Abstract
Glioblastoma multiforme is an invasive primary brain tumor, which evades the current standard treatments. The invasion of glioblastoma cells into healthy brain tissue partly depends on the proteolytic and non-proteolytic activities of the plasminogen activator system proteins, including the urokinase-type (uPA) plasminogen activator, plasminogen activator inhibitor 1 (PAI-1), and a receptor for uPA (uPAR). Here we demonstrate that sphingosine-1-phosphate (S1P), and the inflammatory mediator IL-1, increase the mRNA and protein expression of PAI-1 and uPAR, and enhance the invasion of U373 glioblastoma cells. Although IL-1 enhanced the expression of sphingosine kinase 1 (SphK1), the enzyme that produces S1P, downregulation of SphK1 had no effect on the IL-1-induced uPAR or PAI-1 mRNA expression, suggesting that these actions of IL-1 are independent of S1P production. Indeed, the S1P-induced mRNA expression of uPAR and PAI-1 was blocked by the S1P2 receptor antagonist JTE013, and by the downregulation of S1P2 using siRNA. Accordingly, the inhibition of MEK1/2 and Rho-kinase, two downstream signaling cascades activated by S1P2, blocked the activation of PAI-1 and uPAR mRNA expression by S1P. More importantly, the attachment of glioblastoma cells was inhibited by the addition of exogenous PAI-1 or siRNA to uPAR, while the invasion of glioblastoma cells induced by S1P or IL-1 correlated with their ability to enhance the expression of PAI-1 and uPAR. Collectively, these results indicate that S1P and IL-1 activate distinct pathways leading to the mRNA and protein expression of PAI-1 and uPAR, which are important for glioblastoma invasiveness.
INTRODUCTION
Glioblastoma multiforme (GBM) is one of the most common and most malignant tumors of the central nervous system (1, 2). Due to the invasive phenotype and diffuse penetration of GBM into normal regions of the brain, standard treatments such as surgery and radiotherapy are ineffective (3). It is for these reasons that patients diagnosed with GBM survive an average of 10 to 12 months (4). The invasion of glioblastoma cells requires the degradation of the extracellular matrix (ECM), which depends on the activation/inhibition of proteinases and their inhibitors, respectively. These processes include two main proteolytic systems: the plasminogen activator system (PAS), which controls the activation of the proteinase plasmin from inactive plasminogen, and the matrix metalloproteinases and their inhibitors (5–8).
In the brain, microglia produce inactive plasminogen, while astrocytes and glioma cells produce and secrete the components of the PAS. The PAS includes the plasminogen activators [urokinase-type (uPA), and the tissue-type (tPA)], their inhibitors [plasminogen activator inhibitors (PAI-1, -2, and -3) and protease nexin 1], and a receptor for uPA [urokinase plasminogen activator receptor (uPAR)] (5). The binding of uPA to uPAR leads to the localization of proteolytic activity to the cell surface, the enhancement of plasmin production, and the activation of several signaling pathways via uPAR (9, 10). Significantly, the expression of both uPA and uPAR has been correlated with the invasiveness and migration of several cancer cell lines (11). Moreover, the knockdown of uPAR expression in gliomas, using RNAi, leads to a significant decrease in cell invasion in both Matrigel and spheroid invasion assays (12) Furthermore, transfecting glioblastoma cells with antisense uPA disrupted actin cytoskeleton formation, reduced the amount of cell-bound uPA, and decreased cell migration (13). Surprisingly, high levels of PAI-1, which inhibit uPA, have been associated with highly invasive glioblastomas (14). Similarly, breast cancer patients with high levels of PAI-1 have a poor prognosis for survival (15). Together, these observations support the recent findings that PAI-1 binds to the uPA/uPAR/integrin complex, which promotes the internalization of this complex, and subsequent cell detachment and metastasis (16, 17).
The expression of the components of the PAS is regulated by growth factors and cytokines, such as epidermal growth factor (EGF) and interleukin-1 (IL-1), respectively (18, 24). Importantly, increased glioblastoma invasiveness and decreased patient survival correlates with PAI-1 and EGFR overexpression in tumors (18, 14). Moreover, inhibition of EGFR tyrosine kinase suppresses the invasion of glioblastoma cells, and decreases uPAR protein levels (19). Recently, we have described a novel signaling pathway of EGF-mediated up-regulation of PAI-1 expression in glioblastoma cells, which requires the sequential activation of c-Src, PKCδ, and sphingosine kinase 1 (SphK1) (20). SphK1 produces the potent lipid mediator S1P by phosphorylating sphingosine and its expression correlates with the poor survival of patients with GBM (21). S1P has been shown to be mitogenic for several glioma cell lines, and to enhance their motility and invasiveness (22). S1P acts through five G protein-coupled receptors (S1P1-S1P5) to activate multiple signaling pathways; however, it may also have intracellular actions through mechanisms that are still not understood.
IL-1 is a pro-inflammatory cytokine released by inflammatory cells in the brain due to inflammation caused by an injury or a growing tumor. In addition, glioblastomas have recently been shown to secrete substantial amounts of IL-1 (23), which incites the secretion of other cytokines, such as IL-6 and IL-8, as well as promotes glioblastoma proliferation (24). IL-1 has also been shown to stimulate the expression of several MMPs, tPA, uPA (24), yet nothing is known of its role in invasion of glioblastomas. Because IL-1 has been shown to activate SphK1 in other cell types (25), we investigated the effects of IL-1 and S1P on regulation of the components of the PAS system and invasion in glioblastoma cells. We show that S1P requires S1P2 to activate PAI-1 and uPAR mRNA expression, and although IL-1 markedly upregulated the mRNA expression of SphK1, it acts independently of S1P. Moreover, S1P and IL-1 cooperatively increase the invasion of U373 glioblastoma cells, as well as primary non-established GBM6 glioblastoma cells. Our data highlights the role of S1P and IL-1 in the regulation of PAI-1 and uPAR mRNA and protein expression, which are critical for glioblastoma invasiveness.
MATERIALS AND METHODS
Cell culture
Human glioblastoma U373-MG cells were obtained from the American Type Culture Collection (Herndon, VA), while primary human non-established glioblastoma GBM6 and GBM12 cells were kindly provided by Dr. C. David James (University of California, San Francisco, CA). The U373-TAM67 cells expressing the dominant-negative c-jun(TAM67) were described previously (26). Cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, antibiotics, sodium pyruvate, and non-essential amino acids. For the experiments, cells (5×105/well) were cultured in 6-well culture plates in the presence of 1% serum.
Cytokines and cell stimulation
Cells were stimulated with 10 ng/ml IL-1 (a gift from Immunex Corp., Seattle, WA) or the indicated amounts of S1P (Biomol Research Laboratories, Plymouth Meeting, PA), as described previously (20). For the inhibitor studies, cells were pretreated with 1 μM SP600125, 10 μM SB202190 (Sigma-Aldrich, St. Louis, MO), 1 μM JTE-013 (Tocris, Ellisville, MO), 0.3 μM VPC23019 (Avanti Polar Lipids, Alabaster, Al), 5 μM Y27632, 5 μM Gö6983 (EMD Biosciences, Inc., San Diego, CA), 10 μM LY294002, or 1 μM U0126 (Cell Signaling Technology, Inc., Beverly, MA) 1 hour prior to stimulation.
Northern blot analysis
Total RNA was prepared by phenol extraction exactly as described previously (27). 5 μg samples of RNA were subjected to formaldehyde gel electrophoresis using standard procedures (28) and transferred to Hybond-XL membranes (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) according to manufacturer’s instructions. The filters were prehybridized at 65°C for 3 h in 0.5 M sodium phosphate buffer pH 7.2, 7% SDS, and 1 mM EDTA. Then the filters were hybridized in the same solution with cDNA fragments of PAI-1, uPAR, and uPA labeled by random priming (29). After the hybridization, nonspecifically bound radioactivity was removed by four washes in 40 mM phosphate buffer, 1% SDS, and 1 mM EDTA at 65°C for 20 min each.
Quantitative PCR
uPAR, uPA, PAI-1, SphK1, and GAPDH mRNA levels were measured using TaqMan technology (Applied Biosystems, Foster City, CA) according to the supplier’s instructions. Briefly, one μg of total RNA was reverse-transcribed using the high capacity cDNA archive kit. Subsequently, the cDNA was diluted 10-fold (uPAR, uPA, PAI-1, and SphK1) or 100-fold (GAPDH). For real-time PCR, pre-mixed primer-probe sets and TaqMan Universal PCR Master Mix were purchased from Applied Biosystems, and the cDNA was amplified using an ABI 7900HT cycler. The data are means ± SD from three independent experiments done in triplicate, unless indicated otherwise in the figure legends.
Western blotting and antibodies
Cells (5×105) were lysed in 275 μl of 10 mM Tris pH 7.4, 150 mM sodium chloride, 1 mM EDTA, 0.5% NP-40, 1% Triton X-100, 1 mM sodium orthovanadate, 0.2 mM PMSF, and protease inhibitor cocktail (Roche, Mannheim, Germany). Media were collected and concentrated 6 fold using Microcon 3,000 MWCO filters (Millipore, Billerica, MA) according to the manufacturer’s instructions. The protein amounts were quantified using the BCA assay (Sigma-Aldrich, St. Louis, MO). Subsequently, equal amounts of the proteins (50 μg) were resolved using SDS-PAGE, and electroblotted onto nitrocellulose membranes (Schleicher & Schuell, Keene, NH). Polyclonal anti-ERK, anti-IkBα, anti-uPAR, and anti-PAI-1 antisera were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), while anti-phospho-ERK and anti-phospho-JNK were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Antigen-antibody complexes were visualized by enhanced chemiluminescence according to the manufacturer’s instructions (Pierce, Rockford, IL).
Downregulation with siRNA
S1P2, PAI-1, and uPAR expression was downregulated using SmartPool siRNA purchased from Dharmacon, Inc. (Lafayette, CO). The siRNA (100 nM) was transfected into the cells using Dharmafect 1 (Dharmacon) for 48 hours. Non-targeting Pool1 (Dharmacon) was used as a control.
Attachment assay
Cells were scraped off the dishes in PBS, and dissociated by pipetting. The cells were pelleted by centrifugation, counted, and resuspended in FBS-free DMEM at 100,000/200 μl. Subsequently, the cells were incubated with 1 μM PAI-1 (EMD Biosciences, Inc., San Diego, CA), or 1 μM BSA for 10 minutes at 37°C, and plated onto vitronectin-coated dishes. The cells were allowed to adhere for 10 minutes, the medium containing the non-attached cells was removed, centrifuged, and the cells were counted in the presence of trypan blue using a hemocytometer. The attachment assay performed after transfection with PAI-1 or uPAR siRNA, was performed as described above with minor differences (50,000 cells/200 μl and the cells were allowed to adhere for 5 minutes).
Invasion assay
The cells were stimulated with S1P (100 nM) and/or IL-1 (10 ng/ml) for 12 hours. The invasion of the cells was subsequently measured in a modified Boyden chamber, using polycarbonate filters (25 by 80 mm, 12 μm pore size) coated with Matrigel (BD, Franklin Lakes, NJ) (30). Both IL-1 and S1P were added to both the upper and lower chamber, while the cells were added to the upper chamber at 5×104 cells/well. After 7 h, nonmigratory cells on the upper membrane surface were mechanically removed, and the cells that traversed and spread on the lower surface of the filter were fixed and stained with Diff-Quik (Fisher Scientific, Pittsburgh, PA). The invading cells were counted with an inverted microscope and a 10×objective (31). Each data point is the average number of cells in five random fields, and is the mean ± standard deviation (SD) of three individual wells.
RESULTS
S1P and IL-1 regulate the expression of the PAS in glioblastoma cells
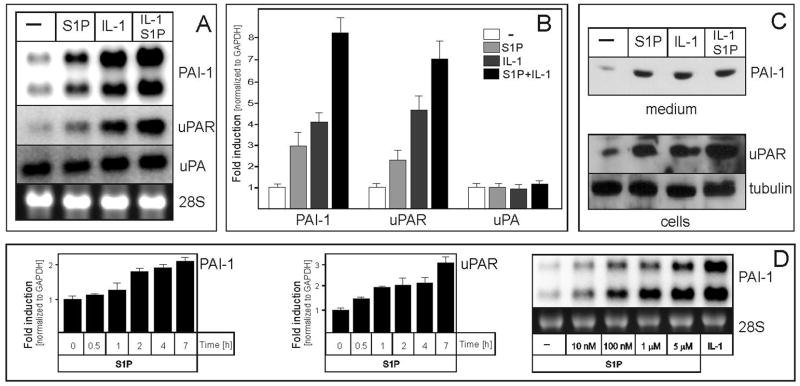
Previous studies have suggested that S1P might regulate invasion of glioblastoma cells (21, 22), yet the mechanism involved remained elusive. To this end, U373 glioblastoma cells were stimulated with both S1P and IL-1, since glioblastomas have recently been shown to secret substantial amounts of this neuroinflammatory cytokine (23), which also activates PAI-1 (32). Both S1P and IL-1 efficiently stimulated the mRNA expression of PAI-1 (both transcripts generated by the alternative cleavage and polyadenylation (33)) and uPAR, while the mRNA expression of uPA was unaffected (Fig. 1A and B). Accordingly, protein levels of PAI-1 secreted into the media, and uPAR present in the cells were upregulated by IL-1 and S1P (Fig. 1C). The effect of exogenous S1P on PAI-1 and uPAR mRNA expression was time- and dose-dependent, with activation observed as early as one hour after stimulation (Fig. 1D). The strongest activation of PAI-1 and uPAR mRNA expression was observed at 5 μM S1P, but the effect was significant at a concentration as low as 10 nM. These results indicate that S1P affects the expression of the PAS components in glioblastoma cells, and its effects are further enhanced by IL-1.
Fig. 1. S1P and IL-1 up-regulate the expression of uPAR and PAI-1 in glioblastoma cells.
U373 cells were treated with 10 ng/ml IL-1 or 5 μM S1P for 18 hours (A-C), stimulated with 5 μM S1P for the indicated times (D, left panels), or stimulated with the indicated amounts of S1P or 10 ng/ml IL-1 (D, right panel). (A, B, and D) RNA was isolated and subjected to analysis either by Northern blotting or qPCR using TaqMan technology, as described in the Materials and Methods. The lower panels in A and D show 28S RNA stained with ethidium bromide on the membrane as a loading control. The qPCR data were normalized to GAPDH mRNA, and expressed as a ratio to mRNA levels in untreated cells. (C) Media were collected, cell lysates prepared, and analyzed by Western blotting as described in the Materials and Methods. Tubulin was used as a loading control.
S1P activates PAI-1 and uPAR mRNA expression via multiple signaling pathways
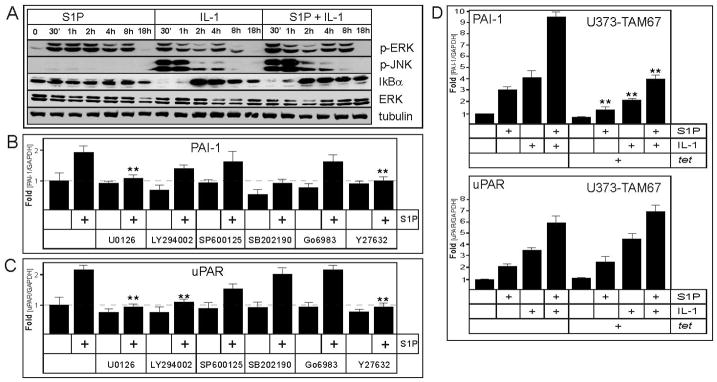
The additive activation of both PAI-1 and uPAR mRNA expression by the combination of S1P and IL-1 (Fig. 1B), suggests that they may regulate the mRNA expression of these genes by distinct pathways. Both S1P and IL-1 have been shown to activate various signaling pathways in many cell types, leading to the reprogramming of gene expression profiles (23, 22). In glioblastoma cells, both S1P and IL-1 induced rapid phosphorylation of ERK1/2; however, the phosphorylation of JNK and the efficient degradation of IkBα, which is important for the activation of NF-kB, were restricted to IL-1 (Fig. 2A). We employed several pharmacological inhibitors in order to identify the signaling pathway(s) regulating PAI-1 and uPAR mRNA expression in response to S1P in U373 cells. The inhibition of PI3K and p38 (using LY294002 and SB202190, respectively) diminished the intrinsic mRNA expression of PAI-1, but did not affect the relative fold activation by S1P (Fig. 2B). In contrast, the inhibition of MEK1/2 and Rho-kinase (using U0126 and Y27632, respectively) blocked PAI-1 and uPAR upregulation by S1P. In addition, the S1P-mediated uPAR activation was also inhibited by LY294002. These results suggest that diverse signaling molecules, including MEK/ERK and Rho-kinase, regulate the mRNA expression of PAI-1 and uPAR in response to S1P. Since both MEK/ERK and Rho-kinase can activate AP-1 and both PAI-1 and uPAR expression are regulated by AP-1 in response to various stimuli (34–36), we tested whether this potent transcription factor may be implicated in the regulation of PAI-1 and uPAR mRNA expression in response to S1P and IL-1. We utilized U373-TAM67 cells inducibly overexpressing dominant-negative c-jun(TAM67), which quenches the expression of AP-1-dependent genes (26). Both the intrinsic mRNA expression of PAI-1, its fold activation by both S1P and IL-1, and the marked increase by their combination were diminished, thus indicating that AP-1 is involved in the regulation of PAI-1 mRNA expression (Fig. 2D). In contrast, the mRNA expression of uPAR was not affected by c-jun(TAM67) overexpression (Fig. 2D). These results argue that while genes encoding PAI-1 and uPAR are regulated by signals initiated by S1P and IL-1, the precise mechanisms of their regulation are distinct.
Fig. 2. Signaling pathways involved in S1P-stimulateed expression of PAI-1 and uPAR.
(A) U373 cells were stimulated with 10 ng/ml IL-1 or 5 μM S1P for the indicated times. The cell lysates were prepared, and analyzed by Western blotting with anti-phospho-ERK, anti-phospho-JNK, anti-IkBα, anti-ERK. Blots were stripped and re-probed with anti-tubulin antibodies to insure equal loading and transfer. (B, C) U373 cells were pretreated with 1 μM U0126, 10 μM LY294002, 1 μM SP600125, 10 μM SB202190, 5 μM Go6983, or 5 μM Y27632 for 1 hour, and subsequently stimulated with 5 μM S1P for 18 hours. RNA was isolated, and the expression of PAI-1 (B) and uPAR (C) was analyzed by qPCR. The data were normalized to GAPDH mRNA, and expressed as a ratio to mRNA levels in untreated cells. Asterisks indicate statistically significant inhibition. (D) U373-TAM67 cells were preincubated with 1μg/ml of tetracycline for 24 hours, and then stimulated with 10 ng/ml IL-1 or 5 μM S1P for 18 hours, RNA was isolated and analyzed as in (B, C).
IL-1-activated PAI-1 and uPAR mRNA expression is SphK1-independent
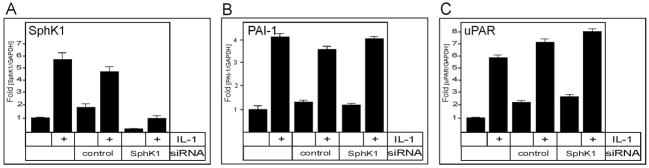
Since IL-1 rapidly activates (37) and upregulates the expression of SphK1 in several other cell types (38), we examined the effect of IL-1 on SphK1 mRNA expression in glioblastoma cells. The mRNA expression of SphK1 was significantly upregulated by IL-1 in U373 cells (Fig. 3A). Thus, the IL-1-induced PAI-1 and uPAR mRNA expression could potentially be due to elevated levels of S1P produced in response to IL-1. Because in many other cell types, S1P produced by agonist-stimulated SphK1 can activate cell surface S1P receptors in an autocrine/paracrine manner (39), this possibility was examined by downregulating the expression of SphK1 with specific siRNA in U373 cells. However, IL-1-induced mRNA expression of PAI-1 and uPAR was not affected by the downregulation of SphK1 (Fig. 3B and C), thus indicating that this activation is SphK1-independent.
Fig. 3. Upregulation of PAI-1 and uPAR mRNA by IL-1 is independent of SphK1.
U373 cells were transfected with control or SphK1 siRNA for 48 hours, and then stimulated with 10 ng/ml IL-1 for 18 hours, as indicated. RNA was isolated, and the expression of SphK1 (A), PAI-1 (B) and uPAR (C) mRNA was analyzed by qPCR. The data were normalized to GAPDH mRNA, and expressed as a ratio to mRNA levels in untreated cells.
S1P activates PAI-1 and uPAR mRNA expression via the S1P2 receptor
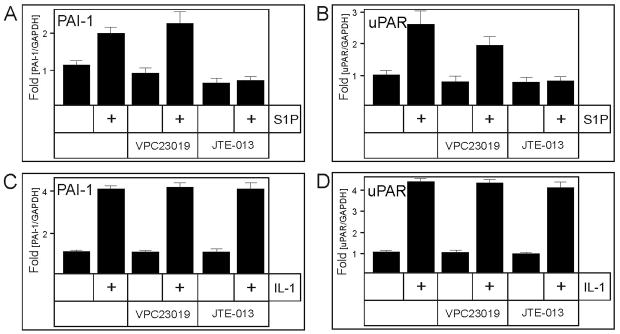
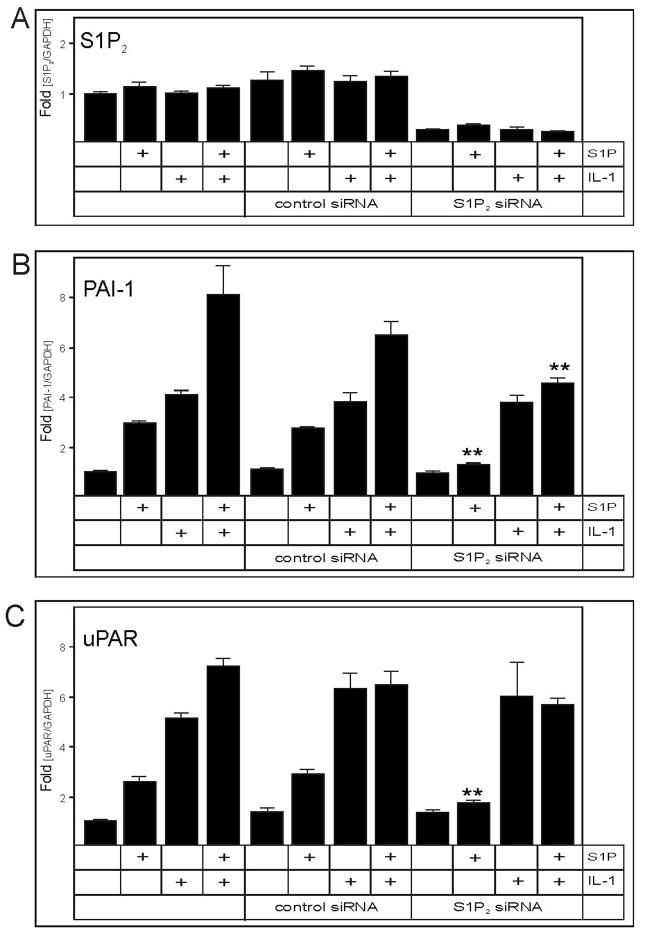
Given that most of the effects of S1P are mediated by binding to specific cell surface receptors (40–42, 22), of which S1P1–3 are expressed by U373 cells (22), it was of interest to identify which of the S1P receptors mediates the activation of PAI-1 and uPAR mRNA expression. To this end, we utilized both pharmacological and molecular approaches. First, antagonists VPC23019 and JTE-013 were used to block the S1P1 and S1P2 receptors, respectively. Inhibition of S1P2 abrogated the activation of PAI-1 and uPAR mRNA expression by S1P, while the inhibition of S1P1 was ineffective (Fig. 4A and B). Moreover, neither the inhibition of S1P1 nor S1P2 had any effect on the IL-1 activated mRNA expression of PAI-1 and uPAR (Fig. 4C and D), thus suggesting that the activation by IL-1 does not require these two receptors. Second, the mRNA expression of S1P2 was down-regulated by more than 80% using S1P2 specific siRNA (Fig. 5A). In agreement with the pharmacological inhibition of S1P2, the downregulation of S1P2 expression abolished the activation of PAI-1 and uPAR mRNA expression by S1P, without affecting the response to IL-1 (Fig. 5B and C). These results indicate that S1P specifically activates PAI-1 and uPAR mRNA expression via the S1P2 receptor.
Fig. 4. Inhibition of S1P2 blocks the activation of PAI-1 and uPAR by S1P.
U373 cells were pretreated with 0.3 μM VPC23019 or 1 μM JTE-013 for 30 min., and subsequently stimulated with 5 μM S1P (A, B) or 10 ng/ml IL-1 (C, D) for 18 hours. RNA was isolated, and the expression of PAI-1 (A, C) and uPAR (B, D) mRNA was analyzed by qPCR. The data were normalized to GAPDH mRNA, and expressed as a ratio to mRNA levels in untreated cells.
Fig. 5. Knock-down of S1P2 expression abrogates S1P-induced upregulation of PAI-1 and uPAR.
U373 cells were transfected with control or S1P2 siRNA for 48 hours. Then the cells were stimulated with 10 ng/ml IL-1 or 5 μM S1P as indicated, for 18 hours. RNA was isolated, and the expression of S1P2 (A), PAI-1 (B) and uPAR (C) mRNA was analyzed by qPCR. The data were normalized to GAPDH mRNA, and expressed as a ratio to mRNA levels in untreated cells. Asterisks indicate statistically significant inhibition.
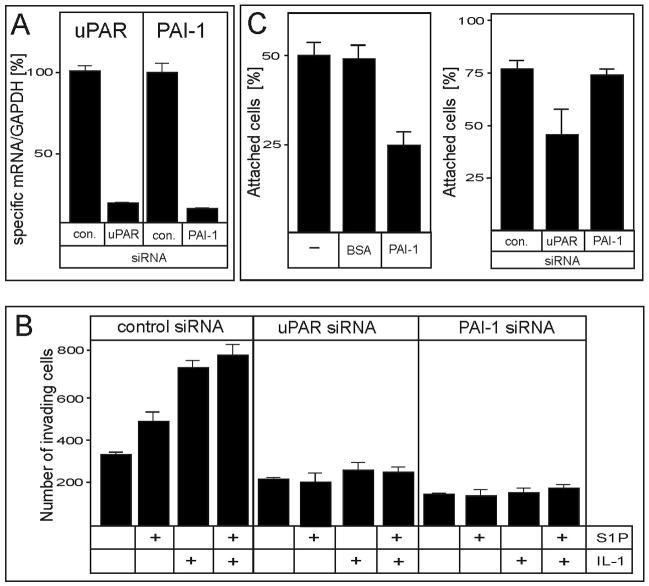
PAI-1 and uPAR are critical for the IL-1- and S1P-induced invasion of glioblastoma cells
Subsequently, we examined the roles of PAI-1, uPAR, S1P, and IL-1 in the invasion of glioblastoma cells. The invasion of U373 cells into Matrigel was significantly increased in response to both S1P and IL-1 (Fig. 6B). More importantly, the S1P- and IL-1-induced invasion was abrogated in U373 cells when the expression of either PAI-1 or uPAR was downregulated (Fig. 6A, B). Thus, both IL-1 and S1P increase the invasion of glioblastoma cells via the activation of PAI-1 and uPAR expression. Since PAI-1 binds to, and induces the internalization of the PAI-1/uPA/uPAR/integrin complex, which cause cell detachment in breast cancer cells (16, 17), we evaluated whether PAI-1 can interfere with the attachment of U373 cells. Indeed, the downregulation of uPAR expression or addition of 1 μM PAI-1 significantly inhibited the attachment of U373 cells to vitronectin-coated dishes (Fig. 6C). These results suggest that uPAR is important for the attachment of glioblastoma cells, while PAI-1 may regulate detachment.
Fig. 6. PAI-1 and uPAR are indispensable for S1P- and IL-1-enhanced invasion of U373 cells.
(A) Duplicate cultures of U373 cells were transfected with control, uPAR, or PAI-1 siRNA for 48 hours, as indicated. RNA was isolated from one of the duplicates, and the expression of uPAR and PAI-1 was analyzed by qPCR. The data were normalized to GAPDH mRNA, and expressed as a ratio to mRNA levels in untreated cells. (B) Cells from the second duplicate were stimulated with 10 ng/ml IL-1 or 100 nM S1P for 12 hours as indicated, and allowed to migrate through polycarbonate filters coated with Matrigel for 7 hours. The invasion was measured as described in the Materials and Methods. The results are means ± SD from three independent experiments. (C) (left panel) U373 cells were incubated with 1 μM PAI-1 or 1 μM BSA for 10 minutes, plated onto vitronectin-coated dishes, and allowed to adhere for 10 minutes. Subsequently, the medium containing the non-attached cells was removed, and the number of cells present in the medium was counted using a hemocytometer. (right panel) Expression of PAI-1 and uPAR was downregulated as described in (A) and the attachment was analyzed as described above. The results are means ± SD from two independent experiments done in triplicate.
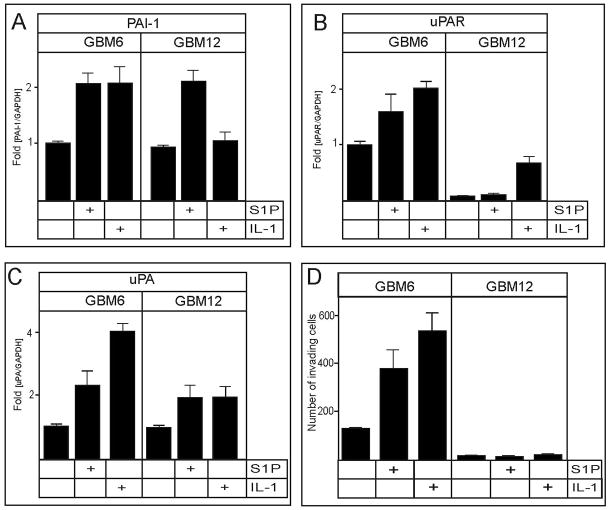
PAI-1 and uPAR mRNA expression is regulated by S1P and IL-1 in primary non-established glioblastomas
Glioblastoma cell lines, commonly used in in vitro studies, are not invasive in in vivo animal models (43, 44). Therefore, we examined the effects of S1P and IL-1 on primary non-established glioblastoma cells (GBM6 and GBM12), which were shown to produce invasive, diffuse tumors in scid mice (43). RNA expression of PAI-1 and uPAR were increased by S1P and IL-1 in GBM6 cells (Fig. 7A, B). However, whereas S1P increased PAI-1 in GBM12 cells, IL-1 increased uPAR without affecting PAI-1 (Fig. 7A, B). In contrast to U373 cells, the expression of uPA mRNA was also upregulated by S1P and IL-1 in both of these non-established GBM cells (Fig. 7C). Moreover, the invasion of GBM6 cells into Matrigel was significantly increased in response to both S1P and IL-1 (Fig. 7D), while GBM 12 cells were not invasive (Fig. 7D), which may be due to the extremely low levels of uPAR in these cells. Thus, both S1P and IL-1 play important roles in regulating the expression of the PAS components, and the invasion of primary glioblastoma cells.
Fig. 7. IL-1 and S1P upregulate the expression of PAI-1 and uPAR mRNA in primary non-established GBMs and stimulate their invasion.
Primary non-established glioblastoma GBM6 and GBM12 cells were stimulated without or with 10 ng/ml IL-1 or 5 μM S1P for 18 hours. RNA was isolated, and the expression of PAI-1 (A), uPAR (B), and uPA (C) mRNA was analyzed by qPCR. The data were normalized to GAPDH mRNA, and expressed as a ratio to mRNA levels in untreated cells. (D) GBM6 and GBM12 cells (50,000 cells/well) were stimulated without or with 10 ng/ml IL-1 or 100 nM S1P, and allowed to migrate through polycarbonate filters coated with Matrigel for 7 hours. The invasion was measured as described in the Materials and Methods. The results are means ± SD from three independent experiments.
DISCUSSION
The invasive phenotype of glioblastoma cells is the major obstacle in the successful treatment of patients diagnosed with GBM. While the precise mechanisms leading to the diffuse penetration of individual cells into normal regions of the brain are not understood, it is known that their invasion is regulated by growth factors, cytokines, and other signaling molecules, including bioactive lipids. Among cytokines and growth factors, IL-1 and EGF have attracted most of the attention. EGF is produced in the brain and readily crosses the blood-brain barrier (45), while its receptor (EGFR) is frequently amplified (46, 47), overexpressed (46, 47), or mutated (48, 46) in glioblastomas. Furthermore, the amplification and overexpression of EGFR is associated with high-grade progression (49), and patients expressing high levels of both EGFR and PAI-1 have a shorter prognosis for survival (14). Similarly to EGF, IL-1 is readily found in the brain, and can be produced by activated microglia and astrocytes surrounding the necrotic center of a glioblastoma tumor. In addition, significant amounts of IL-1 are produced by glioblastomas (23). IL-1, as a potent neuroinflammatory cytokine, activates the expression of many genes in astrocytes and glioblastoma cells, including the genes encoding PAI-1 and SphK1. Similarly, we have recently shown that EGF stimulates PAI-1 expression in glioblastomas via the activation of SphK1 (20), the enzyme that produces S1P.
In this paper, we show that exogenous S1P activates PAI-1 and uPAR expression in both U373 cells (Fig. 1), as well as in primary invasive GBMs (Fig. 7). In addition, S1P enhances uPA mRNA expression in primary GBMs. However, the precise cellular localizations of PAI-1, uPA, and uPAR are likely critical, since the attachment at the leading edge of a migrating cell, and the concurrent detachment at its trailing edge is imperative for efficient migration and invasion. More importantly, the enhanced expression of the PAS components may provide both the attachment, as well as the detachment depending on the ratio of PAI-1, uPA, and uPAR at specific locations on the cell’s membrane. Indeed, uPAR is mainly found at focal adhesion areas, rafts, and caveolae at the leading edge of migrating cells (50, 51). uPAR is bound to catalytically active uPA, leading to the degradation of the ECM, and providing attachment via the uPA/uPAR/integrins complex. In contrast, the enhanced expression of PAI-1 on the trailing edge of migrating cells may induce the internalization of the uPA/uPAR/integrin complex, and result in cell detachment.
Our data indicate that the S1P-induced expression of PAI-1 and uPAR is mediated by S1P2, as shown using pharmacological inhibitors and specific siRNA. S1P2 inhibits glioma cell migration through Rho activation and the Rho-kinase signaling pathway (52), which is probably mediated by PTEN activation (53). However, a recent study demonstrates that S1P2 inhibits glioma cell migration through Rho signaling pathways independent of PTEN (54). In agreement with our results, S1P2 was recently reported to enhance, rather than suppress, invasion most likely by increasing cell adhesion (55). In addition, we show that S1P2 may also control the generation of plasmin, which is critical for the degradation of the ECM, by regulating the expression of PAI-1 and uPAR. The S1P2-mediated upregulation of PAI-1 and uPAR mRNA required functional Rho-kinase and MEK1. The increased mRNA expression of uPAR induced by both S1P and IL-1 was AP-1-independent as shown in U373-TAM67 cells. In contrast, the intrinsic mRNA expression of PAI-1, and the fold stimulation by both S1P and IL-1 was decreased in U373-TAM67 cells, suggesting that this gene is regulated at least in part by AP-1.
Moreover, we showed that IL-1 acts independently of S1P to stimulate PAI-1 and uPAR expression in U373 cells, as well as in primary GBMs. This may be relevant in vivo, since IL-1 is secreted by the majority of GBMs (23). IL-1 can also regulate the levels of S1P in vivo, since it strongly upregulates the mRNA expression of SphK1 (Fig. 3A). However, the IL-1-mediated increase of PAI-1 and uPAR mRNA expression is independent of SphK1, and thus independent of S1P formation. These data are further supported by the observation that the knockdown of S1P2 expression does not affect the IL-1-induced mRNA expression of PAI-1 and uPAR. An additional role for IL-1 may lie in its ability to stimulate the expression of SphK1, thus maintaining a sustained pool of S1P, which is known to be critical for glioblastoma cell growth and survival (21). More import, we demonstrate that the in vitro invasion of both U373 cells, and primary GBM6 cells was increased in response to both S1P and IL-1. Furthermore, the down-regulation of both PAI-1 and uPAR expression abrogated S1P- and IL-1-induced invasion. Therefore, we propose that IL-1 and S1P independently stimulate the invasion of glioblastoma cells via upregulating PAI-1 and uPAR expression. These observations have important implications for development of future therapeutic agents to limit the invasion of GBM cells into surrounding healthy tissue, thus allowing for the effective removal of the tumor.
Footnotes
Acknowledgments: This work was supported by grants from the National Institutes of Health (R01 NSO44118) to T.K., the Pilot Project grant from the Massey Cancer Center, VCU (to T.K.), and in part by R01 CA61774 to S.S. S.M. was supported by the NIMH Intramural Research Program.
The abbreviations used are: AP-1, activating protein-1; ECM, extracellular matrix; ERK, extracellular stress-regulated kinase; IκB, inhibitor of NF-κB; JNK, c-jun N-terminal kinase; IL, interleukin; PAS, plasminogen activator system, PAI-1, plasminogen activator inhibitor-1; SphK1, sphingosine kinase 1; tPA, tissue-type plasminogen activator; uPA, urokinase-type plasminogen activator; uPAR, uPA receptor;
References
- 1.Belda-Iniesta C, de Castro Carpeno J, Casado Saenz E, et al. Molecular biology of malignant gliomas. Clin Transl Oncol. 2006;8:635–41. doi: 10.1007/s12094-006-0033-9. [DOI] [PubMed] [Google Scholar]
- 2.Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–6. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 3.Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–69. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Deb P, Sharma MC, Mahapatra AK, Agarwal D, Sarkar C. Glioblastoma multiforme with long term survival. Neurol India. 2005;53:329–32. [PubMed] [Google Scholar]
- 5.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57:25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landau BJ, Kwaan HC, Verrusio EN, Brem SS. Elevated levels of urokinase-type plasminogen activator and plasminogen activator inhibitor type-1 in malignant human brain tumors. Cancer Res. 1994;54:1105–8. [PubMed] [Google Scholar]
- 7.Levicar N, Nuttall RK, Lah TT. Proteases in brain tumour progression. Acta Neurochir (Wien) 2003;145:825–38. doi: 10.1007/s00701-003-0097-z. [DOI] [PubMed] [Google Scholar]
- 8.Rooprai HK, McCormick D. Proteases and their inhibitors in human brain tumours: a review. Anticancer Res. 1997;17:4151–62. [PubMed] [Google Scholar]
- 9.Binder BR, Mihaly J, Prager GW. uPAR-uPA-PAI-1 interactions and signaling: a vascular biologist’s view. Thromb Haemost. 2007;97:336–42. [PubMed] [Google Scholar]
- 10.Preissner KT, Kanse SM, May AE. Urokinase receptor: a molecular organizer in cellular communication. Curr Opin Cell Biol. 2000;12:621–8. doi: 10.1016/s0955-0674(00)00141-1. [DOI] [PubMed] [Google Scholar]
- 11.Mazar AP, Henkin J, Goldfarb RH. The urokinase plasminogen activator system in cancer: implications for tumor angiogenesis and metastasis. Angiogenesis. 1999;3:15–32. doi: 10.1023/a:1009095825561. [DOI] [PubMed] [Google Scholar]
- 12.Lakka SS, Gondi CS, Dinh DH, et al. Specific interference of urokinase-type plasminogen activator receptor and matrix metalloproteinase-9 gene expression induced by double-stranded RNA results in decreased invasion, tumor growth, and angiogenesis in gliomas. J Biol Chem. 2005;280:21882–92. doi: 10.1074/jbc.M408520200. [DOI] [PubMed] [Google Scholar]
- 13.Rao JS, Gondi C, Chetty C, et al. Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells. Mol Cancer Ther. 2005;4:1399–408. doi: 10.1158/1535-7163.MCT-05-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muracciole X, Romain S, Dufour H, et al. PAI-1 and EGFR expression in adult glioma tumors: toward a molecular prognostic classification. Int J Radiat Oncol Biol Phys. 2002;52:592–8. doi: 10.1016/s0360-3016(01)02699-2. [DOI] [PubMed] [Google Scholar]
- 15.Foekens JA, Peters HA, Look MP, et al. The urokinase system of plasminogen activation and prognosis in 2780 breast cancer patients. Cancer Res. 2000;60:636–43. [PubMed] [Google Scholar]
- 16.Czekay RP, Aertgeerts K, Curriden SA, Loskutoff DJ. Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. J Cell Biol. 2003;160:781–91. doi: 10.1083/jcb.200208117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czekay RP, Loskutoff DJ. Unexpected role of plasminogen activator inhibitor 1 in cell adhesion and detachment. Exp Biol Med (Maywood) 2004;229:1090–6. doi: 10.1177/153537020422901102. [DOI] [PubMed] [Google Scholar]
- 18.Laerum OD, Nygaar SJ, Steine S, et al. Invasiveness in vitro and biological markers in human primary glioblastomas. J Neurooncol. 2001;54:1–8. doi: 10.1023/a:1012565503958. [DOI] [PubMed] [Google Scholar]
- 19.Puli S, Lai JC, Bhushan A. Inhibition of matrix degrading enzymes and invasion in human glioblastoma (U87MG) cells by isoflavones. J Neurooncol. 2006;79:135–42. doi: 10.1007/s11060-006-9126-0. [DOI] [PubMed] [Google Scholar]
- 20.Paugh BS, Paugh SW, Bryan L, et al. EGF regulates plasminogen activator inhibitor-1 (PAI-1) by a pathway involving c-Src, PKCdelta, and sphingosine kinase 1 in glioblastoma cells. Faseb J. 2008;22:455–65. doi: 10.1096/fj.07-8276com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Brocklyn JR, Jackson CA, Pearl DK, et al. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- 22.Van Brocklyn JR, Young N, Roof R. Sphingosine-1-phosphate stimulates motility and invasiveness of human glioblastoma multiforme cells. Cancer Lett. 2003;199:53–60. doi: 10.1016/s0304-3835(03)00334-3. [DOI] [PubMed] [Google Scholar]
- 23.Lu T, Tian L, Han Y, Vogelbaum M, Stark GR. Dose-dependent cross-talk between the transforming growth factor-beta and interleukin-1 signaling pathways. Proc Natl Acad Sci U S A. 2007;104:4365–70. doi: 10.1073/pnas.0700118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller MM, Werbowetski T, Del Maestro RF. Soluble factors involved in glioma invasion. Acta Neurochir (Wien) 2003;145:999–1008. doi: 10.1007/s00701-003-0132-0. [DOI] [PubMed] [Google Scholar]
- 25.Pitson SM, Xia P, Leclercq TM, et al. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gopalan SM, Wilczynska KM, Konik BS, Bryan L, Kordula T. Astrocyte-specific Expression of the {alpha}1-Antichymotrypsin and Glial Fibrillary Acidic Protein Genes Requires Activator Protein-1. J Biol Chem. 2006;281:1956–63. doi: 10.1074/jbc.M510935200. [DOI] [PubMed] [Google Scholar]
- 27.Gopalan S, Kasza A, Xu W, et al. Astrocyte- and hepatocyte-specific expression of genes from the distal serpin subcluster at 14q32.1 associates with tissue-specific chromatin structures. J Neurochem. 2005;94:763–73. doi: 10.1111/j.1471-4159.2005.03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 29.Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 30.Rosenfeldt HM, Hobson JP, Milstien S, Spiegel S. The sphingosine-1-phosphate receptor EDG-1 is essential for platelet-derived growth factor-induced cell motility. Biochem Soc Trans. 2001;29:836–9. doi: 10.1042/0300-5127:0290836. [DOI] [PubMed] [Google Scholar]
- 31.Wang F, Van Brocklyn JR, Hobson JP, et al. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343–50. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- 32.Kasza A, Kiss DL, Gopalan S, et al. Mechanism of plasminogen activator inhibitor-1 regulation by oncostatin M and interleukin-1 in human astrocytes. J Neurochem. 2002;83:696–703. doi: 10.1046/j.1471-4159.2002.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fattal PG, Billadello JJ. Species-specific differential cleavage and polyadenylation of plasminogen activator inhibitor type 1 hnRNA. Nucleic Acids Res. 1993;21:1463–6. doi: 10.1093/nar/21.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim MH, Cho HS, Jung M, et al. Extracellular signal-regulated kinase and AP-1 pathways are involved in reactive oxygen species-induced urokinase plasminogen activator receptor expression in human gastric cancer cells. Int J Oncol. 2005;26:1669–74. [PubMed] [Google Scholar]
- 35.Kim MH, Park JS, Chang HJ, et al. Lysophosphatidic acid promotes cell invasion by up-regulating the urokinase-type plasminogen activator receptor in human gastric cancer cells. J Cell Biochem. 2008 doi: 10.1002/jcb.21696. [DOI] [PubMed] [Google Scholar]
- 36.Nagamine Y, Medcalf RL, Munoz-Canoves P. Transcriptional and posttranscriptional regulation of the plasminogen activator system. Thromb Haemost. 2005;93:661–75. doi: 10.1160/TH04-12-0814. [DOI] [PubMed] [Google Scholar]
- 37.Billich A, Bornancin F, Mechtcheriakova D, et al. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal. 2005;17:1203–17. doi: 10.1016/j.cellsig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Mastrandrea LD, Sessanna SM, Laychock SG. Sphingosine kinase activity and sphingosine-1 phosphate production in rat pancreatic islets and INS-1 cells: response to cytokines. Diabetes. 2005;54:1429–36. doi: 10.2337/diabetes.54.5.1429. [DOI] [PubMed] [Google Scholar]
- 39.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 40.An S, Bleu T, Huang W, et al. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 1997;417:279–82. doi: 10.1016/s0014-5793(97)01301-x. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto H, Takuwa N, Yatomi Y, et al. EDG3 is a functional receptor specific for sphingosine 1-phosphate and sphingosylphosphorylcholine with signaling characteristics distinct from EDG1 and AGR16. Biochem Biophys Res Commun. 1999;260:203–8. doi: 10.1006/bbrc.1999.0886. [DOI] [PubMed] [Google Scholar]
- 42.Van Brocklyn JR, Lee MJ, Menzeleev R, et al. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol. 1998;142:229–40. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giannini C, Sarkaria JN, Saito A, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7:164–76. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarkaria JN, Carlson BL, Schroeder MA, et al. Use of an orthotopic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clin Cancer Res. 2006;12:2264–71. doi: 10.1158/1078-0432.CCR-05-2510. [DOI] [PubMed] [Google Scholar]
- 45.Plata-Salaman CR. Epidermal growth factor and the nervous system. Peptides. 1991;12:653–63. doi: 10.1016/0196-9781(91)90115-6. [DOI] [PubMed] [Google Scholar]
- 46.Libermann TA, Nusbaum HR, Razon N, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–7. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 47.Wong AJ, Bigner SH, Bigner DD, et al. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A. 1987;84:6899–903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–7. [PubMed] [Google Scholar]
- 49.von Deimling A, Louis DN, von Ammon K, et al. Association of epidermal growth factor receptor gene amplification with loss of chromosome 10 in human glioblastoma multiforme. J Neurosurg. 1992;77:295–301. doi: 10.3171/jns.1992.77.2.0295. [DOI] [PubMed] [Google Scholar]
- 50.Cunningham O, Andolfo A, Santovito ML, et al. Dimerization controls the lipid raft partitioning of uPAR/CD87 and regulates its biological functions. Embo J. 2003;22:5994–6003. doi: 10.1093/emboj/cdg588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stahl CW. Theories of international labor migration: an overview. Asian Pac Migr J. 1995;4:211–32. doi: 10.1177/011719689500400203. [DOI] [PubMed] [Google Scholar]
- 52.Lepley D, Paik JH, Hla T, Ferrer F. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res. 2005;65:3788–95. doi: 10.1158/0008-5472.CAN-04-2311. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez T, Thangada S, Wu MT, et al. PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Proc Natl Acad Sci U S A. 2005;102:4312–7. doi: 10.1073/pnas.0409784102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malchinkhuu E, Sato K, Maehama T, et al. S1P(2) receptors mediate inhibition of glioma cell migration through Rho signaling pathways independent of PTEN. Biochem Biophys Res Commun. 2008;366:963–8. doi: 10.1016/j.bbrc.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 55.Young N, Van Brocklyn JR. Roles of sphingosine-1-phosphate (S1P) receptors in malignant behavior of glioma cells. Differential effects of S1P2 on cell migration and invasiveness. Exp Cell Res. 2007;313:1615–27. doi: 10.1016/j.yexcr.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]