Abstract
Chinese Red Yeast Rice (RYR) is a food herb made by fermenting Monascus purpureus Went yeast on white rice. RYR contains a mixture of monacolins, one of which, Monacolin K (MK), is identical to lovastatin. Epidemiological studies show that individuals taking statins have a reduced risk of colon cancer. In the present study, lovastatin decreased cellular proliferation (P<.001) and induced apoptosis (P <.05) in HCT-116 and HT-29 human colon cancer cells. RYR inhibited both tumor cell growth (P <.001) and enhanced apoptosis (P <.05) in HCT-116. The inhibition of proliferation was reversed by mevalonate in lovastatin-treated cells, since lovastatin is a 3-hydroxy-3-methyl-glutaryl CoA reductase (HMGCR) inhibitor. However, RYR with mevalonate did not reverse the observed inhibition of growth. MK-free RYR did not reverse the observed lovastatin-mediated inhibition of cancer cell growth These observations suggest that other components in RYR, including other monacolins, pigments, or the combined matrix effects of multiple constituents may affect intracellular signaling pathways differently than purified crystallized lovastatin in colon cancer cells. RYR was purified into two fractions: pigment-rich (PF-RYR) and monacolin-rich (MF-RYR) fractions. The effect of MF-RYR was similar to that of lovastatin, while the effect of PF-RYR was similar to that of the whole RYR extract in proliferation, apoptosis and mRNA level of HMGCR and sterol response element binding protein-2. These results suggest that matrix effects of RYR beyond MK alone may be active in inhibiting colon cancer growth. RYR with/without MK may be a botanical approach to colon cancer chemoprevention worthy of further investigation.
Keywords: Chinese red yeast rice, colon cancer, lovastatin, monacolins, pigment, cholesterogenesis
1. Introduction
Red yeast fermented on rice is a traditional food spice consumed throughout Asia [1, 2]. It is also known as “red koji”, “angkak” or “red yeast rice (RYR)”, and its food and medicinal value is dates back to more than a thousand years with the first recorded documentation of use in 800 A.D [1, 3, 4]. RYR is derived from rice which has been allowed to ferment with yeast Monascus purpeureus. The fungus Monascus was studied by Dutch scientists in 1884 after discovery its use by villagers in Java [5]. A species isolated from red Koji or Honqu (as red rice yeast is known in East Asia) was named Monascus purpureus Went in 1895 recognizing its purple coloration [6]. RYR contains predominantly rice starches and sugars, and also yeast polyketides, fatty acids, pigments and condensed tannins [7, 8]. The classes of polyketide structures that arise under fermentation process are called monacolins, and the major monacolin found in RYR is monacolin K (MK) which is identical in structure to lovastatin (LV). Other polyketides in RYR are structural analogs of MK [7].
LV is a reversible competitive inhibitor of the key enzyme which controls cholesterol biosynthesis, 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) and it has been used for management of hypercholesterolemia [9, 10]. LV at a dose of 20–40 mg reduces levels of cholesterol and low-density lipoprotein (LDL) cholesterol significantly [11, 12]. RYR has also been shown to reduce blood cholesterol levels in rabbits on an atherogenic diet [13, 14], and RYR can reduce lipid accumulation in 3T3 L1 preadipocytes [15]. In a randomized prospective controlled trial, RYR decreased total cholesterol, triglycerides and apoplipoprotein B in hypercholesterolemic individuals by comparison to a placebo [16].
De novo cholesterogenesis is required for tumor growth, and HMGCR activities are upregulated in colon tumors [17–20]. A growing body of evidence supports the notion that statins including lovastatin may inhibit colon cancer cell growth and thereby have preventive potential for reducing the incidence of colon cancer [21–23]. In a population-based study, statin drug consumption was associated with a 47% reduced risk of colon cancer [24]. The presented study was designed to determine the effect of RYR containing multiple monacolins and lovastatin vs. lovastatin alone on colon cancer cell growth and apoptosis.
Our group has previously shown that RYR administration to hypercholesterolemic individuals at a dose of 2400 mg/day resulted in an 18% decrease in total cholesterol, a 23% decrease in LDL cholesterol and a 15% decrease in triglycerol concentrations [25]. In that study, a dose of 2400 mg of RYR powder daily, containing 0.4% monacolins or 5 to 7.5 mg of MK, reduced cholesterol levels in hypercholesterolemic subjects to a degree that was equivalent to what is typically observed with 20 mg of LV. Therefore, we hypothesized that other constituents in the RYR matrix were bioactive beyond MK alone. In the current study, we examined the efficacy of LV, RYR, MK-free RYR, pigment-rich fraction of RYR (PF-RYR) and monacolin-rich fraction of RYR (MF-RYR) on human colon cancer cell growth, apoptosis and transcription level of HMGCR and sterol response element binding protein-2 (SREBP-2).
2. Materials and methods
2.1. Extract and standard preparation
Chinese Red Yeast Rice (RYR) powder purchased from Botanica BioScience (Ojai, CA) was extracted with methylene chloride and evaporated under vacuum at 40 °C. The monacolin K (MK) concentration of the RYR extract was determined by HPLC-MS analysis (LCQ Classic Finnigan LC-MS/MS Systems, ThermoFinnigan, San Jose, CA) using an authentic standard (AG Scientific, San Diego, CA) (26) (Fig. 1A and 1B). For MK-free RYR, endogenous MK in RYR was removed by injecting a sample of the RYR extract onto a Prep-LC 4000 system coupled with a 490E Programmable Multiwavelength UV detector (Waters Corp., Milford, MA) with conditions as follows: column, Phenomenex Sphereclone (250 × 21.2 mm × 10 mm), isocratic solvent system: methanol: water (8:2), flow 5ml/min, detection λ = 237nm. The fraction collected between 18 to 19 min elution time was eliminated and the remaining fraction collected corresponding to MK-free RYR which was confirmed by analytical HPLC using an authentic standard of MK. For monacolin-rich fraction (MF-RYR) and pigment-rich fraction (PF-RYR) of RYR, RYR was dissolved into a mixture of dichloromethane and acetone (1:1, v/v) solution, mixed with silica gel and dried under vacuum at 40 °C. Flash column chromatographic method was used eluting with hexane and acetone (8:2, v/v), followed by pure acetone. The proportions of PF-RYR and MF-RYR were 10% and 90 % of RYR by weight, respectively.
Fig. 1.
HPLC. (A) Monacolin K (MK) identification using a standard lovastatin (LV). (B) Identification of monacolins in RYR. 1:Monacolin Kanalogue, 2: Monacolin K dehydro analogue, 3: Hydroxy-acid form of Monacolin L, 4: Hydroxy-acid form of Monacolin K, 5: Dihydromonacolin K, 6: Monacolin L, 7: Hydroxy-acid form of Dehydromonacolin K, 8: Monacolin K, 9: Methyl ester of hydroxyl-acid form of Monacolin K, 10: Dehydromonacolin K as previously reported [26]. AU: absorbance.
2.2. Cell culture
Human colon cancer cell lines, HCT-116 and HT-29, were obtained from American Type Culture Collection (ATCC, Manassas, VA), and maintained by seeding 500,000 to 1 million cells weekly into 100 mm dishes using McCoy’s 5A medium (ATCC) containing 10% FBS (Life Technologies, Grand Island, NY), 100 U/ml penicillin (Life Technologies), and 100 μg/ml streptomycin (Life Technologies). All experiments were done within less than 20 passages from the passage number they were upon receipt from ATCC. Cells were kept in a 37°C incubator with 95% air and 5% CO2.
2.3. MTT cell proliferation assay
Cells (5 × 103/well) were seeded in 0.1 ml of the medium in sterile 96 well plates. After 24 h, the medium was removed and replaced with treatment media. For the LV dose curve, cells were treated with LV (5. 93, 20, 40 or 80 μM) for 48 h. The 5.93 μM of LV is equivalent to MK amount in 50 μg/ml of RYR. For the RYR dose experiment, cells were treated with RYR (0 to 300 μg/ml) for 24, 48 or 72 h. To test the function of MK in RYR on colon cancer cell growth, cells were treated with MK-free RYR (0 to 100 μg/ml) for 48 h. To compare the effect of whole RYR, MF-RYR and PF-RYR on cell growth, cells were treated with RYR, MF-RYR (90% of RYR concentration) or PF-RYR (10% of RYR concentration) for 48 h. Mevalonate (MV) (Sigma-Aldrich, St. Louis, MO) in 25 μM or 50 μM was used to test if the effect of RYR and its fraction is by de novo cholesterogenesis. All stock solutions of LV, RYR, MK-free RYR, MF-RYR, PF-RYR and MV were all dissolved in dimethyl sulfoxide (DMSO), and the final concentration of DMSO in media was <0.2 %. Cell proliferation was estimated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich) assay. The MTT assay measures the cellular conversion of a tetrazolium salt into a formazan product, which can be detected by spectrophotometry and provides a relative estimate of cell growth. The absorbance at 570 nm was measured with the SoftMax (Molecular Devices, Sunnyvale, CA). Three replicates per condition were assayed and data averaged from 3–6 separate experiments are presented. Data are expressed as percentage of control (0.2 % DMSO).
2.4. Apoptosis assay
Apoptosis was assessed by measuring DNA fragmentation using Cell Death Detection ELISAPLUS Assay (Roche, Indianapolis, IN). This assay is a photometric enzyme-linked immunoassay (ELISA) that quantitatively measures the internucleosomal degradation of DNA, which occurs during apoptosis. Cells (105/dish) were plated in 60 mm dishes for 24 h then cells were treated with control (0.2 % DMSO), LV (5. 93 μM), RYR (50 μg/ml), MF-RYR (45 μg/ml) or PF-RYR (5 μg/ml) for 48 h. Following treatments, nonadherent cells were collected and pelleted at 200 × g. Adherent cells were washed with PBS (Invitrogen, Carlsbad, CA), trypsinized, collected and combined with nonadherent cells into a total of 1 ml medium. Both live and dead cells were than counted via trypan blue exclusion (Pierce, Rockford, IL), and equal number of cells were added to the microtiter plate for all treatment groups, and apoptosis assay was performed according to the manufacturer’s instructions. Data are expressed as percentage of control at absorbance at 405 nm. Two replicates per condition were assayed and data averaged from 3–4 separate experiments are presented.
2.5. RNA extraction and reverse transcription
Total RNA was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA). Sample RNA content was quantified by measuring the absorbance at 260 nm with a Gene Quant Spectrophotometer (Amersham-Pharmacia Biotech, Piscataway, NJ). Reverse transcription (RT) was performed on 3 μg of RNA by using oligo(dT)12–18 primers (Invitrogen) with SuperScript III Reverse Transcriptase (Invitrogen) according to the manufacturer's instruction.
2.6. Quantitative real time PCR
Gene expression of HMGCR and SREBP-2 were determined using Taqman Universal PCR master mix and primers (Applied Biosystems, Foster City, CA) by quantitative real time polymerase chain reaction (PCR) using the ABI 7900 HT Sequence Detector (Applied Biosystems). The transcription level of target genes was normalized to r18S expression. Every other sample had the RT reaction repeated on a separate occasion, followed by PCR and quantitation to confirm the reproducibility of the assay. In addition, every set of RT reactions contains a minus RT negative control to confirm that no contamination or anomaly has occurred.
2.7. Statistics
Data for the proliferation and apoptosis assays were analyzed by Student’s t test or one-way ANOVA followed by Student-Newman-Keuls (SNK) test with GraphPad PRISM 3.0 (GraphPad Software, San Diego, CA).
3. Results
3.1. Cell proliferation
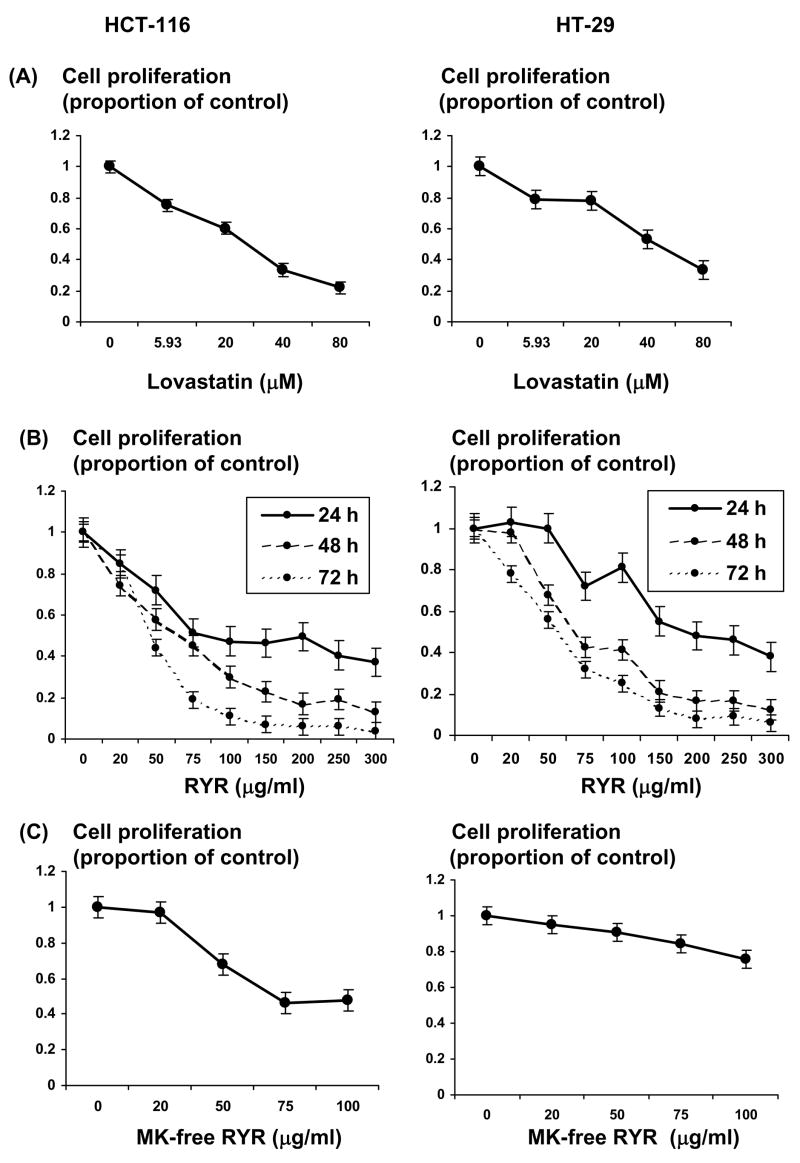
The proliferation of both human colon cancer cell lines, HCT-116 (P<.01) and HT-29 (P <.01), was inhibited by the presence of LV in a dose-dependent manner (Fig. 2A). The 5.93 μM of LV decreased colon tumor cell growth by 25% and 21% in HCT-116 and HT-29, respectively (p<0.01) (Fig. 2A). The 5.93 μM of MK is equivalent to that in 50 μg/ml of RYR. The 50 μg of RYR treatment for 48 h reduced tumor cell growth by 41% and 32% in HCT-116 and HT-29, respectively (p<0.01; Fig. 2B). RYR decreased colon cancer cell growth in a dose-dependent manner with 24, 48 and 72 h treatment (P <.001; Fig. 2B). MK-free RYR treatment still decreased cell proliferation in HCT-116 cells at the level of 50 μg/ml (P <.001) and HT-29 cells at the level of 100 μg/ml (P <.01; Fig. 2C). Addition of MV abolished the inhibitory activity of LV seen at 48 hours at 25 μM in HCT-116 cells (P <.05) and at 50 μM in HT-29 cells (P <.05; Fig. 3A). However, the same concentration of MV had no effect on the anti-proliferative activity of RYR at 48 hours in both cancer cells (Fig. 3B).
Fig. 2.
Lovastatin (LV), RYR and MK-free RYR effects on human colon cancer cell growth. (A) LV treatment for 48 h decreased cell proliferation in a dose-dependent manner in HCT-116 (P <.01) and HT-29 cells (P <.01). (B) RYR decreased cell proliferation of both HCT-116 and HT-29 colon cancer cells in a dose-dependent manner with 24, 48 and 72 h treatment (P <.001). (C) MK-free RYR decreased cell proliferation in both cells (P <.01). MK: monacolin K. Values are Mean ± S.E.M., n=3–6.
Fig. 3.
Mevalonate (MV) effect on lovastatin (LV) or RYR treated colon cancer cell growth. (A) Addition of MV (25 or 50 μM) partially or fully abolished the anti-proliferation activity of LV in HCT-116 and in HT-29 cells. (B) Incubation with MV for 48 h did not reverse the anti-proliferative effect of RYR (50 μg/ml) in HCT-116 and HT-29 cells. Control: 0.2 % DMSO. Values are Mean ± S.E.M., n=3–6. *, **, ***: Significantly different from control at P <.05, P <.01 or P <.001 respectively. †: Significantly different from LV5.93 or LV20 at P <.05.
In order to determine which fraction of RYR exhibited the greatest anti-proliferative potential, the effect of MF-RYR or PF-RYR as well as RYR were compared on tumor cell growth. Both MF-RYR and PF-RYR inhibited cell growth in a dose-dependent manner in both HCT-116 and HT-29 colon cancer cells (P <.001; Fig. 4). However, the degree of anti-proliferation was lower than that of RYR. Treatment of MV (25 μM) with MF-RYR (45 μg/ml) fully reversed the its anti-proliferation effect (P <.01) in HCT-116 cells and partially in HT-29 cells (P <.05; Fig. 5A). However, PF-RYR still suppressed tumor cell growth regardless of MV treatment (P <.05; Fig. 5B).
Fig. 4.
PF-RYR, MF-RYR or RYR effect on colon cancer growth. PF-RYR or MF-RYR treatment for 48 h decreased cell proliferation in both HCT-116 (P <.001) and HT-29 cells (P <.001). However, the degree of anti-proliferation was lower than that of RYR. Values are Mean ± S.E.M., n=3-6. The proportions of PF-RYR and MF-RYR were 10% and 90 % of RYR by weight, respectively. Therefore, for example, 50 μg/ml means 50 μg/ml of RYR, 5 μg/ml of PF-RYR or 45 μg/ml of MF-RYR. PF-RYR: pigment-rich fraction of RYR, MF-RYR: monacolin-rich fraction of RYR.
Fig. 5.
Mevalonate effect on PF-RYR or MF-RYR -treated colon cancer cell growth. (A) Treatment of MV (25 μM) with MF-RYR (45 μg/ml) fully reversed its anti-proliferative effects (P <.01) in HCT-116 cells and partially in HT-29 cells (P <.05). (B) PF-RYR (5 μg/ml) still suppressed tumor cell growth regardless of MV treatment (P <.05). Control: 0.2 % DMSO. MV: mevalonate, PF-RYR: pigment-rich fraction of RYR, MF-RYR: monacolin-rich fraction of RYR. Values are Mean ± S.E.M., n=3–6. *: Significantly different from control at P <.05. †, ††: Significantly different from MF-RYR at P <.05 or P<.01, respectively.
3.2. Apoptosis
An ELISA-based apoptosis assay, which quantitatively detects fragmented DNA, was used to measure the relative amount of induction of apoptosis at 48 hours. LV (5.93 μM) enhanced apoptosis in both HCT-116 and HT-29 cells by 3.8- and 1.6- fold, respectively (P <.001) and incubation with MV nullify the pro-apoptotic action of LV (Fig. 6A). Visual inspection of the cells showed that RYR increased dead (floating) cells, and amount of rounding of the cells was increased (data not shown). Apoptosis was increased with RYR treatment at the level of 50 μg/ml by 2.9 fold in HCT-116 (P <.01; Fig. 6B). Incubation with RYR and MV still increased apoptosis compared to control (P <.001) in HCT-116 cells. MF-RYR fraction showed similar results to that of LV on apoptosis. MF-RYR increased apoptosis in both colon cancer cells by 3- and 1.7-fold, respectively (P <.001) and it was reversed by the incubation with MV (Fig. 6C). PF-RYR enhanced apoptosis by 2.1 fold in HCT-116 cells (P <.01) and increase of apoptosis was also found in MV administration (P <.05; Fig. 6D).
Fig. 6.
Lovastatin (LV), RYR, MF-RYR or PF-RYR effects on apoptosis. (A) LV (5.93 μM) enhanced apoptosis and incubation with mevalonate (MV) nullified the pro-apoptotic action of MK in both HCT-116 and HT-29 cells (P <.001). (B) RYR increased apoptosis regardless of MV presence in HCT-116 (P <.001). In HT-29 cells, there was no effect of RYR with and without treatment of MV. (C and D) The effect of MF-RYR was similar to that of LV, while the effect of PF-RYR was similar to that of RYR on apoptosis. PF-RYR: pigment-rich fraction of RYR, MF-RYR: monacolin-rich fraction of RYR. Values are Mean ± S.E.M., n=3–4. *, **, ***: Significantly different from control at P <.05, P <.01 or P <.001, respectively.
3.3. HMGCR and SREBP-2 expression
LV treatment increased mRNR level of HMGCR and SREBP-2 by greater than 6 fold in HCT-116 cells (p<0.01), and by 3 fold in HT-29 colon cancer cells (P <.01; Fig. 7A and 7B). RYR and PF-RYR did not effect on the expression of HGMCR and SREBP compared to control. MF-RYR increased the transcriptional level of HMGCR and SREBP-2 by greater than 3 fold in HCT-116 and HT-29 colon cancer cells (P <.05; Fig. 7A and 7B).
Fig. 7.
Lovastatin (LV), RYR, MF-RYR or PF-RYR effect on transcription of HMGCR (A) and SREBP-2 (B). LV (5.93 μM) and MF-RYR (45 μg/ml) upregulated HMGCR and SREBP-2 gene expression in both HCT-116 and HT-29. There was not a significant effect of RYR (50 μg/ml) and PF-RYR (5 μg/ml) vs control on mRNR level of HMGCR and SREBP-2. PF-RYR: pigment-rich fraction of RYR, MF-RYR: monacolin-rich fraction of RYR, HMGCR: 3-hydroxy-3-methyl-glutaryl CoA reductase, SREBP-2: sterol response element binding protein-2. Values are Mean ± S.E.M., n=3–6. *, **, ***: Significantly different from control at P <.05, P <.01 or P <.001, respectively.
4. Discussion
There is accumulating evidence that statins may reduce the risk of colon cancer based on observation in vitro [21–23], in vivo [27–29], and in population studies [24, 30, 31]. Our studies were focused on determining the contributions of MK within RYR and the elements in RYR other than MK. For this purpose, we prepared fractions of RYR without MK, a fraction rich in pigments as well as a fraction rich in monacolins but absent of the pigments. In the two human colon cancer cell lines, addition of 25 or 50 μM MV partly or fully reversed the anti-proliferative and pro-apoptotic activity of LV. The selective reversal of LV-mediated inhibition of proliferation and increase of apoptosis as the result of MV supplementation is simply due to the restoration of de novo cholesterogenesis metabolic pathway. On the other hand, RYR effect on cell proliferation and apoptosis was not affected by the addition of MV, even though RYR contained the same range of MK concentrations as the media containing MK alone. Furthermore, MK-free RYR still inhibited cell proliferation. These data suggest that RYR has effects on proliferation and apoptosis which are independent of the MK in RYR. A matrix with other structural analogs and other substances including pigments that were able to inhibit colon cancer cell proliferation and stimulate apoptosis.
RYR contains Monascus pigments as well as MK [7, 8]. Monascus pigments comprise more than 10 compounds, six of which are well known: monascin, ankaflavin, monascorubin, rubropunctatin, monascorubramine and rubropunctamine [32–36]. Recently, it has been reported that derivatives of Monascus pigments have antimicrobial activity [37, 38] and the monascorubrin pigment inhibits skin cancer promotion in mice, either when applied topically or orally [39, 40]. The anti-cancer effect of the pigments was also supported by our current experiment that the pigment-rich fraction of RYR showed anti-proliferation and pro-apoptotic activities. While our studies clearly demonstrate that there are other factors beyond MK mediating some of the effects of RYR, further studies are needed to determine the effects of other active ingredients in RYR including sterols, isoflavones and tannins, on colon cancer cell growth and apoptosis.
It has been reported that lovastatin reduced DNA synthesis by a significant induction of p21WAF1/Cip1 protein expression in vascular smooth muscle cells [41], which may, in part, explain the potential mechanism of RYR on inhibition of cancer cell growth. Simvastatin potentiates tumor necrosis factor α (TNFα)-induced apoptosis through the down-regulation of nuclear factor kappa B (NF-κB) signaling pathway in squamous cell carcinoma SCC4 cells [42], intestinal epithelial cells (IEC) and colon cancer cells (COLO 205) [43]. It was also shown that lovastatin decreased AKT protein expression in SCC6 cells [44] which suggests the involvement of PI-3 Kinase signaling on apoptosis induction. Therefore, RYR which is naturally containing lovastain may enhance apoptosis via downregulation of NFkB and PI3K/AKT signaling.
Lovastatin increased transcription level of HMGCR, the rate limiting enzyme for cholesterogenesis, and transcription of SREBP-2, the response element binds to the promoter region of HMGCR as expected. When cellular cholesterol levels are reduced following statin treatment, SREBPs are released from the endoplasmic reticulum membrane and translocate to the nucleus where they activate SREBP target genes [45]. SREBP-2 primarily regulates the transcription of HMGCR [45, 46]. The increase of HMGCR and SREBP-2 gene expression is a compensatory response designed to restore the reduced levels of cholesterol resulting from statin inhibition of HMGCR. In the present study, lovastatin increased the expression of HMGCR and SREBP-2 while RYR did not increase gene expression of HMGCR and SREBP-2. This is one of the merits in using RYR over the advantage of administration of LV, that it decreases cholesterol level without elevation of gene expression of HMGCR and SREBP-2.
Although the beneficial effects of statins are mediated by their lipid-lowering properties. experimental and clinical studies have suggested that statins also exhibit anti-inflammation activity [47–49]. Cyclooxygeanse (COX-2) expression is closely related to inflammation process and it has an important role in colorectal tumorigenesis [50]. Synergistic interaction between COX-2 inhibitor and statin has been shown in colon cancer cells (colon-26 and CMT-93) [51] and mouse intestine [52]. The rationale of choosing of HCT-116 (no COX-2 expression) and HT-29 (COX-2 expression) [53] human colon adenocarcinoma cell lines was to determine whether the activity of RYR in colon cancer was associated to inhibition of COX-2 related inflammation process. In the present study, RYR inhibited colon cancer cell growth regardless of COX-2 expression. However, some differential response to treatments was also observed. For example, RYR induced apoptosis in HCT-116 cells but not in HT-29 cells. MK-free RYR still decreased cell proliferation in both cells but the degree was much weaker in HT-29 cells. These suggest that with/without COX-2 expression of the cell lines may partially cause the different response to lovastatin and RYR treatment in the two cell lines, and it should be further studied.
The results of the current study are significant enough to warrant for further study. The mechanism of RYR effect on apoptosis, such as via caspase 9, 8 and 3 as well as poly(ADP-ribose) polymerase (PARP) cleavage, and RYR effects on protein level of HMGCR and SREBP are undergoing investigation. In vivo animal studies will be needed to confirm that RYR inhibits colon cancer risk primarily via inhibition of de novo cholesterogenesis.
RYR, a traditional Chinese food herb and a modern dietary supplement, has demonstrated in vitro effects including inhibition of proliferation and stimulation of apoptosis in human colon cancer cells by mechanisms involving MK and the red yeast pigment fraction. The multiple effects of RYR in vitro suggest that further investigation in animal models and ultimately in humans may be warranted given the unique profile of actions of herbal supplements with multiple components compared to purified crystallized drugs containing only a single component.
Acknowledgments
We thank Mr. Rupo Lee for the preparation and performing HPLC of RYR extract. This study was funded by UCLA/NCI Clinical Nutrition Research Unit Grant No. CA 42710. This work was also funded by Dr. M. Y. Hong’s PCRP CDMRP PC060044 grant from Department of Defense.
Funding sources: This study was funded by UCLA/NCI Clinical Nutrition Research Unit Grant No. CA 42710. This work was also funded by Dr. M. Y. Hong’s PCRP CDMRP PC060044 grant from Department of Defense.
Abbreviations
- DMSO
dimethyl sulfoxide
- ELISA
enzyme-linked immunosorbent assay
- HMGCR
3-hydroxy-3-methyl-glutaryl CoA reductase
- HPLC
high performance liquid chromatography
- LDL
low-density lipoprotein
- LV
lovastatin
- MF-RYR
monacolin-rich fraction of Chinese red yeast rice
- MK
monacolin K
- MV
mevalonate
- PCR
polymerase chain reaction
- PF-RYR
pigment-rich fraction of Chinese red yeast rice
- RT
reverse transcription
- RYR
Chinese red yeast rice
- SREBP-2
sterol response element binding protein-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ma J, Li Y, Ye Q, Li J, Hua Y, Ju D, Zhang D, Cooper R, Chang M. Constituents of red yeast rice, a traditional Chinese food and medicine. J Agric Food Chem. 2000;48:5220–25. doi: 10.1021/jf000338c. [DOI] [PubMed] [Google Scholar]
- 2.Simg R-H. Tien-Kung K’ai-Wu. Chinese Technology in the Seventeenth Century; Pennsylvania State University Press. 1966. pp. 292–294. [Google Scholar]
- 3.Haval RJ. Dietary supplement or drug? The case of cholestin. Am J Clin Nutr. 1999;69:175–6. doi: 10.1093/ajcn/69.2.175. [DOI] [PubMed] [Google Scholar]
- 4.Stuart MD. Chinese material medica: Vegetable kindom. Taipai: Republic of China: Southern materials Center; 1979. [Google Scholar]
- 5.Van TP. Monascus, genre nouveau de l’ordre des Ascomycetes. Bull Soc Fr. 1884;31:226–31. [Google Scholar]
- 6.Went FAFC. Monascus purpureus le champignon de l’angquac une nouvelle thelebolee. Ann Soc Nat Bot. 1895;8:1–17. [Google Scholar]
- 7.Martinkova L, Patakova-Juzlova P, Krent V, Kucerova Z, Havlicek V, Olsovsky P, Hovorka O, Rihova B, Vesely D, Vesela D, Ulrichova J, Prikrylova V. Biological activities of oligoketide pigments of Monascus purpureus. Food Addit Contam. 1999;16:15–24. doi: 10.1080/026520399284280. [DOI] [PubMed] [Google Scholar]
- 8.Heber D, Lembertas A, Lu Q-Y, Bowerman S, Go VLW. An analysis of nice proprietary Chinese Red Yeast Rice dietary supplements: Implications of variability in chemical profile and contents. J Altern Complement Med. 2001;7:133–9. doi: 10.1089/107555301750164181. [DOI] [PubMed] [Google Scholar]
- 9.Retterstol K, Stugaard M, Gorbitz C, Ose L. Results of intensive long-term treatment of familial hypercholesterolemia. Am J Cardiol. 1996;78:1369–74. doi: 10.1016/s0002-9149(96)00649-2. [DOI] [PubMed] [Google Scholar]
- 10.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–42. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 11.Lewis-Barned NJ, Ball MJ. Beneficial effect of simvastatin in patients with drug resistant familial hypercholesterolaemia. N Z Med J. 1992;105:284–6. [PubMed] [Google Scholar]
- 12.Jenkins DJ, Kendall CS, Marchie A, Faulkner DA, Wond JM, de Souza R, Emam A, Parker TL, Vidgen E, Lapsley KG, Trautwein EA, Josse RG, Leiter LA, Connelly PW. Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. JAMA. 2003;290:502–10. doi: 10.1001/jama.290.4.502. [DOI] [PubMed] [Google Scholar]
- 13.Wei W, Li C, Want Y, Su H, Zhu J, Kritchevsky D. Hypolipidemic and anti-atherogenic effects of long-term Cholestin (Monascus purpureus-fermented rice, red yeast rice) in cholesterol fed rabbits. J Nutr Biochem. 2003;14:314–8. doi: 10.1016/s0955-2863(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 14.Lee CL, Tsai TY, Wang JJ, Pan TM. In vivo hypolipidemic effects and safety of low dosage Monascus powder in a hamster model of hyperlipidemia. Appl Microbiol Biotechnol. 2006;705:533–40. doi: 10.1007/s00253-005-0137-0. [DOI] [PubMed] [Google Scholar]
- 15.Jeon T, Hwang SG, Hirai S, Matsui T, Yano H, Kawada T, Lim BO, Park DK. Red yeast rice extracts suppress adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 cells. Life Sci. 2004;75:3195–203. doi: 10.1016/j.lfs.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Lin CC, Li TC, Lai MM. Efficacy and safety of Monascus purpureus Went rice in subjects with hyperlipidemia. Eur J Endocrino. 2005;153:679–86. doi: 10.1530/eje.1.02012. [DOI] [PubMed] [Google Scholar]
- 17.Buchwald H. Cholesterol inhibition, cancer, and chemotherapy. Lancet. 1992;339:1154–6. doi: 10.1016/0140-6736(92)90744-n. [DOI] [PubMed] [Google Scholar]
- 18.Hentosh P, Yuh SH, Elson CE, Peffley DM. Sterol-independent regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in tumor cells. Mol Carcinog. 2001;32:154–66. doi: 10.1002/mc.1074. [DOI] [PubMed] [Google Scholar]
- 19.Notarnicola M, Messa C, Pricci M, Guerra V, Altomare DF, Montemurro S, Caruso MG. Up-regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in left-sided human colon cancer. Anticancer Res. 2004;24:3837–42. [PubMed] [Google Scholar]
- 20.Caruso MG, Notarnicola M, Cavallini A, Di Leo A. 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity and low-density lipoprotein receptor expression in diffuse-type and intestinal-type human gastric cancer. J Gastroenterol. 2002;37:504–8. doi: 10.1007/s005350200078. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal B, Halmos B, Feoktistov AS, Protiva P, Ramey WG, Chen M, Pothoulakis C, Lamont JT, Holt PR. Mechanism of lovastatin-induced apoptosis in intestinal epithelial cells. Carcinogenesis. 2002;23:521–8. doi: 10.1093/carcin/23.3.521. 2002. [DOI] [PubMed] [Google Scholar]
- 22.Ukomadu C, Dutta A. p21-dependent inhibition of colon cancer cell growth by mevastatin is independent of inhibition of G1 cyclin-dependent kinases. J Biol Chem. 2003;278:43586–94. doi: 10.1074/jbc.M307194200. [DOI] [PubMed] [Google Scholar]
- 23.Lin WY, Song CY, Pan TM. Proteomic analysis of caco-2 cells treated with monacolin K. J Agric Food Chem. 2006;54:6192–200. doi: 10.1021/jf061060c. [DOI] [PubMed] [Google Scholar]
- 24.Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, Low M, Greenson JK, Rennert G. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–92. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 25.Heber D, Yip I, Ashley JM, Elashoff DA, Elashoff RM, Go VL. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Clin Nutr. 1999;69:231–6. doi: 10.1093/ajcn/69.2.231. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Seeram NP, Lee R, Thames G, Minutti C, Wang HJ, Heber D. Plasma clearance of lovastatin versus chinese red yeast rice in healthy volunteers. J Altern Complement Med. 2005;11:1031–8. doi: 10.1089/acm.2005.11.1031. [DOI] [PubMed] [Google Scholar]
- 27.Reddy BS, Wang CX, Kong AN, Khor TO, Zheng X, Steele VE, Kopelovich L, Rao CV. Prevention of azoxymethane-induced colon cancer by combination of low doses of atorvastatin, aspirin, and celecoxib in F 344 rats. Cancer Res. 2006;66:4542–6. doi: 10.1158/0008-5472.CAN-05-4428. [DOI] [PubMed] [Google Scholar]
- 28.Swamy MV, Patlolla JM, Steele VE, Kopelovich L, Reddy BS, Rao CV. Chemoprevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APCMin mice. Cancer Res. 2006;66:7370–7. doi: 10.1158/0008-5472.CAN-05-4619. [DOI] [PubMed] [Google Scholar]
- 29.Naito Y, Katada K, Takagi T, Tsuboi H, Isozaki Y, Handa O, Kokura S, Yoshida N, Ichikawa H, Yoshikawa T. Rosuvastatin, a new HMG-CoA reductase inhibitor, reduces the colonic inflammatory response in dextran sulfate sodium-induced colitis in mice. Int J Mol Med. 2006;17:997–1004. [PubMed] [Google Scholar]
- 30.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen TR, Berg K, Cook TJ, Faergeman O, Haghfelt T, Kjekshus J, Miettinen T, Musliner TA, Olsson AG, Pyorala K, Thorgeirsson G, Tobert JA, Wedel H, Wilhelmsen L. Safety and tolerability of cholesterol lowering with simvastatin during 5 years in the Scandinavian Simvastatin Survival Study. Arch Intern Med. 1996;156:2085–92. [PubMed] [Google Scholar]
- 32.Chen FC, Manchand PS, Whalley WB. The chemistry of fungi. LXIV The structure of monascin: the relative stereochemistry of the azaphilones. J Chem Soc. 1971;21:3577–9. doi: 10.1039/j39710003577. [DOI] [PubMed] [Google Scholar]
- 33.Manchand PS, Whally WB, Chen FC. Isolation and structure of ankaflavin. Phytochem. 1973;12:2531–2. [Google Scholar]
- 34.Kuromo M, Nakanishi K, Shindo K, Tada M. Biosynthesis of monascorubrin and monascoflavin. Chem Pharm Bull (Tokyo) 1963;11:358–62. [Google Scholar]
- 35.Hadfield JR, Holker JSE, Stanway DN. The biosynthesis of fungal metabolites. Part II The β-oxo-lactone equivalences in rubropunctatin and monascorubrin. J Chem Soc. 1967;19:751–5. [Google Scholar]
- 36.Fowell ADG, Robertson A, Whalley WB. Monascorubramin. J Chem Soc (Special Publ) 1956;5:27–35. [Google Scholar]
- 37.Kim C, Jung H, Kim YO, Shin CS. Antimicrobial activities of amino acid derivatives of monascus pigments. FEMS Microbiol Lett. 2006;264:117–24. doi: 10.1111/j.1574-6968.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 38.Journoud M, Jones PJ. Red yeast rice: a new hypolipidemic drug. Life Sci. 2004;74:2675–2683. doi: 10.1016/j.lfs.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Yasukawa K, Takahashi M, Natori S, Kawai K, Yamazaki M, Takeuchi M, Takido M. Azaphilones inhibit tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mice. Oncology. 1994;51:108–12. doi: 10.1159/000227320. [DOI] [PubMed] [Google Scholar]
- 40.Yasukawa K, Takahashi M, Yamanouchi S, Takido M. Inhibitory effect of oral administration of Monascus pigment on tumor promotion in two-stage carcinogenesis in mouse skin. Oncology. 1996;53:247–9. doi: 10.1159/000227568. [DOI] [PubMed] [Google Scholar]
- 41.Muller C, Kiehl MG, van de Loo J, Koch OM. Lovastatin induces p21WAF1/Cip1 in human vascular smooth muscle cells: influence on protein phosphorylation, cell cycle, induction of apoptosis, and growth inhibition. Int J Mol Med. 1999;3:63–8. doi: 10.3892/ijmm.3.1.63. [DOI] [PubMed] [Google Scholar]
- 42.Ahn KS, Sethi G, Aggarwal BB. Simvastatin potentiates TNF-alpha-induced apoptosis through the down-regulation of NF-kappaB-dependent antiapoptotic gene products: role of IkappaBalpha kinase and TGF-beta-activated kinase-1. J Immunol. 2007;178:2507–16. doi: 10.4049/jimmunol.178.4.2507. [DOI] [PubMed] [Google Scholar]
- 43.Lee JY, Kim JS, Kim JM, Kim N, Jung HC, Song IS. Simvastatin inhibits NF-kappaB signaling in intestinal epithelial cells and ameliorates acute murine colitis. Int Immunopharmacol. 2007;7:241–8. doi: 10.1016/j.intimp.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Mantha AJ, Hanson JEL, Goss G, Lagarde AE, Lorimer IA, dimitroulakos J. Targeting the mevalonate pathway inhibits the function of the epidermal growth factor receptor. Clin Cancer Res. 2005 Mar 15;11(6):2398–407. doi: 10.1158/1078-0432.CCR-04-1951. [DOI] [PubMed] [Google Scholar]
- 45.Mascaro C, Ortiz JA, Ramos MM, Haro D, Hegardt FG. Sterol regulatory element binding protein-mediated effect of fluvastatin on cytosolic 3-hydroxy-3-methylglutaryl-coenzyme A synthase transcription. Arch Biochem Biophys. 2000;374:286–92. doi: 10.1006/abbi.1999.1600. [DOI] [PubMed] [Google Scholar]
- 46.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–31. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naito Y, Katada K, Takagi T, Tsuboi H, Isozaki Y, Handa O, Kokura S, Yoshida N, Ichikawa H, Yoshikawa T. Rosuvastatin, a new HMG-CoA reductase inhibitor, reduces the colonic inflammatory response in dextran sulfate sodium-induced colitis in mice. Int J Mol Med. 2006;17:997–1004. [PubMed] [Google Scholar]
- 48.Musial J, Undas A, Gajewski P, Jankowski M, Sydor W, Szczeklik A. Anti-inflammatory effects of simvastatin in subjects with hypercholesterolemia. Int J Cardiol. 2001;77:247–53. doi: 10.1016/s0167-5273(00)00439-3. [DOI] [PubMed] [Google Scholar]
- 49.Devaraj S, Chan E, Jialal I. Direct demonstration of an anti-inflammatory effect of simvastatin in subjects with the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:4489–96. doi: 10.1210/jc.2006-0299. [DOI] [PubMed] [Google Scholar]
- 50.Sinicrope FA. Targeting cyclooxygenase-2 for prevention and therapy of colorectal cancer. Mol Carcinog. 2006;45:447–54. doi: 10.1002/mc.20232. [DOI] [PubMed] [Google Scholar]
- 51.Feleszko W, Jalili A, Olszewska D, Mlynarcznuk I, Grzela T, Giermasz A, Jakobisiak M. Synergistic interaction between highly specific cyclooxygenase-2 inhibitor, MF-tricyclic and lovastatin in murine colorectal cancer cell lines. Oncol Rep. 2002;9:879–85. [PubMed] [Google Scholar]
- 52.Swamy MV, Patlolla JM, Steele VE, Kopelovich L, Reddy BS, Rao CV. Chemoprevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APCMin mice. Cancer Res. 2006;66:2370–7. doi: 10.1158/0008-5472.CAN-05-4619. [DOI] [PubMed] [Google Scholar]
- 53.Palozza P, Serini S, Maggiano N, Tringali G, Navarra P, Ranelletti FO, Calviello G. beta-Carotene downregulates the steady-state and heregulin-alpha-induced COX-2 pathways in colon cancer cells. J Nutr. 2005;135:129–36. doi: 10.1093/jn/135.1.129. [DOI] [PubMed] [Google Scholar]