (1.576 g/L Tris, 8.776 g/L sodium chloride, 0.5 mL/L Tween 20) and probed with primary antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibodies as indicated.
Annexin V staining
Vybrant Apoptosis Assay Kit (V13241) was purchased from Molecular Probes (Eugene, OR). Panc-1 cells were seeded on Lab-Tek Chambered coverglass and allowed to attach overnight. After treatment with the desired compounds, cells were washed with cold PBS twice and incubated with Annexin V conjugate and propidium iodide for 15 min according to the manufacturer’s instruction. The cells were then washed with the Annexin-binding buffer twice and detected for fluorescence with Zeiss LSM 510 confocal microscope (Germany).
Animals and orthotopic implantation of tumor cells
Male athymic nude mice (NCI-nu) were purchased from the Animal Production Area of the National Cancer Institute, Frederick Cancer Research and Development Center (Frederick, MD). The mice were housed and maintained under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the United States Department of Agriculture, United States Department of Health and Human Services, and the NIH. The mice were used in accordance with institutional guidelines when they were 8–12 wk old.
To produce tumors, L3.6pl cells were harvested from subconfluent cultures by a brief exposure to 0.25% trypsin and 0.02% EDTA. Trypsinization was stopped with medium containing 10% fetal bovine serum, and the cells were washed once in serum-free medium and resuspended in HBSS. Only suspensions consisting of single cells with >90% viability were used for the injections. Injection of cells into the pancreas was performed as described previously (29, 30). Seven days after implantation of tumor cells into the pancreas of each mouse, 5 mice were killed to confirm the presence of tumor lesions. Mice were randomized (5 per group) to receive by oral gavage control (corn oil) or 25 mg/kg/d DIM-C-pPhBr. Treatments were continued for 4 wk and the mice were sacrificed on day 35 and subjected to necropsy. Tumor volumes were calculated by using the following formula: 0.5 × (length) × (width)2. The size and weight of the primary pancreatic tumors were recorded. For immunohistochemistry and histological staining procedures, tumor tissues were fixed in formalin and embedded in paraffin. Organ and body weights and histopathology on these tissues were determined.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay and histologic studies
For H&E staining and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay, tumor tissue was fixed in formalin and embedded in paraffin, one section was processed for H&E staining, and the others were used for TUNEL assay. TUNEL staining was carried out using DeadEnd Colorimetric TUNEL System (Promega). Paraffin-embedded sections (4–6 μM thick) were processed per manufacturer’s protocol. Briefly, sections were deparaffinized in xylene and then treated with a graded series of alcohol [100%, 95%, 85%, 70%, and 50% ethanol (v/v) in double-distilled water] and rehydrated in PBS (pH 7.5). Tissues were then treated with proteinase K solution for permeabilization and then refixed with 4% paraformaldehyde solution. Slides were then treated with recombinant terminal deoxynucleotidyl transferase reaction mix and incubated at 37°C for 1 hr. Reaction was terminated by immersing the slides in 2x SSC solutions for 15 min at room temperature. After blocking the endogenous peroxidases activity (by 0.3% hydrogen peroxide), slides were washed with PBS and then incubated with streptavidin horseradish peroxidase solution for 30 min at room temperature. After washing, slides were incubated with 3,3′-diaminobenzidine (substrate) solution until a light brown background appears (10 min) and then rinsed several times in deionized water. After mounting, slides were observed by light microscope.
RESULTS
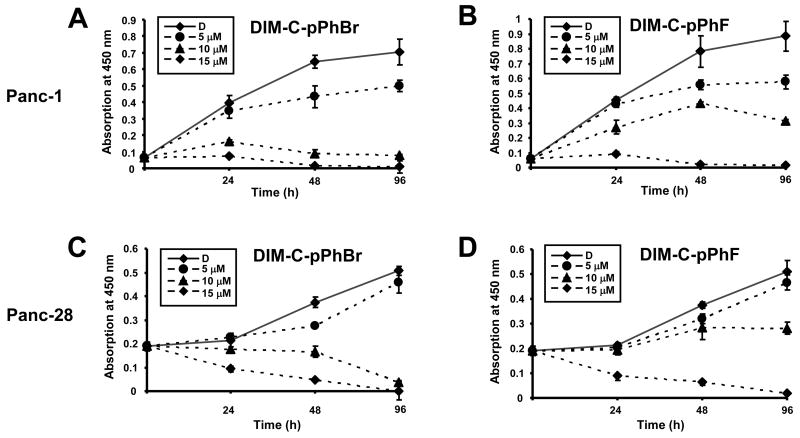
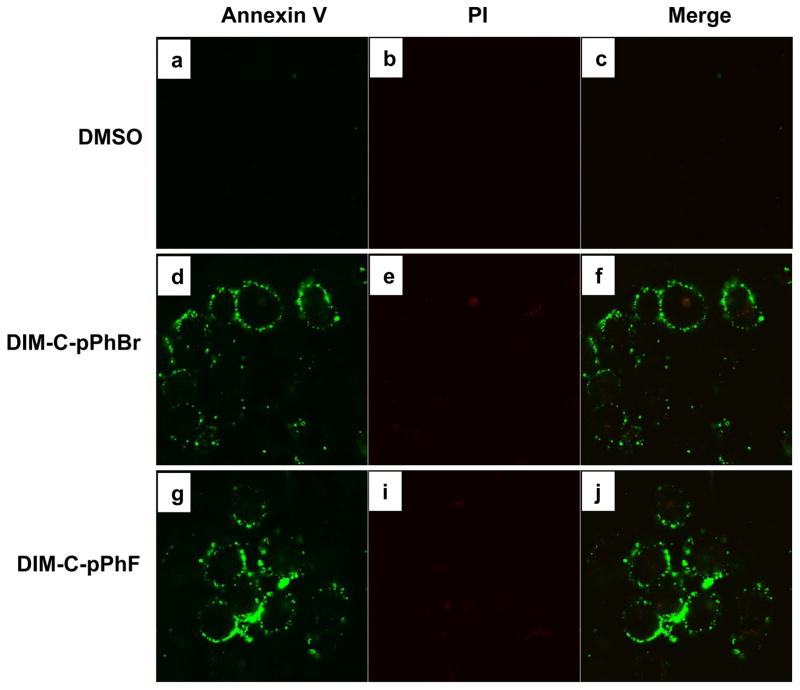
Previous studies in this laboratory showed that DIM-C-pPhBr and DIM-C-pPhF are cytotoxic to cancer cells but do not activate PPARγ or Nur77 (21, 24, 27). Results in Figures 1A and 1B illustrate that both DIM-C-pPhBr and DIM-C-pPhF inhibited Panc-1 cell proliferation with IC50 values of 7.2 μM and 9.3 μM, respectively. Similar results were obtained in Panc-28 cells (Figs. 1C and 1D); however, the DIM-C-pPhF analog was slightly less cytotoxic in Panc-28 cells. Higher concentrations of both compounds (10 and 15 μM) caused a considerable increase in floating cells consistent with activation of cell death pathways. Figure 2 summarizes the effects of DMSO, DIM-C-pPhBr and DIM-C-pPhF on Annexin V and propidium iodine (PI) staining in Panc-1 cells after treatment for 18 hr. Positive Annexin V staining indicates the transfer of phosphatidylserine from the inner to outer leaflet of the plasma membrane while negative PI staining shows membrane integrity. The merged staining results show that both compounds induced apoptosis in this cell line.
Figure 1. Growth inhibitory effects of C-DIMs.
Effects of DIM-C-pPhBr and DIM-C-pPhBr on Panc-1 (A, B) and Panc-28 (C, D) cell growth. Panc-1 or Panc-28 cells were treated with 5, 10 or 15 μM C-DIM compounds or control vehicle DMSO (D), and cell growth was determined every 24 hr as described in the Materials and Methods. Significant (p<0.05) growth inhibition was observed at all concentrations of DIM-C-pPhBr and 10 and 15 μM DIM-C-pPhF after treatment 24 hr. Results are means ± SD for at least 3 replicate experiments for each treatment group.
Figure 2. Induction of apoptosis by DIM-C-pPhBr and DIM-C-pPhF.
Panc-1 cells were treated with DMSO (solvent control), 15 μM DIM-C-pPhBr or DIM-C-pPhF for 18 hr and stained for Annexin V (a, d, g) and propidium iodide (b, e, h) as described in the Materials and Methods. Both DIM-C-pPhBr and DIM-C-pPhF induced the translocation of phosphatidylserine from the inner to outer leaflet of the plasma membrane which in combination with negative PI staining as seen in the merged images (c, f, i) indicates the induction of apoptosis.
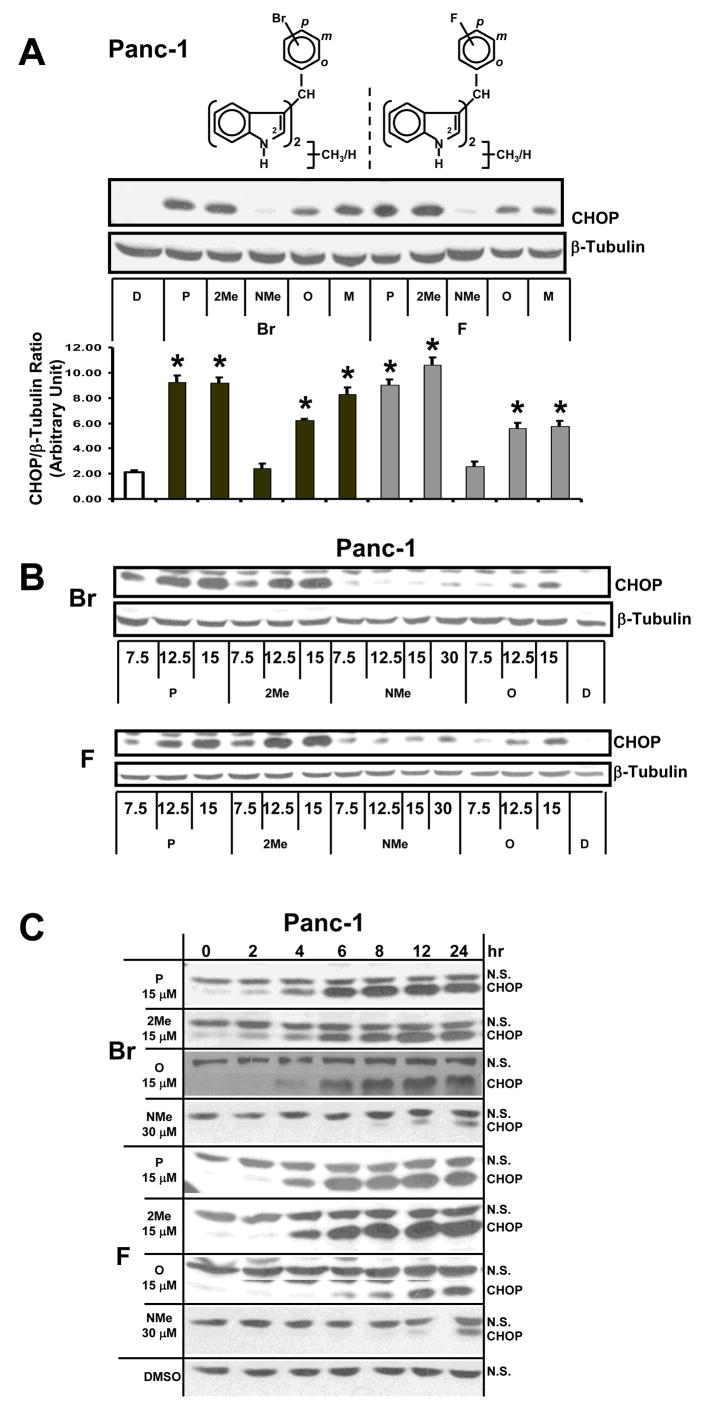
DIMs and C-DIMs activate the extrinsic apoptosis pathway in colon, pancreatic and ovarian cancer cells though induction of CHOP and CHOP-dependent activation of DR5 (23, 25, 28). Using CHOP as an endpoint assay, we investigated the induction of this protein by DIM-C-pPhBr (P) and the corresponding ortho-(O) and meta-(M) bromophenyl C-DIM isomers as well as the 2-methyl (2-Me) and N-methyl (NMe) derivatives of DIM-C-pPhBr (Fig. 3A). Panc-1 cells were treated with 15 μM concentrations of these compounds for 24 hr, and a significant induction of CHOP was observed in cells after treatment with all compounds except the NMe derivatives of DIM-C-pPhBr. The same series of fluoro-substituted C-DIM analogs was also investigated and, with the exception of the NMe derivative of DIM-C-pPhF, the remaining compounds all induced CHOP in Panc-1 cells. The para-F/Br analogs and the 2-Me derivatives exhibited the highest activity, whereas 15 μM concentrations of the NMe derivatives did not significantly induce expression of CHOP protein. These structure-dependent effects on CHOP expression show the importance of a free indole N group for maximum activity.
Figure 3. Structure-dependent activation of CHOP by DIM-C-pPhBr and DIM-C-pPhF analogs.
Structure-(A), dose-(B) and time-(C) dependent activation of CHOP in Panc-1 cancer cells. Cells were treated with either DMSO (D) or 15/30 μM concentrations of the C-DIM derivatives as indicated for 12 hr (A, B) or different periods of time (C), and changes in protein expression were determined by Western blot analysis of whole cell lysates as described in the Materials and Methods. Protein levels were measured with Image J and normalized to β-tubulin. All experiments were carried out at least 3 times and results in (A) are means ± SD for 3 replicate determinations and significant (p< 0.5) induction of CHOP is indicated by an asterisk.
Figure 3B illustrates the concentration-dependent effects (7.5, 12.5 and 15 μM) of some of the same bromo- and fluoro-substituted analogs, and the results show that DIM-C-pPhBr/DIM-C-pPhF (P) and the 2-methyl derivatives induced CHOP at the lowest concentration. Their corresponding ortho-fluoro/bromo-substituted analogs were less active and minimal induction was observed for the NMe derivatives. In these experiments, Panc-1 cells were treated for 24 hr. The time course induction of CHOP in Panc-1 cells (Fig. 3C) by the same set of compounds showed that DIM-C-pPhBr and the 2-methyl analog induced CHOP within 2 hr after treatment. Maximal induction was observed within 6–8 hr and this was maintained for up to 24 hr. In contrast, the ortho isomer (DIM-C-oPhBr) exhibited a delay in the induction of CHOP (4–6 hr) and induction by the NMe derivative was only observed at later time points and never induced the levels of CHOP protein observed for the C-DIMs containing a free indole group. Moreover, in this study, the concentration of the NMe analog was increased to 30 μM in order to see induction of CHOP since lower concentrations did not induce its expression (Figs. 3A and 3B). The DIM-C-pPhF series of compounds were somewhat less potent as inducers of CHOP than the bromo analogs; however the structure-dependent differences in the time-course induction of CHOP in Panc-1 cells by the fluoro-substituted analogs were comparable to those observed for the bromo compounds.
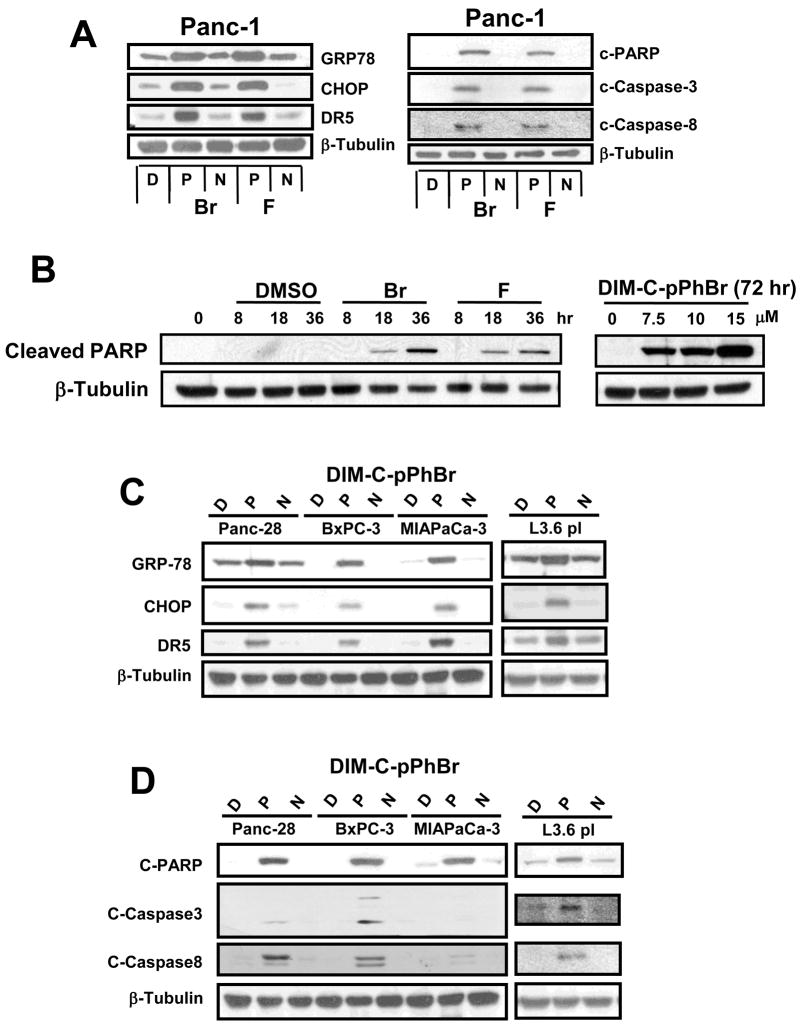
Previous studies with C-DIMs have demonstrated their activation of ER stress in pancreatic and ovarian cancer cells (23, 25) but not in colon cancer cell lines (28). Results in Figure 4A show that, in Panc-1 cells, 15 μM DIM-C-pPhBr and DIM-C-pPhF (P) induced expression of the ER stress protein GRP78 and this was accompanied by enhanced expression of both CHOP and DR5. In contrast, treatment with DMSO (D) and the NMe derivates (N) did not affect expression of GRP78, CHOP or DR5 proteins in Panc-1 cells. ER stress-dependent activation of DR5 by DIM-C-pPhBr and DIM-C-pPhF in Panc-1 cells was also accompanied by activation of caspases 8 and 3 and induction of caspase-dependent PARP cleavage consistent with activation of the extrinsic apoptotic pathway. The NMe (N) analogs were inactive as inducers of apoptosis and served as a negative control for these effects. DIM-C-pPhBr induced PARP cleavage at concentrations from 7.5 – 15 μM, and results of a time course study showed that 15 μM DIM-C-pPhBr and DIM-C-pPhF induced PARP cleavage within 8 hr after treatment (Fig. 4B). Using a similar approach, Panc-28, BxPC-3, L3.6pl and MIAPaCa-2 pancreatic cancer cells were treated with DMSO, 15 μM DIM-C-pPhBr and DIM-C-pPhF or their NMe analogs (N) for 24 hr, and the structure-dependent induction of GRP78, CHOP and DR5 (Fig. 4C), caspase activation and PARP cleavage (Fig. 4D) were also observed in these pancreatic cancer cell lines. This suggests that treatment with DIM-C-pPhBr, DIM-C-pPhF and related compounds induces comparable ER stress-dependent activation of apoptosis in pancreatic cancer cells and this is consistent with previous studies in Panc-1 and ovarian cancer cells which showed that PPARγ-active C-DIM analogs (23, 25) also induce ER stress that is receptor-independent.
Figure 4. Activation of ER stress and pro-apoptotic pathways by C-DIM compounds in pancreatic cancer cells.
(A) Induction of ER stress and apoptosis in Panc-1 cells. Panc-1 cells were treated with DMSO (D), 15 μM DIM-C-pPhBr or DIM-C-pPhF (P) or their corresponding N-methyl analogs (N) for 24 hr, and whole cell lysates were analyzed by western blots as described in the Materials and Methods. (B) Concentration- and time-dependent induction of PARP cleavage. Panc1 cells were treated with different concentrations of DIM-C-pPhBr (for 24 hr) or treated with DIM-C-pPhBr and DIM-C-pPhF for different times. Whole cell lysates were analyzed for cleaved PARP by Western blots as described in the Materials and Methods. Induction of ER stress (C) and apoptosis (D) by DIM-C-pPhBr and related compound in pancreatic cancer cells. Panc-28, BxPC3, MIAPaCa-3 and L3.6pl cells were treated and analyzed by western blots essentially the same as described in A/B above. β-Tubulin served as a loading control for all experiments and the blots illustrated in A–D were observed in 2 or more separate experiments.
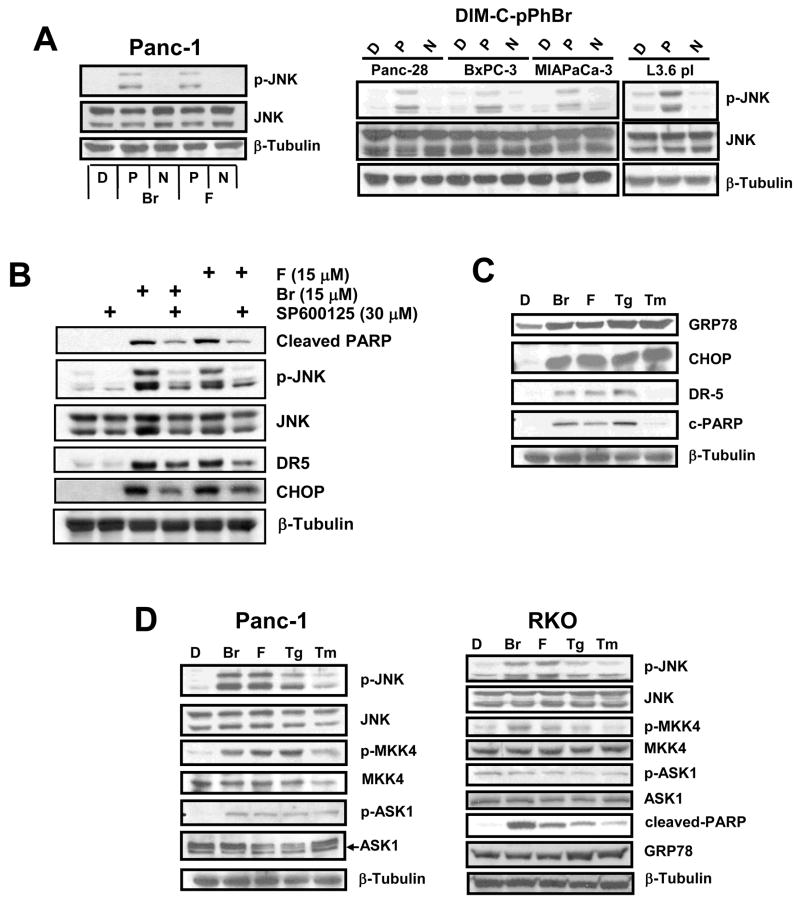
C-DIMs also induce JNK phosphorylation in ovarian (25) and colon (28) cancer cells and in the latter cell line, this response was ER stress-independent. Therefore, we investigated the possible induction of JNK phosphorylation in Panc-1 cells by 15 μM DIM-C-pPhBr/DIM-C-pPhF and their NMe analogs, and the results (Fig. 5A) demonstrate that both C-DIMs but not their NMe analogs enhanced levels of phospho-JNK. Moreover, 15 μM DIM-C-pPHBr but not the NMe analog also induced phospho-JNK in Panc-28, BxPC-3, MiaPaCa-2 and L3.6pl pancreatic cancer cell lines (Fig. 5A), suggesting that this may be a common C-DIM-induced response in pancreatic cancer cells. The effects of C-DIM-induced JNK phosphorylation on downstream responses were determined in Panc1 cells treated with DIM-C-pPhBr and DIM-C-pPhF alone or in combination with the JNK inhibitor SP60015. C-DIM-induced JNK phosphorylation, CHOP and DR5 induction, and PARP cleavage were all inhibited by SP600125 (Fig. 5B). We also investigated the comparative induction of ER stress by DIM-C-pPhBr and DIM-C-pPhF and the classical ER stress inducers thapsigargin (Tg) and tunicamycin (Tm) in Panc-1 cells (Fig. 5C). Results show that 15 μM DIM-C-pPhBr and DIM-C-pPhF and 5 μM Tg induced GRP78, CHOP, DR5 and PARP cleavage. Although 5 μg/ml Tm induced comparable levels of GRP78 and CHOP proteins, levels of DR5 and PARP cleavage were lower than observed for the other three compounds. This was probably due to the concentration of Tm since higher concentrations enhanced DR5 levels and PARP cleavage (data not shown). Figure 5D compares the effects of the same compounds in Panc-1 and RKO (colon) cancer cells on the induction of phospho-JNK and the upstream kinases MKK4 and ASK1 that are associated with ER stress-dependent activation of this pathway through inostiol-requiring enzyme 1 (IRE1) (31). In Panc-1 cells, DIM-C-pPhBr, DIM-C-pPhF, Tg and Tm enhance phospho-JNK expression and this is accompanied by activation of the upstream kinases MKK4 and ASK-1 in which their phosphorylation was also increased. The comparative effects of DIM-C-pPhBr/DIM-C-pPhF, Tg and Tm in activation of markers of ER stress in RKO colon cancer cells illustrates subtle but consistent cell context and compound-dependent difference. DIM-C-pPhBr and DIM-C-pPhF did not activate the ER stress marker GRP-78 in RKO cells but induced apoptosis and JNK and MKK4 but not ASK-1 phosphorylation. In constrast, Tg and Tm induced phosphorylation of JNK and proapoptotic responses in RKO cells and this was accompanied by induction of the ER-stress protein GRP78. Based on the induction of this stress protein, DIM-C-pPhF and DIM-C-pPhBr induce ER stress-dependent apoptosis in pancreatic and ER stress-independent apoptosis in colon cancer cells.
Figure 5. Activation of stress-dependent JNK and proapoptotic pathway.
(A) Activation of JNK pathway in Panc-1 and other pancreatic cancer cells. Panc-1, Panc-28, BxPC3, MIAPaCa-3 and L3.6pl cells were treated with DMSO (D), 15 μM DIM-C-pPhBr and DIM-C-pPhF (P) or the N-methyl analogs (N) for 24 hr, and whole cell lysates were analyzed by western blots as described in the Materials and Methods. (B) Effects of SP600125. Panc1 cells were treated with DMSO, 30 μM SP600125, 15 μM DIM-C-pPhBr or DIM-C-pPhF alone or in combination with SP600125 for 24 hr. Whole cell lysates were analyzed by Western blots as described in the Materials and Methods. Comparative activation of apoptosis and stress in Panc-1 cells (C) and activation of kinases in Panc-1 and RKO cells (D). Cells were treated with DMSO (D), 15 μM DIM-C-pPhBr (Br), 15 μM DIM-C-pPhF (F), 5 μM Tg or 5 μg/ml Tm for 24 hr, and whole cell lysates were analyzed by western blots as described in the Materials and Methods. β-Tubulin served as a loading control and blots illustrated in A–D were observed in 2 or more experiments.
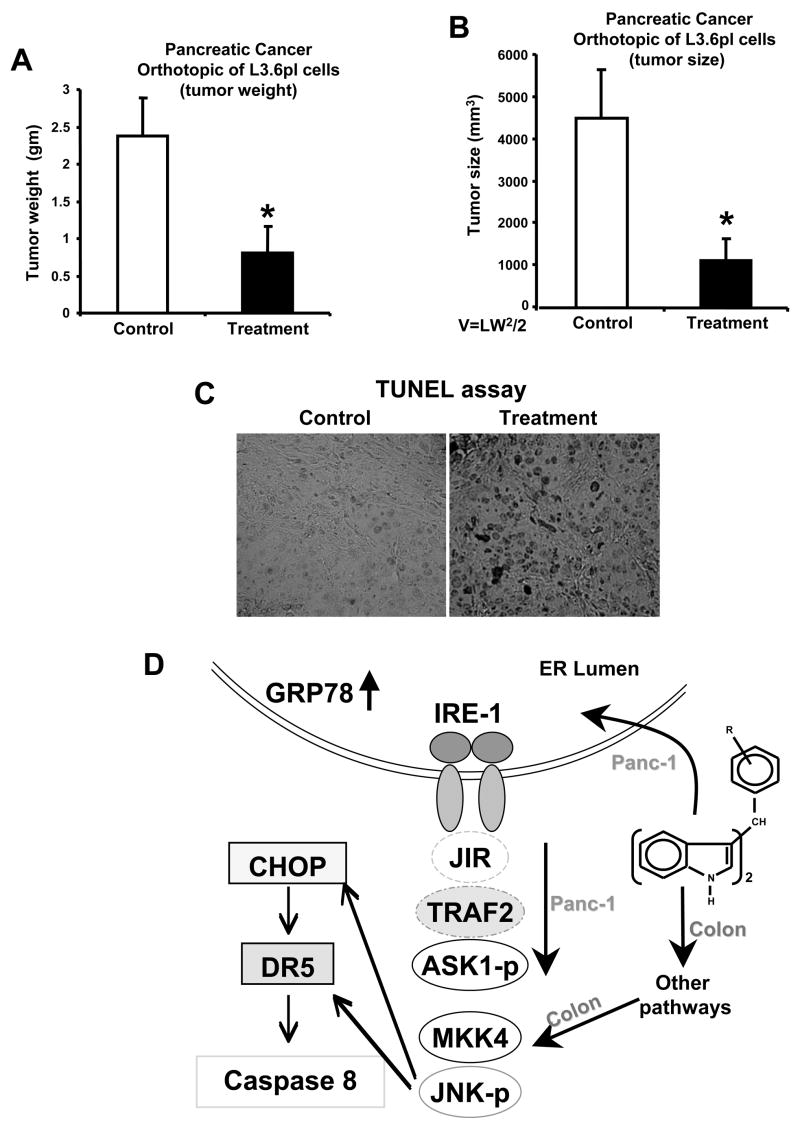
The in vivo anticarcinogenic activity of DIM-C-pPhBr was determined in athymic nude mice in which the L3.6pl pancreatic cancer cells were injected directly into the pancreas. In this orthotopic mouse model for pancreatic cancer, DIM-C-pPhBr (25 mg/kg/d) was administered 5 days after injecting the cells and continued for 4 weeks. Results show that DIM-C-pPhBr significantly decreased tumor size (Fig. 6A) and weight (Fig. 6B). No changes in organ or body weights were observed (data now shown) and a comparison of tumors from treated vs. untreated animals showed increased TUNEL staining in tumors from mice treated with DIM-C-pPhBr (Fig. 6C). This is consistent with the observed proapoptotic effects of this compound in pancreatic cancer cell lines.
Figure 6. DIM-C-pPhBr inhibits tumor growth in an orthotopic model for pancreatic cancer.
Tumor weights (A) and volumes (B). Male athymic nude mice bearing L3.6 pl cell xenografts were treated with DIM-C-pPhBr (25 mg/kg/d) or control (corn oil) for 4 weeks, sacrificed for necropsy and tumor weight and volumes were determined as described in the Materials and Methods. Asterisk indicates significant (p<0.05) decrease in tumor weight and volume in animals treated with DIM-C-pPhBr. (C) TUNEL assay. Fixed tumor sections from DIM-C-pPhBr treated animals exhibited increased apoptosis in the TUNEL assay as described in the Materials and Methods. (D) Model for C-DIM induced responses. The model depicts activation of ER stress-dependent and independent activation of JNK in pancreatic and colon cancer cells respectively and also stress-dependent activation of DR5 in pancreatic cancer cells.
DISCUSSION
The unfolded protein response (UPR) or ER stress is an important pathway that responds to ER dysfunction due to accumulation of mis-folded proteins and other stressors including inappropriate nutrients, abnormal calcium levels, and induced alterations of cellular homeostasis (32). The ER stress response involves coordinate induction of chaperone protein synthesis, activation of an ER-associated degradation pathway to remove proteins from the ER, and decreased protein translation to limit ER processing of proteins. Failure to respond to ER stress results in activation of cell death pathways and removal of severely damaged cells. There is evidence that tumors which grow under hypoxic conditions have an elevated ER stress response, and GRP78, an important marker for ER stress, is upregulated in many cancers and is a prognostic factor for cancer recurrence and poor rates of survival (33). It has been suggested that since GRP78 has antiapoptotic properties and contributes to cancer progression, metastasis agents that target this protein may be effective for cancer chemotherapy (33).
In contrast, drugs that activate ER stress and GRP78 expression are also being developed for clinical treatment of cancer alone or in combination with other therapies (34). For example, bortezomib (PS-341, Velcade) alone induces ER stress and apoptosis in pancreatic and human non-small lung cancer cells, and also sensitizes cells to the apoptotic effects of other chemotherapeutic drugs and the death receptor ligand TRAIL which is also being developed for treatment of some tumors (34). Several reports show that other ER stress inducers sensitize cancer cells and tumors to TRAIL-induced apoptosis and this combination is dependent, in part, on upregulation of DR5 and/or decreased expression of the cellular FLICE inhibitory protein (c-FLIP) (34–39).
Previous studies show that in several different cancer cell lines, 20 μM DIM alone does not induce activation of caspase 8 or apoptosis but enhances the activity of TRAIL by downregulation of C-FLIP (36). However, in Panc-1 and Panc-28 cells, 20 μM DIM induce DR5 and the extrinsic pathway of apoptosis (23, 25). Our recent studies in colon cancer cells show that DIM-C-pPhF and DIM-C-pPhBr induce CHOP, DR5 and apoptosis but this is not accompanied by induction of the ER stress marker GRP78 (28). The results suggest that induction of apoptosis in colon cancer cell is due to ER stress-independent activation of the JNK pathway. In contrast, PPARγ-active C-DIM compounds induce ER stress in ovarian and pancreatic cancer cells through enhanced expression of CHOP which in turn induces DR5 and activation of the extrinsic apoptotic pathway which is dependent on the induction of CHOP and activation of JNK (23, 25). In this study, we used DIM-C-pPhF and DIM-C-pPhBr, which are not PPARγ agonists, to further investigate the structure-dependent activation of ER stress and apoptosis in pancreatic cancer cells and to determine the in vivo potency of DIM-C-pPhBr as an anticancer agent.
Treatment of Panc-1 or Panc-28 cells with DIM-C-pPhBr or DIM-C-pPhF inhibited cell proliferation and induced apoptosis (Fig. 1 and 2). Structure-activity studies among a series of C-DIM analogs gave results (Fig. 3) which were similar to those observed in colon cancer cells (28). DIM-C-pPhBr, DIM-C-pPhF and their 2-methyl analogs (15 μM) were the most active inducers of CHOP in Panc-1 cells and CHOP expression was induced within 2–4 hr after treatment. There were structure-dependent differences among the ortho, meta and para DIM-C-PhBr and DIM-C-PhF isomers; however, the most striking differences were observed for the N-methyl analogs which induced minimal CHOP expression and only at higher concentrations (30 μM) (Fig. 3), indicating that maximal activity required a free indole group. The structure-dependent pattern of CHOP induction by DIM-C-pPhBr and DIM-C-pPhF was also observed for induction of DR5 and cleaved PARP, caspase-8 and caspase-3 in Panc-28, BxPC-3, L3.6pl and MIAPaCa-2 cells (Fig. 4). Moreover, in this study, we also showed that in Panc1 cells inhibition of C-DIM-induced JNK phosphorylation with SP600125 decreased the induction of CHOP and DR5 and PARP cleavage, indicating that JNK plays a major role in mediating ER stress responses.
The structure-dependent induction of CHOP, DR5 and PARP cleavage by DIM-C-pPhBr, DIM-C-pPhF and related compounds were comparable in colon and pancreatic cancer cells; however, there were important differences in their mechanism of action. The effects of DIM-C-pPhBr and DIM-C-pPhF in pancreatic cancer cells were similar to those observed for the classical ER stress inducers Tg and Tm. These compounds induce the classical ER stress markers GRP78 and also enhance expression of phospho-ASK-1 (Figs. 4 and 5) which is downstream from IRE-1 and is responsible for ER-stress-dependent activation of JNK (40). In contrast, DIM-C-pPhBr and DIM-C-pPhF did not enhance phosphorylation of ASK-1 in colon cancer cells (28) (Fig. 5D), whereas activation of MKK4, which is upstream from JNK, was increased in both colon and pancreatic cancer cells. Thus, in pancreatic cancer cells, DIM-C-pPhBr and DIM-C-pPhF induced JNK phosphorylation through the classical ER-stress pathway, whereas in colon cells JNK phosphorylation was ER stress-independent (Fig. 5D) and required activation of MKK4 through other pathways (Fig. 6D) which are currently being investigated.
We also determined the in vivo effects of DIM-C-pPhBr in an orthotopic model of pancreatic cancer in which L3.6pl pancreatic cells (29, 30) are injected directly into the pancreas. The results (Fig. 6) clearly show that DIM-C-pPhBr (25 mg/kg/day) inhibited pancreatic tumor growth and these effects were not accompanied by changes in body or organ weights or histopathology as previously observed for C-DIMs in other in vivo studies in mice (21, 24, 25). In addition, an increase in TUNEL staining was observed in tumors from mice treated with DIM-C-pPhBr compared to mice treated with the vehicle control, demonstrating activation of apoptotic cell death in vivo which complements results obtained after treatment of L3.6pl cells in culture with DIM-c-pPhBr (Fig. 4C and 4D).
In summary, this study shows that DIM-C-pPhBr and related compounds activate ER stress pathways in pancreatic cancer cells leading to upregulation of DR5 and induction of apoptosis. Previous studies show that activation of this pathway is an important element for overcoming TRAIL resistance (34–39); however, our results show that DIM-C-pPhBr alone is a highly effective inhibitor of pancreatic cell and tumor growth, and this compound and other C-DIMs exhibit minimal toxicity in in vivo mouse models (21, 24, 28). Current studies are focused on development of C-DIMs as a novel class of anticancer drugs for clinical treatment of pancreatic cancer either alone or in combination with other agents including TRAIL.
Acknowledgments
Financial Support: This research was supported by the National Institutes of Health (ES09106 and CA108718) and the Texas Agricultural Experiment Station.
References
- 1.American Cancer Society. Cancer facts and figures - 2006. Atlanta, GA: American Cancer Society; 2006. [Google Scholar]
- 2.Evans DB, Abbruzzese JL, Willett CG. Cancer of the pancreas. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott, Williams & Wilkins; 1997. pp. 1126–61. [Google Scholar]
- 3.Hruban RH. Pancreatic cancer: from genes to patient care. J Gastrointest Surg. 2001;5:583–7. doi: 10.1016/s1091-255x(01)80099-8. [DOI] [PubMed] [Google Scholar]
- 4.Li D. Molecular epidemiology of pancreatic cancer. Cancer J. 2001;7:259–65. [PubMed] [Google Scholar]
- 5.Gold EB, Goldin SB. Epidemiology of and risk factors for pancreatic cancer. Surg Oncol Clin N Am. 1998;7:67–91. [PubMed] [Google Scholar]
- 6.Weiderpass E, Partanen T, Kaaks R, et al. Occurrence, trends and environment etiology of pancreatic cancer. Scand J Work Environ Health. 1998;24:165–74. doi: 10.5271/sjweh.295. [DOI] [PubMed] [Google Scholar]
- 7.Silverman DT, Dunn JA, Hoover RN, et al. Cigarette smoking and pancreas cancer: a case-control study based on direct interviews. J Natl Cancer Inst. 1994;86:1510–6. doi: 10.1093/jnci/86.20.1510. [DOI] [PubMed] [Google Scholar]
- 8.Duell EJ, Holly EA, Bracci PM, et al. A population-based, case-control study of polymorphisms in carcinogen-metabolizing genes, smoking, and pancreatic adenocarcinoma risk. J Natl Cancer Inst. 2002;94:297–306. doi: 10.1093/jnci/94.4.297. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Abbruzzese JL, Friess H, et al. DNA adducts in human pancreatic tissues and their potential role in carcinogenesis. Cancer Res. 1998;58:38–41. [PubMed] [Google Scholar]
- 10.Anderson KE, Sinha R, Kulldorff M, et al. Meat intake and cooking techniques: associations with pancreatic cancer. Mutat Res. 2002;506–507:225–31. doi: 10.1016/s0027-5107(02)00169-0. [DOI] [PubMed] [Google Scholar]
- 11.Risch HA. Etiology of pancreatic cancer, with a hypothesis concerning the role of N-nitroso compounds and excess gastric acidity. J Natl Cancer Inst. 2003;95:948–60. doi: 10.1093/jnci/95.13.948. [DOI] [PubMed] [Google Scholar]
- 12.Klein AP, Hruban RH, Brune KA, Petersen GM, Goggins M. Familial pancreatic cancer. Cancer J. 2001;7:266–73. [PubMed] [Google Scholar]
- 13.Jaffee EM, Hruban RH, Canto M, Kern SE. Focus on pancreas cancer. Cancer Cell. 2002;2:25–8. doi: 10.1016/s1535-6108(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 14.Haller DG. Chemotherapy for advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2003;56:16–23. doi: 10.1016/s0360-3016(03)00448-6. [DOI] [PubMed] [Google Scholar]
- 15.McKenna S, Eatock M. The medical management of pancreatic cancer: a review. Oncologist. 2003;8:149–60. doi: 10.1634/theoncologist.8-2-149. [DOI] [PubMed] [Google Scholar]
- 16.Cohen SJ, Ho L, Ranganathan S, et al. Phase II and pharmacodynamic study of the farnesyltransferase inhibitor R115777 as initial therapy in patients with metastatic pancreatic adenocarcinoma. J Clin Oncol. 2003;21:1301–6. doi: 10.1200/JCO.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Moore M, Hamm J, Eisenberg P, et al. A comparison between gemcitabine and the matrix metalloproteinase inhibitor BAY12-9566 in patients with advanced pancreatic cancer. 2000:930. [Google Scholar]
- 18.Bramhall SR, Rosemurgy A, Brown PD, et al. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol. 2001;19:3447–55. doi: 10.1200/JCO.2001.19.15.3447. [DOI] [PubMed] [Google Scholar]
- 19.Abbruzzese JL, Rosenberg A, Xiong Q, et al. Phase II study of anti-epidermal growth factor receptor antibody cetuximab (IMC-C225) in combination with bemcitabine in patients with advanced pancreatic cancer. 2001:518. [Google Scholar]
- 20.Hotz HG, Reber HA, Hotz B, et al. Angiogenesis inhibitor TNP-470 reduces human pancreatic cancer growth. J Gastrointest Surg. 2001;5:131–8. doi: 10.1016/s1091-255x(01)80024-x. [DOI] [PubMed] [Google Scholar]
- 21.Cho SD, Yoon K, Chintharlapalli S, et al. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and independent pathways. Cancer Res. 2007;67:674–83. doi: 10.1158/0008-5472.CAN-06-2907. [DOI] [PubMed] [Google Scholar]
- 22.Chintharlapalli S, Papineni S, Safe SH. 1,1-Bis(3′-indolyl)-1-(p-substitutedphenyl)methanes inhibit growth, induce apoptosis, and decrease the androgen receptor in LNCaP prostate cancer cells through PPARγ-independent pathways. Mol Pharmacol. 2007;71:558–69. doi: 10.1124/mol.106.028696. [DOI] [PubMed] [Google Scholar]
- 23.Lei P, Abdelrahim M, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit ovarian cancer cell growth through peroxisome proliferator-activated receptor-dependent and independent pathways. Mol Cancer Ther. 2006;5:2324–36. doi: 10.1158/1535-7163.MCT-06-0184. [DOI] [PubMed] [Google Scholar]
- 24.Chintharlapalli S, Papineni S, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through PPARγ-dependent and PPARγ-independent pathways. Mol Cancer Ther. 2006;5:1362–70. doi: 10.1158/1535-7163.MCT-06-0002. [DOI] [PubMed] [Google Scholar]
- 25.Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S. 3,3′-Diindolylmethane (DIM) and derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis. 2006;27:717–28. doi: 10.1093/carcin/bgi270. [DOI] [PubMed] [Google Scholar]
- 26.Chintharlapalli S, Papineni S, Baek SJ, Liu S, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substitutedphenyl)methanes are peroxisome proliferator-activated receptor gamma agonists but decrease HCT-116 colon cancer cell survival through receptor-independent activation of early growth response-1 and NAG-1. Mol Pharmacol. 2005;68:1782–92. doi: 10.1124/mol.105.017046. [DOI] [PubMed] [Google Scholar]
- 27.Qin C, Morrow D, Stewart J, et al. A new class of peroxisome proliferator-activated receptor γ (PPARγ) agonists that inhibit growth of breast cancer cells: 1,1-bis(3′-indolyl)-1-(p-substitutedphenyl)methanes. Mol Cancer Therap. 2004;3:247–59. [PubMed] [Google Scholar]
- 28.Lei P, Abdelrahim M, Cho SD, et al. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through activation of c-Jun N-terminal kinase. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruns CJ, Harbison MT, Kuniyasu H, Eue I, Fidler IJ. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia. 1999;1:50–62. doi: 10.1038/sj.neo.7900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker CH, Solorzano CC, Fidler IJ. Blockade of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling for therapy of metastatic human pancreatic cancer. Cancer Res. 2002;62:1996–2003. [PubMed] [Google Scholar]
- 31.Nishitoh H, Matsuzawa A, Tobiume K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–55. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–80. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 33.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–9. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida T, Shiraishi T, Nakata S, et al. Proteasome inhibitor MG132 induces death receptor 5 through CCAAT/enhancer-binding protein homologous protein. Cancer Res. 2005;65:5662–7. doi: 10.1158/0008-5472.CAN-05-0693. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Yue P, Chen S, et al. The proteasome inhibitor PS-341 (bortezomib) up-regulates DR5 expression leading to induction of apoptosis and enhancement of TRAIL-induced apoptosis despite up-regulation of c-FLIP and survivin expression in human NSCLC cells. Cancer Res. 2007;67:4981–8. doi: 10.1158/0008-5472.CAN-06-4274. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Shen HM, Ong CN. Down-regulation of c-FLIP contributes to the sensitization effect of 3,3′-diindolylmethane on TRAIL-induced apoptosis in cancer cells. Mol Cancer Ther. 2005;4:1972–81. doi: 10.1158/1535-7163.MCT-05-0249. [DOI] [PubMed] [Google Scholar]
- 37.Higuchi H, Grambihler A, Canbay A, Bronk SF, Gores GJ. Bile acids up-regulate death receptor 5/TRAIL-receptor 2 expression via a c-Jun N-terminal kinase-dependent pathway involving Sp1. J Biol Chem. 2004;279:51–60. doi: 10.1074/jbc.M309476200. [DOI] [PubMed] [Google Scholar]
- 38.Joo JH, Liao G, Collins JB, Grissom SF, Jetten AM. Farnesol-induced apoptosis in human lung carcinoma cells is coupled to the endoplasmic reticulum stress response. Cancer Res. 2007;67:7929–36. doi: 10.1158/0008-5472.CAN-07-0931. [DOI] [PubMed] [Google Scholar]
- 39.Vaculova A, Hofmanova J, Andera L, Kozubik A. TRAIL and docosahexaenoic acid cooperate to induce HT-29 colon cancer cell death. Cancer Lett. 2005;229:43–8. doi: 10.1016/j.canlet.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]