Abstract
Background
We have shown that continuous, systemic delivery of interferon-β (IFN-β) remodels dysfunctional tumor vasculature, thereby improving tumor perfusion and enhancing delivery and efficacy of chemotherapeutic drugs. We hypothesized that because of their inherent tumor-tropism, neural progenitor cells (NPCs) engineered to express IFN-β could also effect maturation of tumor vasculature without generating high systemic levels of IFN-β.
Methods
Mice with luciferase-expressing, disseminated human neuroblastoma were divided into four groups of equal tumor burden by bioluminescence imaging: (1)untreated controls (2)NPC-IFN-β only (3)cyclophosphamide only and (4)NPC-IFN-β in combination with cyclophosphamide. Two million NPC-IFN-β cells were given twice, seven days apart, starting twenty-one days after tail vein administration of tumor cells. Cyclophosphamide was given every six days for three doses. Mice were euthanized at six weeks, livers and kidneys weighed, and tissue harvested for immunohistochemistry for endothelial cells (CD34), pericytes (α-SMA), apoptosis (TUNEL), and diI-labeled NPCs.
Results
Fluorescent-labeled NPCs confirmed localization to tumors. The α-SMA/CD34 ratio, a marker for vascular maturation, significantly increased in NPC-IFN-β treated tumors compared to controls. Bioluminescent signal from luciferase-expressing tumor cells, reflecting tumor burden, was lower with combination therapy than control or either monotherapy, and combination therapy resulted in significantly less tumor burden by weight in the kidneys and liver.
Conclusions
Targeted delivery of IFN-β with NPCs produced low circulating levels of IFN-β, yet the maturing effect on the tumor vasculature and the enhanced efficacy of adjuvant therapy was maintained. Thus, combination therapy of NPC-IFN-β with cyclophosphamide warrants further investigation for the treatment of high-risk neuroblastoma patients.
Keywords: Neuroblastoma, Neural progenitor cells, Interferon-beta, Cyclophosphamide, Angiogenesis
Introduction
Neuroblastoma (NB) is the most common solid tumor diagnosed in infants and the most common extracranial solid tumor of childhood.1 The course of the disease can be extremely variable, but patients with high-risk NB have a very poor prognosis, with the 5-year survival being less than 40%, despite aggressive multimodality therapy.2 New treatment strategies are needed for these patients.
Type I interferons have been shown to be effective in the treatment of some solid tumors, and pre-clinical work in our lab indicates that interferon has significant direct anti-tumor activity against NB in heterotopic, orthotopic, and disseminated NB models.3–5 Previous work in our lab has also demonstrated that continuous systemic delivery of type I interferons alters the tumor vasculature in pre-clinical models of human NB.3–5 Tumor angiogenesis normally produces vessels with an abnormal phenotype. These vessels are leaky and dysfunctional, resulting in inefficient tumor perfusion. As early as the 1970’s investigators showed that angiogenesis inhibitors could enhance the anti-tumor effect of other chemotherapeutic agents when administered in conjunction with these agents.6,7 Normalization of the tumor vasculature provides one potential explanation for this enhancement.6,8 This process occurs as modulators of angiogenesis remodel the tumor vasculature, destroying the immature, dysfunctional vessels, while leaving the more normal, mature vessels relatively unperturbed. This, in turn, results in improved perfusion of the tumors and can facilitate delivery of the systemically-administered chemotherapeutic agents to the malignant cells within a tumor. In our previous experience, tumors treated with continuous delivery of interferon-beta (IFN-β) had a much more normal appearing architecture with an increased number of vessel-stabilizing perivascular cells compared to untreated tumors.9 This normalization of the NB vasculature also appeared to affect improved perfusion of the tumors, as measured by contrast-enhanced ultrasound.5,9 Finally, we have also shown that this improved tumor perfusion improves the delivery of systemically-administered chemotherapeutic agents.10,11
While interferon has been effective in pre-clinical trials, outcomes in clinical trials have often been limited by systemic side effects from the high-dose administration which was thought to be required for effective therapy.12 Pharmacokinetic studies have demonstrated that daily intravenous and subcutaneous dosing are associated with a systemic half-life of less than five hours,13 perhaps limiting efficacy by exposing tumor to only short bursts of therapy. Delivery in a manner that provides local or low, continuous levels of IFN-β therapy is needed to reduce these adverse effects.
Neural progenitor cells (NPCs) may provide a means of delivering IFN-β directly to the tumor in continuous but low levels. NPCs have been shown to have a remarkable capacity to migrate toward sites of pathology, including tumors, after introduction at local sites of disease or following intravascular injection.14,15 This migration was originally thought to only occur toward tumors within the brain, but NPCs have now also been shown to migrate toward extracranial tumors.16 Consequently, NPCs have potential as a vehicle for delivering gene-mediated therapy directly to the tumor.17–19 We hypothesized that because of their inherent tumor-tropism, NPCs engineered to express IFN-β could also effect maturation of tumor vasculature through local delivery of IFN-β and that this would improve tumor response to cyclophosphamide (CTX).
Materials and Methods
Cell Lines
The F3.C1 NPC line was originally derived from the primary dissociated cell culture of a 15 week gestation human fetal telencephalon by Seung Kim with permission from the Clinical Screening Committee of the University of British Columbia. Primary cultures were initially grown in UBC1 serum-free medium containing insulin, transferrin, selenium, hydrocortisone, T3, and bFGF, as reported previously.20 The F3.C1 clonal cell line was immortalized by retroviral transduction with v-myc, also as described previously.20 The cell line was maintained in Dulbecco’s minimum essential medium supplemented with 10% fetal bovine serum in a humidified atmosphere with 10% CO2. The human NB cell line, NB1691, was provided by P. Houghton (Memphis, TN). These cells were engineered to constitutively express firefly luciferase, as previously described.21
Transduction of NPCs with IFN-β gene
To transduce the NPCs with the human IFN-β gene, an adenoviral vector was constructed carrying the hIFN-β gene, as previously described.19 The resulting E1-E3 deleted vector expressed hIFN-β via a cytomegalovirus promoter/human b-globin intron and polyadenylation sequence cassette.22 The adenoviral vector was then used to transduce NPCs in log growth at a multiplicity of infection of 20, 24 hours before they were to be injected.
Animal model
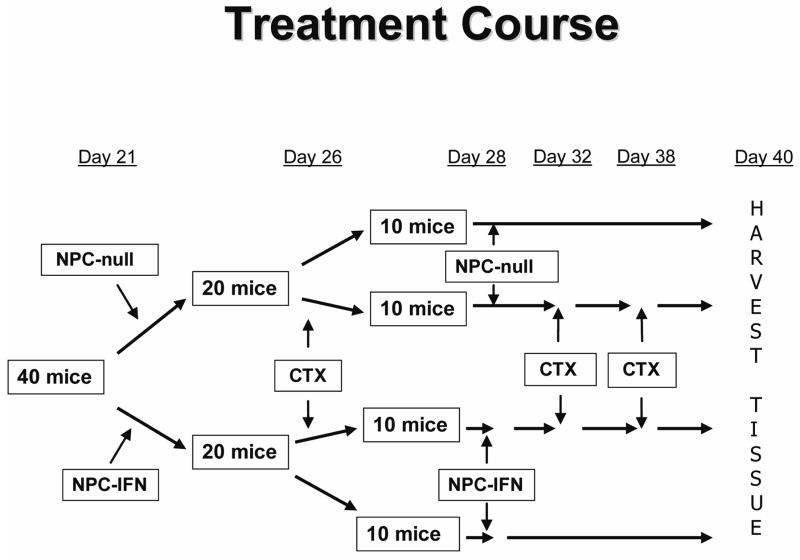
All murine experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of St. Jude Children’s Research Hospital. A model of disseminated NB was established by intravenous (IV) injection of 2 × 106 luciferase-expressing NB1691 human NB cells into 4- to 6-week-old male CB-17 SCID mice (Charles’ River Laboratories, Wilmington, Mass). Disease burden was measured from whole body images using a CCD camera (Xenogen, USA) by quantitating the bioluminescent signal emitted by the tumor cells following intraperitoneal administration of the substrate D-luciferin (Caliper Life Sciences, Hopkinton, MA). Using this bioluminescence imaging (BLI), mice were divided into four groups of equal tumor burden (n=10mice/group). BLI signal was measured again after three weeks of treatment and was divided by baseline BLI for each mouse to determine the relative tumor burden. The four groups of mice were treated as follows: (1) untreated controls received NPCs that had not been transduced with the IFN-β gene (2) NPC-IFN-β only (3) CTX only and (4) NPC-IFN-β in combination with CTX. Two million NPC-IFN-β cells were given twice, 7 days apart, starting 21 days after tumor injection. CTX (160mg/kg) was given subcutaneously every 6 days for 3 doses, starting on day 26 after tumor injection. Mice were euthanized at 6 weeks, livers and kidneys weighed, and tumor harvested for immunohistochemistry. The mean weight of liver and kidneys from tumor-naive mice (n=5) was subtracted from the weight of these organs in each tumor-bearing mouse to determine the tumor burden within the liver and kidney.
NPC labeling
A separate cohort of mice was used to confirm NPC localization to sites of tumor growth. The cells were labeled with the lipophilic tracer CellTracker CM-DiI (C-7000; Invitrogen Molecular Probes, Eugene, Ore) immediately before injection, as previously described.19 Four days later, mice were sacrificed and tumors were harvested for histology. DAPI and hematoxylin and eosin–stained sections were viewed and digitally photographed using an Olympus U-SPT microscope (Olympus, Center Valley, PA) as previously described.19
Immunohistochemistry
Mice in the treatment groups were sacrificed after three weeks of treatment, and tumors were fixed in 10% formalin and paraffin embedded. Four micrometer sections were cut and stained for CD34 and α-smooth muscle actin (α-SMA) immunoreactivity, as described previously, using rat anti-mouse CD34 (RAM 34, PharMingen, San Diego, CA) and mouse anti-human α-SMA (clone 1A4, DAKO, Carpenteria, CA) antibodies.23 Sections were viewed and digitally photographed using an Olympus U-SPT light microscope with an attached CCD camera. Images were taken of each tumor section at 400 power magnification with care to avoid areas of necrosis. Images were processed in Adobe Photoshop (Adobe Systems, Inc., San Jose, CA). Positive staining was quantified using NIH image analysis software, Image J, and was reported as the mean number of positive pixels/tumor section. The ratio of pericytes to endothelial cells was calculated by dividing the α-SMA positive pixels by the CD34 positive pixels. Apoptosis within each tumor section was analyzed by staining with a terminal deoxyribonucleotide transferase–mediated nick-end labeling (TUNEL) assay using the Dead End kit (Promega, PRG7130, Madison, WI) adapted for use on a DAKO Autostainer. Results were reported as the percentage of positive cells, with at least 2,000 nuclei being counted per section. Care was taken to avoid areas of necrosis.
Reverse transcriptase–polymerase chain reaction analysis
The femurs of euthanized mice were excised, and the medullary cavities were flushed with ice-cold PBS to obtain bone marrow samples from the mice. The resulting bone marrow suspension was centrifuged at 2,000 × gravity for 5 minutes. The supernatant was discarded and the cell pellet of bone marrow cells was homogenized in RNA STAT-60 (Tel-Test, Inc, Friendswood, Tex). The presence of NB tumor cells was detected by performing reverse transcriptase–polymerase chain reaction (RT-PCR) as previously described,21 except MYCN-specific primers (Hs00232074_m1, Applied Biosystems, Foster City, CA) were used for amplification.
Interferon levels
Systemic levels of hIFN-β in mouse plasma were measured using commercially available immunoassays (ELISA, Fujirebio Inc, Tokyo, Japan).
Statistical Analysis
Results were reported as mean ± SE. The Sigmaplot program (SPSS, Inc, Chicago, IL) was used to graphically present the data and analyze statistical differences in the results using an unpaired Student’s t-test. A P value of <0.05 was considered statistically significant.
Results
NB1691 human NB cells injected into the tail vein of SCID mice established disseminated tumors in ninety percent of the mice injected. The largest foci of tumors formed predominantly in the liver, kidneys, bone marrow of the femurs, mandible, and retroperitoneal lymph nodes. Tumors were allowed to grow for three weeks before the presence of tumor was confirmed by BLI, and mice were divided into four treatment groups of equal tumor burden as measured by BLI (n=10 mice per group). Two groups were treated with monotherapy (either three doses of CTX or two doses of NPC cells expressing IFN-β). Another group was treated with both the two doses of NPCs expressing IFN-β and the three doses of CTX. The remaining group served as untreated controls and received two doses of unmodified NPCs. A flow chart of the treatment schedule is shown in Figure 1.
Figure 1. Treatment course.
Flow chart illustrating the timing of each treatment dose for the four treatment groups.
NPC localization to tumor
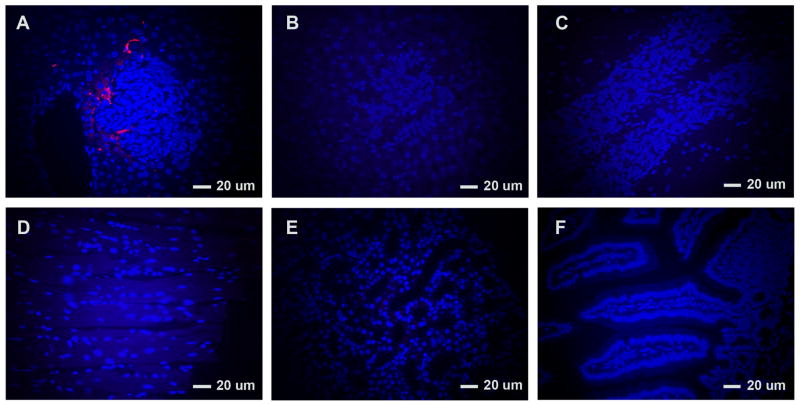
The localization of NPCs carrying IFN-β to tumors was confirmed with micrographs of fluorescent-labeled NPCs. NPCs labeled with the lipophilic tracer, CM-DiI, were injected into four tumor-bearing mice. Four days later tumor-bearing liver was harvested from the mice, and labeled NPCs were evident in the tumor (Figure 2) confirming their migration to tumor-bearing tissue. Tumor from two mice not injected with labeled-NPCs showed no background red fluorescence in the tumor tissue. Likewise, disease-free organs from tumor-bearing mice also showed no evidence of labeled-NPCs (Figure 2).
Figure 2. Labeled neural progenitor cells (NPCs) present in tumor.
Four days after administration of NPCs labeled with CM-DiI to mice with disseminated neuroblastoma, tumors were harvested and evaluated for the presence of CM-DiI–labeled NPCs at 400×. These cells (red) were detectable in tumor against a background of DAPI stained tumor cells. No background red fluorescence was evident in the tumor of mice not given NPCs. No red-labeled cells were evident in the tumor-free tissues shown here, after mice were injected with labeled NPCs. (A=tumor in mice injected with labeled-NPCs; B=tumor in mice without labeled-NPCs; C-F=brain, heart, kidney, and intestine, respectively, without tumor in mice injected with labeled-NPCs.)
Plasma interferon levels
Five days after injection with NPCs carrying the IFN-β gene, blood was drawn from the retro-orbital venous plexus, plasma was isolated, and levels of IFN-β assessed. Human IFN-β was detectable in all NPC-IFN-β treated mice with a mean of 71.1pg/ml.
Tumor angiogenesis inhibition
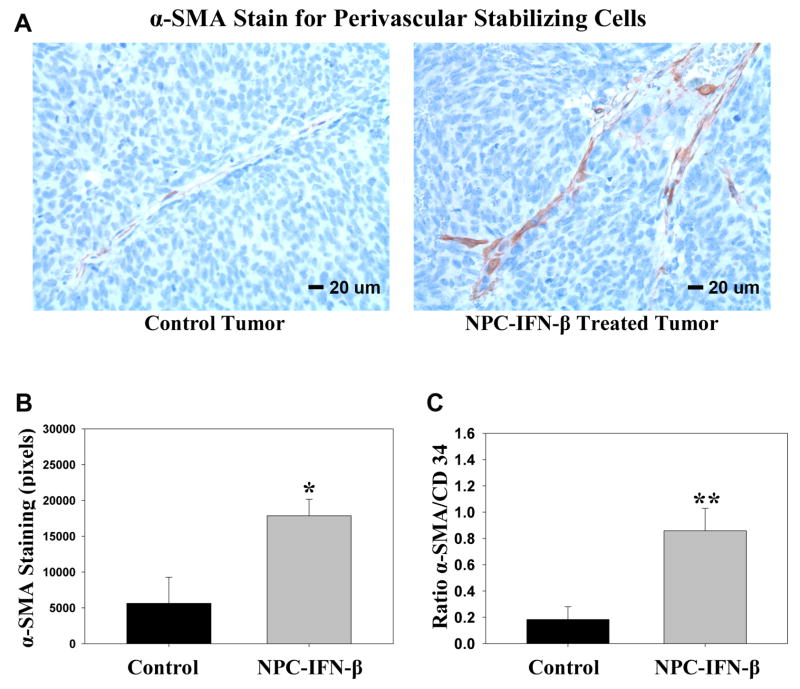
To confirm that this lower level of IFN-β remained effective in normalizing the tumor vasculature as we have previously seen with higher levels of IFN-β,9 tumor was harvested at euthanasia and immunohistochemical analysis was performed for endothelial cells and perivascular smooth muscle cells (Figure 3a). The ratio of perivascular cells (α-SMA positivity) to endothelial cells (CD34 positivity), a marker for vascular maturation24, was determined. As we have previously noted with IFN-β treatment, there was not a significant difference in the amount of CD34+ staining, or endothelial cell count, between the NPC-IFN-β treated tumors and the untreated tumors (p=0.645). However, there was a significant increase in the amount of α-SMA staining, suggesting a significant increase in stabilizing perivascular cells, after treatment with NPC-IFN-β (17,854.3 ± 2299.6pixels) compared to untreated controls (5,628.6 ± 3631.6pixels, p=0.032). Similarly, the α-SMA/CD34 ratio was significantly increased in NPC-IFN-β treated tumors compared to untreated controls (0.858 ± 0.17 vs 0.184 ± 0.1, p<0.009), indicating that the tumor vascular normalization occurred with this local expression of IFN-β (Figure 3b).
Figure 3. α-Smooth muscle actin (α-SMA) stain for perivascular cells.
(A) Micrographs from control tumors and tumors treated with NPC-IFN-β taken at 400× after staining for α-SMA (brown) against a background of tumor cells (blue). (B) Bar graph comparing α-SMA staining of tumors in control and NPC-IFN-β treated mice. (*p value = 0.032 vs control) (C) Bar graph comparing the ratio of perivascular cells (α-SMA+) to endothelial cells (CD34+) in NPC-IFN-β treated tumors versus untreated control tumors. (**p value <0.009) (NPC-IFN-β=neural progenitor cell-delivered interferon-β)
Tumor cell apoptosis
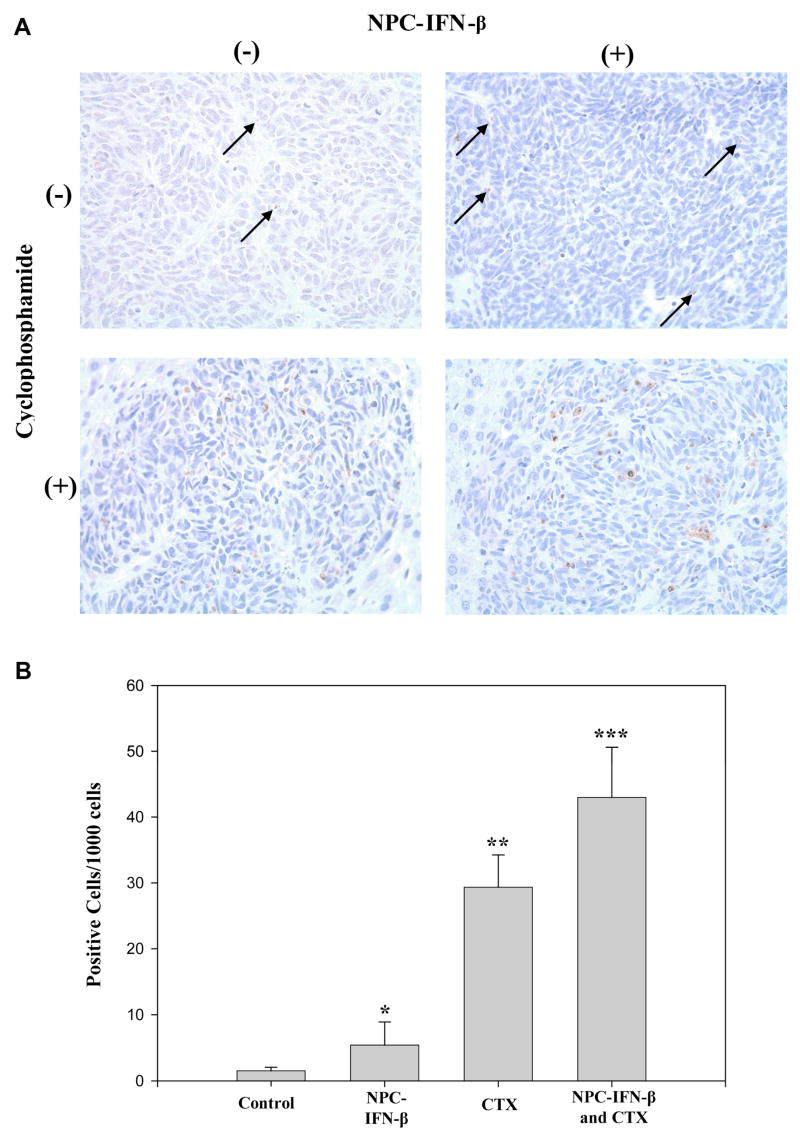
Sections of tumor tissue from each of the treatment groups were also analyzed with TUNEL staining, a marker for apoptosis (Figure 4a). The number of apoptotic cells was increased in tumors treated with either NPC-IFN-β alone (5.7 ± 1.5 apoptotic cells/1000cells, p=0.07) or CTX alone (29.4 ± 2.5 apoptotic positive cells/1000 cells, p<0.001) compared to control tumors (1.5 ± 0.3 apoptotic positive cells/1000 cells). Combination therapy produced significantly more apoptosis in tumors (43 ± 5.4 apoptotic cells/1000cells) than was seen in control-treated tumors (p<0.001) or tumors treated with either monotherapy (p=0.05) (Figure 4b).
Figure 4. Apoptotic cells in the four treatment groups.
(A) Micrographs from each of the four treatment groups taken at 400× after TUNEL staining (brown) for apoptotic cells against a background of tumor cells (blue). (B) Bar graph comparing the rate of apoptosis in each of the four treatment groups. (NPC-IFN-β=neural progenitor cell-delivered interferon-beta, CTX=cyclophosphamide) *p value vs. control = 0.07, vs. combination = 0.05; **p value vs. control <0.001, vs. combination = 0.05; ***p value vs. control <0.001.
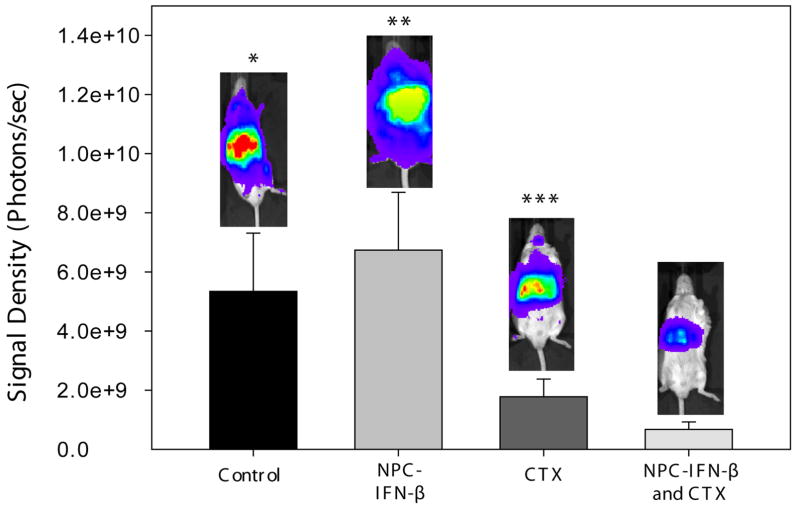
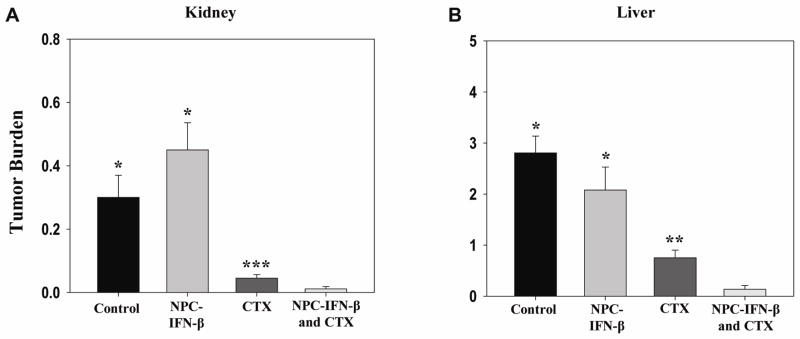
Tumor burden
The tumor burden within each mouse was then evaluated in three different ways (BLI, presence of bone marrow disease, and the relative increase in the weight of tumor-bearing organs) to determine whether there was a reduction in tumor progression and burden of disease with either monotherapy or the combination therapy. Overall, combination therapy resulted in the least tumor burden. Prior to euthanasia, the overall tumor burden in the mice was measured with BLI. Dickson et al. showed that BLI signal correlated well with tumor burden in NB-bearing mice.21 Bioluminescent signal from luciferase-expressing tumor cells was lower (Figure 5) with combination therapy (6.73×108 ± 2.54×108photons/sec) than control (5.34×106 ± 1.97×109, p=0.018), or monotherapy (6.74×109 ± 1.96×109[NPC-IFN-β], p=0.007 or 1.78×109 + 5.99×108[CTX], p=0.121). Bone marrow obtained from the femurs of euthanized mice was evaluated for the presence of tumor cells by RT-PCR. Eighty percent of untreated mice had detectable levels of tumor cells within the bone marrow. NPC-IFN-β and CTX treated mice had tumor cells in 67% and 60% of available femur bone marrow, respectively. Combination therapy with NPC-IFN-β and CTX resulted in the lowest percentage (25%) of tumor-positive bone marrow. The tumor burden in each mouse liver and each mouse kidney was also evaluated by weighing each organ and subtracting the mean weight of those organs in an age-matched, non-tumor bearing mouse. Combination therapy resulted in significantly less tumor burden by weight in the kidneys (0.01 ± 0.01g) than controls (0.3 ± 0.07g, p<0.001). This was also significantly less than the kidney tumor burden in mice treated with monotherapy with either NPC-IFN-β (0.45 ± 0.09g, p<0.001) or CTX (0.05 ± 0.01g, p=0.02). Combination therapy resulted in significantly less tumor burden by weight in the liver (0.14 ± 0.07g) than controls (2.81 ± 0.33g, p<0.001), as well. Again, there appeared to be enhancement of the monotherapy antitumor activity when the two treatments were combined, as combination therapy significantly decreased tumor burden compared to either monotherapy with NPC-IFN-β (2.08 ± 0.45g, p<0.001) or CTX (0.75 ± 0.15g, p=0.002) (Figure 6).
Figure 5. Tumor burden by bioluminescent signaling.
Bar graph showing the tumor burden in each of the four treatment groups at the completion of therapy. (NPC-IFN-β =neural progenitor cell-delivered interferon-β, CTX=cyclophosphamide) *p value vs. combination = 0.018; **p value vs. combination = 0.007; ***p value vs. combination = 0.121
Figure 6. Tumor burden by organ weight.
(A) Bar graph comparing the mean tumor burden in the kidneys of each of the four treatment. Tumor burden was calculated by subtracting the weight of a normal kidney from the kidneys of each of the tumor-bearing mice. (B) Bar graph comparing the mean tumor burden in the liver of each of the four treatment groups. Tumor burden was calculated by subtracting the weight of a normal liver from the livers of each of the tumor-bearing mice. (NPC-IFN-β =neural progenitor cell-delivered interferon-β, CTX=cyclophosphamide) *p value vs. combination < 0.001; **p value vs. combination = 0.002; ***p value vs. combination = 0.02
Discussion
NPCs provide a unique delivery system for targeting therapy directly to sites of pathology. In this pre-clinical model of disseminated neuroblastoma, using NPCs to deliver IFN-β enhanced the effect of cyclophosphamide, a chemotherapeutic agent commonly used in the treatment of advanced stage NB. Type I IFNs are multi-functional regulatory cytokines which control cell function and regulation, and they have been effective in the treatment of several types of cancer.25–27 Type I IFNs hinder tumor growth through a variety of mechanisms, including inhibition of cell growth by promoting cell cycle arrest,28 immunomodulation,29 induction of tumor apoptosis,30 and effects on angiogenesis.9 This model used SCID mice, likely eliminating the immune modulation mechanism. The mean tumor burden by BLI in mice treated with NPC-IFN alone does appear higher than the mean tumor burden in control mice, but there was no significant difference between the two groups (p=0.629). Despite the low circulating levels of IFN-β protein, there was an increase in apoptosis in tumors treated with NPC-IFN-β compared to control tumors. Bone marrow disease and liver tumor burden were also less in the NPC-IFN-β treated group compared to controls. In addition, the low levels of IFN-β seem to have been sufficient to alter the vascular phenotype within the tumor, resulting in a higher ratio of stabilizing pericytes to endothelial cells. Paradoxically, this vascular normalization may be beneficial in hindering tumor growth, especially when used in combination with a cytotoxic agent. The increased edema or interstitial pressure produced by abnormal tumor vasculature is thought to collapse small vessels and hinder perfusion. Shifting the phenotype toward thicker-walled, less-permeable vessels produces less edema and should enhance tumor perfusion. This may also reduce the development of necrotic areas where tumor cells can “hide” from the body’s immune system and exogenous therapeutic agents. Indeed, we have shown previously that IFN-β-mediated normalization of neuroblastoma vasculature correlates with increased delivery of therapeutic agents,11 and that is likely the mechanism whereby the modest effect of NPC-IFN-β significantly enhances the effect of CTX over CTX monotherapy.
Although IFNs have great promise for the treatment of solid neoplasms including NB, clinical trials have often been limited by systemic side effects from the high-dose administration which was thought to be required for effective therapy.12 Furthermore, previous research has shown that optimization of the dose and frequency of IFN delivery is necessary for maximal anti-tumor efficacy.32 Gene-therapy mediated delivery provides continuous levels of IFN-β and may address the limitations of current IFN clinical protocols while avoiding some of the systemic side-effects. NPC-delivered IFN-β has the added benefit of providing treatment directly at the site of pathology, which appears to produce a therapeutic effect without generating high systemic levels of IFN-β. In this study, human IFN-β was detectable in mouse plasma after NPCs carrying the human IFN-β were injected into the mice, but systemic levels of human IFN-β were low (71.1pg/ml) compared to the plasma level of IFN-β drawn from mice treated with IFN-β delivered by an adeno-associated virus vector, which has been our previous method of providing continuous IFN-β treatment for NB.9,11 This adeno-associated virus delivery system produced systemic IFN-β levels greater than 18,000pg/ml. The reduction in systemic levels of IFN-β with NPC-mediated delivery would likely significantly reduce the toxicity of IFN-β.
NPCs have great clinical potential as a unique vehicle for targeting chemotherapeutic agents directly to tumors. Specifically, NPC delivery of IFN-β to NB allows targeted delivery of an effective agent, providing direct tumor cell toxicity, as well as angiogenesis inhibition which appears to enhance the delivery and efficacy of other chemotherapeutic agents. At the same time, systemic levels of IFN-β are low, perhaps allowing IFN-β to be used clinically without producing toxic side effects. Approval has recently been granted for clinical trials utilizing NPCs, and studies are underway to further clarify how NPCs target and migrate toward tumors. Further studies are also needed to optimize NPC-IFN-β dosing and to clarify the long-term effects of NPC-mediated delivery.
In conclusion, targeted delivery of IFN-β with NPCs produced low circulating levels of IFN-β. Yet, the maturing effect on the tumor vasculature was maintained, and NPC delivery of IFN-β enhanced the efficacy of CTX, improving the anti-tumor response of combination therapy significantly over either treatment agent alone. Because of their synergy, IFN-β and CTX therapy may be effective in the clinical treatment of NB. Thus, NPC-IFN-β with CTX warrants further investigation for the treatment of high-risk NB patients.
Acknowledgments
This work was supported by the Assisi Foundation of Memphis, the US Public Health Service Childhood Solid Tumor Program Project Grant No. CA23099, the Cancer Center Support Grant No. 21766 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Miller RW, Young JL, Jr, Novakovic B. Childhood cancer. Cancer. 1995;75(1 Suppl):395–405. doi: 10.1002/1097-0142(19950101)75:1+<395::aid-cncr2820751321>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341(16):1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 3.Streck CJ, Ng CY, Zhang Y, Zhou J, Nathwani AC, Davidoff AM. Interferon-mediated anti-angiogenic therapy for neuroblastoma. Cancer Lett. 2005;228(1–2):163–170. doi: 10.1016/j.canlet.2004.11.063. [DOI] [PubMed] [Google Scholar]
- 4.Streck CJ, Zhang Y, Miyamoto R, Zhou J, Ng CY, Nathwani AC, Davidoff AM. Restriction of neuroblastoma angiogenesis and growth by interferon-alpha/beta. Surgery. 2004;136(2):183–189. doi: 10.1016/j.surg.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 5.McCarville MB, Streck CJ, Dickson PV, Li CS, Nathwani AC, Davidoff AM. Angiogenesis inhibitors in a murine neuroblastoma model: quantitative assessment of intratumoral blood flow with contrast-enhanced gray-scale US. Radiology. 2006;240(1):73–81. doi: 10.1148/radiol.2401050709. [DOI] [PubMed] [Google Scholar]
- 6.Le Serve AW, Hellmann K. Metastases and the normalization of tumour blood vessels by ICRF 159: a new type of drug action. Br Med J. 1972;1(5800):597–601. doi: 10.1136/bmj.1.5800.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teicher BA, Holden SA, Ara G, Sotomayor EA, Huang ZD, Chen YN, Brem H. Potentiation of cytotoxic cancer therapies by TNP-470 alone and with other anti-angiogenic agents. Int J Cancer. 1994;57(6):920–925. doi: 10.1002/ijc.2910570624. [DOI] [PubMed] [Google Scholar]
- 8.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7(9):987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 9.Dickson PV, Hamner JB, Streck CJ, et al. Continuous delivery of IFN-beta promotes sustained maturation of intratumoral vasculature. Mol Cancer Res. 2007;5(6):531–542. doi: 10.1158/1541-7786.MCR-06-0259. [DOI] [PubMed] [Google Scholar]
- 10.Dickson PV, Hamner JB, Sims TL, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13(13):3942–3950. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 11.Dickson PV, Hagedorn NL, Hamner JB, et al. Interferon-β mediated improved efficacy of systemically administered topotecan in a murine neuroblastoma model. J Pediatr Surg. 2007;42:160–165. doi: 10.1016/j.jpedsurg.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 12.Einhorn S, Grander D. Why do so many cancer patients fail to respond to interferon therapy? J Interferon Cytokine Res. 2006;16(4):275–281. doi: 10.1089/jir.1996.16.275. [DOI] [PubMed] [Google Scholar]
- 13.Salmon P, Le Cotonnec JY, Galazka A, Abdul-Ahad A, Darragh A. Pharmacokinetics and pharmacodynamics of recombinant human interferon-beta in healthy male volunteers. J Interferon Cytokine Res. 1996;16(10):759–764. doi: 10.1089/jir.1996.16.759. [DOI] [PubMed] [Google Scholar]
- 14.Bendetti S, Pirola B, Pollo B, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 15.Aboody KS, Najbauer J, Schmidt NO, et al. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro Oncol. 2006;8(2):119–126. doi: 10.1215/15228517-2005-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown A, Yang W, Schmidt N, et al. Intravascular delivery of neural stem cell lines to target intracranial and extracranial tumors of neural and non-neural origin. Hum Gene Therapy. 2003;14:1777–1785. doi: 10.1089/104303403322611782. [DOI] [PubMed] [Google Scholar]
- 17.Aboody K, Brown A, Rainov N, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97(23):12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danks MK, Yoon KJ, Bush R, et al. Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer Res. 2007;67(1):22–25. doi: 10.1158/0008-5472.CAN-06-3607. [DOI] [PubMed] [Google Scholar]
- 19.Dickson PV, Hamner JB, Burger R, et al. Intravascular administration of tumor tropic neural progenitor cells permits targeted delivery of interferon-β and restricts tumor growth in a murine model of disseminated neuroblastoma. J Pediatr Surg. 2007;42:48–53. doi: 10.1016/j.jpedsurg.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Nakagawa E, Hatori K, Nagai A, Lee M, Bang J. Production of immortalized human neural crest stem cells. Methods Mol Biol. 2002;198:55–65. doi: 10.1385/1-59259-186-8:055. [DOI] [PubMed] [Google Scholar]
- 21.Dickson PV, Hamner B, Ng CY, et al. In vivo bioluminescence imaging for early detection and monitoring of disease progression in a murine model of neuroblastoma. J Pediatr Surg. 2007;42(7):1172–1179. doi: 10.1016/j.jpedsurg.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 22.Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci U S A. 1996;93(21):11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spurbeck WW, Ng CY, Strom TS, Vanin EF, Davidoff AM. Enforced expression of tissue inhibitor of matrix metalloproteinase-3 affects functional capillary morphogenesis and inhibits tumor growth in a murine tumor model. Blood. 2002;100(9):3361–3368. doi: 10.1182/blood.V100.9.3361. [DOI] [PubMed] [Google Scholar]
- 24.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 25.Lo C, Liu C, Chan S, et al. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;24(6):831–842. doi: 10.1097/01.sla.0000245829.00977.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moschos S, Kirkwood J. Present role and future potential of type I interferons in adjuvant therapy of high-risk operable melanoma. Cytokine Growth Factor Review. 2007;18(5–6):451–458. doi: 10.1016/j.cytogfr.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Wakabayashi T, Natsume A, Hashizume Y, Fujii M, Mizuno M, Yoshida J. A phase I clinical trial of interferon-beta gene therapy for high-grade glioma: novel findings from gene expression profiling and autopsy. J Gene Med. 2008 doi: 10.1002/jgm.1160. [DOI] [PubMed] [Google Scholar]
- 28.Tanabe T, Kominsky S, Subramaniam P, Johnson H, Torres B. Inhibition of the glioblastoma cell cycle by type I IFNs occurs at both the G1 and S phases and correlates with the upregulation of P21 (WAF1/CIP1) J Neurooncol. 2000;48:225–232. doi: 10.1023/a:1006476408190. [DOI] [PubMed] [Google Scholar]
- 29.Kirkwood JM, Richards T, Zarour H, et al. Immunomodulatory effects of high-dose and low-does interferon alpha2b in patients with high-risk resected melanoma: the E2690 laboratory corollary of intergroup adjuvant trial E1690. Cancer. 2002;95:1101–1112. doi: 10.1002/cncr.10775. [DOI] [PubMed] [Google Scholar]
- 30.Chen Q, Gong B, Mahmoud-Ahmed A, Zhou A, Hsi E, Hussein M, Almasan A. Apo2L/TRAIL and Bcl-2-related proteins regulate type I interferon-induced apoptosis in multiple myeloma. Blood. 2001;98:2183–2192. doi: 10.1182/blood.v98.7.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slaton JW, Perrotte P, Inoue K, Dinney CP, Fidler IJ. Interferon-alpha-mediated down-regulation of angiogenesis-related genes and therapy of bladder cancer are dependent on optimization of biological dose and schedule. Clin Cancer Res. 1999;5(10):2726–2734. [PubMed] [Google Scholar]