Abstract
Once hypoxic–ischemic (HI) injury ensues in the human neonate at birth, the resulting brain damage lasts throughout the individual’s lifetime, as no ameliorative treatments are currently available. We have recently shown that intracerebral transplantation of multipotent adult progenitor cells (MAPCs) results in behavioral improvement and reduction in ischemic cell loss in neonatal rat HI-injury model. In an attempt to advance this cellular therapy to the clinic, we explored the more practical and less invasive intravenous administration of MAPCs. Seven-day-old Sprague–Dawley rats were initially subjected to unilateral HI injury, then 7 days later received intracerebral or intravenous injections of allogeneic rat MAPCs. On post-transplantation days 7 and 14, the animals that received MAPCs via the intracerebral or intravenous route exhibited improved motor and neurologic scores compared with those that received vehicle infusion alone. Immunohistochemical evaluations at day 14 after transplantation revealed that both intracerebrally and intravenously transplanted MAPCs were detected in the ischemic hippocampal area. The degree of hippocampal cell preservation was almost the same in the two treatment groups and greater than that in the vehicle group. These results show that intravenous delivery of MAPCs is a feasible and efficacious cell therapy with potential for clinical use.
Keywords: transplantation, stem cells, neural progenitors, cell migration, animal behavior
Introduction
We have recently shown that the delivery of multipotent adult progenitor cells (MAPCs) by injection into the brain of surgically induced neonatal hypoxic–ischemic (HI) injury results in behavioral improvement and reduction in ischemic cell loss (Yasuhara et al, 2006a, b). Specifically, we showed equivalence of therapeutic benefits of syngeneic and allogeneic rat MAPCs after intracerebral transplantation into the neonatal rat hippocampal ischemic area. This finding supports our clinical goal of using allogeneic adult stem cell transplantation in treating human neonates suffering from HI injury. Furthermore, histologic data revealed that these stem cells display robust cell differentiation potential, that is, they exhibit early as well as mature neuronal phenotypic markers, suggesting that neuronal differentiation of the transplanted stem cells participates in tissue repair and behavioral recovery. In our desire to bring this therapy to the clinic, we posit that if we can produce a similar degree of behavioral benefit and neuronal rescue of the ischemic area via a minimally invasive delivery of the stem cells, then we will be able to reduce the risk associated with direct intracerebral transplantation. Moreover, such a minimally invasive route, that is, intravenous, may allow a more efficient scheduling of the transplantation procedure immediately after the diagnosis of the disease. In the clinical situation, we envision that the cells would be delivered via the intravenous route shortly after the time of brain injury.
The rat neonatal HI model is relevant to the type of brain injury occurring in infants and young children. Perinatal asphyxia is estimated to occur in 0.5% of live births (Wu et al, 2004), and although many of these infants do not suffer long-term morbidity or mortality, a number do (Davenport and Dennis, 2000). The infants destined to exhibit significant injury could be predicted in advance based on clinical characteristics observed at the time of injury. Even in this population alone, there are a large number of infants who would benefit from cell therapy. There are other brain insults in infants and children of similar pathophysiology where cell therapy could also be used. Thus, the potential for benefit is high in infants and children just as it is for adults with stroke. Hence, we will test this methodology in the neonatal HI model with the long-term aim of using stem cell therapy in this population. The purpose of this study is to compare the behavioral benefits, cell survival, migration, and differentiation of allogeneic stem cells after their delivery via either the intracerebral or intravenous route.
Materials and methods
Animals and Hypoxic–Ischemic Injury Surgical Procedure
This study was performed according to the approved NIH guidelines for use of animals in research. All surgical procedures in this study were conducted under aseptic conditions. Each litter of three Sprague–Dawley rats (Harlan Inc., Indianapolis, IN, USA) was housed under standard conditions. Offspring were reared with their dams until the time of surgery, and then until weaning at 3 weeks of life. The HI-injury surgery was performed in accordance with our previous reports (Yasuhara et al, 2006a, b). Briefly, rat pups (n = 33) underwent permanent ligation of the right common carotid artery at post-natal day of life 7 (Rice et al, 1981) under anesthesia with 2% isoflurane. A midline cervical incision was made to expose the right common carotid artery, which was ligated by a single suture. The incision was closed with interrupted 6-0 silk sutures. The pups were then placed with the dams for 2 h before placement in an 8% oxygen chamber partially immersed in a water bath at 37°C for 2.5 h. The animals were then placed on a temperature-controlled blanket until they recovered from anesthesia.
Cell Preparation
The MAPCs used in this study (provided by Athersys Inc., Cleveland, OH, USA) were previously characterized by Verfaille and coworkers (Keene et al, 2003). Cryopreserved βgal-labeled MAPCs were thawed at 37°C just before transplantation surgery. Viability was determined using the trypan blue dye exclusion method and cell concentration was adjusted to 200,000/3 μL for transplantation. A minimum of 85% viability after thawing was used as a criterion for using the cells for transplantation.
Multipotent Adult Progenitor Cell Injection
Three rat pups were excluded from this study before cell transplantation because of death or growth insufficiency. At day 7 after HI injury, 10 anesthetized (equithesin 300 mg/kg intraperitoneal) animals were fixed in stereo-taxic apparatus (Kopf instruments, Tujunga, CA, USA) and implanted with MAPCs directly into the hippocampus (1.2mm posterior to bregma, 1.5mm lateral to midline, and 1.8mm below the dural surface), using a 28-gauge implantation cannula (Borlongan et al, 1998). Another group of 10 randomly selected HI-injured animals received intravenous infusion (via the jugular vein) of MAPCs. Both intracerebrally and intravenously transplanted animals received 200,000 viable MAPCs. The remaining group of 10 HI-injured animals received an intravenous infusion of vehicle. Based on parallel transplantation studies in adult stroke rats, we found that immunosuppression is not required for allogeneic transplantation of MAPCs. Accordingly, all animals in this study received no immunosuppression.
Behavioral Testing
The HI-injured rats were tested by elevated body swing test (EBST) and Rotarod treadmill test at days 7 and 14 post-transplantation. The EBST provided a motor asymmetry parameter and involved handling the animal by its tail and recording the direction of the biased body swings (Borlongan and Sanberg, 1995). The EBST consisted of 20 trials with the number of swings ipsilateral and contralateral to the ischemic hemisphere recorded and expressed in percentage to determine the biased swing activity. Sensorimotor functions were evaluated using the Rotarod treadmill (Accuscan Inc., Columbus, OH, USA). The average score (total time spent on treadmill divided by 5 trials) was calculated for each animal on days 7 and 14. Previous studies have shown that animals that underwent the HI protocol perform significantly worse in this Rotarod task up to at least 5 weeks after HI surgery (Jansen et al, 1997).
Immunohistochemical and Histologic Analyses
Animals were killed for immunohistochemical analysis of grafted cells and preserved hippocampal neurons. Two weeks after MAPC injection, animals were killed (equithesin 500 mg/kg intraperitoneal) and perfused through the ascending aorta with 100mL of cold phosphate-buffered saline, followed by 75mL of 4% PFA in phosphate-buffered saline. Brains were removed and post-fixed in the same fixative for 24 h followed by 30% sucrose in phosphate buffer for 1 week. Six series of coronal sections were cut at a thickness of 25 μm in a freezing microtome (Leica, Bannockburn, IL, USA) and stored at −20°C. Free-floating sections were incubated overnight at 4°C with an anti-βgal antibody (1:200, mouse monoclonal IgG; Cell Signaling Technologies, Danvers, MA, USA) and an anti-MAP2 antibody (1:500, rabbit polyclonal IgG; Chemicon, Temecula, CA, USA) with 10% normal horse serum. After several rinses, the sections were incubated for 1 h in goat anti-mouse IgG Alexa Fluor 488 conjugate (1:1000; Molecular Probes, Eugene, OR, USA) and goat anti-rabbit IgG Alexa Fluor 594 conjugate (1:1000) with Hoechst33342 (1:1000; Sigma, St Louis, MO, USA). The sections were then washed, mounted on Superfrost Plus glass slides (Erie Scientific, Portsmouth, NH, USA), and embedded in mounting medium (Biomeda, Foster City, CA, USA). Control studies included exclusion of primary antibody substituted with 10% normal horse serum in phosphate-buffered saline. No immunoreactivity was observed in these controls. βgal-Positive cells were explored in the entire hippocampal area of every six sectioned slices and summed up. For estimation of neuronal cell viability of the hippocampal CA3 region, Nissl staining was performed using cresyl violet solution (Sigma), and randomly selected visual fields of the CA3 region and the corresponding contralateral intact CA3 in three sections were photographically captured (Axio-phot2; Carl Zeiss, Thornwood, NY, USA). The cells were quantified by counting per high-power field view selected at random (28,800 μm2). The percentages of preserved neurons in damaged CA3 relative to the intact side were calculated and used for statistical analyses. All the counted numbers were corrected by the Aber-crombie formula. Additionally, confocal analysis was performed using Zeiss LSM 510 confocal laser-scanning microscope.
Statistical Analyses
The behavioral scores were analyzed using repeated measures of ANOVA (combining both test periods) and single ANOVA (test periods analyzed separately). The evaluation of cell loss was analyzed using single ANOVA. The level of significance was set at P < 0.05. Post hoc t-tests were performed for pairwise comparisons between treatment conditions.
Results
Amelioration of Locomotor Function in Intracerebrally and Intravenously Transplanted HI-Injured Rats
Transplantation with intracerebral and intravenous MAPCs or vehicle infusion did not cause any overt behavioral side effects (i.e., no exacerbation of HI injury). Both intracerebrally and intravenously transplanted HI-injured animals displayed a similar degree of behavioral recovery in both tests during the post-transplantation test period (repeated measures of ANOVA: EBST, F2,27 = 5.2 and P = 0.012; Rotarod, F2,27 = 7.3 and P = 0.003; Figure 1). At day 7 post-transplantation, MAPC-transplanted HI-injured animals exhibited a trend of less motor asymmetry (intracerebral: 68.5%; intravenous: 67.5%) and longer time spent on the Rotarod (intracerebral: 19.05 secs; intravenous: 21.63 secs) compared with vehicle-infused injured animals (EBST: 75%; Rotarod: 17.49 secs). At day 14 post-transplantation, MAPC-transplanted animals exhibited significantly reduced motor asymmetry (intracerebral: 62.5%; intravenous: 59%) and longer time spent on the Rotarod (intracerebral: 29.11 secs; intravenous: 34.11 secs) than animals that received vehicle infusion (EBST: 80.5%; Rotarod: 20.75 secs). Both intracerebrally and intravenously MAPC-transplanted animals did not differ significantly in their behavioral improvements during both test periods.
Figure 1.
The EBST and Rotarod test at 1 and 2 weeks after intracerebral or intravenous injection of MAPC. (A) EBST, (B) Rotarod. Significant behavioral recovery of locomotor tasks in transplanted HI-injured animals (P<0.05 versus control) appears by week 2. Intracerebrally (IC) and intravenous (IV) transplanted HI-injured animals did not differ significantly in their behavioral recovery. Data are shown as mean values+s.e. *P<0.05 versus vehicle-treated rats.
Survival and Differentiation of Intravenously and Intracerebrally Grafted MAPCs
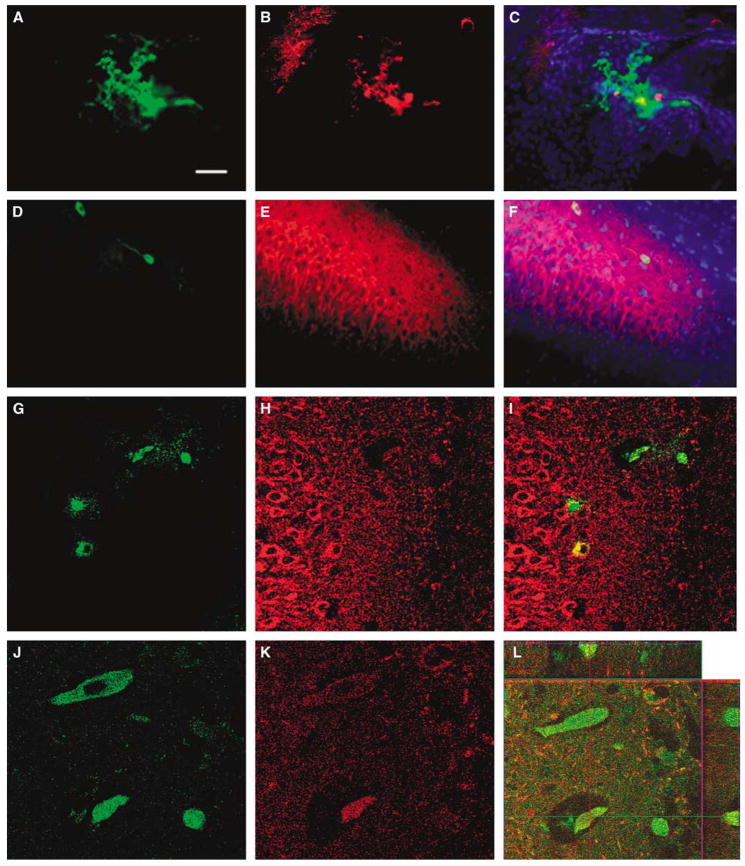
Grafted MAPCs were detected in the brains of the HI-injured animals (Figure 2). β-gal-Positive allogeneic cells, delivered via either the intracerebral or the intravenous route, were detected in the hippocampal CA3 ischemic area and the adjacent CA2 region, which colabeled with Hoechst33342. Percentages of graft survival of intracerebrally and intravenously transplanted cells were 0.51%± 0.15% and 0.18%±0.02%, respectively, at 14 days post-transplantation. Of note, both intracerebrally and intravenously transplanted cells exhibited the neuronal phenotypic marker MAP2 (Figure 2). Furthermore, some intravenously transplanted MAPCs were found in the vessels in the hippocampal CA3 region (Figure 2).
Figure 2.
Multipotent adult progenitor cell (MAPC) graft survival in HI brains of behaviorally recovered animals. Representative images of βgal and MAP2 staining of MAPC after intracerebral and intravenous transplantation are shown (A–C: intracerebral graft; D–L: intravenous graft). βgal-Positive allogeneic grafts were detected in the hippocampal CA3 region and the adjacent CA2 area, which colabeled with the nuclei marker Hoechst in rats receiving both intracerebral (A–C: A, βgal; B, MAP2; C, merged with Hoechst) and intravenous (D–F: D, βgal; E, MAP2; F, merged with Hoechst) grafts. A higher magnification, using confocal microscopy, further reveals the colocalization of intravenously delivered βgal-labeled MAPC with the neuronal marker MAP2 (G–I). Some MAPCs (J) colocalized with MAP2 (K) seemed to reside inside vessels in the hippocampus, which was confirmed by confocal z-stacked imaging (L). These data indicate that transplanted allogeneic MAPCs survived in the ischemic hippocampus (in the case of intravenous route, migrated into the ischemic hippocampus), and expressed a neuronal phenotypic marker, which could have promoted recovery from motor deficits associated with HI injury. Bars: A–F, 30μm; G–I, 10μm; J–L, 2.5 μm.
Improved Cell Survival in the Hippocampal Region
Both intracerebral and intravenous transplantation promoted significant cell preservation in the hippocampal region compared with vehicle-infused HI-injured animals (single ANOVA: F2,25 = 12.9 and P = 0.0001; post hoc t-tests of P-values < 0.05: intracerebral, 80.8%±3.6% and intravenous, 74.5%± 3.2% and vehicle, 55.2%±4.2% relative to the intact side; Figure 3).
Figure 3.
Intracerebral (IC) and intravenous (IV) MAPC grafts reduce CA3 cell loss. Representative images of low (A1–D1) and high-magnification (A2–D2) Nissl staining of CA3 are shown (A: intact; B: vehicle; C: intravenous; D: intracerebral; A1–D1: bar=50 μm; A2–D2: bar=15 μm). Cell counts of Nissl-stained cells along the ischemic hippocampal CA3 region revealed that intracerebral and intravenous grafts significantly rescued the damaged neuronal cells compared with vehicle-infused HI-injured animals (E). Data are shown as mean values+s.e. expressed as percentages relative to the intact side. *P<0.05 versus vehicle-treated rats.
Discussion
This study tested the feasibility of intravenous transplantation of allogeneic rat MAPCs in a rat model of neonatal HI injury to promote neurologic benefit and cell engraftment at a 2-week endpoint. The administration of allogeneic MAPCs via the intravenous route replicates the behavioral benefits produced by direct intracerebral injection into the HI-injured neonatal rat brain (Yasuhara et al, 2006a, b). Both intravenous and intracerebral delivery routes of MAPCs provide statistically significant improvement in both locomotor tests when compared with vehicle-only control treated animals at 2 weeks. Histologic examinations revealed that allogeneic MAPCs are present at the site of injury and injection (the hippocampus). Although the percentage of graft survival is small, early phenotypic neuronal differentiation is present. Furthermore, significant cell preservation is observed in the hippocampal region of transplanted animals compared with the ischemic hippocampus of animals that received vehicle infusion.
Two established motor tasks, namely EBST and Rotarod test, were employed in this study. For EBST, the normal motor activity was 50% (i.e., equal swings ipsilateral and contralateral to the ischemic hemisphere), while a 75% or higher activity represents our criterion of abnormal motor activity. Both intracerebrally and intravenously transplanted animals showed about 60% swing activity, whereas the vehicle-infused animals displayed about 80% swing activity. The 20% reduction in the motor asymmetry of transplanted animals compared with vehicle-infused animals equate to 25% improvement. Similarly, the Rotarod test reveals that transplanted animals significantly stayed longer on the rotating rod (30 to 35 secs) than vehicle-infused animals (20 secs). The extra 10 to 15 secs of staying on the rod exhibited by the transplanted animals compared with the vehicle-infused animals equate to about 30% improvement. A review of the literature reveals that 20% to 30% behavioral improvement after ischemic injury is consistently considered a robust therapeutic benefit (Chen et al, 2001; Borlongan et al, 1998, 2004, 2005), but we recognize that more than 50% behavioral improvement can be achieved after a longer post-transplantation period (i.e., 9 weeks; Jansen et al, 1997). Of note, the present study period was limited to 14 days after the HI injury, and experiments examining such long-term characterization of behavioral improvement are ongoing. For the ischemic cell loss reduction, the vehicle-infused and transplanted animals showed 55% and 78% surviving cells in the CA3 region, respectively. This 23% higher survival in transplanted animals is equal to about 25% reduction in ischemic cell loss compared with vehicle-infused animals, which closely parallels the degree of behavioral improvement seen in these transplanted animals. Thus, a closer examination of the data reveals robust benefits exerted by MAPC transplantation in both the behavioral and histologic parameters examined here.
The fact that intravenous administration is equivalent to intracerebral administration further advances the potential of using adult stem cells for clinical use. Although either route of administration could possibly be employed in human neonates, intravenous usage is more likely to be accepted in clinical practice. A similar response has been observed with intravenous injection of marrow stem cells or umbilical cord blood cells in rodent models of adult stroke (Chen et al, 2001; Borlongan et al, 2004). Although the reasons for the equivalency between intracerebral and intravenous administration are unclear, several cell-based mechanisms can be speculated. First, the major effect of the cells is likely trophic and not related to their retention in brain tissue in significant quantity. The fact that the actual count of transplanted cells in the brain tissue is low supports this notion. Second, the injured brain secretes a number of chemokines or cell attractants in response to injury, perhaps enabling the incorporation of transplanted cells via either the vascular or direct injection route. An example of chemokine upregulation is seen with SDF-1 in neonatal HI injury (Miller et al, 2005). Such migratory cues from the injured brain may facilitate even the few incorporated MAPC grafts to exert a positive influence on brain remodeling (Chopp et al, 2008).
Both intracerebral and intravenous injection resulted in a surprisingly rapid transformation of the transplanted stem cells into cells exhibiting neuronal markers. This process was much more pronounced than what we observed in the adult stroke model using CD133 + human bone marrow cells (Borlongan et al, 2005). The differences in the phenotypic potential between MAPC and CD133 + cells, in part, could explain the discrepancies in the fate of the transplanted cells. Alternatively, this expedited adaptation of the transplanted cells to the host microenvironment suggests that the neonatal animal, compared with the adult animal, is particularly more susceptible to the benefit of cell therapy.
Another possible explanation for the behavioral improvement seen with either intracerebral or intravenous transplantation appears to involve MAPC-facilitated endogenous neurorestoration, as evidenced by the marked preservation of intrinsic hippocampal cells in transplanted animals. These findings attribute recovery from ischemic injury to the production of trophic factors mediating reduction of inflammation and neurorestoration, as well as stimulation of neoangiogenesis. At this time, it appears that neurogenesis occurs in the hippocampus, but the extent of formation of newly born cells and their subsequent neuronal differentiation in other HI-injured brain regions warrant additional studies to reveal these endogenous neurorestorative mechanisms. Furthermore, although we did not examine the behavioral effects of MAPC grafts over an extended period, the much greater survival of the endogenous hippocampal cells in the transplanted animals augurs well for sustained benefit.
For the safe and effective clinical application of MAPCs, further preclinical experiments, such as intravenous dose response to booster injections or functional benefit assessment at longer timescales, will be needed. Similarly, the long-term safety of the cells in animals needs to be shown.
Acknowledgments
DCH is supported by VA Merit Review Award and NIH R21- NS043487-02, JEC by NIH R21-43439 and AHA Grant-In-Aid Award, and CVB by VA Merit Review Award and NIH 1U01NS055914-01. This study is directly supported by NIH STTR Phases I and II Grants (1R41NS055606-01 and 2R42NS055606-02). DCH, JEC, and CVB received research funds from Athersys Inc.
References
- Borlongan CV, Evans A, Yu G, Hess DC. Limitations of intravenous human bone marrow CD133+ cell grafts in stroke rats. Brain Res. 2005;1048:116–22. doi: 10.1016/j.brainres.2005.04.087. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuro-protection in stroke. Stroke. 2004;35:2385–9. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Sanberg PR. Elevated body swing test: a new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J Neurosci. 1995;15:5372–8. doi: 10.1523/JNEUROSCI.15-07-05372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Cerebral ischemia and CNS transplantation: differential effects of grafted fetal rat striatal cells and human neurons derived from a clonal cell line. NeuroReport. 1998;9:3703–9. doi: 10.1097/00001756-199811160-00025. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemic injury in rats. Stroke. 2001;32:1005–11. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y, Zhang J. Plasticity and remodeling of brain. J Neurol Sci. 2008;265:97–101. doi: 10.1016/j.jns.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Davenport R, Dennis M. Neurological emergencies: acute stroke. J Neurol Neurosurg Psychiatry. 2000;68:277–88. doi: 10.1136/jnnp.68.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen EM, Solberg L, Underhill S, Wilson S, Cozzari C, Hartman BK, Faris PL, Low WC. Transplantation of fetal neocortex ameliorates sensorimotor and locomotor deficits following neonatal ischemic–hypoxic brain injury in rats. Exp Neurol. 1997;147:487–97. doi: 10.1006/exnr.1997.6596. [DOI] [PubMed] [Google Scholar]
- Keene CD, Ortiz-Gonzalez XR, Jiang Y, Largaespada DA, Verfaillie CM, Low WC. Neural differentiation and incorporation of bone marrow-derived multipotent adult progenitor cells after single cell transplantation into blastocyst stage mouse embryos. Cell Transplant. 2003;12:201–13. doi: 10.3727/000000003108746768. [DOI] [PubMed] [Google Scholar]
- Miller J, Bartley JH, Wimbourne HJC, Walker AL, Hess DC, Hill WD, Carroll JE. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly upregulated by reactive astrocytes in brain following neonatal hypoxic–ischemic injury. BMC Neurosci. 2005;6:63. doi: 10.1186/1471-2202-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JE, III, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic–ischemic brain damage in the rat. Ann Neurol. 1981;9:131–41. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Wu YW, Backstrand KH, Zhao S, Fullerton HJ, Johnston SC. Declining diagnosis of birth asphyxia in California: 1991 to 2000. Pediatrics. 2004;114:1584–90. doi: 10.1542/peds.2004-0708. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Matsukawa N, Yu G, Xu L, Mays RW, Kovach J, Deans R, Hess DC, Carroll JE, Borlongan CV. Transplantation of cryopreserved human bone marrow-derived multipotent adult progenitor cells for neonatal hypoxic–ischemic injury: targeting the hippocampus. Rev Neurosci. 2006a;17:215–25. doi: 10.1515/revneuro.2006.17.1-2.215. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Matsukawa N, Yu G, Xu L, Mays RW, Kovach J, Deans RJ, Hess DC, Carroll JE, Borlongan CV. Behavioral and histological characterization of intrahippocampal grafts of human bone marrow-derived multipotent progenitor cells in neonatal rats with hypoxic–ischemic injury. Cell Transplant. 2006b;15:231–8. doi: 10.3727/000000006783982034. [DOI] [PubMed] [Google Scholar]