Abstract
Rationale
Drugs with addictive liability have a high probability of co-abuse in many addicts. For example, cocaine users are several times more likely to smoke cigarettes than non-cocaine users, and smoking increases during cocaine use. Previous work has provided evidence that nicotine and cocaine have interactive neurochemical effects, particularly with regard to dopamine (DA) transmission.
Objectives
The present study examined the impact of nicotine treatment on the reinforcement efficacy of self-administered cocaine and non-reinforced responding for cocaine in rats.
Methods
Rats were trained to self-administer cocaine (i.v.) on a progressive ratio (PR) schedule of reinforcement. Self-administration training continued until stable responding was obtained. Acute nicotine pretreatment consisted of a subcutaneous injection (0.15, 0.3 and 0.6 mg/kg) 3 min prior to cocaine access. In the repeated treatment condition, a separate group of animals was given nicotine (0.6 mg/kg, s.c.) 3 min prior to cocaine access for 14 consecutive days. During extinction trials, these animals were injected with nicotine (0.6 mg/kg, s.c.) after 45 min of non-reinforced responding.
Results
Acute nicotine treatment produced an inverted U-shaped dose–response function with lower doses increasing and the highest dose decreasing the number of cocaine infusions obtained during a session. Animals treated repeatedly with the highest dose of nicotine showed a significant increase in the number of cocaine infusions by day 8 of nicotine treatment. During extinction sessions when cocaine was not available, injections of nicotine in these animals caused a reinstatement of the previously rewarded lever-press behavior.
Conclusions
These findings indicate that nicotine can facilitate cocaine reinforcement, may contribute to the transition from moderate drug-taking to an escalation of drug intake which is characteristic of addiction, and may trigger relapse.
Keywords: Psychostimulant, Intravenous, Self-administration, Progressive-ratio, Nicotine, Addiction, Cholinergic system, Acetylcholine
Introduction
The compulsive need to take a drug and the inability to control the amount of drug consumed are characteristic of drug addiction in humans (American Psychiatric Association 1994; McLellan et al. 2000). Addiction is also associated with a high probability of relapse to drug taking after periods of abstinence. In fact, many former drug addicts remain highly vulnerable to return to drug abuse even after many years of abstinence. Often, drug craving and potentially relapse to drug taking can be triggered by seemingly minor stimuli that were repeatedly experienced during drug-taking episodes (Childress et al. 1988; O’Brien and McLellan 1996). Understanding which factors contribute to the escalation of drug intake and relapse to drug taking after abstinence are critical steps toward establishing effective treatments for addiction.
Drugs with addictive liability have a high probability of co-abuse in many addicts. Psychostimulants (such as cocaine), alcohol and nicotine are among the most likely drugs to be co-administered. For example, cocaine users are several times more likely to smoke cigarettes than non-cocaine users, and smoking increases during cocaine use (Higgins et al. 1994; Roll et al. 1996). Reid and colleagues have shown that cocaine addicts report increased cue-induced craving for cocaine following nicotine treatments and reduced craving following treatment with a nicotinic antagonist (Reid et al. 1998, 1999). These findings might be explained by reports by humans that nicotine and cocaine have similar subjective effects (Jones et al. 1999) and studies showing that nicotine substitutes for cocaine in a discriminative stimulus paradigm in rats (Desai et al. 1999). Clearly, these findings suggest that the reinforcing effects of cocaine may be modulated by exposure to nicotine.
It has been firmly established that many drugs of abuse share a common mechanism in their ability to increase dopamine (DA) levels in the nucleus accumbens (NAc) (Imperato et al. 1986; Wise and Bozarth 1987; Koob and Bloom 1988). While the role of DA has been thoroughly investigated, there is mounting evidence that other neurotransmitters, including serotonin (McGregor et al. 1993), glutamate (Dworkin et al. 1995), gamma-aminobutyric acid (Peris 1996), and the cholinergic system (Mark et al. 1999b) also contribute to the function of the mesocorticolimbic system in drug reward.
The cholinergic system impacts mesolimbic nuclei at several levels. Cholinergic interneurons are present in the NAc and nicotinic acetylcholine receptors (nAChRs) are present on both the cell bodies of dopaminergic neurons in the ventral tegmental area (VTA) and their terminals in the NAc (Lehmann and Langer 1983; Clarke and Pert 1985). Functional studies using micro-dialysis have shown that nicotine activates the mesolimbic system and causes an increase in DA output (Damsma et al. 1989; Mifsud et al. 1989). Cocaine self-administration also results in increased synaptic availability of DA and stimulates ACh release in the NAc that can activate nAChRs (Kalivas and Duffy 1988; Mark et al. 1999a). Moreover, when cocaine and nicotine are administered simultaneously, their effects on NAc DA levels can be additive (Zernig et al. 1997; Gerasimov et al. 2000). Since DA is an integral component of drug reward, these findings suggest that nicotine has the potential to amplify the reinforcing properties of other abused drugs.
In the studies reported here, we sought to examine the interaction of nicotine and cocaine reward. The objectives of the experiments were twofold. First, we wanted to examine the effects of acute and repeated exposure to nicotine on the reinforcing efficacy of cocaine. To do this, we measured lever-press responding in rats for i.v. cocaine on a progressive ratio (PR) schedule of reinforcement before and after systemic injections of nicotine. The second objective was to determine whether exposure to nicotine during cocaine abstinence would reinstate a previously reinforced cocaine-seeking behavior.
Methods
Subjects
Thirty male Sprague-Dawley rats weighing approximately 300 g were used in these studies and maintained according to the guidelines set forth in the NIH publication “Principles of laboratory animal care” (1996) under the approval of the University’s Institutional Animal Care and Use Committee. Animals were housed individually following surgery in a temperature-controlled environment (22°C) with a 12-h/12-h day/night schedule initiated at 0600 hours. All animals had free access to food and water while in their home cages throughout the experiment.
Drug dosage and preparation
Cocaine HCl was generously supplied by the National Institute on Drug Abuse and was dissolved in physiological saline (0.9%). (–)-Nicotine di-D-tartrate (Sigma, St Louis, Mo.) was dissolved in physiological saline (0.9%) and the pH adjusted to 7.3 with dilute NaOH. The doses administered refer to the salt.
Surgery
Animals were anesthetized with pentobarbital (20 mg/kg, i.p.) supplemented by ketamine (40 mg/kg, i.p.) and implanted with intrajugular catheters assembled based on methods previously described elsewhere (Caine et al. 1993; Parsons et al. 1998). Briefly, the catheters were constructed of micro-renathane tubing (0.025 mm OD × 0.012 mm ID; Braintree Scientific Inc., Braintree, Mass.) connected to L-shaped external guide cannulae (Plastics One Inc., Roanoke, Va.), which were fastened to polypropylene mesh with cranioplastic cement. The tip of the catheter was inserted into the right or left jugular vein approximately 27 mm while the distal end was threaded subcutaneously to an exit point between the scapulae. Animals were allowed to recover for a minimum of 5 days. Catheters were flushed with 0.2 ml physiologic saline containing heparin (70 U/ml) and an antibiotic (ticarcillin: Timentin, 100 mg/ml; Smithkline Beecham, Philadelphia, Pa.) daily. Catheter patency was tested when necessary by infusion of the fast-acting barbiturate Brevital (methohexital sodium; 1 mg/0.1 ml; Lilly, Indianapolis, Ind.). Catheters were deemed patent in animals showing profound loss of muscle tone within 5 s of infusion.
Self-administration training
Following recovery from surgery, animals were placed in an operant conditioning chamber and the i.v. catheters were attached to an infusion line. This line was connected to a syringe that contained a cocaine solution and was mounted on a syringe pump. Animals were trained to bar press for an acquisition dose of cocaine (0.25 mg per infusion, i.v.) on a fixed ratio (FR) 1 schedule of reinforcement and maintained at that dose until stable responding was maintained for three consecutive days (±10%). The reinforcement schedule was then changed to the PR schedule of reinforcement described by Parsons et al. 1998 with a maximum session of length of 5 h.
To determine the effect of unit dose (i.e. the amount of cocaine received per infusion) on drug intake, we established dose–response functions using both FR and PR reinforcement schedules. Eight rats were trained to bar press for a maintenance dose of cocaine (0.25 mg per infusion) on a FR 1 reinforcement schedule until stable responding was obtained (3 days of ±10%). Then, two test doses (0.03 mg and 0.10 mg per infusion) were administered in counterbalanced order for one 3-h session per dose per day. One day of access to the maintenance dose of cocaine was intercalated between the two test doses. After the FR cocaine dose–response function was established, the reinforcement schedule was changed to a PR reinforcement schedule, and animals were allowed to bar press daily for a maintenance dose of cocaine (0.10 mg per infusion) until stable responding was obtained (3 days of ±10%). For this dose–response function, the maintenance dose was reduced from the 0.25-mg per infusion dose used previously to prevent a ceiling effect on responding encountered in pilot studies. The PR reinforcement schedule session continued until 1 h had lapsed without reinforcement with a maximum session length of 5 h. After stable responding for the maintenance dose of cocaine was obtained (3 days of ±10%), two test doses (0.03 mg and 0.30 mg per infusion) were administered in counterbalanced order for one daily session per dose. Between the two test doses, animals were given access to the maintenance dose for three daily sessions. In order to establish a more accurate log/linear relationship between doses, the highest dose for the PR dose–response function was increased slightly above that used for the FR dose–response function (0.30 mg vs 0.25 mg per infusion).
Experimental procedures
Experiment 1: cocaine self-administration after initial nicotine treatment
Six rats were trained to bar press for i.v. cocaine on a PR schedule as described above. After 3 days of baseline measurement (±10%), rats were injected with nicotine (0.15, 0.30, and 0.60 mg/kg, s.c.) or saline 3 min prior to a self-administration session, during which they were allowed to bar press for i.v. cocaine (0.25 mg per infusion). All of the nicotine doses were administered to each animal in counterbalanced order and were chosen based on previous studies that examined the locomotor effects of s.c. nicotine (Clarke and Kumar 1983; Ksir et al. 1987). Rats typically vocalized and showed increased respiration rate after initial nicotine injections. These behaviors diminished with repeated injection in the second experiment.
Experiment 2: cocaine self-administration after repeated nicotine
Twenty-two animals were trained to respond on a PR schedule of reinforcement for i.v. cocaine as described above (six of these rats had been used previously to establish dose–response functions). After stable responding was obtained (±10%), 15 animals were given a nicotine injection (0.6 mg/kg, s.c.) 3 min prior to each daily self-administration session, during which they were allowed to bar press for cocaine (0.10 mg per infusion) on a PR schedule of reinforcement for 14 consecutive days. The remaining seven animals included in this experiment received saline injections just prior to cocaine self-administration sessions for 14 consecutive days.
Experiment 3: nicotine induced non-reinforced responding for cocaine after repeated nicotine
After completion of the second experiment, the eleven remaining animals from the nicotine group in experiment 2 were subjected to six extinction trials. Rats that received 14 days of nicotine prior to cocaine access were first given four daily 1-h sessions of cocaine extinction training. In these sessions, rats received injections of nicotine (0.6 mg/kg) and were then placed in the chamber where cocaine was no longer available and allowed to bar press without consequence for 60 min. By the end of the forth session, rats usually ceased responding within 30 min of the start of the session. On extinction day 5 and day 6, no injection of nicotine was given before the session, and animals were allowed to bar press without consequence for 45 min after which they were injected s.c. with either nicotine or saline (counterbalanced on consecutive days) and again allowed to bar press for 45 min. A separate control group of nine animals was treated exactly as described above with the exception that they received saline injections just prior to cocaine access for 14 consecutive days rather than nicotine. These rats received nicotine injections for the first time during testing on extinction day 5 or day 6.
Statistical analyses
Data were normalized to a percentage of the baseline score for treatment days. The baseline was calculated as the average of the 3 days of self-administration just prior to the test day. Data were analyzed using two-way analysis of variance (ANOVA) followed by appropriate one-way ANOVAs and Tukey’s HSD t-tests.
Results
Figure 1 shows the mean (±SEM) number of cocaine infusions obtained during daily self-administration sessions where animals bar pressed for varying doses of cocaine on either a FR 1 (a) or a PR (b) schedule of reinforcement. One-way within-subjects ANOVA for cocaine dose on a FR 1 schedule of reinforcement demonstrated a significant main effect of dose on the number of cocaine infusions (F2,14=41.81, P<0.001). Post-hoc tests revealed that rats received more infusions at the 0.10-mg per infusion dose than at the 0.03-mg and 0.25-mg per infusion doses (Tukey’s HSD; P<0.001). One-way within-subjects ANOVA for dose on a PR schedule of reinforcement revealed a significant effect of cocaine dose on the number of infusions (F2,14=46.400, P<0.001). Post-hoc tests revealed significant differences in the number of cocaine infusions among all of the cocaine doses.
Fig. 1.
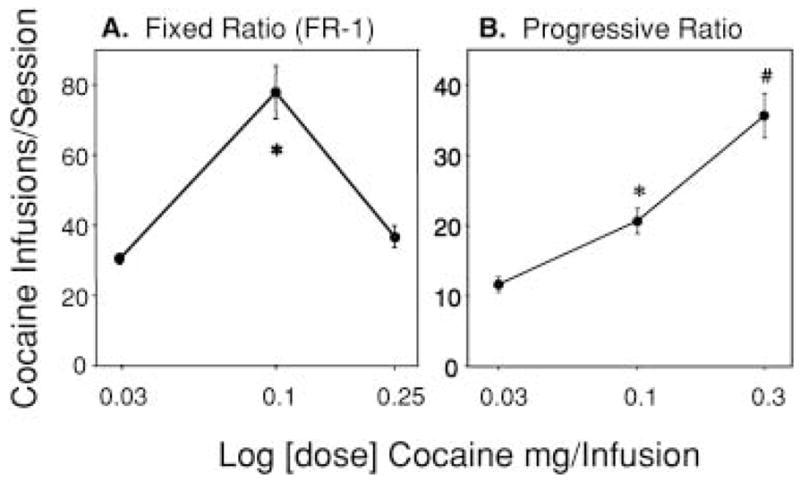
A Dose-related effects of cocaine dose for a fixed ratio (FR) 1 schedule of reinforcement (3-h session) on the mean (±SEM) number of cocaine infusions obtained during a 3-h session (n=8). Asterisk denotes a significant difference from the other two cocaine concentrations (0.03 mg and 0.25 mg per infusion; P<0.001). B Dose-related effects of cocaine dose for a progressive ratio (PR) schedule of reinforcement (maximum session length = 5 h) on the mean (±SEM) number of cocaine infusions obtained before 1 h of non-reinforcement (n=8). Asterisk denotes a significant difference from the 0.03 mg and 0.3 mg per infusion cocaine concentrations and # indicates significant difference from 0.03 mg and 0.1 mg per infusion cocaine concentrations (P<0.01; Tukey’s HSD after one-way, repeated-measures ANOVA)
Experiment 1: effect of acute nicotine on cocaine self-administration
Figure 2 shows the effect of nicotine (0.15, 0.30, and 0.60 mg/kg) pretreatment on the mean number of cocaine infusions obtained under a PR schedule of reinforcement. A one-way ANOVA demonstrated a significant effect of nicotine dose on the number of cocaine infusions (F3,20=13.286, P<0.001). Post-hoc tests revealed a significant decrease from the baseline number of cocaine infusions in animals pretreated with 0.6 mg/kg nicotine and a significant increase from the baseline in animals pretreated with 0.3 mg/kg nicotine relative to animals pretreated with saline (Tukey’s HSD; P=0.03 and P=0.04, respectively). Responses on the inactive lever did not differ from the baseline number of responses after saline or nicotine treatment (data not shown).
Fig. 2.
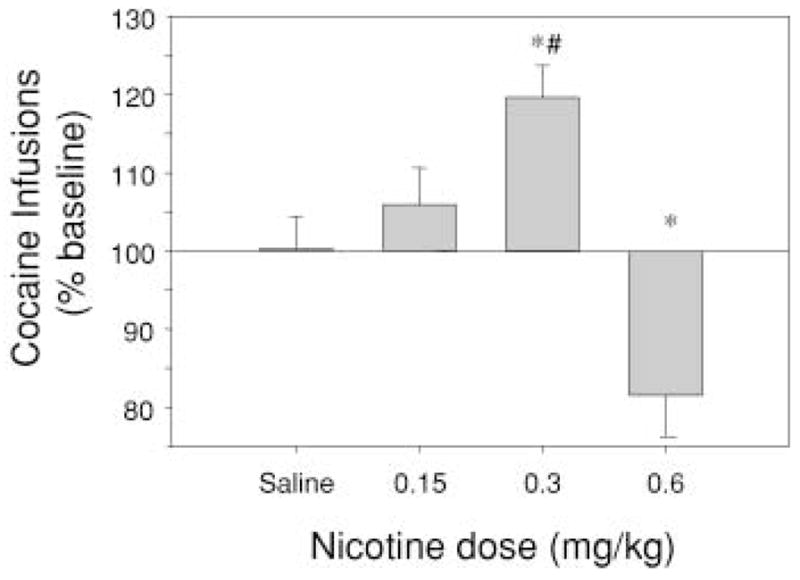
Effects of nicotine (0.15, 0.3 and 0.6 mg/kg, s.c.) pretreatment on the mean (±SEM) number of cocaine infusions (% baseline) on a progressive ratio (PR) schedule of reinforcement obtained before 1 h of non-reinforcement (n=6). Asterisks denote a significant difference from saline treatment (P<0.05) and # denotes a significant difference from the highest dose of nicotine (0.6 mg/kg; P<0.05; Tukey’s HSD after one-way, repeated-measures ANOVA)
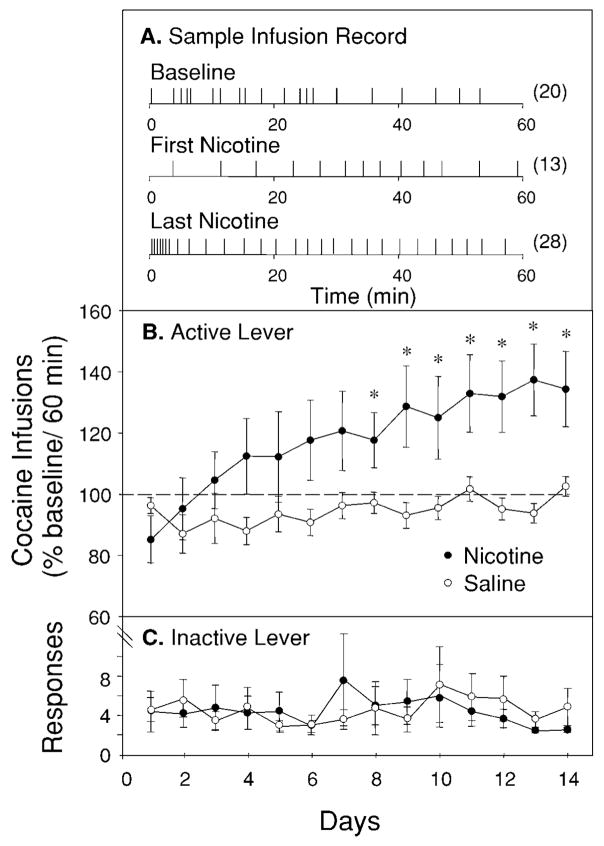
Experiment 2: effect of repeated nicotine on cocaine self-administration
Figure 3A shows representative infusion records from the first and fourteenth self-administration sessions for a nicotine-treated animal. Initial nicotine treatments typically resulted in fewer infusions during the beginning of the session accompanied by a greater time between infusions than during the baseline distribution. However, by the last nicotine treatment, an increase in the number of infusions during the beginning of the session and a decreased time between infusions was characteristic. Figure 3B shows the mean (±SEM) number of cocaine infusions (% baseline) obtained during the first 60 min of PR reinforcement schedule sessions prior to which animals were treated with either nicotine or saline daily for 14 days. Two-way, repeated-measures ANOVA yielded a significant treatment × day interaction (F13,260=3.38, P<0.0001). Post-hoc analyses revealed a significant difference in the number of cocaine infusions between nicotine- and saline-treated animals on days 8–14 of nicotine treatment (Tukey’s HSD; 12–14, P<0.05). Figure 3C shows responses on the inactive lever over the 14 days of treatment. Responses on this lever did not differ from baseline number after saline or nicotine treatment.
Fig. 3.
Effects of nicotine (0.60 mg/kg) on cocaine self-administration on a progressive ratio (PR) schedule of reinforcement. A Example of an infusion record showing the effect of initial nicotine treatment and the effect of nicotine after repeated treatment. Numbers in parentheses indicate infusions obtained during the first 60 min of the session. B Effect of repeated nicotine on cocaine self-administration. Animals injected with nicotine (n=15) 3 min before daily cocaine access show a significant increase in number of cocaine infusions relative to animals injected with saline (n=7) beginning at day 8. Data are from the first 60 min of each session and are reported as the percentage (mean±SEM) of 3 days prior to the beginning of injections (i.e., baseline). C Animals treated with nicotine did not differ from those treated with saline in responding on the inactive lever. Data are number of responses (mean±SEM) during the first 60 min of each session. *P<0.05 [Tukey’s HSD after mixed two-way ANOVA; drug (between subjects) × day (within subjects)]
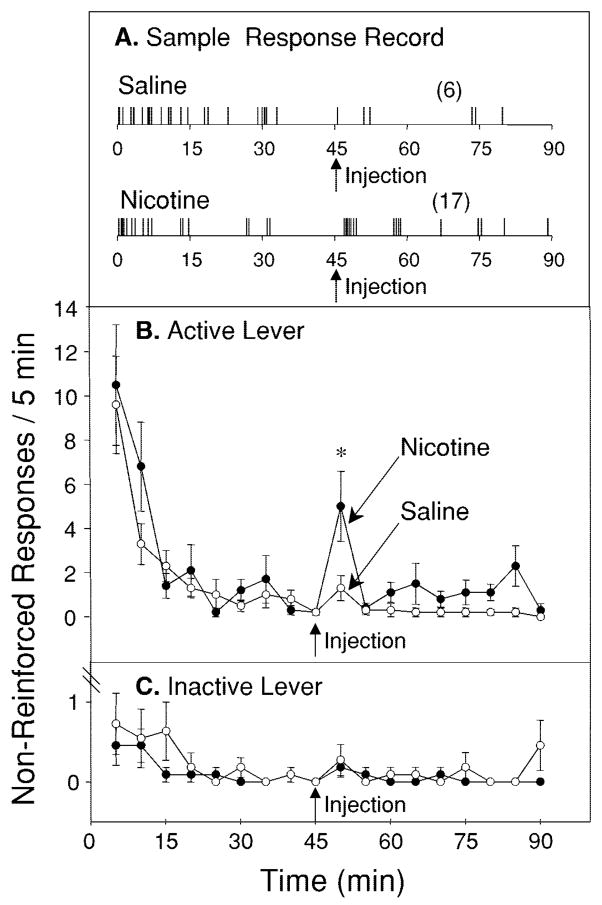
Experiment 3: effect of nicotine on non-reinforced responding for cocaine after repeated nicotine
Figure 4A shows representative response records during extinction sessions of an animal treated with nicotine. Animals typically emitted a burst of responding just after a nicotine injection that was not observed following a saline injection. Figure 4B shows the mean (±SEM) number of non-reinforced response on the active lever pooled over 5-min bins during the 90-min extinction trials in animals that were repeatedly given nicotine injections just prior to cocaine access. Two-way, repeated-measures ANOVA revealed a significant main effect of treatment (F1,20=4.44, P<0.05). Post-hoc tests revealed a significant increase in the number of non-reinforced responses during the 5-min period just after nicotine injections relative to that period just after saline (Tukey’s HSD; P<0.05). Figure 4C shows the responses on the inactive lever during the extinction trials. Responding on this lever after nicotine was not different from that after saline.
Fig. 4.
A Example of a response record showing the effect of a nicotine injection during an extinction session. Numbers in parentheses indicate the responses made during the 45-min period after the injection. B The effect of nicotine administered during an extinction trial on non-reinforced responding for cocaine in animals previously treated with nicotine 3 min prior to cocaine self-administration sessions. Data are presented as responses per 5 min (mean±SEM). During the extinction session, animals reached near-zero levels of responding. However, when animals were injected with nicotine (arrow) after 45 min of non-reinforced responding, they showed a significant increase in responding during the 5-min period following the injection relative to when they were injected with saline (n=11). C Responding on the inactive lever was not significantly different when animals were treated with nicotine relative to when they were treated with saline. Data are presented as the number of responses per 5 min (mean±SEM). *P<0.001 [Tukey’s HSD after repeated-measures, two-way ANOVA analyses (drug × time) across 90 min]
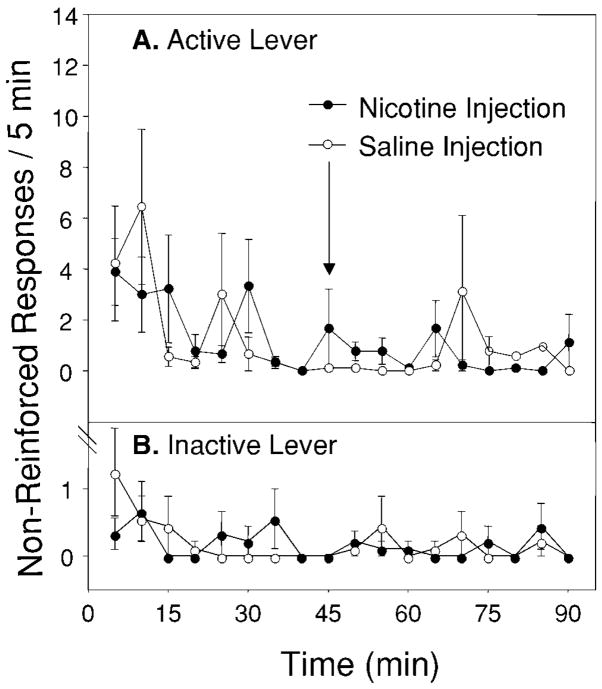
Figure 5A shows the mean (±SEM) number of non-reinforced responses on the active lever (pooled over 5-min bins) during the 90-min extinction trials in animals that were given saline injections just prior to cocaine access for 2 weeks before extinction testing. Figure 5B shows the mean (±SEM) number of responses on the inactive. In contrast to rats that received 2 weeks of nicotine, the number of bar presses on the active and inactive levers did not differ when these animals were injected with nicotine relative to when they were injected with saline.
Fig. 5.
A The effect of nicotine administered during an extinction trial on non-reinforced responding for cocaine in animals previously treated with saline 3 min prior to cocaine self-administration sessions for 2 weeks. Data are presented as responses per 5 min (mean±SEM). No differences were observed when animals were injected with nicotine (closed circles; injections indicated by the arrow) after 45 min of non-reinforced responding relative to saline (open circles). B Responding on the inactive lever was not significantly different when animals were treated with nicotine relative to when they were treated with saline. Data are presented as the number of responses per 5 min (mean±SEM)
Discussion
In these experiments, we found that 0.60 mg/kg of acutely administered nicotine reduced cocaine intake on a PR reinforcement schedule. When nicotine was given repeatedly before cocaine access, however, rats demonstrated an escalation in daily cocaine self-administration. After several days of extinction training, nicotine injections reinstated responding for cocaine in these rats.
We compared responding on FR and PR schedules of reinforcement and found that the PR schedule yielded a nearly linear relationship between the cocaine unit dose and the number of cocaine infusions. This finding is consistent with previous reports (Roberts et al. 1989; Parsons et al. 1998) and is interpreted as representing the increase in cocaine’s reinforcing effect with increases in dose. Responding on this PR schedule, rats showed a modest decrease in cocaine infusions when they were injected with an acute dose of 0.6 mg/kg nicotine, which suggests that the reinforcing value of cocaine was reduced following nicotine. In contrast, after acute injections of low doses of nicotine (0.3 mg/kg), rats increased responding, which implies that the reinforcing efficacy of cocaine may have been moderately augmented. Alternatively, both of these findings may reflect the acute locomotor effects of nicotine when it is administered to nicotine-naive animals. Previous studies have shown that a high dose of nicotine similar to the high dose used in the current study suppressed locomotor activity (Clarke and Kumar 1983; Ksir et al. 1987), whereas lower doses increased locomotion (Ksir et al. 1987). It is possible that suppression of locomotor activity following 0.6 mg/kg nicotine accounted for the decrease in responding for cocaine, and the increase in responding following lower doses of nicotine may have been due to non-specific locomotor activation. We did not detect a difference in responses on the inactive lever between any dose of nicotine and saline treatments which suggests that increased cocaine intake was not due to general activation. Still, because activity on this lever was consistently very low, a reduction in responding following a high dose of nicotine would have been difficult to detect so we cannot rule out locomotor suppression as the cause of decreased cocaine intake in this experiment.
Repeated exposure to nicotine has been reported to result in tolerance to its locomotor suppressant effects so, in the second study, animals were treated daily with nicotine before access to cocaine. Over the course of 2 weeks and beginning as early as a few days, exposure to nicotine caused a progressive increase in cocaine self-administration. The temporal pattern of this effect was consistent with our first experiment and with previous reports showing that acute exposure to nicotine in drug-naive rats causes reduced locomotor activity that dissipates with repeated exposure (Clarke and Kumar 1983; Ksir et al. 1987). Animals treated with nicotine significantly increased their cocaine intake by day 8, while the level of daily cocaine intake remained relatively stable in vehicle-treated rats. The progressive increase in cocaine responding across days in nicotine-treated rats is consistent with nicotine having increased the reinforcement value of cocaine. It is unlikely that nicotine’s effects were due to its general locomotor activating effects since the number of responses on the inactive lever did not differ between nicotine- and saline-treated rats.
When these rats received an injection of nicotine during the extinction test session they emitted a “burst” of non-reinforced responses immediately after nicotine which did not occur when they were injected with saline. This finding shows that nicotine can elicit cocaine-seeking behavior. Previous studies have shown that prior exposure to nicotine facilitates acquisition of cocaine self-administration (Horger et al. 1992). However, consistent with the results from our control group (Fig. 5), Schenk and Partridge have shown that nicotine does not elicit non-reinforced responding for cocaine in nicotine-naive rats (Schenk and Partridge 1999). It is possible that in experiments 2 and 3 of the present study, tolerance developed to the acute effects of nicotine in rats given repeated nicotine treatment (Fig. 3b, Fig. 4b). If this tolerance occurred, nicotine may have acquired the potential to reinstate previously extinguished responding for cocaine because it became a discriminative stimulus (or cue) that signaled the onset of cocaine access. As the association between the psychological effects of nicotine and cocaine strengthened, exposure to nicotine in the absence of cocaine may have induced a state similar to that of cocaine “craving” (de Wit and Stewart 1981; Markou et al. 1993). Our results represent an animal model consistent with recent data showing that human cocaine addicts report increased cue-induced cocaine craving after a nicotine treatment and, conversely, decreased cue-induced cocaine craving after administration of a nicotinic antagonist (Reid et al. 1998, 1999). This model may have relevance for the development of effective intervention strategies for treating co-addiction to multiple drugs.
One feature of the transition from drug use to addiction in humans is a loss of control over the amount of drug that is consumed in a given drug-taking episode such that addicts take increasing amounts of drug over time (Gawin 1991; American Psychiatric Association 1994; McLellan et al. 2000). In our study, rats given nicotine along with cocaine showed a similar escalation in drug intake, which may represent an animal model of this loss of control. Under certain drug-reinforcement schedules (such as the progressive ratio schedule used here), rats typically exhibit a stable level of cocaine intake over many days (Roberts and Richardson 1992; Stafford et al. 1998), which is what we found in the control group that was given daily saline injections before cocaine access. With longer access times (and simpler, fixed-ratio reinforcement schedules) rats show a gradual increase in daily cocaine intake (Ahmed and Koob 1998). Repeated exposure to nicotine results in a similar transition to heightened intake despite the difficulty of the progressive ratio response requirements. It has been argued that the switch from relatively stable drug intake to increasingly higher levels of consumption is driven by a change in a ‘hedonic set-point’ (termed allostasis) that an organism seeks to defend by escalating drug self-administration (Koob and Le Moal 1997, 2001). This allostatic process may be induced by the combined exposure to two stimulant drugs (i.e., cocaine and nicotine) and it is reasonable to suspect that this occurs because they act on a common neurobiological substrate. In this case, the ability of nicotine to augment cocaine reinforcement may be due to the fact that both drugs increase DA transmission in the mesolimbic system, an event that is central to the reinforcing properties of many drugs and natural stimuli (e.g., food, water, sex; Imperato et al. 1986; Wise and Bozarth 1987; Koob and Bloom 1988). Nicotine activates DA neurons in the VTA that project to the limbic forebrain including the NAc and medial prefrontal cortex (Pidoplichko et al. 1997; Grillner and Svensson 2000). Therefore, an explanation for nicotine’s facilitation of cocaine reinforcement (as indexed by escalation of intake) is that nicotine directly activated VTA neurons leading to increased release of DA in the NAc and, therefore, cocaine, as a reuptake inhibitor, was consequently perceived as more rewarding. In support of this idea, results from microdialysis studies have shown that nicotine (Imperato et al. 1986; Schilstrom et al. 1998) and cocaine (Carboni et al. 1989; Weiss et al. 1992) increase DA concentrations in the NAc and, when given in combination, their facilitative effects on extra-cellular DA are at least additive (Zernig et al. 1997) and sometimes synergistic (Gerasimov et al. 2000).
The neurobiological factors that contribute to drug craving and relapse are slowly being elucidated. Imaging studies in humans have identified several key neural substrates of drug and drug cue-induced craving including limbic pathways that mediate motivation, emotion, memory, and reward (Volkow et al. 1991; Grant et al. 1996; Childress et al. 1999). In animals, it is known that stimulation of the ventral subiculum in the hippocampus reinstates cocaine-seeking behavior via a glutamate-dependent mechanism localized in the VTA (Vorel et al. 2001). Our findings show that stimulation of nicotinic receptors, which are prominent in the hippocampus and VTA, could be the trigger that activates (and/or potentiates) the circuit described by Vorel and colleagues. Moreover, both nicotine and ventral subiculum stimulation increase VTA neuron activity and NAc DA along similar time courses (Gerasimov et al. 2000; Vorel et al. 2001). Stimuli previously associated with access to cocaine can stimulate cocaine seeking for up to 2 months following drug withdrawal (Grimm et al. 2001). Since nicotinic activation is important for many forms of associative learning, it is tempting to consider that nicotine dependence might also exacerbate this phenomena. Because of the prominent role of nicotinic receptors in drug reward circuits, our findings suggest that a successful strategy for the treatment of psychostimulant addiction should include concurrent treatment for nicotine dependence.
Acknowledgments
We are grateful to Drs. John Belknap, John Crabbe, Christopher Cunningham, Deborah Finn, Jennifer Mitchell, and Tamara Phillips for advice and critical comments on earlier versions of this manuscript. We thank Ms. Melani Helm for help with data analysis and graphics. This work was supported by USPHS grants DA11203 and DA14639 to G.P.M. A.J.B. was supported by Institutional NRSA grant DA07262.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Caine SB, Lintz R, Koob GF. Intravenous drug self-administration techniques in animals. In: Sahgal A, editor. Behavioral neuroscience: a practical approach. Oxford University Press; New York: 1993. pp. 117–143. [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extra-cellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, McLellan AT, O’Brien CP. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr. 1988;81:74–80. [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Brit J Pharmacol. 1983;78:329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Pert A. Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain Res. 1985;348:355–358. doi: 10.1016/0006-8993(85)90456-1. [DOI] [PubMed] [Google Scholar]
- Damsma G, Day J, Fibiger HC. Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. Eur J Pharmacol. 1989;168:363–368. doi: 10.1016/0014-2999(89)90798-x. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. Asymmetric generalization between the discriminative stimulus effects of nicotine and cocaine. Behav Pharmacol. 1999;10:647–656. doi: 10.1097/00008877-199911000-00011. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Co C, Smith JE. Rat brain neurotransmitter turnover rates altered during withdrawal from chronic cocaine administration. Brain Res. 1995;682:116–126. doi: 10.1016/0006-8993(95)00327-m. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Rice O, Schiffer WK, Dewey SL. Synergistic interactions between nicotine and cocaine or methylphenidate depend on the dose of dopamine transporter inhibitor. Synapse. 2000;38:432–437. doi: 10.1002/1098-2396(20001215)38:4<432::AID-SYN8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner P, Svensson TH. Nicotine-induced excitation of midbrain dopamine neurons in vitro involves ionotropic glutamate receptor activation. Synapse. 2000;38:1–9. doi: 10.1002/1098-2396(200010)38:1<1::AID-SYN1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Hughes JR, Bickel WK, Lynn M, Mortensen A. Influence of cocaine use on cigarette smoking. J Am Med Assoc. 1994;272:1724. [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology. 1992;107:271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Jones HE, Garrett BE, Griffiths RR. Subjective and physiological effects of intravenous nicotine and cocaine in cigarette smoking cocaine abusers. J Pharmacol Exp Ther. 1999;288:188–197. [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effects of daily cocaine and morphine treatment on somatodendritic and terminal field dopamine release. J Neurochem. 1988;50:1498–1504. doi: 10.1111/j.1471-4159.1988.tb03036.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation or reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Ksir C, Hakan RL, Kellar KJ. Chronic nicotine and locomotor activity: influences of exposure dose and test dose. Psycho-pharmacology. 1987;92:25–29. doi: 10.1007/BF00215474. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Langer SZ. The striatal cholinergic interneuron: synaptic target of dopaminergic terminals? Neuroscience. 1983;10:1105–1120. doi: 10.1016/0306-4522(83)90102-1. [DOI] [PubMed] [Google Scholar]
- Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology. 1999a;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- Mark GP, Kinney AE, Grubb MC, Keys AS. Involvement of acetylcholine in the nucleus accumbens in cocaine reinforcement. Ann NY Acad Sci. 1999b;877:792–795. doi: 10.1111/j.1749-6632.1999.tb09324.x. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- McGregor A, Lacosta S, Roberts DC. L-tryptophan decreases the breaking point under a progressive ratio schedule of intravenous cocaine reinforcement in the rat. Pharmacol Biochem Behav. 1993;44:651–655. doi: 10.1016/0091-3057(93)90181-r. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. J Am Med Assoc. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Mifsud J-C, Hernandez L, Hoebel BG. Nicotine infused into the nucleus accumbens increases synaptic dopamine as measured by in vivo microdialysis. Brain Res. 1989;478:365–367. doi: 10.1016/0006-8993(89)91518-7. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, McLellan AT. Myths about the treatment of addiction. Lancet. 1996;347:237–240. doi: 10.1016/s0140-6736(96)90409-2. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Weiss F, Koob CF. Serotonin lB receptor stimulation enhances cocaine reinforcement. J Neurosci. 1998;18:10078–10089. doi: 10.1523/JNEUROSCI.18-23-10078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J. Repeated cocaine injections decrease the function of striatal gamma-aminobutyric acid, receptors. J Pharmacol Exp Ther. 1996;276:1002–1008. [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend. 1998;49:95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20:297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psycho-pharmacology. 1989;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Richardson NR. Self-administration of psychomotor stimulants using progressive ratio schedules of reinforcement. In: Wu P, Boulton A, Baker GB, editors. Neuro-methods: animal models of drug addiction. Humana Press; Clifton: 1992. pp. 263–269. [Google Scholar]
- Roll JM, Higgins ST, Tidey JW. Cocaine use can increase cigarette smoking: evidence from laboratory and naturalistic settings. Exp Clin Psychopharmacol. 1996;5:262–268. doi: 10.1037//1064-1297.5.3.263. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Cocaine-seeking produced by experimenter-administered drug injections: dose–effect relationships in rats. Psychopharmacology. 1999;147:285–290. doi: 10.1007/s002130051169. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Svensson HM, Svensson TH, Nomikos GG. Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroscience. 1998;85:1005–1009. doi: 10.1016/s0306-4522(98)00114-6. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Weiss F, Paulus MP, Lorang MT, Koob GF. Increases in extracellular dopamine in the nucleus accumbens by cocaine are inversely related to basal levels: effects of acute and repeated administration. J Neurosci. 1992;12:4372–4380. doi: 10.1523/JNEUROSCI.12-11-04372.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Zernig G, O’Laughlin IA, Fibiger HC. Nicotine and heroin augment cocaine-induced dopamine overflow in nucleus accumbens. Eur J Pharmacol. 1997;337:1–10. doi: 10.1016/s0014-2999(97)01184-9. [DOI] [PubMed] [Google Scholar]