Abstract
Little is known about dietary diversity of children residing in areas of high HIV prevalence. This study examined dietary diversity in 381 children ages 6−24 mo in rural South Africa. Twenty-eight (7.3%) children and 170 mothers (44.6%) were HIV infected. Home visits were conducted weekly and a detailed history of dietary intake obtained. A dietary diversity score was computed based on the weekly consumption of 8 food classes. Low dietary diversity was defined as falling within the lowest quartile of the diversity scale. There were 22,772 child weeks of observation: 1369 for HIV-infected children, 8876 for HIV-uninfected children born to HIV-infected mothers, and 12,527 for HIV-uninfected children born to HIV-uninfected mothers. Low dietary diversity was more common in HIV-infected children [crude odds ratio (OR), 2.59; 95% CI, 1.52 to 4.41) compared with children born to HIV-uninfected mothers. In a multiple logistic regression analysis adjusting for socioeconomic and health status, HIV-infected children had lower dietary diversity (conditional OR, 1.76; 95% CI, 1.06 to 2.94) than HIV-uninfected children. HIV-infected children consumed less in 6 of 8 food classes compared with HIV-uninfected children, with the 2 exceptions being breast milk and formula milk. In rural South Africa, HIV-infected children's diets are significantly less diverse than those of HIV-uninfected children. This may be a factor contributing to increased morbidity and poorer survival in these children.
Introduction
Childhood malnutrition remains a problem in Africa. This is in part because of the homogeneous postweaning diet, which in many areas of Africa consists mainly of diluted porridges (1). HIV infection may exacerbate this problem. In addition to directly affecting the nutritional status of children with HIV infection, it can also cause social and family disruption, thereby contributing to household economic and food insecurity (2).
Current WHO-United Nations Children's Fund recommendations are that HIV-infected women and women with unknown HIV status not breast-feed their child after 6 mo if safe and sustainable alternatives are available. Dietary diversity, the consumption of an adequate variety of food groups, is an aspect of dietary quality and can be considered an indicator of general nutritional adequacy. Low dietary diversity has been associated with specific nutrient deficiencies (3) and with stunting, an important health indicator, independently of socioeconomic status (4). Dietary diversity is endangered if breast-feeding is abruptly replaced by poor replacement feeding (5). It is therefore important to have knowledge of dietary diversity in areas where HIV is highly prevalent.
Measuring dietary diversity can, however, be difficult. It is especially difficult to determine dietary patterns over time. Determination of longitudinal dietary patterns is important for infants and young children, because they should be moving rapidly from a predominantly milk-based diet to a diet with increasingly diverse complementary foods.
The dietary assessment tools most often used in epidemio-logical studies of diet are cross-sectional, e.g. 24-h recall and FFQ. Indices of dietary quality and variety in infants and children have been developed, but these instruments tend to be detailed and thus their iterative use to determine long-term diet becomes time consuming and costly (6–8). If long-term diet is to be determined, there needs to be a balance between the frequency and the detail of the assessment that is done. In this study, we used a locally adapted dietary questionnaire that listed only foods most commonly consumed in the area, rather than all possible foods, and categorized them into 8 common food types. This simplification of the questionnaire allowed us to administer it weekly at homes of the children enrolled in the study and to determine daily food intake of these children.
This study used these tools to describe the dietary diversity of 381 children living in an area of very high HIV prevalence and analyzed the effect of HIV infection in children and mothers on dietary diversity.
Subjects and Methods
Study area
The study was conducted in rural northern KwaZulu-Natal Province, South Africa. The study area consists of scattered homesteads, with the exception of a small black township that is adjacent to a market and sugar mill town where, under apartheid, residence had been restricted to whites. Median household incomes are less than one-half the national average, 80% of the population lives below the national poverty line, and unemployment among adults is 75% (9). Eighty percent of the population lack access to electricity or clean water in their homes (10). As a consequence of colonial and apartheid policies, there is little subsistence farming in this area despite 98% of households being categorized as rural. Food insecurity within households is common (9). The main source of income is from migrant labor and old-age pensions from the government. Antenatal HIV prevalence in KwaZulu-Natal Province is 39% (11).
Study subjects
This study reports on 381 children who were part of a randomized trial examining the efficacy of 3 micronutrient supplements, given daily from 6 to 24 mo of age, in reducing diarrhea and respiratory disease prevalence (12,13). The 3 supplement groups were: vitamin A alone, vitamin A plus zinc, and multiple vitamins and minerals, including vitamin A and zinc. Three cohorts of children were included in the study: 28 children were HIV infected; 142 were HIV-uninfected children born to an HIV-infected mother; and 211 were HIV-uninfected children born to an HIV-uninfected mother. Nearly all mothers knew their child's HIV status, but none of the children were receiving antiretroviral therapy. Although it is possible that some of the 142 HIV uninfected children from HIV-infected mothers seroconverted, this group was analyzed “as initially classified” based on the premises that only a small portion of vertical transmission of HIV is detected after age 6 mo of age and that breast-feeding declines rapidly after age 6 mo in this population. Study subjects were enrolled at 1 of 5 government primary health care clinics and were excluded if they had received vitamin or mineral supplements in the 3 mo before enrolment, if they intended to breast-feed exclusively beyond the age of 6 mo, if they had persistent diarrhea, or if they were severely malnourished (<60% of median weight-for-age using the National Center for Health Statistics standards) (14). At enrolment, study nurses counseled all mothers on breast-feeding and complementary feeding.
Collection of dietary data
Before the start of data collection, 17 fieldworkers received intensive training on interview skills and questionnaire completion. Refresher training was conducted every 2 mo. Fieldworkers administered morbidity and dietary questionnaires at weekly home visits. If an informant was not available for the scheduled visit, a follow-up visit was made the same week. If both visits were unsuccessful, then dietary information was obtained at the next weekly scheduled visit for which an informant was available. Dietary information was obtained for up to 28 d after the day for which information was sought.
Similar to a standard FFQ, the questionnaire used in this study contained a list of specific foods thought to be important components of the diet of children in this region (Table 1). Instead of reporting usual frequency, however, we asked the caregiver about consumption of these foods on each day since the last visit. All foods were asked about separately. Foods that were consumed but not listed in the questionnaire were also recorded. We also asked mothers to complete food diaries. The diaries had pictures of breast milk, fruits, vegetables, formula milk, cereals, and a picture of a plate with a typical family dish. Mothers were requested to complete the diary daily and the diaries were used as a reminder during the dietary interview.
TABLE 1.
Food classes, and foods within those classes, used in the study questionnaire1
| Food class | Foods included in class |
|---|---|
| Breast milk | Breast milk |
| Formula milk | Formula milk |
| Dairy products | Cow's milk, yogurt, cheese, milk powder |
| Vegetable protein | Dried beans, canned beans |
| Nonmilk animal protein | Meat, chicken, fish, eggs |
| Snacks and sweetened beverages | Flavored drinks containing juice extracts, tea, biscuits, chips, candy, cake |
| Fruit and vegetables | Fresh or canned fruit or vegetables |
| Complex carbohydrates | Bread, cereal porridge, maize meal |
Values of median diversity score are percentages of the maximum diversity score. Other values are percentages of 22,772 observed weeks.
Data handling
The data system used and the data quality obtained in the study have been described in detail elsewhere (13,15). Briefly, data were entered into a Structured Query Language database (16) using an electronic image recognition and translation system (17). This software permits internal validation checks before entry into the electronic database. Data that were not valid were queried and only entered into the database after resolution of the query. Remaining errors were screened and when possible corrected during postentry data cleaning.
Measurement reliability of food items was mostly good (13). For example, intra- and interobserver reliability for consumption of breast milk were excellent (all κ values >0.85). For previous-day consumption of fruits, the intraobserver κ was 0.94 (excellent), but the interobserver κ was 0.53 (weak).
Dietary diversity assessment
Foods were grouped into 8 classes based on similarities in nutrient content and source (Table 1). For each child observation week, we computed a raw (unadjusted) diversity score based on consumption of foods from the 8 predefined food classes. The unadjusted diversity score was calculated as a percentage of the maximum food diversity that could occur in a week, with the maximum diversity being defined as consumption of each of the 8 food categories every day. A value of 0 meant that since the last visit the child had eaten nothing from any of the 8 food groups; a value of 100 meant all 8 food groups had been consumed every day.
To adjust for the potential effects of age, sex, and season, we computed a dietary diversity Z-score, using the internal age, sex, and seasonal distribution of raw score values as a reference. The modeling of the distributions was conducted using the LMS method (18) and Growth Analyzer software (19).
Data analysis and statistical methods
Cross-sectional associations with weekly dietary diversity
To assess how dietary diversity changes with age, we calculated median and quartiles of unadjusted diversity in 3-mo age groups from 6 to 24 mo. We evaluated the contribution of individual food classes to changes in food diversity by plotting age trends of frequency of consumption of specific food classes and tabulating frequency of consumption of specific food classes for weeks with low diversity (defined as unadjusted dietary diversity in the lowest quartile) and weeks with higher diversity (top 3 quartiles). We used the Kruskal-Wallis test to determine the significance of differences in raw diversity scores between age groups, sexes, and seasons.
In a cross-sectional analysis, we examined the association of HIV status and other potential predictors with low weekly dietary diversity as an outcome. This was done independent of age, sex, and season by using the adjusted diversity Z-scores calculated as described. Low diversity was defined here as falling within the lowest quartile of diversity Z-score. Using single-predictor logistic regression analyses, we calculated crude odds ratios (OR) for low diversity according to HIV status, recall period of the dietary interview, location of residence (defined as township, rural, or deep rural), and whether the mother was the main caregiver. The category deep rural was assigned to areas located away from the main roads. We also examined if the prevalence of low dietary diversity differed during episodes of diarrhea or respiratory infections and whether it was different according to the type of micronutrient supplement given during the study (none, vitamin A alone, vitamin A plus zinc, or multi-vitamins and minerals). Diarrhea was considered to be present on any day the stool was reported by the caretaker to be more frequent or looser than normal or the stool contained water or blood. Upper respiratory tract infection was defined as the history (or presence during the field worker visit) of cough or runny nose. All factors from the bivariate analyses were subsequently examined together in a multiple logistic regression analysis and adjusted (conditional) OR calculated. All OR (crude and adjusted) were recalculated using a random effects model to adjust for multiple observations from each subject not being independent.
To explore which food classes contributed to differences in dietary diversity according to HIV status of the child, we tabulated the frequency of consumption of specific food classes by age group and HIV status.
P-values <0.05 were considered significant. The Statistical Package for the Social Sciences (20) and Stata (21) were used for data analysis.
Ethical oversight
The study was approved by the Tufts-New England Medical Center's Institutional Review Board and the University of KwaZulu-Natal Ethics Committee. Written informed consent to participate in the study was obtained from the mothers of all children participating in the study.
Results
Twelve (3.1%) of the 381 children enrolled in the study died before they completed the study, 62 (16.3%) moved out of the study area during the study and could not be followed, 2 (0.5%) dropped out of the study because the child did not like the taste of the tablets, and 38 (10.0%) dropped out for other or unspecified reasons. During the 18 mo of study there were 22,772 observed weeks. The mean number of observed weeks per child was 60 of the 78 expected and was lower for HIV-infected children (49 wk) because of their higher mortality.
Information was collected on dietary intake for 159,642 study days. Information on daily dietary intake was obtained within 7 d for 70% of days included in the analysis; 30% was obtained during a follow-up visit within 30 d because an informant was not available during the scheduled visit.
The median unadjusted dietary diversity score was 37.5% (25th and 75th percentiles, 28.6% and 45.8%). Median unadjusted dietary diversity scores increased rapidly between 6 and 9 mo, from 25.0 to 37.5% (P < 0.001) and remained relatively stable thereafter. The increasing diversity between 6 and 9 mo of age resulted from increasing consumption of dairy products, nonmilk animal protein, vegetable protein, and snacks and sweetened beverages (Fig. 1). Consumption of breast milk and formula milk decreased after 9 mo of age, but dietary diversity was maintained by increased intake of snacks/sweetened beverages and of nonmilk animal protein. Unadjusted dietary diversity did not differ by gender or season (P > 0.05).
FIGURE 1.
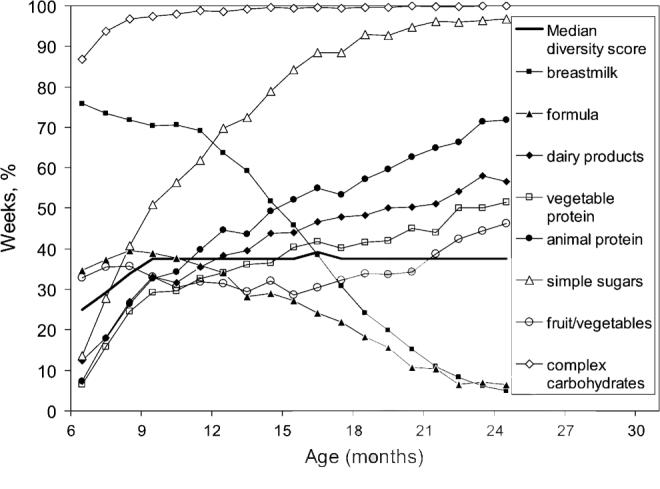
Age trend of median unadjusted diet diversity scores and percentage of observed weeks when items belonging to specific food classes were consumed by 381 6- to 24-mo-old children in rural South Africa.
The pattern of food intake leading to a low dietary diversity differed by age (Table 2). At 6 mo of age, lack of intake of fruits, vegetables, dairy products, snacks/sweetened beverages, and complex carbohydrates accounted for poor dietary diversity. At age 18 mo, lack of breast milk and formula and of vegetable protein contributed to the low dietary diversity.
TABLE 2.
Weekly food class consumption by age and level of diet diversity in 381 children aged 6−24 mo in rural South Africa1
| Age, mo2 | Dietary diversity level3 | Breast milk | Formula milk | Dairy products | Vegetable protein | Nonmilk animal protein | Snacks and sweetened beverages | Fruit and vegetables | Complex carbohydrates |
|---|---|---|---|---|---|---|---|---|---|
| Weeks when food, % | |||||||||
| 6 mo | Normal | 78.4 | 44.0 | 24.1 | 8.8 | 14.0 | 28.4 | 57.8 | 100.0 |
| Low | 74.3 | 29.5 | 5.7 | 5.2 | 3.8 | 5.2 | 19.0 | 79.5 | |
| 9 mo | Normal | 73.4 | 43.3 | 42.3 | 36.7 | 42.5 | 64.5 | 43.3 | 98.8 |
| Low | 63.2 | 28.4 | 10.4 | 10.9 | 8.9 | 17.5 | 8.1 | 93.8 | |
| 12 mo | Normal | 66.8 | 35.3 | 45.6 | 38.4 | 52.4 | 81.4 | 38.1 | 99.8 |
| Low | 50.9 | 28.7 | 10.0 | 16.8 | 14.3 | 23.3 | 4.7 | 93.9 | |
| 18 mo | Normal | 29.1 | 21.9 | 60.5 | 48.2 | 67.3 | 96.9 | 42.5 | 100.0 |
| Low | 8.3 | 6.5 | 9.4 | 20.2 | 24.9 | 79.8 | 6.5 | 97.8 | |
| 24 mo | Normal | 6.2 | 8.3 | 69.5 | 61.2 | 82.8 | 99.6 | 55.6 | 100.0 |
| Low | 0.5 | 0.0 | 13.9 | 19.7 | 36.1 | 87.5 | 15.4 | 100.0 | |
Values are percentages of 22,772 observed weeks.
Midpoint of 3-mo age interval.
Low dietary diversity was considered present when the raw diversity score was in the in lowest quartile. Normal dietary diversity was considered to be present when the diversity score was in the top 3 quartiles.
In the bivariate analyses of risk factors for low dietary diversity (Table 3), the crude OR for a low diversity Z-score increased for observation weeks from HIV-infected children (OR, 2.59; 95% CI, 1.52−4.41) in comparison with observation weeks coming from HIV-uninfected children born to HIV-uninfected mothers. Low dietary diversity was also more frequent during diarrhea episodes, when the child took micro-nutrient supplements (as opposed to not taking supplements) and when the recall period was longer than 1 wk. Dietary diversity was greater during respiratory infections, when tap water was not used for food preparation, when the place of residence was deep rural, and when the mother was not the caregiver that week.
TABLE 3.
Risk of low dietary diversity of 381 children by clinical and socioeconomic characteristics in 381 children aged 6−24 mo in rural South Africa
| Child characteristic | Wk of observation, n (%) | Crude OR (95% CI) of a low diversity Z-score1 | Adjusted OR (95% CI) of a low diversity Z-score2 |
|---|---|---|---|
| Child HIV uninfected | |||
| Mother uninfected | 12,527 (55.0) | (reference group) | (reference group) |
| Mother infected | 8876 (39.0) | 1.25 (0.94 to 1.67) | 1.10 (0.82 to 1.45) |
| Child HIV infected | 1369 (6.0) | 2.59 (1.52 to 4.41) | 1.76 (1.06 to 2.94) |
| Diarrhea that week | |||
| No | 21,148 (92.9) | (reference group) | (reference group) |
| Yes | 1624 (7.1) | 1.14 (1.00 to 1.31) | 1.18 (1.02 to 1.34) |
| Respiratory infection that week | |||
| No | 18,644 (82.5) | (reference group) | (reference group) |
| Yes | 3963 (17.5) | 0.84 (0.76 to 0.92) | 0.85 (0.77 to 0.94) |
| Caregiver is mother | |||
| No | 1399 (6.1) | (reference group) | (reference group) |
| Yes | 21,373 (93.9) | 1.79 (1.50 to 2.13) | 2.15 (1.78 to 2.60) |
| Source of water used for preparing meals | |||
| Tap | 14,245 (62.6) | (reference group) | (reference group) |
| River or pond | 1216 (5.3) | 0.68 (0.56 to 0.83) | 0.69 (0.53 to 0.84) |
| Tanker or bottle | 944 (4.1) | 0.51 (0.39 to 0.66) | 0.54 (0.42 to 0.71) |
| No water used | 6367 (28.0) | 3.87 (3.57 to 4.18) | 3.91 (3.60 to 4.24) |
| Residence: | |||
| Semi-urban | 9860 (43.3) | (reference group) | (reference group) |
| Rural | 4353 (19.1) | 0.88 (0.61 to 1.27) | 0.90 (0.63 to 1.28) |
| Deep rural | 8559 (37.6) | 0.66 (0.48 to 0.89) | 0.81 (0.61 to 1.09) |
| Study tablet composition | |||
| None | 2166 (9.5) | (reference group) | (reference group) |
| Vitamin A | 7111 (31.2) | 1.63 (1.00 to 2.67) | 1.45 (0.88 to 2.39) |
| Vitamin A + Zinc | 7024 (30.8) | 1.90 (1.16 to 3.11) | 1.68 (1.03 to 2.74) |
| Multivitamins and minerals | 6471 (28.4) | 1.68 (1.02 to 2.75) | 1.44 (0.88 to 2.36) |
| Recall | |||
| 1 wk | 15,902 (69.9) | (reference group) | (reference group) |
| >1 wk | 6870 (30.1) | 2.18 (2.02 to 2.34) | 2.00 (1.85 to 2.16) |
Low diversity of each observed week defined as an age-, sex-, and season-adjusted dietary diversity score (diversity Z-score) falling within the lowest quartile. Crude OR obtained by single predictor logistic regression analysis with adjustment for clustering of observed weeks within individual children.
Conditional OR obtained using multiple logistic regression analysis with adjustment for clustering of observed weeks within individual children.
In multiple logistic regression analysis with adjustment for within-subject clustering (Table 3), child HIV infection was still independently and significantly associated with low dietary diversity (OR, 1.76; 95% CI, 1.06−2.96). Low diet diversity was also associated with diarrhea episodes, absence of respiratory infection, absence of use of water for food preparation, the mother being the caregiver, and recall longer than 1 wk. The associations in bivariate analysis with deep rural residence and overall micronutrient supplementation use disappeared, except for a remaining association between low diversity and supplementation with tablets containing vitamin A and zinc.
Among HIV-infected children, the lack of diversity relative to HIV-uninfected children was significant at all ages and was most pronounced at 6 mo of age (Table 4), when HIV-infected children were less likely to consume all classes of food except for formula. This pattern changed at 9 mo, when all food classes were consumed less frequently except breast milk. At later ages, HIV-infected children consistently consumed fewer dairy products than HIV-uninfected children.
TABLE 4.
Weekly food class consumption by age and HIV status of child in 381 children aged 6−24 mo in rural South Africa1
| Age, mo2 | Child's HIV status | Mean diversity Z-score | Breast milk | Formula milk | Dairy products | Vegetable protein | Nonmilk animal protein | Snacks and sweetened beverages | Fruits and vegetables | Complex carbohydrate |
|---|---|---|---|---|---|---|---|---|---|---|
| Weeks food class consumed, % | ||||||||||
| 6 | Infected | −0.52 | 60.8 | 44.0 | 6.4 | 8.0 | 8.8 | 16.8 | 24.0 | 87.2 |
| Uninfected | −0.07* | 75.1 | 36.2 | 17.7 | 14.4 | 16.3 | 25.4 | 35.8 | 92.8 | |
| 9 | Infected | −0.19 | 73.6 | 33.9 | 26.1 | 24.1 | 27.1 | 41.7 | 23.4 | 91.9 |
| Uninfected | 0.06* | 70.7 | 39.1 | 30.9 | 28.1 | 31.4 | 50.1 | 33.7 | 97.8 | |
| 12 | Infected | −0.17 | 74.2 | 25.0 | 25.8 | 39.1 | 45.7 | 61.1 | 28.7 | 97.5 |
| Uninfected | 0.04* | 63.4 | 33.2 | 38.6 | 34.0 | 42.5 | 68.4 | 31.0 | 98.9 | |
| 18 | Infected | −0.33 | 27.0 | 17.9 | 34.7 | 49.0 | 65.3 | 83.7 | 36.2 | 99.0 |
| Uninfected | −0.01* | 24.8 | 18.7 | 49.6 | 40.7 | 56.2 | 91.8 | 33.1 | 99.5 | |
| 24 | Infected | −0.16 | 6.7 | 8.4 | 46.2 | 58.8 | 78.2 | 97.5 | 40.3 | 100.0 |
| Uninfected | 0.07** | 5.0 | 6.3 | 58.3 | 50.1 | 70.9 | 96.8 | 45.3 | 100.0 | |
Values are percentage of 22,772 observed weeks.
Different from infected, P < 0.01
different from infected, P < 0.05.
Midpoint of 3-mo age interval.
3 Kruskal-Wallis test comparing observed weeks between HIV-infected and HIV-uninfected children.
Discussion
This study has a number of important findings on the dietary patterns of children living in an area with an extremely high prevalence of HIV and on the methods used to determine dietary diversity. Chief among these is that HIV-infected children have lower dietary diversity between 6 and 24 mo of age than HIV-uninfected children. This difference was present both in bivariate analysis when compared with HIV-uninfected children born to HIV-uninfected mothers and when controlling for socioeconomic status, recall period, and diarrheal and respiratory morbidity. Children without HIV infection born to HIV-infected mothers had a diminished dietary diversity compared with children born to mothers without HIV infection, but this difference did not reach significance.
The association of HIV infection and lower dietary diversity in a multiple-regression including diarrhea and respiratory infection as covariates suggests that the lower dietary diversity in HIV-infected children cannot be explained only by increased frequency of disease episodes. It might be related to difficult to conditions (especially in infants and young children), such as poorer appetite, or unmeasured aspects of socioeconomic status, such as household income. Poor appetite with inadequate dietary intake has been well documented in HIV-infected children (22,23). Increased levels of tumor necrosis factor-a may be involved in the appetite inhibition (24). Neurodevelopmental delay and decreased maternal well-being may be additional factors leading to low energy intake and concomitant low dietary diversity. Because we did not measure energy intake, we were unable to determine whether the association between HIV and dietary diversity disappears when energy intake is introduced in the model. If that were the case, it would suggest that energy intake reduction was indeed the main mechanism by which HIV exerts its effect on dietary diversity.
HIV-infected children in this study, compared with HIV-uninfected children born to either HIV-infected or HIV-uninfected mothers, consumed fewer food classes in the first year of life with the exception of breast milk. This may be because children who were known to be HIV-infected at 6 mo of age were encouraged to continue breast-feeding. However, we cannot exclude a survival bias affecting this finding, because breast-fed, HIV-infected children may have been more likely to survive to 6 mo of age, when our observations began.
The pattern of dietary diversity identified in this study is one of rapidly increasing diversity in the seecond half of the first year of life in infants in all 3 HIV status groups. Although infants progressively abandon breast milk and formula, diversity increases because they replace it with nonmilk sources of protein, snacks, and sweetened beverages. Although increasing diversity is often thought of as beneficial in and of itself, the decline in breast-feeding after 6 mo of age has important health implications even though overall dietary diversity is increasing. The health benefit of continued breast-feeding after 6 mo of age for children of HIV-uninfected mothers is well documented (25). Dietary diversity could be further enhanced in this population by reinforcing breast-feeding by HIV-uninfected mothers. Indeed, the observed pattern of breast-feeding was not optimal. Already at age 6 mo, 1 of 4 HIV-uninfected children was no longer receiving any breast milk. In y 2 of life, breast milk consumption continued to decrease rapidly so that by age 18 mo, 3 of 4 children were not receiving breast milk.
Another concern in the diet of this population is the low consumption of fruits and vegetables. In more than one-half of the observed child weeks, no fruit or vegetable was consumed. Although there may have been some underreporting of fruit and vegetable consumption, this is unlikely to explain the low consumption. The area where this study was conducted has little subsistence farming and is very poor. Poor households are known to spend a disproportionate portion of their income on staple foods, primarily maize meal, and fruits and vegetables are generally considered less essential and are more expensive. This may increase the risk of micronutrient deficiencies (4). The South African government has initiated a staple food fortification program to decrease the risk of micronutrient deficiencies in the population (26). Nevertheless, campaigns to promote subsistence farming could be very helpful to increase the consumption of fruits, vegetables, and animal-source food (27).
The reliance on purchased foods in this population may also explain why we did not find any seasonal variation in dietary diversity. This is not a typical situation in sub-Saharan Africa where periodic food insecurity is frequent. For example, in a study of adult women in Burkina Faso, diversity of the family diet was found to fluctuate heavily due to periods of general food shortage (28).
An intriguing finding of this study was that dietary diversity increased when the mother was not the main caregiver. This may appear counterintuitive and could in part be due to respondent bias by the replacement caregivers. Alternative explanations are that mothers who travel for whatever reason tend to make provisions to ensure an optimal diet for their child during their absence, that if the mother is away it is more likely that she is working and there is more family income to make these provisions possible, or that the replacement caregivers, often the grandmothers, tend to “spoil” the children. The latter explanation seems a good possibility, because in the study area, old age pensions constitute a major source of family income.
There are no universally accepted standardized tools for studying dietary diversity. Reports of studies that have described the association between dietary diversity and energy or nutrient intake have also invariably recommended the development and refinement of indices of dietary diversity (6). The method we have introduced to assess dietary diversity in young children should be relatively easy to reproduce in other settings, because the questionnaire is relatively short and locally adaptable. If this survey was to be adapted for use in other settings, an initial pilot study of the target population is needed to identify foods that are commonly consumed within each of the 8 food classes. An internal standardization of the weekly dietary diversities for age, sex, and season should be easy to perform using the same method as we describe.
Other advantages of the method we used include the fact that it allows collection of daily dietary intake information. It also allows monitoring of dietary diversity over longer periods than with more traditional dietary surveys. Those traditional surveys are more intensive and require longer to complete and thus are more difficult to sustain over time. The diets of children <2 y of age are constantly changing as more foods become available to the maturing child. This makes it more relevant to collect information daily or weekly to overcome large between- and within-person variation.
This study focused on exploring determinants of dietary diversity in young children, primarily examining the effect of HIV status. The functional validity and usefulness of dietary diversity measures needs further study both in children and adults (6,29,30). Little is known about the possible effects of low dietary diversity on growth and health outcomes. Such studies should ideally also quantify total energy intake so that the independent effects of diet diversity and energy intake can be assessed. It has been suggested that exploration of such relationships should also adjust for socioeconomic variables (31,32). An 11-country demographic survey found that dietary diversity was significantly associated with attained height even after adjusting for socioeconomic and other confounding factors (4).
In conclusion, this study shows that the diets of HIV-infected children are significantly less diverse than those of HIV-uninfected children born to HIV-uninfected mothers and of those of HIV-infected children born to HIV-infected mothers. Such lack of diversity may contribute to the development of specific nutrient deficiencies (3,33) and may be a factor contributing to increased morbidity and poorer survival in these children.
Acknowledgment
We thank Lungeleni Phakathi for assistance with data entry.
Footnotes
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2−3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
Supported by grants from the United States NIH (1 UO1 AI45508-01, 1 K24 bAI/HDO1671-01, D43TW05572-01) and by the Wellcome Trust (063009, 62925).
Author disclosures: N. Mpontshane, J. Van den Broeck, M. Chhagan, K. K. A. Luabeya, A. Johnson, and M. L. Bennish, no conflicts of interest.
Literature Cited
- 1.Anonymous Solving the weanling's dilemma: power-flour to fuel the gruel? Lancet. 1991;338:604–5. doi: 10.1016/0140-6736(91)90611-r. [DOI] [PubMed] [Google Scholar]
- 2.Hunter LM, Twine W, Patterson L. “Locusts are now our beef”: adult mortality and household dietary use of local environmental resources in rural South Africa. Scand J Public Health Suppl. 2007;69:165–74. doi: 10.1080/14034950701356385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faber M. Complementary foods consumed by 6−12-month-old rural infants in South Africa are inadequate in micronutrients. Public Health Nutr. 2005;8:373–81. doi: 10.1079/phn2004685. [DOI] [PubMed] [Google Scholar]
- 4.Arimond M, Ruel MT. Dietary diversity is associated with child nutritional status: evidence from 11 demographic and health surveys. J Nutr. 2004;134:2579–85. doi: 10.1093/jn/134.10.2579. [DOI] [PubMed] [Google Scholar]
- 5.Becquet R, Leroy V, Ekouevi DK, Viho I, Castetbon K, Fassinou P, Dabis F, Timite-Konan M, ANRS 1201/1202 Ditrame Plus Study Group Complementary feeding adequacy in relation to nutritional status among early weaned breastfed children who are born to HIV-infected mothers: ANRS 1201/1202 Ditrame Plus, Abidjan, Cote d'Ivoire. Pediatrics. 2006;117:e701–10. doi: 10.1542/peds.2005-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kranz S, Hartman T, Siega-Riz AM, Herring AH. A diet quality index for American preschoolers based on current dietary intake recommendations and an indicator of energy balance. J Am Diet Assoc. 2006;106:1533. doi: 10.1016/j.jada.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Sawadogo PS, Martin-Prevel Y, Savy M, Kameli Y, Traissac P, Traore AS, Delpeuch F. An infant and child feeding index is associated with the nutritional status of 6- to 23-month-old children in rural Burkina Faso. J Nutr. 2006;136:656–63. doi: 10.1093/jn/136.3.656. [DOI] [PubMed] [Google Scholar]
- 8.Knol LL, Haughton B, Fitzhugh EC. Food group adherence scores assess food patterns compared to US Department of Agriculture Food Guide. J Am Diet Assoc. 2006;106:1201–8. doi: 10.1016/j.jada.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Provide Project 2005 [2006 Jun 30];A profile of KwaZulu-Natal: demographics, poverty, inequality and unemployment. Available from: http://www.elsenburg.com/provide/documents/BP2005_1_5%20Demographics%20KZ.pdf.
- 10.Development Bank of Southern Africa [2006 Jun 1];Statistical infrastructure review of the new demarcated municipalities. 2002 Available from: http://www.dbsa.org/document/StatisticalsInfrastructureReview.pdf.
- 11.National HIV and Syphilis Prevalence Survey South Africa2005 South Africa Department of Health. 2006 Available from: http://www.doh.gov.za/docs/reports/2005/hiv.pdf.
- 12.Luabeya KK, Mpontshane N, Mackay M, Ward H, Elson I, Chhagan M, Tomkins A, Van den Broeck J, Bennish ML. Zinc or multiple micronutrient supplementation to reduce diarrhea and respiratory disease in South African children: a randomized controlled trial. PloS ONE. 2007;2:e541. doi: 10.1371/journal.pone.0000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van den Broeck J, Chhagan M, Mackay M, Mpontshane N, Luabeya A, Bennish ML. Maintaining data integrity in a rural clinical trial. Clin Trials. 2007;4:572–82. doi: 10.1177/1740774507084106. [DOI] [PubMed] [Google Scholar]
- 14.Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF, Moore WM. Physical growth: National Centre for Health Statistics percentiles. Am J Clin Nutr. 1979;32:607–29. doi: 10.1093/ajcn/32.3.607. [DOI] [PubMed] [Google Scholar]
- 15.Van den Broeck J, Argeseanu-Cunningham S, Eeckels R, Herbst K. Data cleaning: detecting, diagnosing and editing data abnormalities. PLoS Med. 2005;2:e267. doi: 10.1371/journal.pmed.0020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Microsoft 2007 Availablefrom: http://www.microsoft.com/sql/default.mspx.
- 17.Teleform . Cardiff Software Inc.; San Marcos (CA): 2005. Available from: http://www.verity.com/products/teleform/index.html. [Google Scholar]
- 18.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–19. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 19.Analyser G. version 3.0. Dutch Growth Foundation; Rotterdam: 2006. Available from: www.growthanalyser.org. [Google Scholar]
- 20.SPSS Statistical Package for the Social Sciences version 12.0. 2005 Available from: www.spss.com/
- 21.Stata Corporation . Stata Statistical Software, release 7.0. Stata Corporation; College Station (TX): 2001. Available from: http://www.stata.com/ [Google Scholar]
- 22.Miller TL. Nutrition in paediatric human immunodeficiency virus infection. Proc Nutr Soc. 2000;59:155–62. doi: 10.1017/s0029665100000185. [DOI] [PubMed] [Google Scholar]
- 23.Arpadi SM. Consultation on Nutrition and HIV/AIDS in Africa: evidence, lessons and recommendations for Action. WHO; Geneva: 2005. Growth failure in HIV-infected children. pp. 1–20. [Google Scholar]
- 24.Van Rossum AM, Gaakeer MI, Verweel S, Hartwig NG, Wolfs TF, Geelen SP, Lamberts SW, de Groot R. Endocrinologic and immunologic factors associated with recovery of growth in children with human immunodeficiency syndrome virus type 1 infection treated with protease inhibitors. Pediatr Infect Dis J. 2003;22:70–6. doi: 10.1097/00006454-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Alvarado BE, Zunzunegui MV, Delisle H, Osorno J. Growth trajectories are influenced by breastfeeding and infant health in an Afro-Colombian community. J Nutr. 2005;135:2171–8. doi: 10.1093/jn/135.9.2171. [DOI] [PubMed] [Google Scholar]
- 26.Ministry of Health South Africa. Regulations relating to the fortification of certain foodstuffs [2006 Jul];2003 Available from: http://www.doh.gov.za/docs/regulations/2003/ffortification.html.
- 27.Allen LH. Interventions for micronutrient deficiency control in developing countries: past, present and future. J Nutr. 2003;133:S3875–8. doi: 10.1093/jn/133.11.3875S. [DOI] [PubMed] [Google Scholar]
- 28.Savy M, Martin-Prevel Y, Traissac P, Eymard-Duvernay S, Delpeuch F. Dietary diversity scores and nutritional status of women change during the seasonal food shortage in rural Burkina Faso. J Nutr. 2006;136:2625–32. doi: 10.1093/jn/136.10.2625. [DOI] [PubMed] [Google Scholar]
- 29.Drewnowski A, Henderson SA, Driscoll A, Rolls BJ. The Dietary Variety Score: assessing diet quality in healthy young and older adults. J Am Diet Assoc. 1997;97:266–71. doi: 10.1016/s0002-8223(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 30.Kant AK, Schatzkin A, Harris TB, Ziegler RG, Block G. Dietary diversity and subsequent mortality in the first National Health and Nutrition Examination Survey epidemiological follow-up study. Am J Clin Nutr. 1993;57:434–40. doi: 10.1093/ajcn/57.3.434. [DOI] [PubMed] [Google Scholar]
- 31.Ruel MT. Operationalizing dietary diversity: a review of measurement issues and research priorities. J Nutr. 2003;133:S3911–26. doi: 10.1093/jn/133.11.3911S. [DOI] [PubMed] [Google Scholar]
- 32.Eckhardt CL, Suchindran C, Gordon-Larsen P, Adair LS. The association between diet and height in the post-infancy period changes with age and socio-economic status in Filipino youths. J Nutr. 2005;135:2192–8. doi: 10.1093/jn/135.9.2192. [DOI] [PubMed] [Google Scholar]
- 33.Friis H. Micronutrient interventions and HIV infection: a review of current evidence. Trop Med Int Health. 2006;11:1849–57. doi: 10.1111/j.1365-3156.2006.01740.x. [DOI] [PubMed] [Google Scholar]


