Abstract
In a model for neuronal movement, PC12 cells undergo fast migration in response to nerve growth factor (NGF) and phorbol ester (PMA). We previously showed that NGF increases intracellular cAMP via activation of soluble adenylyl cyclase (sAC). In this report, we demonstrate that sAC activation is an essential component of NGF- +PMA-induced fast migration in PC12 cells. Interestingly, PMA also raises intracellular cAMP but does so by stimulating transmembrane adenylyl cyclases (tmAC); however, this tmAC-generated cAMP does not contribute to fast migration. Therefore, cells must possess independent pools of cAMP capable of modulating distinct functions.
Keywords: adenylyl cyclase, nerve growth factor, neuron, compartmentalization, protein kinase C
Neuronal migration and differentiation constitute key processes in the development of the central nervous system. The rat pheochromocytoma cell line PC12 has been extensively used as a model for the morphological changes that accompany both processes. When treated with nerve growth factor (NGF) for long periods (48–72 hr), PC12 cells extend neurites in a process analogous to differentiation of sympathetic neurons (Levi-Montalcini and Angeletti, 1968; Greene and Tischler, 1982; Vaudry et al., 2002). Conversely, when PC12 cells, plated on laminin-coated surfaces, are simultaneously stimulated with NGF and phorbol 12-myristate-13-acetate (PMA), the cell body recedes away from points of attachment to the plate surface, and the cells assume a crescent shape (Glowacka and Wagner, 1990). The latter phenomenon is rapid (observable within 1 hr of NGF + PMA addition) and has been considered a model for “fast neuronal migration.”
The signaling cascades that mediate the fast migration process have not been adequately described. Specifically, it is known that PMA exerts its effects via the activation of protein kinase C (PKC), which was independently shown to be essential for cell motility in primary neuronal cultures (Choe et al., 2003). NGF has been shown to promote the survival and differentiation of multiple neuronal populations within the central nervous system (Levi-Montalcini and Angeletti, 1968), but the signaling mechanisms involved in NGF-induced neuritogenesis remain controversial (Vaudry et al., 2002).
We recently identified “soluble” adenylyl cyclase (sAC) as the source of cAMP downstream of NGF and a mediator of NGF-induced activation of the monomeric GTPase Rap1 in PC12 cells (Stessin et al., 2006). sAC is a ubiquitously expressed cAMP source in mammalian cells, distinct from the classically described trans-membrane adenylyl cyclases (tmACs) in its subcellular localization and biochemical profile. In this report, we demonstrate that the NGF-sAC signaling axis is also required for fast migration. We also show that stimulation of PC12 cells with PMA results in cAMP elevation via tmACs, but, unlike sAC-generated cAMP, tmAC-generated cAMP does not contribute to the fast migration phenomenon. These results reveal the existence of distinct pools of cAMP, which are generated by independent sources and do not serve the same functions.
MATERIALS AND METHODS
Materials
PMA, laminin, 2′–5′ dideoxyadenosine (2′–5′-ddAdo), isobutyl-methylxanthine (IBMX), and Ro 31-8220 were purchased from Sigma (St. Louis, MO). NGF was purchased from Harlan Bioproducts for Science (Indianapolis, IN). Calphostin C and 8-bromo-cAMP were purchased from Biomol International (Plymouth Meeting, PA). Blasticidin was purchased from Invitrogen (Carlsbad, CA). KH7 and KH7.15 were synthesized by Chemical Diversity, Inc. (San Diego, CA) and by the Abby and Howard Milstein Synthetic Chemistry Core Facility of Weill Cornell Medical College. sAC protein was detected by immunoblotting with monoclonal antibody R21 (Zippin et al., 2003), and actin was detected by using commercially obtained polyclonal antisera (Santa Cruz Biotechnology, Santa Cruz, CA).
Cell Culture
PC12 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) plus 10% fetal bovine serum (FBS), 5% donor horse serum (DHS), 1% L-glutamine, and antibiotics at 37°C in 5% CO2. Low-serum media contained DMEM plus 2% FBS, 1% DHS, 1% L-glutamine, and antibiotics.
sAC-overexpressing stable PC12 cells were generated by infection with a lentivirus expressing the full-length isoform of human sAC (Wu et al. 2006), and control, lacZ-overexpressing stable PC12 cells were generated by infection with a lentivirus expressing LacZ protein. Both mutant PC12 cell lines were selected and maintained in selection media containing 1 μg/ml blasticidin.
Fast Migration Assay
Tissue culture plates (35 mm) were coated with laminin by incubating with a laminin-containing solution (20 μl/ml laminin, 127 mM NaCl, 5.3 mM KCl, 18.2 mM HEPES, pH 7.2) at 37°C for 2 hr. Cells were plated at a density of 0.5–1.0 × l04 cells/ml, and 24 hr later the media were replaced with low-serum media. Cells were treated with 200 ng/ml NGF + 200 nM PMA, along with 4 μM Ro 31-8220, 1 μM calphostin C, 50 μM 2′–5′-ddAdo, or 50 μM KH7 in the presence or absence of 0.5 mM 8Br-cAMP (as indicated) 24 hr poststarvation and counted 1.5 hr after treatment. The percentage cell migration was determined by counting 200–250 cells in random fields; only cells bearing processes 1–1.5 times the length of the cell body were counted as positive. Cells with multiple processes were counted only once. Experiments were repeated until at least 1,000 cells were counted for each condition.
The sAC-overexpressing PC12 mutant cells (or lacZ-expressing control cells) were plated in blasticidin (1 μg/ml) selection media at a density of 0.5–1.0 × l04 cells/ml and 24 hr later were fed with low-serum media without blasticidin. Twenty-four hours later, cells were treated with 200 ng/ml NGF ± 200 nM PMA ± 0.5 mM 8Br-cAMP as indicated. Cells were counted using the same methods as described for wild-type PC12 cells.
cAMP Measurements
Cellular cAMP accumulation was measured in PC12 cells that were seeded at 2.0 × 105 density in 24-well plates; serum-starved for 16 hr (see under Cell culture); and treated for 3 min with 500 μM IBMX and 200 nM PMA, 4 μM Ro 31-8220, 1 μM calphostin C, 50 μM ddAdo, or 50 μM KH7 as indicated in the figure legends; then lysed in 0.1 N HCl. cAMP was quantitated in cell lysates using the Correlate-EIA cAMP assay (Assay Design, Ann Arbor, MI), and the results were analyzed by using the paired t-test in Prism software.
Adenylyl cyclase activity in cellular extracts was assayed in the presence of 2.5 mM ATP, 10 mM MgCl2, 50 mM Tris-HCl (pH 7.5), 500 μM IBMX, 20 mM creatine phosphate, 100 U/ml creatine phosphokinase (CPK), and 1 mM dithiothreitol (DTT) in the presence or absence of 40 mM NaHCO3 or 10 μM forskolin. cAMP generated was measured after 30 min at 30°C using a Correlate-EIA cAMP assay as described above.
siRNA Reagents and Transfection
siRNA against sAC corresponded to nucleotides 692–710 (CCAAGTGTATGGCCTTCAT) relative to the first nucleotide of the start codon of rat sAC (GenBank accession No. AF081941). As a control, we utilized a commercially available siRNA control nonsilencing sequence (Qiagen, Valencia, CA). PC12 cells were transfected with siRNA (control or sAC-specific) using Gene Silencer Transfection Reagent (Gene Therapy Systems, San Diego, CA), following the manufacturer’s protocol. Cells were used for experiments approximately 48 hr after siRNA transfection.
RESULTS
Fast Migration of PC12 Cells Is Partially Blocked by sAC Inhibition
We recently utilized the small molecule sAC inhibitor KH7 as well as sAC-specific siRNA to show that sAC is uniquely responsible for NGF-elicited cAMP generation and mediates NGF-induced Rap1 activation (Stessin et al., 2006). Therefore, we explored the role of sAC in the fast neuronal migration triggered by costimulation of PC12 cells with NGF and PMA (Glowacka et al., 1992). In the absence of any inhibitors, migration was observed in 140 (±15.9) of every field of 200 cells treated with the combination of NGF + PMA (Fig. 1). As previously reported (Glowacka and Wagner, 1990; Glowacka et al., 1992), NGF alone and PMA alone resulted in no observable fast migration (data not shown). Inhibition of sAC with KH7 decreased the number of NGF- + PMA-induced migrating PC12 cells to 80 per 200 (±16.5; Fig. 1B). Negative controls included vehicle alone and a KH7-related compound (KH7.15; 132 per 200 cells, ±8.2), which does not inhibit mammalian sAC. The inhibitory effects of KH7 were rescued by the addition of a cell-permeable cAMP analog (8-Br-cAMP; 140 per 200 cells, ±2.5).
Fig. 1.
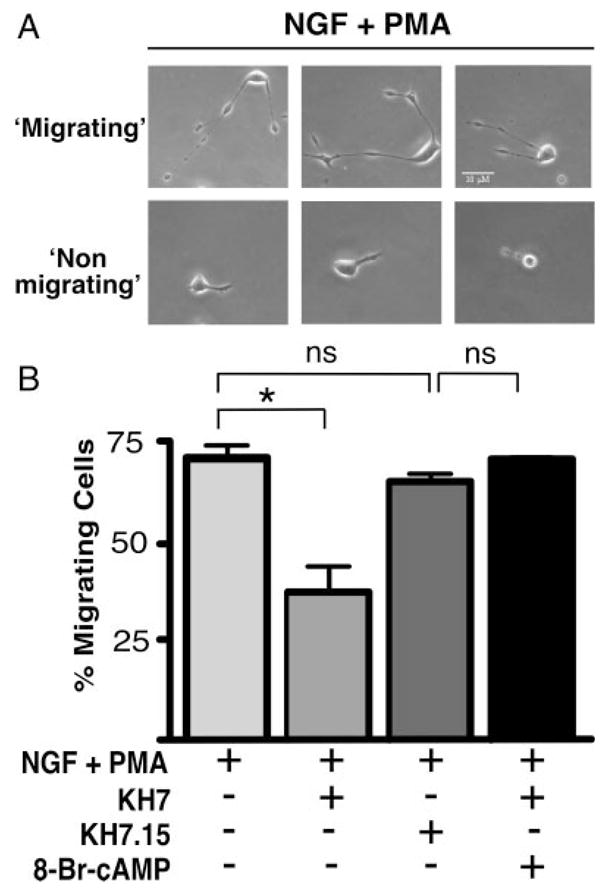
Fast migration of PC12 cells is partially blocked by inhibiting sAC. A: Phase-contrast photography of three fields with representative examples of cells scored as migrating or nonmigrating. B: Cells plated on laminin were treated with NGF (200 ng/ml) + PMA (200 nM) in the presence or absence of KH7 (50 μM), KH7.15 (50 μM), or KH7 (50 μM) + 8-Br-cAMP (0.5 mM). Bars represent the percentage of migrating cells scored 1.5 hr after treatment. Values are based on a minimum of 1,000 cells per condition counted from at least three separate experiments with SEM indicated. *P < 0.05; ns, indicates not statistically significant (P > 0.2).
The specificity of KH7 and the role of sAC in fast migration were confirmed using RNAi. In PC12 cells, sAC-specific siRNA caused substantial knockdown of the sAC protein (Fig. 2A, inset) and abrogated sAC-dependent, bicarbonate-stimulated cyclase activity without affecting tmAC-dependent, forskolin-stimulated activity (Fig. 2A). The seemingly complete knockdown of sAC activity (in both in vitro adenylyl cyclase activity assay and cellular migration assay) is inconsistent with the partial knockdown of sAC protein detectable by immunoblotting. This discrepancy likely reflects inherent limitations in the sensitivities of these two assays. As seen with KH7, inhibition of sAC by RNAi caused decrease in NGF- + PMA-induced cell migration from 127 per 200 cells (±10.6) to 71 per 200 cells (±5.7), and this inhibition was rescued by addition of 8-Br-cAMP (119 per 200 cells, ±8.4; Fig. 2B). Addition of KH7 to the sAC-specific siRNA transfectants had no further effect on fast migration (65 per 200 cells, ±4.9; Fig. 2B); thus, the observed effects of KH7 are solely mediated through sAC inhibition.
Fig. 2.
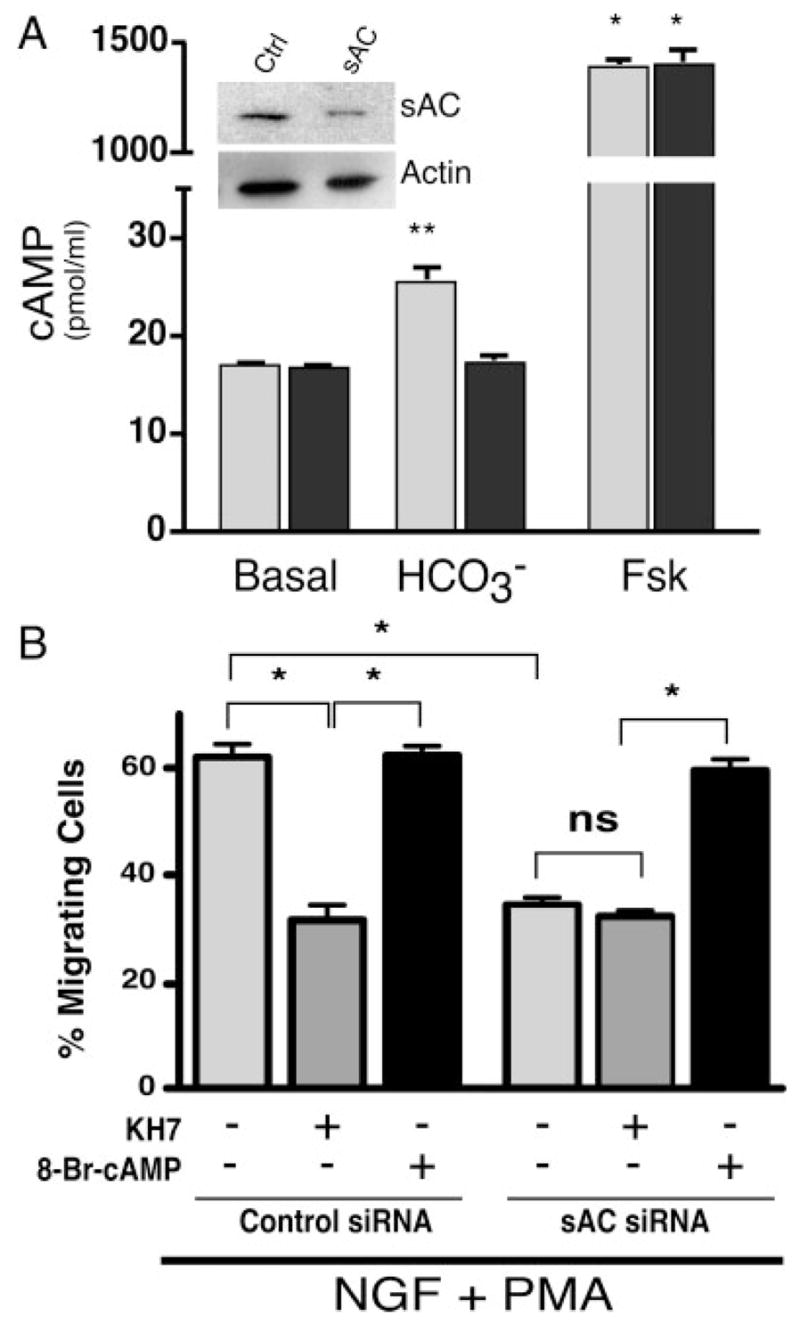
sAC-specific siRNA abrogates sAC activity and blocks cell migration. A: Cells were transfected with either nonsilencing control sequence (ctrl, light gray bars) or sAC-specific sequence (sAC, dark gray bars). Whole-cell extracts were immunoblotted with sAC-specific monoclonal antibody R21 (inset, top panel); equal loading was confirmed by subsequent immunoblotting with polyclonal antisera against actin (inset, bottom panel). Whole-cell extracts were also assayed for in vitro adenylyl cyclase activity in the presence or absence of the sAC-specific activator bicarbonate (40 mM) or the tmAC-specific activator forskolin (10 μM). Values represent averages of at least triplicate determinations repeated at least three times with SE indicated. *P < 0.001, **P < 0.005; all other values were not statistically different. B: Cells, transfected with either nonsilencing sequence (left) or sAC-specific sequence (right), were treated with NGF (200 ng/ml) + PMA (200 nM) in the presence or absence of KH7 (50 μM) or 8-Br-cAMP (0.5 mM). Bars represent the percentage of migrating cells scored 1.5 hr after treatment. There was no statistical difference in migration between untransfected cells and cells transfected with control siRNA. Values are based on a minimum of 1,000 cells per condition with SEM indicated. *P < 0.05; ns, not statistically significant (P > 0.2).
NGF-Triggered Fast Migration Signals Include, but Are Not Limited to, sAC Activation
Glowacka and Wagner (1990) demonstrated that fast migration in PC12 was dependent on NGF and phorbol ester; cAMP alone was insufficient to induce migration. PC12 cells treated with PMA + 8-Br-cAMP exhibited migration rates of 68 per 200 cells (±8.9), or one-half of the rates that were observed in cells treated with NGF + PMA (136 per 200 cells, ±9.8; Fig. 3), suggesting that NGF exerts its effects on fast migration via multiple effectors. We tested this hypothesis by stably overexpressing sAC protein in PC12 cells to determine whether sAC-generated cAMP could supplant the requirement for NGF. PMA alone induced no migration above basal level in control PC12 cells, stably expressing lacZ protein (16 per 200 cells, ±5.6). In contrast, PMA increased migration of PC12 cells that stably overexpress sAC protein (64 per 200 cells, ±4.1). The migration frequencies in sAC-overexpressing cells matched those in control cells treated with PMA + 8-Br-cAMP (68 per 200 cells, ±8.9), and the migration frequency of PMA-treated sAC-overexpressing cells was unaffected by addition of exogenous 8-Br-cAMP (60 per 200 cells, ±4.3). Thus, it appears that sAC is capable of providing all of the cAMP relevant to fast migration in PC12 cells but that stimulation of sAC is not the only contributor downstream of NGF.
Fig. 3.
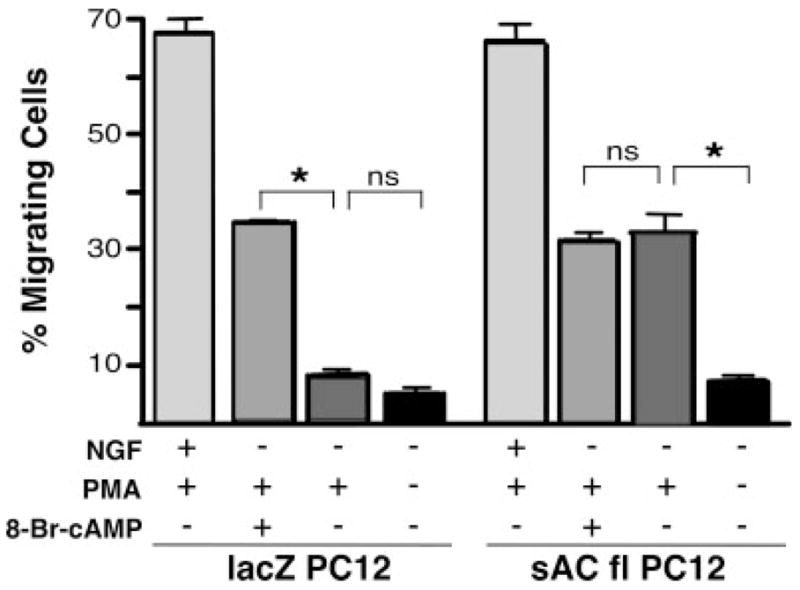
NGF-triggered fast migration signals include, but are not limited to, sAC activation. LacZ-expressing control PC12 cells (lacZ PC12) or PC12 cells overexpressing sAC (sAC fl PC12) were plated on laminin and treated with NGF (200 ng/ml) + PMA (200 nM), PMA (200 nM) + 8-Br-cAMP (0.5 mM), PMA (200 nM) alone, or vehicle. Bars represent the percentage of migrating cells scored 1.5 hr after treatment. Values are based on a minimum of 1,000 cells per condition, accumulated over at least three experiments, with SEM indicated. *P < 0.05; ns, not statistically significant (P > 0.2).
Fast Migration Is Unaffected by PMA-Elicited cAMP Elevations
Among the known consequences of PMA stimulation of PKC is the elevation of intracellular cAMP; PKC can specifically stimulate the activity of a number of tmAC isoforms (Choi et al., 1993; Jacobowitz et al., 1993; Yoshimura and Cooper, 1993; Jacobowitz and Iyengar, 1994; Kawabe et al., 1994, 1996; Morimoto and Koshland, 1994). We performed cAMP accumulation assays to determine whether PMA treatment might also modulate cAMP levels in PC12 cells. PMA treatment induced a significant increase in cAMP over basal levels (Fig. 4A). As predicted, this elevation was blocked by the tmAC-selective inhibitor 2′–5′-dideoxyadenosine (2′–5′-ddAdo) as well as by the PKC inhibitors Ro 31-8220 (McKenna and Hanson, 1993) and calphostin C (Kobayashi et al., 1989), but not by the sAC inhibitor KH7. Thus, PMA signaling via PKC elevates the levels of tmAC-generated cAMP without affecting the pool of sAC-generated cAMP.
Fig. 4.
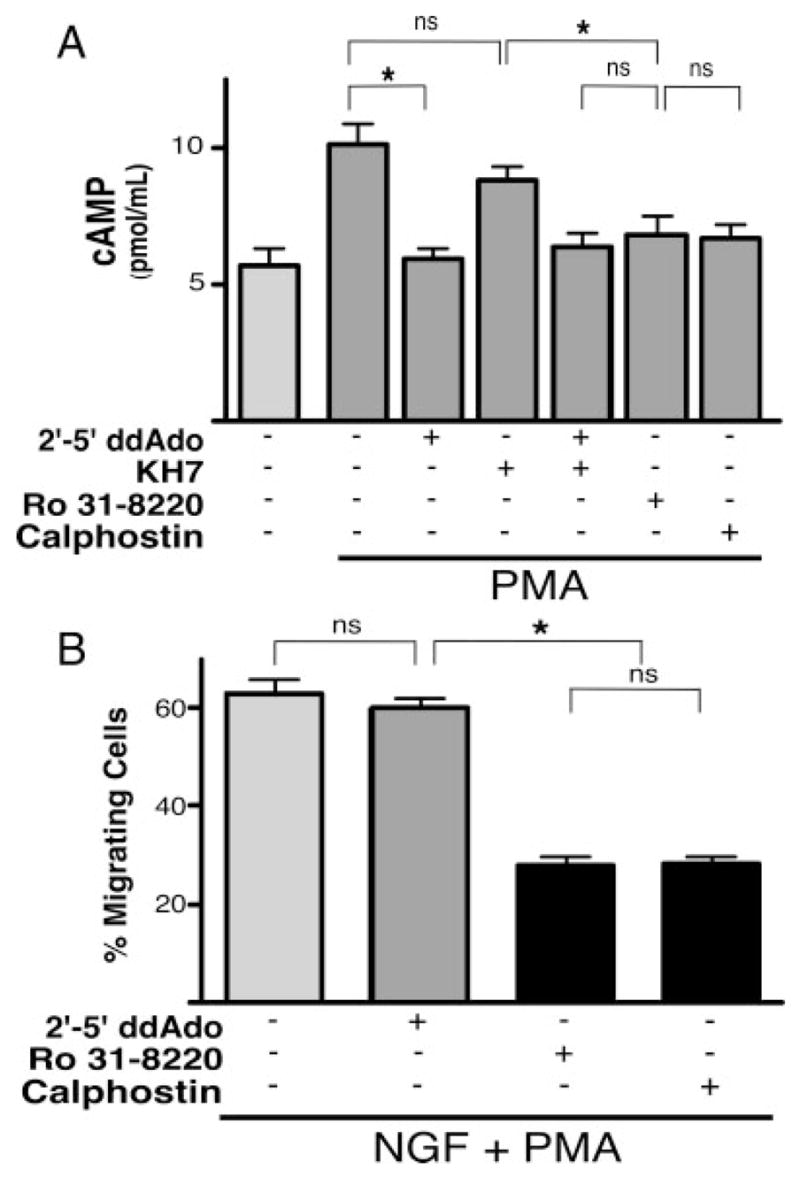
Fast migration is unaffected by PMA-elicited cAMP elevations. A: Accumulated cAMP was measured in whole cells in the presence of IBMX (500 μM) and PMA (200 nM) in the presence or absence of 2′–5′-ddAdo (50 μM), KH7 (50 μM), 2′–5′-ddAdo (1 μM) + KH7 (50 μM), Ro 31-8220 (4 μM), or calphostin C (1 μM). Bars represent the average values for at least two independent experiments, each performed in triplicate. *P < 0.05; ns, indicates not statistically significant (P > 0.2). B: Cells, plated on laminin, were treated with NGF (200 ng/ml) + PMA (200 nM) in the presence or absence of 2′–5′-ddAdo (50 μM), Ro 31-8220 (4 μM), or calphostin C (1 μM). Bars represent the percentage of migrating cells scored 1.5 hr after treatment. Values are based on a minimum of 1,000 cells per condition with SEM indicated.
We next explored the contribution of the PMA-induced, tmAC-generated cAMP to the process of fast migration. In contrast to the results with PMA (Fig. 3), NGF did not synergize with 8-Br-cAMP nor with sAC overexpression to induce fast migration (data not shown); therefore, addition of cAMP cannot replace PMA treatment. Furthermore, we found the NGF + PMA-induced migration (130 per 200 cells, ±15.4) to be unaffected by the addition of the tmAC-selective inhibitor, 2′–5′-ddAdo (128 per 200 cells, ±11.7; Fig. 4B). On the other hand, migration was inhibited by the PKC inhibitors Ro 31-8220 (56 per 200 cells, ±5.8) and calphostin C (55 per 200 cells, ±5.2). These results indicate that, although cAMP is being generated via tmACs in response to PMA treatment, the resultant cAMP pool is not capable of supporting fast migration. Instead, PMA contributes to migration via cAMP-independent factors downstream of PKC.
DISCUSSION
Classically, cAMP was thought to be generated solely by tmACs at the plasma membrane; the second messenger was postulated to diffuse freely throughout the cytosol to reach its effectors, which can be localized to distal regions of the cell. Recent studies have undermined this model by showing that diffusion of physiologically generated cAMP is restricted to short distances (Zaccolo and Pozzan, 2002; Mongillo et al., 2004, 2006). These results demand cAMP sources to reside within close proximity to their various targets (Zippin et al., 2003, 2004; Bundey and Insel, 2004; Bornfeldt, 2006). We now show that cAMP pools, generated by different sources, do not possess the same function. Both PMA and NGF elicit cAMP production; NGF activates sAC (Stessin et al., 2006), whereas PMA stimulation of PKC activates tmACs. However, the two pools of cAMP have very different consequences. NGF-induced, sAC-generated cAMP is essential for fast migration, whereas PMA-induced, tmAC-generated cAMP is of unknown function. It will be interesting to utilize modern biophysical methods for localizing cAMP generation in vivo to determine the subcellular distribution of these distinct pools. For example, it seems likely the sAC-generated pool of cAMP will be situated in a position to modulate relevant NGF targets, such as Rap1 (Stessin et al., 2006).
As revealed in Figure 2, whole cell-based cAMP measurements can be misleading with regard to cellular processes controlled by cAMP. Application of a reagent such as the tmAC-specific activator forskolin yields very large changes in whole-cell cAMP, which may have little physiological relevance to a particular biological process. In contrast, the microdomain model of cAMP action dictates that subtle changes in whole-cell cAMP, as can be observed when using bicarbonate to stimulate endogenous sAC activity in an in vitro assay or during NGF treatment of PC12 cells (Stessin et al., 2006), can have profound physiological consequences. In the future, great care should be exercised when using pharmacological agents that alter cAMP.
Both NGF and PMA must trigger other signaling pathways, which contribute to fast migration. Exogenous cAMP or sAC overexpression only partially replaces NGF treatment; thus, sAC mediates only one of the arms of NGF signaling that is essential for fast migration. Both PMA-induced activation of tmACs and the effects of PMA on fast migration are mediated via PKC; however, tmACs do not appear to play a role in the migration. Therefore, PKC can be thought of as a branching point, transducing signals in two directions: one via undetermined intermediates to elicit fast migration and the other via tmACs to exert a different set of biological effects.
Investigation of NGF and PKC signaling in the context of cell migration carries wide implications for our understanding of central nervous system development. Both PKC and NGF have been implicated in the morphological changes associated with CNS development and maintenance. In particular, PKC has been shown to promote neuronal migration via activation of focal adhesion proteins (Choe et al., 2003), whereas NGF is known to promote the survival and differentiation of multiple neuronal subtypes by its regulation of the MAP kinase and phosphoinositide kinase cascades (Chao et al., 2006). NGF was also recently shown to play a role in the shaping of the neural tube in chick embryos (Bhargava, 2007). Interestingly, one study proposed that NGF-induced neuronal differentiation may require inhibition of PKC-delta (Gallagher et al., 2000). Clearly, the roles of different PKC isoforms in migration and differentiation processes in various neuronal populations demand further study.
NGF and other neurotrophins are known to exert their actions via two types of receptors: the high-affinity tropomyosin-related tyrosine kinase (Trk) receptors and the low-affinity receptor p75NTR, a member of the tumor necrosis factor (TNF) receptor family. Interestingly, it has been suggested that Trk and p75NTR may act either as subunits of a single receptor complex or as independent transducers of distinct neurotrophin functions (Chao, 1994). Although it is generally thought that the NGF-induced activation of Rap1, which we have shown to be mediated via sAC (Stessin et al., 2006), involves TrkA, rather than p75NTR, we have also found sAC to mediate the activation of Rap1 by TNF in neutrophils (Han et al., 2005). Thus, the roles of TrkA and p75NTR in the activation of sAC by NGF are a subject for future studies.
By quantifying the number of cells with long processes, we are able to report responses of populations of cells. This approach was established in earlier studies, where the investigators had confirmed that quantification at 90 min yielded all the information gleaned from time-lapse microscopy (Glowacka and Wagner, 1990; Glowacka et al., 1992). The technique, which we currently use, allows us to examine a far greater number of cells than would be feasible with time-lapse microscopy; thus, the statistical significance of our findings can be evaluated in a more robust manner.
It is accepted that the magnitude of the migratory response can vary from experiment to experiment. This variability is likely to be caused by differences in the batches of tissue culture plastic and laminin used in the different experiments. Laminin is essential for the migratory response (Glowacka et al., 1992), but it is likely that the level of laminin binding to the plastic may vary with different batches of culture plates and different lots of laminin. Differences among batches of serum or the inevitable small variations in exact culture conditions of the PC12 cells are also likely to have influences on the magnitude of the migratory response. As we had discovered in earlier studies, the rate of migration increases rapidly after addition of NGF and PMA, but migration is relatively stable for many hours (Glowacka and Wagner, 1990; Glowacka et al., 1992; Altun-Gultekin, 1997). Importantly, we have found the inevitable differences in experimental conditions to affect the early rates of migration to a greater extent than those observed 75–120 min after stimulation. Thus, we chose to quantify the migratory response after 90 min of treatment to minimize the experiment-to-experiment variations observed at the very early time points.
Fast migration in PC12 cells is a paradigm for studying the interplay between separate signaling cascades, in that they synergize to elicit a single morphological response. Our results reveal that PMA contributes to fast migration via cAMP-independent pathways involving PKC, whereas NGF acts via multiple cascades, one of which requires sAC activation.
Acknowledgments
Contract grant sponsor: MSTP (to A.M.S.); Contract grant sponsor: NIH; Contract grant number: HD42060 (to J.B.); Contract grant number: GM62328 (to J.B.); Contract grant number: AI64842; Contract grant number: NS55255; Contract grant sponsor: American Diabetes Association; Contract grant sponsor: Hirschl Weil-Caulier Trust (to L.R.L.); Contract grant sponsor: Milstein Foundation (to J.B. and L.R.L.).
J.J.Y. thanks Ruth Gotian, Renee Horton, Elaine Velez, Kymora Scotland, and Olaf S. Andersen for support and guidance. We thank Dr. Lavoisier Ramos for assistance in quantitating cell migration, Dr. Francis S. Lee for helpful suggestions, and the members of the Levin-Buck Laboratory for critical reading of the manuscript. The authors declare that they have no competing financial interests.
References
- Altun-Gultekin ZA. Signal transduction mechanisms and the role of cytoskeleton in PC12 migration: regulation by SRC, RAS, and RAC. New York: Cornell University Press; 1997. p. 172. [Google Scholar]
- Bhargava S. Role of nerve growth factor and its receptor in the morphogenesis of neural tube in early chick embryo. Gen Comp Endocrinol (in press) 2007 doi: 10.1016/j.ygcen.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Bornfeldt KE. A single second messenger: several possible cellular responses depending on distinct subcellular pools. Circ Res. 2006;99:790–792. doi: 10.1161/01.RES.0000247760.34779.f5. [DOI] [PubMed] [Google Scholar]
- Bundey RA, Insel PA. Discrete intracellular signaling domains of soluble adenylyl cyclase: camps of cAMP? Sci STKE. 2004;2004(231):pe19. doi: 10.1126/stke.2312004pe19. [DOI] [PubMed] [Google Scholar]
- Chao MV. The p75 neurotrophin receptor. J Neurobiol. 1994;25:1373–1385. doi: 10.1002/neu.480251106. [DOI] [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci. 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- Choe Y, Jung H, Khang I, Kim K. Selective roles of protein kinase C isoforms on cell motility of GT1 immortalized hypothalamic neurones. J Neuroendocrinol. 2003;15:508–515. doi: 10.1046/j.1365-2826.2003.01023.x. [DOI] [PubMed] [Google Scholar]
- Choi EJ, Wong ST, Dittman AH, Storm DR. Phorbol ester stimulation of the type I and type III adenylyl cyclases in whole cells. Biochemistry. 1993;32:1891–1894. doi: 10.1021/bi00059a001. [DOI] [PubMed] [Google Scholar]
- Gallagher HC, Odumeru OA, Regan CM. Regulation of neural cell adhesion molecule polysialylation state by cell-cell contact and protein kinase C delta. J Neurosci Res. 2000;61:636–645. doi: 10.1002/1097-4547(20000915)61:6<636::AID-JNR7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Glowacka D, Wagner JA. Role of the cAMP-dependent protein kinase and protein kinase C in regulating the morphological differentiation of PC12 cells. J Neurosci Res. 1990;25:453–462. doi: 10.1002/jnr.490250403. [DOI] [PubMed] [Google Scholar]
- Glowacka D, Ginty DD, Wagner JA. Synergistic effects of nerve growth factor and phorbol 12-myristate 13-acetate on rapid motility and process formation in PC12 cells: the role of laminin. J Neurosci Res. 1992;31:263–272. doi: 10.1002/jnr.490310207. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. PC12 pheochromocytoma cultures in neurobiological research. Adv Cell Neurobiol. 1982;3:373–414. [Google Scholar]
- Han H, Stessin AM, Roberts J, Hess KC, Gautam N, Kamenetsky M, Lou O, Hyde E, Nathan N, Muller WA, Buck J, Levin LR, Nathan C. Role of calcium-sensing soluble adenylyl cyclase in TNF signal transduction in human neutrophils. J Exp Med. 2005;202:353–361. doi: 10.1084/jem.20050778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobowitz O, Iyengar R. Phorbol ester-induced stimulation and phosphorylation of adenylyl cyclase 2. Proc Natl Acad Sci U S A. 1994;91:10630–10634. doi: 10.1073/pnas.91.22.10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobowitz O, Chen J, Premont RT, Iyengar R. Stimulation of specific types of Gs-stimulated adenylyl cyclases by phorbol ester treatment. J Biol Chem. 1993;268:3829–3832. [PubMed] [Google Scholar]
- Kawabe J, Iwami G, Ebina T, Ohno S, Katada T, Ueda Y, Homcy CJ, Ishikawa Y. Differential activation of adenylyl cyclase by protein kinase C isoenzymes. J Biol Chem. 1994;269:16554–16558. [published erratum appears in J Biol Chem 1994 Sep 9;269:22912] [PubMed] [Google Scholar]
- Kawabe J, Ebina T, Toya Y, Oka N, Schwencke C, Duzic E, Ishikawa Y. Regulation of type V adenylyl cyclase by PMA-sensitive and -insensitive protein kinase C isoenzymes in intact cells. FEBS Lett. 1996;384:273–276. doi: 10.1016/0014-5793(96)00331-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Angeletti PU. Nerve growth factor. Physiol Rev. 1968;48:534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- McKenna JP, Hanson PJ. Inhibition by Ro 31-8220 of acid secretory activity induced by carbachol indicates a stimulatory role for protein kinase C in the action of muscarinic agonists on isolated rat parietal cells. Biochem Pharmacol. 1993;46:583–588. doi: 10.1016/0006-2952(93)90541-4. [DOI] [PubMed] [Google Scholar]
- Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, Huston E, Hannawacker A, Lohse MJ, Pozzan T, Houslay MD, Zaccolo M. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ Res. 2004;95:67–75. doi: 10.1161/01.RES.0000134629.84732.11. [DOI] [PubMed] [Google Scholar]
- Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, Zaccolo M. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res. 2006;98:226–234. doi: 10.1161/01.RES.0000200178.34179.93. [DOI] [PubMed] [Google Scholar]
- Morimoto BH, Koshland D., Jr Conditional activation of cAMP signal transduction by protein kinase C. The effect of phorbol esters on adenylyl cyclase in permeabilized and intact cells. J Biol Chem. 1994;269:4065–4069. [PubMed] [Google Scholar]
- Stessin AM, Zippin JH, Kamenetsky M, Hess KC, Buck J, Levin LR. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J Biol Chem. 2006;281:17253–17258. doi: 10.1074/jbc.M603500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Stork PJ, Lazarovici P, Eiden LE. Signaling pathways for PC12 cell differentiation: making the right connections. Science. 2002;296:1648–1649. doi: 10.1126/science.1071552. [DOI] [PubMed] [Google Scholar]
- Wu KY, Zippin JH, Huron DR, Kamenetsky M, Hengst U, Buck J, Levin LR, Jaffrey SR. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat Neurosci. 2006;9:1257–1264. doi: 10.1038/nn1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Cooper DMF. Type-specific stimulation of adenylylcyclase by protein kinase C. J Biol Chem. 1993;268:4604–4607. [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003;17:82–84. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- Zippin JH, Farrell J, Huron D, Kamenetsky M, Hess KC, Fischman DA, Levin LR, Buck J. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J Cell Biol. 2004;164:527–534. doi: 10.1083/jcb.200311119. [DOI] [PMC free article] [PubMed] [Google Scholar]


