Abstract
Our previous studies demonstrated that urine contains DNA derived from the circulation and that this DNA originated, in part, from organ sites and tumors distal to the urinary tract. To explore the potential use of DNA from urine as compared to other body fluids as a source for circulating DNA for cancer detection, the DNA concentration and the frequency of detection of mutated Kristin-ras (K-ras) DNA in serum, plasma, and urine were examined. The concentration of DNA in the urine was similar to that in the serum, but the DNA concentration in plasma was significantly lower than in either urine or serum (P < 0.05). When DNA derived from 10 μL of body fluid was used in each mutation assay, the detection frequency of mutated K-ras DNA was comparable among serum, plasma, and urine. However, when DNA derived from 200 μL of body fluid was used, the incidence of detecting mutated K-ras DNA in urine was significant higher (95%) than in either serum (35%) or plasma (40%) (P < 0.0005), suggesting that inhibitory factors in serum/plasma may be more limiting than in urine. The use and practicality of urine as a source of circulating DNA for cancer detection are discussed.
Keywords: urine DNA, circulating DNA, cancer biomarkers, K-ras mutations, cancer detection
Introduction
Urinalysis for tumor DNA has been suggested for detection of tumors located in or close to the urinary tract, such as kidney or bladder cancer.1–7 We8 and others9,10 have shown that short-length, tumor-derived DNA from non–urinary tract can be filtered through the kidney barrier and can be excreted into urine. Two distinct size categories of DNA can be noted by ethidium bromide–stained agarose gel electrophoresis of total urine DNA, as previously described.8 They are the high-molecular-weight (MW) DNA, which was derived from sloughed-off urinary tract cells, and the low-MW DNA that was previously suggested to be mostly derived from the circulation.8 Thus, we reason that analysis of DNA in urine should not be limited to the diseases of the urinary tract, but could also be a valuable tool for virtually every cancer entity with known DNA alterations.
Potential applications of urine DNA analysis are similar to those expected from the analysis of plasma DNA11–20 and include cancer screening, cancer detection, monitoring of tumor growth, evaluation of the effectiveness of tumor chemotherapy or radiation therapy, and monitoring of tumor recurrence. Urine collection is an absolutely noninvasive procedure, can be done in remote geographic areas, and requires no special facility or equipment apart from sterile collection containers, as compared to the requirements for serum or plasma collection. The amount of urine that can be collected from patients readily exceeds the amount of serum or plasma normally collected. Thus, it was of interest to explore the potential of using urine in the area of noninvasive cancer screening or detection.
We have previously used the K-ras codon 12 mutation as a marker for circulating DNA to demonstrate that genetic alterations that occurred in the colon could be detected in urine.8 To further evaluate the potential of using urine as a source of circulating DNA for detection of colorectal cancer (CRC) biomarkers, we compared the DNA quantity in the blood (serum and plasma) and urine, and the incidence of detection of mutated K-ras DNA in serum, plasma and urine from individuals with a diagnosis of colorectal disease containing K-ras mutations.
The K-ras mutation was chosen for two reasons: (1) this is an important DNA biomarker for CRC21–24 and adenomatous polyps,25–27 a precursor of colon cancer, and (2) the incidence of K-ras gene mutation occurring in cancer of the urinary tract is rare.28 Thus, mutated K-ras DNA can be used as a representative of a mutation occurring at a tumor site outside of the urinary tract. Here we report that the ability to detect mutated K-ras DNA in urine could be significantly higher than in serum or plasma of patients with known K-ras mutations in their disease tissues, when a larger volume (200 μL) of body fluid is used in the assay.
Materials and Methods
Study Subjects and Selection
Participants were recruited from the Great Lakes–New England Clinical Epidemiological Center, which includes the University of Michigan, Dana-Farber Harvard Cancer Center, M.D. Anderson Cancer Center, and the University of Toronto. Cancer patients were enrolled from the surgical or oncological services (prior to initiation of chemo- or radiation therapy or surgery). Patient samples were obtained under IRB strictures.
Collection of Body Fluid
Urine Collection
Urine samples were collected from patients during office visits. Freshly collected urine was immediately mixed with 0.5 M EDTA, pH 8.0 to a final concentration of 10 mM EDTA, in order to inhibit possible nuclease activity in the urine sample; this was stored at −70°C. To isolate total urine DNA, frozen urine samples were thawed at room temperature, and then placed immediately on ice prior to DNA isolation. Thawed urine was processed for DNA isolation within an hour.
Serum and Plasma Collection
Fifty milliliters of blood were collected in heparinized containers from informed and consenting subjects. The samples were permitted to sit at room temperature for a minimum of 30 minutes (and a maximum of 60 minutes) to allow the clot to form in the red-top tubes; they were then centrifuged at 1,300 × g at 4°C for 20 minutes. The serum or plasma was transferred to a polypropylene capped tube, and frozen on dry ice.
DNA Isolation
Five milliliters (mL) of urine samples were mixed with 1.5 volume of 6M guanidine thio-cyanate (Sigma, St. Louis, MO, USA) by inverting eight times. One milliliter of resin (Wizard Plus Mini-Prep DNA Purification System, Promega, Madison, WI, USA) was added into the urine lysate and incubated overnight at room temperature with gentle mixing. The resin–DNA complex was centrifuged, transferred to a mini-column (provided from the kit), and washed with a buffer provided by the manufacturer. Then, the DNA was eluted with H2O. 0.5 mL of serum and plasma DNA was isolated, as for urine, except for using a 2.5 volume of 6M guanidine thiocyanate. DNA from paraffin-embedded tissue sections was isolated using the MasterPure DNA Kit (Epicentre, Madison, WI, USA) according to the manufacturer’s instruction.
K-ras Codon 12 Mutation Assay
A restriction enriched polymerase chain reaction (RE-PCR) described previously8,29 was used to assay for mutated K-ras DNA. In brief: 20 cycles of Hot-Start PCR were performed as follows: DNA, with 1.5 mM MgCl2, 1× PCR buffer (Qiagen, Valencia, CA, USA), 0.5 U Hot-Start Taq (Qiagen), 200 μM dNTP, 0.1 μM primers [L-Ext (5′ GCT CTT CGT GGT GTG GTG TCC ATA TAA ACT TGT GGT AGT TGG ACC T 3′) and R-Ext (5′ gct ctt cgt ggt gtg gtg tcc cgt cca caa aat gat tct ga 3′)] were mixed and denatured at 95°C for 15 min; this was followed by 20 cycles at 94°C, 30 sec; 52°C, 30 sec; and 1 cycle at 72°C for 5 min. After the first round of PCR, the amplified products were digested with BstNI (New England Biolab, Beverly, MA, USA) to eliminate amplified products derived from wild-type DNA. 1/20 of digested product was then subjected to the second Hot-Start PCR with the 1 μM primers L-Bst (5′ ACT GAA TAT AAA CTT GTG GTA GTT GGA CCT 3′) and R-Bst (5′ GTC CAC AAA ATG ATC CTG GAT TAG C 3′) under the following conditions: 95°C, 15 min; 40 cycles at 94°C, 30 sec; 56°C, 30 sec; and 1 cycle at 72°C 5 min. The amplified products (87 bp) of the second PCR were digested to completion with BstNI (as shown by disappearance of the 87-bp DNA fragment), and resolved through polyacrylamide gels. Each sample was assayed 2–3 times. The samples were scored as “positive” for K-ras mutation when the 71-bp DNA fragment appeared after the second BstNI digestion of the PCR products in 2 of 2 or 2 of 3 repeated assays. As validation controls, HepG2 cell DNA, containing wild-type K-ras reconstituted with various amounts of K-ras codon 12–mutated SW480 DNA, was included in each assay.
Size Fractionation of Urine DNA and DNA Quantification
Total urine DNA was fractionated by 2% agarose gel electrophoresis. The agarose slices corresponding to desired ranges of DNA sizes were excised and subjected to DNA isolation using the QIAquick Gel Extraction Kit (Qiagen) and resuspended in 20 μL of H2O. DNA was quantified by real-time PCR using K-ras Q-primers (forward: 5′ AGG CCT GCT GAA AAT GAC TG 3′, reverse: 5′ TTG GAT CAT ATT CGT CCA CAA 3′, product size of 116 bp), with the hybridization probes (0.15 μM Sensor LC Red640-TTG CCT ACG CCA CAA GGT CCA a-P; 0.3 μM Anchor CCA CAA AAT GAT TCT GAA TTA GCT GTA TCG TCA AGG CAC-X). These quantification reactions were performed using the LightCycler thermocycler (Roche Biochemical, Mannheim, Germany) at 95°C for 10 min, 45 cycles at 95°C for 5 s, 70°C for 15 s, 55°C for 10 s, and 72°C for 10 s. As calibrators for quantification, serially diluted genomic DNA was used.
Results
The Quantity of DNA in Urine, Serum, and Plasma from Each Individual
Previously, we and others have suggested that urine can be a source for circulating DNA.8,29 Thus, it was of interest to compare the sensitivity of detecting genetic mutations in urine with that of two other body fluids, plasma and serum, that have been extensively used as a source of circulating DNA. The circulating DNA marker chosen in this study is mutated K-ras DNA, which occurs rarely in tumors of the urinary tract.28 In order to compare the incidence of K-ras mutations in three body fluids, we used the body fluids from 20 patients having known mutant K-ras colorectal diseases (adenomatous ployps with or without hyper-plastic polyps, or colorectal cancer). The diagnosis, gender, and age information of the human subjects used in this study are listed in Table 1. The existence of the K-ras mutation in the diseased tissue was first verified by performing RE-PCR K-ras mutation assay, as described in the Materials and Methods section (data not shown).
TABLE 1.
Study Subjects and Detection of Mutated K-ras DNA in Urine, Serum, and Plasma of Each Subject
| Total DNAb |
Mutant K-rasd |
|||||
|---|---|---|---|---|---|---|
| (ng/mL body fluidc)
|
Urine/Serum/Plasma
|
|||||
| Patienta | Urine | Serum | Plasma | 200 μLe | 10 μLe | Diagnosis/Gender/Age |
| 1 | < 1.5 | 6.7 | < 1.5 | +/−/+ | −/−/− | CRC/M/73 |
| 2 | 1.9 | 94.8 | 5.8 | +/+/− | −/+/+ | CRC/M/85 |
| 3 | 7.2 | 6.2 | < 1.5 | +/+/− | +/−/− | CRC/M/69 |
| 4 | < 1.5 | 19.2 | 14.6 | +/+/− | −/+/+ | CRC/F/70 |
| 5 | 18.7 | 20.9 | 1.8 | +/+/− | +/+/+ | CRC/F/57 |
| 6 | 62.2 | 171.8 | 1.5 | +/−/− | +/−/+ | CRC/F/76 |
| 7 | 5.4 | 11.2 | < 1.5 | +/−/− | −/−/− | CRC/M/87 |
| 8 | 15.1 | 1.7 | 4.1 | +/−/+ | +/+/+ | Adnms/F/53 |
| 9 | 26.1 | 4.1 | 3.2 | +/−/+ | −/−/− | Adnms/F/55 |
| 10 | 3.3 | 1.8 | 8.1 | +/+/+ | −/+/+ | Adnms/M/70 |
| 11 | 14.4 | 3.1 | 7.6 | +/−/− | +/+/+ | Adnms/F/67 |
| 12 | 2.7 | 95.1 | < 1.5 | +/−/− | −/−/− | Adnms/F/67 |
| 13 | 138.3 | 4.2 | 15.1 | −/+/− | −/−/− | Adnms/F/63 |
| 14 | 18.3 | 11.3 | 5.8 | +/−/+ | +/−/+ | Adnms/M/66 |
| 15 | 2.2 | 24.2 | < 1.5 | +/−/− | −/−/+ | Adnms/M/73 |
| 16 | 33.5 | 107.2 | 12.9 | +/−/− | −/−/+ | Adnms/F/57 |
| 17 | 34.9 | 55.8 | 21.4 | +/+/− | +/−/− | Adnms & Hypl/M/51 |
| 18 | 26.8 | 12.7 | 9.9 | +/−/+ | +/−/− | Adnms & Hypl/M/59 |
| 19 | 25.1 | < 1.5 | 27.3 | +/−/+ | −/−/− | Adnms & Hypl/M/50 |
| 20 | 31.1 | 7.7 | 2.8 | +/−/+ | −/−/+ | Adnms & Hypl/F/84 |
Patients were recruited under IRB approval from the Great Lakes–New England Clinical Epidemiological Center led by Dr. Brenner, University of Michigan, Ann Arbor, MI. The disease tissue of each patient was known to contain K-ras mutation and the mutation was verified by K-ras RE-PCR assay as described in Materials and Methods on the DNA isolated from the paraffin-fixed tissue specimens.
DNA concentration (ng/mL) of each body fluid was determined by real-time PCR assay using albumin primers as previously described.30 The limit of the quantitation was 0.015 ng/μL/rxn and was calculated as 1.5 ng/mL of body fluid. “<1.5” represents the DNA concentration below the level of detection.
Three body fluids, urine, serum, and plasma, from each individual were collected under IRB approval.
The samples scored as “+” or “−” for K-ras mutation by RE-PCR assays were described in the Materials and Methods section.
The amount of DNA used in each assay was derived from either 200 μL or 10 μL of each body fluid.
CRC = colorectal cancer; Adnms = adenomatous polyps; Hypl = hyperplasia.
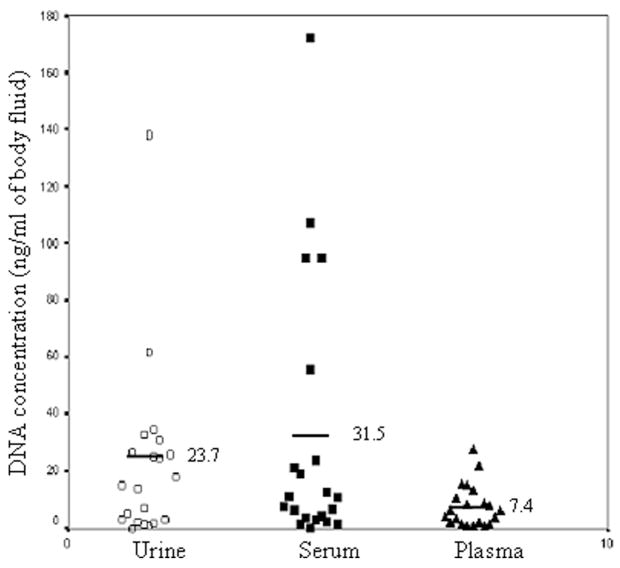
It was first of interest to determine the DNA yield from the three body fluids—urine, serum, and plasma. Three body fluids from each individual were collected according to the IRB-approved protocol. Total DNA was isolated and quantified by real-time PCR using primers specific for the albumin gene, as described previously.30 The limit of the assay was 1.5 ng/mL body fluid. The DNA concentrations in each body fluid from different individuals are listed in Table 1 and summarized in Figure 1 along with the average DNA concentration of each body fluid from 20 individuals. The concentration of DNA in urine and serum varied widely among individuals, ranging from <1.5 ng/mL (the limit of detection) to 200 ng/mL of body fluid. In contrast, the DNA concentration detected in plasma fell within a narrower range (<1.5 ng/mL to 30 ng/mL).
Figure 1.
Comparison of DNA concentration in urine, plasma, and serum from 20 individuals. DNA was isolated from urine, plasma, and serum from 20 individuals diagnosed with K-ras mutated CRC or adenomatous polyps as described in Materials and Methods. Every data point represents the DNA concentration (ng/mL) from each individual specimen as determined by real-time PCR using the albumin primers. The average (the bar and the number) of DNA concentrations for each body fluid is also indicated.
To compare the DNA concentrations of these three body fluids, the Kruskal–Wallis test of ANOVA was performed. This analysis showed there were statistical differences (P = 0.046). To compare the DNA concentration between any two body fluids, the matched-pairs Wilcoxon test was performed. The P-values were 0.4524, 0.01755, and 0.004285 for urine versus serum, serum versus plasma, and urine versus plasma, respectively. The P-values suggest that the concentration of DNA in plasma is significantly lower than that in urine or serum (P < 0.05), but there was no significant difference between the DNA concentration in urine and in serum (P = 0.4524). Collectively, the DNA concentration in plasma is significantly less than that in serum or urine; the DNA concentrations between serum and urine were not significantly different.
Incidence of Detection of Mutated K-ras DNA
Since the DNA concentration varied across a wide range, and might be complicated by how much cell debris was collected in urine or serum, to compare the ability to detect mutated K-ras DNA in three body fluids, a certain volume of each body fluid, instead of an absolute amount of DNA (ng DNA), was used in each mutation assay. DNA derived from two different volumes, 10 μL or 200 μL, was subjected to an RE-PCR K-ras mutation assay as described in Materials and Methods. The RE-PCR assay for mutated K-ras DNA is a qualitative assay with an assay sensitivity of 2 copies/input sample. The results are listed as positive (+), representing ≥ 2 copies of mutated K-ras DNA detected, or negative (−) for less than 2 copies of mutated DNA present in the input DNA (Table 1). Analysis of the results is summarized in Table 2.
TABLE 2.
Detection of Mutated K-ras DNA in Body Fluids from 20 Patients with Detectable K-ras Mutation in Colorectal Disease Tissues
| 200 μL body fluid
|
10 μL body fluid
|
|||||
|---|---|---|---|---|---|---|
| Urine | Serum | Plasma | Urine | Serum | Plasma | |
| No. of samples detected | 19 | 7 | 8 | 8 | 6 | 11 |
| Percent of detection | 95% | 35% | 40% | 40% | 30% | 55% |
| P-value | – | 0.00014 | 0.00043 | – | 0.741 | 0.527 |
DNA derived from 200 μL or 10 μL of urine, plasma, and serum of each individual was subjected to RE-PCR K-ras mutation assay. Statistical analysis was performed by the Fisher exact two-tailed test. The P-values were obtained as compared to the results of urine to serum or urine to plasma.
When 10 μL of serum, plasma, or urine was used, mutated K-ras was detected in approximately 35–40% of the samples (Table 2). When DNA derived from 200 μL of body fluid was used in each assay, the incidence of mutated K-ras DNA in urine was 19 of 20 samples, or 95%. In contrast, when 200 μL, instead of 10 μL, of serum or plasma was used, the incidence of mutated K-ras DNA was still only 35% to 40%. Using increased volumes of serum and plasma did not improve the efficiency of detection of mutated K-ras, and the reasons for this are considered in the discussion.
If 200 μL of body fluid was used in each assay, the ability to detect mutated K-ras DNA using DNA isolated from urine was significantly higher than that of serum and plasma, with P-values of 0.00014 and 0.00043, respectively, as determined by the Fisher exact two-tailed test. If 10 μL of fluid was used, the detection frequency of mutated K-ras DNA in urine (40%), serum (30%), and plasma (55%) was comparable. These data suggest that urine could be a better source of circulating DNA for detecting K-ras mutations as compared to serum or plasma, if a larger volume of body fluid can be used in the assay.
Distribution of Mutated K-ras DNA in Size-Fractionated Urine DNA
We have previously shown that mutated K-ras DNA was more concentrated in the subpopulation of urine DNA ranging from 100 bp to 400 bp compared with the urine DNA larger than 1 kb. To more comprehensively study the size-wise distribution of mutated K-ras DNA in the urine, we fractionated total urine DNA from two patients (A and B) into 10 fractions by agarose gel electrophoresis as described in Materials and Methods. The urine DNA from these two patients was chosen because of the high amount of mutated K-ras DNA present in their urine specimens. 1.0 μL of each fraction (prepared as described in Materials and Methods) was subjected to DNA quantification by real-time PCR using K-ras Q-primers and to K-ras mutation assay by RE-PCR as described in Materials and Methods. The DNA quantification using K-ras Q-primers instead of albumin primers was to determine the number of DNA templates that contain the K-ras gene sequences. The results are summarized in Table 3.
TABLE 3.
Distribution of Mutated K-ras DNA in Fractionated Urine DNA in Two Patients
| Patient A
|
Patient B
|
|||
|---|---|---|---|---|
| Fractions | DNA concentration
(ng/μL) |
Mutated K-ras DNA | DNA concentration
(ng/μL) |
Mutated K-ras DNA |
| <150 bp | ND | + | ND | + |
| 150–300 bp | 0.010 | + | 0.016 | + |
| 300–500 bp | 0.013 | + | 0.043 | + |
| 500–700 bp | 0.024 | + | 0.043 | + |
| 700 bp–1 kb | ND | + | 0.013 | − |
| 1–1.5 kb | ND | − | 0.010 | − |
| 1.5–2 kb | 0.016 | − | ND | − |
| 2–3 kb | ND | − | ND | − |
| 3–4 kb | ND | − | ND | − |
| 4–10 kb | ND | − | ND | − |
| 10 kb–well | 0.03 | + | ND | + |
DNA concentration was determined by the real-time PCR assay using K-ras Q-primers as described in Materials and Methods. The limit of detection of the assay is 0.010 ng/μL/rxn; ND: below the limit of detection.
Mutated K-ras DNA was detected by RE-PCR (≥2copies per reaction). The positive sign (+) shows that the mutated DNA was detected and the negative (−) indicates that the mutated K-ras DNA was not detected.
As expected, the mutated K-ras DNA was enriched in lower-MW DNA fractions (less than 700 bp). Surprisingly, although the number of DNA templates that contained K-ras gene sequences in the fraction of less than 150 bp was below the limit of detection [0.015 ng (or 2 copies)/μL/rxn] by real-time PCR quantification, mutated K-ras DNA was detected in this fraction from both patients by RE-PCR (≥2 copies/rxn). In addition to the small fragmented DNA fractions, mutated K-ras DNA was also detected in the fraction of ≥10 kb DNA. There was no detectable mutated K-ras DNA in the fractions ranging from 1 kb to 10 kb. Collectively, these data indicate that the majority of mutated K-ras DNA detected in urine was less than 700 bp.
Discussion
The possibility of using circulating DNA isolated from serum or plasma in the diagnosis and prognosis of disease has been explored extensively.11,13–18,31–33 Less is known about urine-derived DNA. In this study, we compared, for the first time, how volumes of body fluids can influence the sensitivity of detection of specific mutations in the DNA derived from those fluids. Mutated K-ras DNA was used as a marker for colorectal disease–derived DNA, and urine, serum, and plasma–derived DNAs were compared for the frequency of mutation detection. The results suggest that urine could be a better source than serum or plasma for detecting mutated circulating DNA for diagnosis of disease. This was demonstrated by a significantly higher incidence of detecting mutated K-ras DNA in urine than in serum or plasma when DNA derived from 200 μL of body fluid was used in each assay.
It was interesting that the relative ability to detect mutated K-ras DNA in urine, serum, and plasma was very different with different volumes of body fluid used in each assay. When DNA derived from 10 μL of body fluid from individuals with colorectal cancer or adenomatous polyps containing mutated ras proto-oncogenes was used, the detection of mutated K-ras DNA in urine, serum, and plasma was comparable. Mutated ras was detected in each body fluid approximately 40% of the time. However, when DNA derived from 200 μL of urine was used, the incidence of detecting mutated K-ras DNA increased from 40% to 95%. This was not the case for serum or plasma. The efficiency in detecting mutated K-ras DNA from 10 μL or 200 μL of serum or plasma was similar.
It is possible that low levels of contaminants exist in the DNA isolated from serum or plasma that are either not present or are much less abundant than in urine. In this model, the amount of contaminants is low, such that their interferences with PCR is limited in the small volumes of sample, but becomes a much more significant factor in larger amounts of plasma or serum.
Previously, we reported that the sensitivity of detection of circulating tumor DNA in serum varied with the methods of DNA isolation.30 We found that methods that preferentially isolate small DNA provide better substrates for detecting mutated K-ras DNA than methods that do not discriminate between large and small DNA. Urine contains small-MW circulating DNA filtered through the kidney barriers. Macromolecules, such as proteins, and circulating long DNA, are retained in the circulation under normal physiological conditions. These macromolecules could possibly interfere with PCR amplification. DNA isolated from urine could be less contaminated with possible PCR inhibitors as compared with DNA isolated directly from the circulation; thus, the incidence of detecting a target sequence increases when a greater amount of the substrate is used.
By monitoring the appearance of mutated K-ras DNA in each fraction of the total urine DNA separated by size, we show that the size of DNA derived from the circulation present in the urine was predominately less than 700 bp. Of interest, we can also detect a limited amount of mutated K-ras DNA in two higher-MW DNA fractions (larger than 700 bp, as shown in Table 2). It is possible that this is due to an incomplete separation of low MW from high MW by agarose gel electrophoresis. The other possibility is that the limited amount of mutated K-ras DNA detected in higher-MW DNA fractions was indeed derived from high-MW DNA templates. This would suggest that high-MW DNA can pass through the kidney barrier, or be actively transported into urine, or that the K-ras mutation derives from the dying cells of the urinary tract. There has been no evidence to distinguish between these possibilities yet.
Although the amount of DNA in the fraction less than 150 bp was below the level of detection by our real-time PCR assay, mutated K-ras DNA was detected in this fraction from both patients by RE-PCR. Note that the amplicon generated in RE-PCR is only 76 nt, whereas the amplicon generated by the K-ras Q-primers for DNA quantification is 116 bp. Assuming circulating DNA found in urine was generated by random breakage, the possibility that a DNA molecule in the fraction <150 bp contains both RE-PCR primer sequences is higher than that of K-ras Q-primer sequences. Therefore, it is likely that the RE-PCR assay was more sensitive because more templates contained sequences corresponding to both primers used in this assay, even though some of these templates would not be amplified by the K-ras Q-primers in the quantification assay.
The quantity of circulating DNA in plasma and serum has been shown to increase in patients with variety of medical conditions such as autoimmune disease, chronic inflammatory states, infectious disease, and cancer.20,34–38 In addition, the circulating DNA concentration can vary with methods of blood collection, processing, and analysis. In order to investigate whether the frequency of detecting the mutated DNA in urine, serum, and plasma is associated with the quantity of DNA, we compared the quantity of genomic DNA among these three body fluids. The concentration of DNA in urine and serum varied widely among individuals, ranging from undetectable to 200 ng/mL of body fluid. In contrast, the DNA concentration detected in plasma is in a narrower range (below the level of detection to 30 ng/mL). These ranges are within those reported previously.20,32,35 Interestingly, our results indicate that the quality rather than the quantity of DNA might contribute to a significantly higher detection rate (95%) of mutated K-ras DNA in urine as compared to two other body fluids, serum (35%) and plasma (40%), when DNA isolated from a larger volume (200 μL) of body fluid was used in each assay.
It has been suggested by several investigators that the major source for tumor-derived circulating DNA is apoptotic cells and necrotic cells.19,39,40 Apoptosis of tumor cells can be due to the elimination of tumor cells by the immune system or mutations of anti-apoptotic genes, such as the K-ras gene. During the early stage of carcinogenesis, the rate of apoptosis increased as suggested.41–43 Of interest, a typical apoptotic DNA pattern, a nucleosome-sized DNA ladder, was observed for the DNA isolated from the circulation previously by us8 and others.39 Studies by Jahr et al.39 suggested that the sizes of DNA derived from necrotic cells were of higher MW, approximately larger than 10 kb. Here, we show that the circulating DNA found in urine is most likely in sizes less than 700 bp; thus, the DNA from necrotic cells might not be excreted into urine unless further degradation of DNA occurs in the circulation. This could be one limitation of using urine DNA as a source for circulating DNA if one requires the DNA substrate from the necrotic cells. Nevertheless, the presence of circulating tumor-derived DNA in urine holds great promise, encouraging further exploration of the potential of using urine DNA biomarkers for noninvasive disease screening, diagnosis, and prognosis.
Acknowledgments
This work was supported, in part, by the Early Detection Research Network (EDRN) of the National Cancer Institute, the Hepatitis B Foundation of America, and an appropriation from The Commonwealth of Pennsylvania. The authors acknowledge the Great Lakes–New England Clinical Epidemiological Centers (within the EDRN, NCI, Grant CA86400) for providing clinical specimens.
Footnotes
Conflicts of Interest The authors declare no conflicts of interest.
References
- 1.Utting M, et al. Microsatellite analysis of free tumor DNA in urine, serum, and plasma of patients: a minimally invasive method for the detection of bladder cancer. Clin Cancer Res. 2002;8:35–40. [PubMed] [Google Scholar]
- 2.Hoque MO, et al. High-throughput molecular analysis of urine sediment for the detection of bladder cancer by high-density single-nucleotide polymorphism array. Cancer Res. 2003;63:5723–5726. [PubMed] [Google Scholar]
- 3.Cairns P. Detection of promoter hypermethylation of tumor suppressor genes in urine from kidney cancer patients. Ann NY Acad Sci. 2004;1022:40–43. doi: 10.1196/annals.1318.007. [DOI] [PubMed] [Google Scholar]
- 4.Dulaimi E, et al. Promoter hypermethylation profile of kidney cancer. Clin Cancer Res. 2004;10:3972–3979. doi: 10.1158/1078-0432.CCR-04-0175. [DOI] [PubMed] [Google Scholar]
- 5.Dulaimi E, et al. Detection of bladder cancer in urine by a tumor suppressor gene hypermethylation panel. Clin Cancer Res. 2004;10:1887–1893. doi: 10.1158/1078-0432.ccr-03-0127. [DOI] [PubMed] [Google Scholar]
- 6.Cairns P, et al. Molecular detection of prostate cancer in urine by GSTP1 hypermethylation. Clin Cancer Res. 2001;7:2727–2730. [PubMed] [Google Scholar]
- 7.Papadopoulou E, et al. Cell-free DNA and RNA in plasma as a new molecular marker for prostate cancer. Oncol Res. 2004;14:439–445. doi: 10.3727/0965040041791473. [DOI] [PubMed] [Google Scholar]
- 8.Su YH, et al. Human urine contains small, 150 to 250 nucleotide-sized, soluble DNA derived from the circulation and may be useful in the detection of colorectal cancer. J Mol Diagn. 2004;6:101–107. doi: 10.1016/S1525-1578(10)60497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botezatu I, et al. Genetic analysis of DNA excreted in urine: a new approach for detecting specific genomic DNA sequences from cells dying in an organism. Clin Chem. 2000;46:1078–1084. [PubMed] [Google Scholar]
- 10.Serdyuk OI, et al. Detection of mutant k-ras sequences in the urine of cancer patients. Bull Exp Biol Med. 2001;131:283–284. doi: 10.1023/a:1017624120807. [DOI] [PubMed] [Google Scholar]
- 11.Taback B, Hoon DSB. Circulating nucleic acids and proteomics of plasma/serum: clinical utility. Ann NY Acad Sci. 2004;1022:1–8. doi: 10.1196/annals.1318.002. [DOI] [PubMed] [Google Scholar]
- 12.Chan AKC, et al. Cell-free nucleic acids in plasma, serum and urine: a new tool in molecular diagnosis. Ann Clin Biochem. 2003;40:122–130. doi: 10.1258/000456303763046030. [DOI] [PubMed] [Google Scholar]
- 13.Lo YM, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- 14.Nawroz H, et al. Microsatellite alterations in serum DNA of head and neck cancer patients. Nat Med. 1996;2:1035–1037. doi: 10.1038/nm0996-1035. [DOI] [PubMed] [Google Scholar]
- 15.Chen XQ, et al. Microsatellite alterations in plasma DNA of small cell lung cancer patients. Nat Med. 1996;2:1033–1035. doi: 10.1038/nm0996-1033. [DOI] [PubMed] [Google Scholar]
- 16.Goessl C, et al. Microsatellite analysis of plasma DNA from patients with clear renal carcinoma. Cancer Res. 1998;58:4728–4732. [PubMed] [Google Scholar]
- 17.Wong IHN, et al. Detection of aberrant p16 methylation in the plasma and serum of liver cancer patients. Cancer Res. 1999;59:71–73. [PubMed] [Google Scholar]
- 18.Esteller M, et al. Detection of abbertant promoter hypermethylation of tumor suppressor genes in serum DNA from non-small cell lung cancer patients. Cancer Res. 1999;59:67–70. [PubMed] [Google Scholar]
- 19.Pathak AK, et al. Circulating cell-free DNA in plasma/serum of lung cancer patients as a potential screening and prognostic tool. Clin Chem. 2006;52:1833–1842. doi: 10.1373/clinchem.2005.062893. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro B, et al. Determination of circulating DNA Levels in patients with benign of malignant gastrointestinal disease. Cancer. 1983;51:2116–2120. doi: 10.1002/1097-0142(19830601)51:11<2116::aid-cncr2820511127>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Maltzman T, et al. Ki-ras proto-oncogene mutations in sporadic colorectal adenomas: relationship to histologic and clinical characteristics. Gastroenterology. 2001;121:302–309. doi: 10.1053/gast.2001.26278. [DOI] [PubMed] [Google Scholar]
- 22.Ahlquist DA, et al. Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology. 2000;119:1219–1227. doi: 10.1053/gast.2000.19580. [DOI] [PubMed] [Google Scholar]
- 23.Rashid A, et al. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: a population-based study in China. Clin Cancer Res. 2002;8:3156–3163. [PubMed] [Google Scholar]
- 24.Vogelstein B, et al. Genetic alterations during colorectal-tumor development. New Engl J Med. 1998;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 25.Andersen S, et al. Villous, hypermucinous mucosa in long standing ulcerative colitis shows high frequency of K-ras mutations. Gut. 1999;45:686–692. doi: 10.1136/gut.45.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otori K, et al. High frequency of K-ras mutations in human colorectal hyperplastic polyps. Gut. 1997;40:660–663. doi: 10.1136/gut.40.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takayama T, et al. Analysis of K-ras, APC, and B-catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis. Gastroenterology. 2001;121:599–611. doi: 10.1053/gast.2001.27203. [DOI] [PubMed] [Google Scholar]
- 28.Przybojewska B, et al. H-RAS, K-RAS, and N-RAS gene activation in human bladder cancers. Cancer Genet Cytogenet. 2000;121:73–77. doi: 10.1016/s0165-4608(00)00223-5. [DOI] [PubMed] [Google Scholar]
- 29.Su YH, et al. Transrenal DNA as a diagnostic tool: important technical notes. Ann NY Acad Sci. 2004;1022:81–89. doi: 10.1196/annals.1318.014. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, et al. Preferential isolation of fragmented DNA enhances the detection of circulating mutated k-ras DNA. Clin Chem. 2004;50:211–213. doi: 10.1373/clinchem.2003.026914. [DOI] [PubMed] [Google Scholar]
- 31.Chan KCA, et al. Molecular characterization of circulating EBV DNA in the plasma of nasopharyngeal carcinoma and lymphoma patients. Cancer Res. 2003;63:2028–2032. [PubMed] [Google Scholar]
- 32.Taback B, et al. Quantification of circulating DNA in the plasma and serum of cancer patients. Ann NY Acad Sci. 2004;1022:17–24. doi: 10.1196/annals.1318.004. [DOI] [PubMed] [Google Scholar]
- 33.Jorg HH, et al. Patterns of circulating hepatitis B virus serum nucleic acids during lamivudine therapy. Ann NY Acad Sci. 2004;1022:271–281. doi: 10.1196/annals.1318.042. [DOI] [PubMed] [Google Scholar]
- 34.Koffler D, et al. The occurrence of single-stranded DNA in the serum of patients with systemic lupus erythematosus and other diseases. J Clin Invest. 2006;52:198–204. doi: 10.1172/JCI107165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leon SA, et al. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1997;37:646–650. [PubMed] [Google Scholar]
- 36.Steinman CR. Circulating DNA in polyarteritis nodosa and related syndromes. Arthritis Rheum. 1982;25:1425–1430. doi: 10.1002/art.1780251206. [DOI] [PubMed] [Google Scholar]
- 37.Steinman CR. Circulating DNA in systemic lupus erthematosus. J Clin Invest. 1984;73:832–841. doi: 10.1172/JCI111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neurath AR, et al. Strategies for detection of transfusion-transmitted viruses eluding identification by conventional serologic tests II. Detection of host DNA in human plasmas with elevated alanine aminotransferase. J Virol Methods. 1994;8:73–86. doi: 10.1016/0166-0934(84)90042-9. [DOI] [PubMed] [Google Scholar]
- 39.Jahr S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 40.Anker P, et al. Detection of circulating tumor DNA in the blood (plasma/serum) of cancer patients. Cancer Metast Rev. 1999;18:65–73. doi: 10.1023/a:1006260319913. [DOI] [PubMed] [Google Scholar]
- 41.Arai N, et al. Enhanced epithelial cell turnover associated with p53 accumulation and high p21WAF1/CIP1 expression in ulcerative colitis. Modern Pathol. 1999;12:604–611. [PubMed] [Google Scholar]
- 42.Nakamura T, et al. Cell death in colorectal polyps as evaluated by in situ 3′-tailinig reaction and its relationship to BCL-2 expression. Pathol Int. 1995;45:721–728. doi: 10.1111/j.1440-1827.1995.tb03388.x. [DOI] [PubMed] [Google Scholar]
- 43.Schulte-Hermann R, et al. Role of active cell death (apoptosis) in multi-stage carcinogenesis. Toxicol Lett. 1995;82/83:143–148. doi: 10.1016/0378-4274(95)03550-8. [DOI] [PubMed] [Google Scholar]