Abstract
Epidermal growth factor receptor (EGFR), a member of the ErbB family of receptor tyrosine kinases (RTKs), is highly expressed in head and neck squamous cell carcinoma (HNSCC) where increased EGFR expression levels in tumors are associated with decreased survival. HNSCC patient responses to EGFR-targeted monotherapies in clinical trials, though significant, have been limited. Tumor signaling pathway components that work in cooperation with EGFR or provide compensation for the loss of EGFR-initiated signaling will be ideal targets for therapies to be used in combination with EGFR-targeted agents. Based upon current understanding of molecular signaling pathways and available agents, ErbB family-targeted and Src family-targeted agents represent strategies for further exploration. Here, we discuss agents targeting ErbB and Src family kinases in clinical development, provide an overview of completed and ongoing clinical trials, and outline a molecular rationale for combining ErbB- and Src-targeted therapeutics.
Introduction
The epidermal growth factor receptor (EGFR), a member of the ErbB family of receptor tyrosine kinases (RTKs), has been identified as a therapeutic target for head and neck squamous cell carcinoma (HNSCC), and several EGFR-targeted agents have been developed and tested in HNSCC clinical trials. Clinical response to EGFR-targeted therapeutics in phase II and III clinical studies though modest have been significant, and the anti-EGFR antibody cetuximab (Erbitux, C225; Imclone Systems, Branchburg, New Jersey), was FDA-approved for treatment of HNSCC in 2006. Efforts to increase clinical responses have resulted in the development and testing of dual specificity and pan-ErbB inhibitors as well as combinations of targeted therapeutics.
There are many potential combinatorial approaches and selection of the most likely combinations to test empirically will require an increased understanding of complex molecular pathways and functional consequences of individual targeted agents. The majority of head and neck cancers express EGFR and EGFR-targeted therapies have demonstrated clinical effectiveness. For these reasons, it is logical to consider targeted therapeutics to be used in combination with an EGFR-targeted agent. Based upon current understanding of molecular signaling pathways and available agents, ErbB family-targeted and Src family-targeted agents represent strategies for further exploration. ErbB family members can homodimerize and heterodimerize to form signaling complexes. EGFR molecules are inactivated and downregulated following EGFR inhibition, suggesting that signaling by other ErbB receptors may be critical to tumor survival and growth. Src family kinases can mediate EGFR-initiated signals, EGFR-independent pathways, and even serve as upstream activators of EGFR. Below, we summarize clinical and preclinical findings for EGFR- and Src-targeted therapeutics in HNSCC and provide a molecular signaling context and rationale for pursuing EGFR dual specificity inhibitors as well as EGFR and Src combined therapies for the treatment of HNSCC.
ErbB family of receptors and head and neck cancers
ErbB family of type I receptor tyrosine kinases are expressed in many tissues of epithelial, mesenchymal and neuronal origin and are important for development, growth and differentiation. The ErbB family members, EGFR, ErbB2/HER2/Neu, HER3, and HER4, form ligand-dependent homo- and hetero-dimers resulting in tyrosine phosphorylation of receptor cytoplasmic residues and activation of multiple signal transduction pathways involved in proliferation, survival, motility and angiogenesis.
EGFR is overexpressed in many cancers including HNSCC. EGFR activating mutations have been reported in lung cancers (1) where these mutations correlate with response to EGFR tyrosine kinase inhibitors (TKI) (2). However, activating mutations of the EGFR kinase are rare in head and neck cancers (3, 4). Overexpression of EGFR in HNSCC is an important mechanism for enhanced EGFR signaling, as EGFR mRNA is overexpressed in up to 90% of HNSCC (5). Several studies evaluating the association between EGFR overexpression and survival have reported that increased tumor EGFR protein expression as assessed by immunohistochemistry (IHC) is associated with reduced HNSCC survival (6–8) and poorer response to radiotherapy (9). Studies evaluating HNSCC EGFR levels by radioligand binding assays also reported significant reduction in survival with increased EGFR tumor expression (10–12). More recently, EGFR gene amplification and altered copy number have been associated with reduced HNSCC survival in two independent studies (13) (14). Notably, EGFR gene amplification was found to not relate to EGFR mRNA levels or EGFR protein levels in one report (13). EGFR has seven known ligands including TGF-α, amphiregulin, and epidermal growth factor (EGF) (15). TGF-α mRNA has been reported to be overexpressed in 87% of HNSCC (5). Thus, the majority of HNSCC exhibit autocrine/paracrine EGFR stimulation.
HER2 is overexpressed in a variety of tumors including breast, colon, ovarian and non-small cell lung carcinoma (NSCLC) and is an important therapeutic target for the treatment of breast cancer (16). HER2 protein has been reported by several groups to be present in 17% to 53% of HNSCC as assessed by IHC (17–19). A subset of these studies found HER2 tumor expression to be associated with reduced survival (19) (17). Though no ligand for HER2 has been identified, HER2 is able to form heterodimers with EGFR, HER3 and HER4. HER2 protein levels in HNSCC have been reported to be correlated with EGFR levels in oral squamous cell carcinoma (20). These studies suggest a role for HER2 in HNSCC, especially as a signaling partner of EGFR. The importance of HER3 and HER4 is head and neck cancer is less clear. HER3 was reported to be present in 35% of 111 oral squamous cell carcinomas with HER3 tumor protein levels significantly correlated with EGFR protein (20); however, an independent study found that HER3 and HER4 expression levels in oral squamous cell carcinoma were not distinct from levels in normal epithelium (21).
Targeting EGFR for treatment of HNSCC
The rationale for the development of EGFR-targeted therapies for treatment of HNSCC includes the following: 1) EGFR is highly expressed in many head and neck cancers, 2) EGFR overexpression in HNSCC is associated with reduced survival in several independent studies, and 3) EGFR-targeting in HNSCC preclinical models demonstrated anti-tumor efficacy (22–24). Several classes of EGFR-targeted therapies are currently in clinical use and/or are undergoing active investigation for the treatment of HNSCC. Anti-EGFR antibodies recognize the ligand-binding domain of EGFR and interfere with receptor activation while EGFR TKIs bind to the cytoplasmic region of EGFR and interfere with ligand-binding induced signaling events. Two recently published reviews describe EGFR-targeted therapeutic clinical studies in head and neck cancers (25, 26). An overview of published and ongoing EGFR-targeted studies in HNSCC is provided below.
Anti-EGFR antibody therapies
Cetuximab (Erbitux, C225; Imclone Systems), a humanized mouse anti-EGFR IgG1 monoclonal antibody, was approved by the Federal Drug Administration (FDA) in March of 2006 for the treatment of unresectable HNSCC in combination with radiation therapy following a phase III HNSCC clinical trial with 424 subjects that demonstrated a significant survival benefit for HNSCC patients receiving cetuximab plus radiation therapy (RT) compared to HNSCC patients receiving RT alone (27, 28). Median overall survivals for the two arms were 49.0 months and 29.3 months, respectively (28). A separate phase III clinical trial for HNSCC demonstrated a significant improvement in objective response rates, defined as complete response and partial response rates, for subjects treated with cetuximab in combination with chemotherapy (CT) versus placebo plus CT. Objective response rates for the two arms were 26% and 10%, respectively, but no significant difference in survival was observed (29). A randomized phase III study involving 442 subjects with recurrent and/or metastatic HNSCC reported a survival benefit for patients treated with cetuximab in addition to platinum-based chemotherapy, cisplatin or carboplatin with 5-fluorouracil, compared to subjects treated with platinum-based chemotherapy alone (30). Median survival time, which differed significantly between the two study arms, was 10.1 months for the cetuximab plus platinum-based CT study arm versus 7.4 months for the study arm with platinum-based CT alone (30). In general, objective response rates to combined cetuximab plus platinum-based therapies in subjects with recurrent or metastatic cancers have been between 6% and 26% (29, 31, 32). A recent open-label, multicenter phase II clinical trial evaluating cetuximab as a single agent for treatment of patients with recurrent and/or metastatic HNSCC who failed CT (n=103) reported an objective response rate of 13% (33).
Cetuximab-related toxicities are generally confined to skin rash, and one study reported improved survival for patients who develop skin rash in response to cetuximab (34). Of note, therapeutic response to cetuximab has not been found to be positively correlated with the EGFR tumor protein levels in HNSCC. Though few HNSCC clinical studies for cetuximab have reported EGFR tumor protein level molecular correlates (28, 34), the lack of association between elevated EGFR tumor levels and clinical response to cetuximab are similar to those reported for colorectal cancer (35, 36).
Other more extensively humanized or fully humanized anti-EGFR antibodies have been and/or are currently being evaluated in phase II/III clinical trials for HNSCC including panitumumab (ABX-EGF, Amgen, Thousand Oaks, California), which was FDA-approved for the EGFR-expressing metastatic colorectal cancer with disease progression following CT treatment (37), zalutumumab (Hu-Max-EGFr, ZF8; Genmab, Copenhagen K, Denmark), and nimotuzumab (h-R3; YM Biosciences, Inc., Mississauga, Ontario, Canada) (Table 1). Panitumumab, which blocks ligand binding and causes receptor internalization, is undergoing a HNSCC two-arm, multicenter phase II trial evaluating docetaxol and cisplatin combination CT with and without panitumumab as a first-line treatment for patients with metastatic or recurrent HNSCC (I.D. NCT00454779, ClinicalTrials.gov) and a randomized phase III trial of CT with or without panitumumab in patients with metastatic and/or recurrent HNSCC (I.D. NCT00460265, ClinicalTrials.gov). Zalutumumab, which blocks EGF and TGF-α binding to EGFR and induces antibody-dependent cell mediated cytotoxicity in vitro (38), is currently being evaluated in a randomized phase III clinical trial with best supportive care for HNSCC patients with non-curable HNSCC who have failed standard platinum-based CT (I.D. NCT00382031, ClinicalTrials.gov). Zalutumumab is also undergoing a phase I/II clinical trial as a first line therapy in combination with chemoradiotherapy (CRT) for patients with HNSCC (I.D. NCT00401401, ClinicalTrials.gov). A dose response to nimotuzumab was reported in HNSCC patients in a phase I/II study with no associated skin toxicities (39).
Table 1.
Anti-EGFR Antibody Agents in HNSCC Clinical Trials
| Agent | Sponsor | Isotype | Components | Cancersa in phase II or III clinical study | FDA Approval (Date) | HNSCC Clinical Phasea | References |
|---|---|---|---|---|---|---|---|
| Cetuximab | Imclone | IgG1 | chimeric human/mouse | lung, breast, prostate, colon, liver, cervical, ovarian, pancreas, head and neck | colon (Feb. 2004) and head and neck (Mar. 2006) cancers | II/III | [27] |
| Panitumumab | Amgen | IgG2 | human | prostate, colon, head and neck | colon cancers (Sept. 2006) | II/III | [36] |
| Zalutumumab | Genmab | IgG1 | human | lung, head and neck | --- | II/III | |
| Matuzumab | EMD Pharmaceuticals | IgG1 | humanized mouse | lung cancer, esophageal and gastric, ovarian, | --- | I | |
| Nimotuzumab | YM Biosciences | IgG1 | humanized mouse | colon | --- | I |
ClinicalTrials.gov solid tumors
EGFR-targeted TKIs
Two reversible competitive EGFR-selective TKIs, Gefinitib (Iressa, ZD1839; AstraZeneca Pharmaceuticals, Wilmington, Deleware) and Erlotinib (Tarceva, OSI_774; Genetech, South San Francisco, California) (Table 2), have been evaluated as monotherapies for patients with recurrent and/or metastatic HNSCC with therapeutic response rates of 4–10% (40–42). Erlotinib, which has been approved for treatment of patients with locally advanced non-small cell lung cancer following failure of at least one prior CT treatment and for treatment of advanced pancreatic cancer in combination with gemcitabine (Gemzar, Eli Lilly and Company, Indianapolis, Indiana) (43), has been and is currently being evaluated in clinical trials combining platinum-based CT and/or RT for HNSCC. A phase I/II trial combining erlotinib and cisplatin for treatment of patients with recurrent or metastatic HNSCC reported an intention-to-treat response rate of 21% (10–30%, 95% C.I.) (44), suggesting that elotinib may be more effective when combined with CRT or RT for metastatic or recurrent HNSCC. Phase III trials will be required to determine whether erlotinib or gefitinib improve survival compared to standard of care. Currently ongoing is a phase III trial to evaluate the effectiveness of erlotinib in combination with CRT or RT for patients with resected HNSCC (I.D. NCT00412217, ClinicalTrials.gov) and a phase II/III trial evaluating erlotinib in combination with standard platinum-based CT for advanced HNSCC (I.D. NCT00448240, ClinicalTrials.gov). Gefitinib, which was approved for new labeling by the FDA in June 2005 to limit indicated use following the failure of two clinical trials to show survival benefit (45), is currently being evaluated in a phase II trial for locally advanced HNSCC in combination with CRT followed by gefitinib adjuvant therapy (46). In this study, 91% of evaluable subjects were reported to have a complete response, and 77% of subjects were alive without progressive disease at two years (46). A phase III study involving 486 patients with recurrent HNSCC reported no improvement in overall survival and no improvement in objective response rates with the addition of gefitinib at either 250 mg/day or 500 mg/day to methotrexate treatment compared to methotrexate treatment alone (47). A phase III study evaluating docetaxel with or without gefitinib for the treatment of metastatic or locally advanced recurrent HNSCC is currently ongoing (I.D. NCT00088907, ClinicalTrials.gov).
Table 2.
Targeted TKIs in clinical development for HNSCC
| Agent | Sponsor | Target (IC-50)a | Irrever sible | Cance rsb in phase II or III clinical study | FDA Approva I (Date) | HNSCC Clinical Phasea | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Erlotinib | Tarceva, CP 358774, OSI 774, NSC 718781, R 1415 | OSI Pharmaceuticals | EGFR (2 nM) | No | lung, prostate, breast, colon, liver, bladder, kidney, brain and CNS, pancreatic, head and neck | lung (Nov. 2004) and pancreatic (Nov. 2005) cancers | III | [40] |
| Gefitinib | Iressa, ZD1839 | AstraZeneca | EGFR (33 nM) | No | lung, prostate, breast, colon, liver, bladder, kidney, pancreatic, cervical, ovarian, head and neck | lung cancer (new labeling June 2005) | III | [39] |
| Lapatinib | GW572016 | GlaxoSmithKline | EGFR (10.8 nM); HER2 (9.3 nM) | No | breast, prostate, colon, kidney, ovarian, cervical, brain and CNS, head and neck | breast cancer (Mar. 2007) | III | [51] |
| Dasatinib | BMS-354825 | Bristol-Myers Squibb | Src (0.50 nM); Abl(<1.0 nM); c-Kit(5.0 nM); PDGF(28 nM); | No | colon | chronic myeloid leukemia (June 2006) | I | [70] |
| AZD0530 | AstraZeneca | Src (1–5 nM); Abl(30 nM) | No | pancreatic | --- | I | [98] |
Cell-free in vitro IC-50
ClinicalTrials.gov solid tumors
EGFR-targeted antisense
There have been several reports of EGFR-targeted nucleotide-based therapeutic efficacy in preclinical in vivo HNSCC models as a monotherapy (23) and in combination with cytotoxic agents (48, 49). There are as yet no published reports from HNSCC clinical trials implementing EGFR-targeted nucleotide-based therapies. However, our group recently conducted an EGFR antisense (AS) phase I clinical trial and enrolled seventeen patients with recurrent HNSCC refractory to standard therapy (I.D. NCT00009841, ClinicalTrials.gov). Five subjects, including 2 complete responders and 3 partial responders, achieved a clinical response (29%; 10 – 56%, 95% C.I.) (unpublished observations). Though the study population size was small, we did observe a higher response rate than has been reported in other EGFR targeted therapies used as monotherapies in similar patient populations. In addition, no grade 3/4 or dose-limiting toxicities were observed (unpublished data). Molecular correlate biomarker characterization for this trial is currently underway.
Dual specificity ErbB TKIs
HER2 has been found to be elevated in a portion of HNSCC where HER2 tumor protein levels have been correlated with EGFR expression levels (20). Furthermore, EGFR and HER2 heterodimerize to form functional signaling complexes suggesting that combined targeting of HER2 and EGFR may result in enhanced clinical responses compared to EGFR-targeted therapies alone. Lapatinib (GW 572016; GlaxoSmithKline, Research Triangle Park, North Carolina), which was FDA-approved March 13, 2007 for use in combination with capecitabine (Xeloda; Roche, Basel, Switzerland) for the treatment of advanced or metastatic breast cancers overexpressing HER2 (50), is a dual specificity, reversible tyrosine kinase inhibitor of EGFR and HER2 (Table 2). In cell-free systems, lapatinib IC50 values for EGFR and HER2 were 10.8 and 9.3 nM, respectively (51). Unlike erlotinib and gefinitib, which bind the active conformation of EGFR, lapatinib binds EGFR in its inactive state (52).
In a phase II multi-institution study of lapatinib in patients with recurrent/metastatic HNSCC, no objective responses were observed among the 42 subjects treated with lapatinib at 1500 mg oral dose (53). However, several lapatinib phase II trials are ongoing in HNSCC (I.D. NCT00371566, I.D. NCT00114283, and I.D. NCT00114283, ClinicalTrials.gov). One phase III double-blind placebo-controlled multicenter trial is ongoing to evaluate lapatinib versus placebo in the adjuvant setting. Labatinib or placebo will be administered post-operatively in combination with CRT followed by lapatinib or placebo adjuvant treatment for one year (I.D. NCT00424255, ClinicalTrials.gov). Additional agents targeting EGFR and HER2 are currently being tested in clinical trials for cancers other than HNSCC. Two irreversible EGFR-HER2 dual inhibitors, HKI-272 (Wyeth; Madison, New Jersey) and BIBW-2992 (Boehringer-Ingelhelm; Germany), are undergoing phase II testing for breast and non-small cell lung cancer, and prostate cancer, respectively (54). In addition to the predicted enhanced antitumor activity of irreversible TKIs, irreversible TKIs also have the theoretical advantage that the effective dosing scheme would likely require less prolonged exposure than the reversible TKI (55).
HNSCC patient responses to EGFR-targeted monotherapies in clinical trials, though significant, have been limited. This is likely due to the presence or activation of compensatory tumor-survival cell signaling pathways. Tumor signaling pathway components that work in cooperation with EGFR or provide compensation for the loss of EGFR-initiated signaling will be ideal targets for therapies to be used in combination with cetuximab or other EGFR-targeted therapies for HNSCC treatment. The selection of appropriate combinations of therapies requires an understanding of the direct and indirect effects of the therapy as well as tumor compensatory changes, and these effects are not expected to be uniform across HNSCC patients or tumors. A detailed molecular characterization of signaling pathway components in HNSCC tumors prior to therapy and alterations resulting from targeted therapies combined with patient clinical response data is expected to have the following impacts on HNSCC treatment: (1) define subpopulations of responsive patients, (2) improve combination therapy selection, and (3) identify novel therapeutic targets. Therefore, clinical trials with correlative molecular studies will be critical for the identification of optimal combination therapies.
EGFR and c-Src signaling in HNSSC
Four primary signaling pathways have been implicated in mediating EGFR signaling. These pathways have been previously described to include the following: (1) Ras—mitogen-activated protein kinase (MAPK), (2) phosphotidylinositol-3-kinase (PI3K)—Akt, (3) phospholipase-C gamma (PLC-γ)—protein kinase C, and (4) signal transducers and activators of transcription (STAT) pathways (56). The integration of these activated pathways results in cell proliferation, survival, angiogenesis, migration and adhesion. Importantly, Src kinases, which have been reported to be activated in many cancers with high EGFR levels, have been shown to potentiate EGFR signaling (57, 58). c-Src potentiation of EGFR growth has been demonstrated to be associated with c-Src-dependent phosphorylation of the EGFR receptor and complex formation between c-Src and EGFR (57, 58). In addition to focal adhesion kinase (FAK), which is involved in the regulation of adhesion and migration, PI3K and STAT3 are also substrates for c-Src (59).
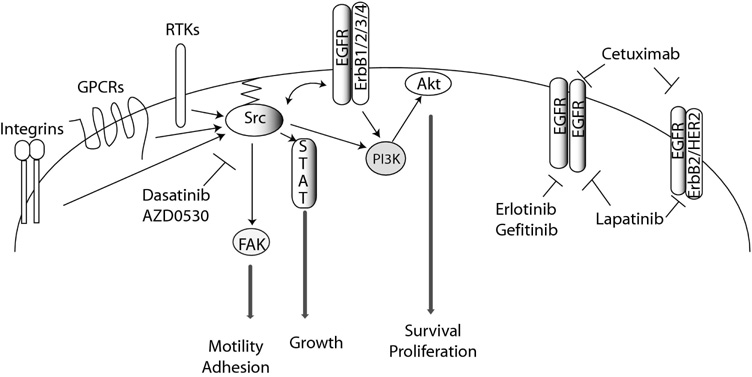
In addition to EGFR, c-Src is activated in response to stimulation of many other receptor tyrosine kinases (RTKs) platelet-derived growth factor receptor (PDGFR), insulin-like growth factor- (IGF-) 1 receptor (IGF-1R), and others, as well as G-protein-coupled receptors (GPCRs), cytokines, integrins, cell adhesion complexes, and others (60). Because c-Src is activated following stimulation of several other receptor tyrosine kinases and steroid receptors in addition to EGFR, PI3K—Akt and STAT pathways may be activated in a Src-dependent manner following EGFR inhibition (Figure 1).
Figure 1. EGFR-dependent and –independent activation of Src-mediated pathways.
Receptor tyrosine kinases (RTKs), G-protein-coupled receptors (GPCRs), and integrins can activate Src kinases independent of EGFR activation. Primary pathways mediated by Src kinases include the signal transducers and activators of transcription (STAT) pathway and PI-3 kinase (PI3K)—Akt axis in addition to the activation of focal adhesion kinase (FAK). Primary pathway components, relevant inhibitors, and cellular consequences of activation are presented. Arrows indicate activation either direct or indirect
The PI3K pathway is involved in cell proliferation, growth, survival, invasion and migration, and aberrant PI3K—Akt pathway activation has been described for many cancers (61). PI3K phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) at the inositol ring 3’ position, converting PIP2 to PIP3; PIP3 recruits Akt to the plasma membrane where Akt is activated by phosphorylation (61, 62). Activated Akt suppresses apoptosis by phosphorylating pro-apoptotic transcription factors of the FOXO family of forkhead transcription factors, resulting in their retention in the cytoplasm (61). In addition, Akt promotes cell cycle progression through the phosphorylation and inactivation of glycogen synthase kinase 3 (GSK3). Inactivation of GSK3 leads to the stablization of cyclin D1 and myc, promoting cell cycle progression (63, 64). In addition to GSK3 and forkhead transcription factors, Akt phosphorylates many other factors including I- κB kinase that lead to promotion of cell survival, cell cycle progression and growth (61).
Many human cancers and cell lines, including head and neck cancers, have been found to have constitutively activated STAT proteins, which reside in the cytoplasm until activated by cytokine, growth factor, or hormone signaling events. Of the seven identified STAT proteins, STAT1, STAT3 and STAT5 are activated in many different tissues, play roles in embryogenesis, are important regulators of cell cycle progression and apoptosis, and contribute to oncogenesis. STAT proteins are activated by specific phosphorylation events following EGFR stimulation to form homo- and hetero-dimers, translocate to the nucleus, and activate transcription of genes involved in differentiation, proliferation, cell survival, apoptosis, and angiogenesis. STAT3 has been found to be a Src-dependent mediator of EGFR-stimulated growth of HNSCC in vitro and decreased apoptosis and increased tumor growth in vivo (65, 66) (67).
Studies indicate that in addition to mediating signals from RTKs, Src kinases play roles in the activation of RTKs through the release of RTK ligands following GPCR stimulation, resulting in the autocrine or paracrine HNSCC stimulation (68, 69). Src kinases can also modulate RTK activity by directing the formation of active multi-protein signaling complexes. A recent study using breast cancer cell lines reported that c-Src directed the heterodimerization of HER2 with HER3, resulting in the formation of a Src kinase-dependent active signaling complex (70). Because Src kinases play critical functions in many tumorigenic and tumor progression processes and have been shown to potentiate EGFR signaling as well as mediate critical tumorigenic pathways independently of EGFR, Src may be a candidate for HNSCC targeted therapeutics.
Src kinases in head and neck cancer
Eight Src family nonreceptor protein tyrosine kinase (PTK) members, c-Src, c-Yes, Fyn, Blk, Fgr, Hck, Lck, and Lyn, are expressed in mammals. c-Src, c-Yes and Fyn are broadly expressed, while Blk, Fgr, Hck, Lck and Lyn are primarily expressed in cells of hematopoietic lineage (71). Of the Src family kinases, c-Src is most often implicated in cancer. Though Src is rarely mutated in cancer (72–74), Src kinases, especially c-Src, are frequently overexpressed and/or aberrantly activated in epithelial and non-epithelial cancers (75). Activated c-Src is commonly found in colorectal and breast cancers, and elevated c-Src protein and/or c-Src kinase activity have also been reported in many human cancers including lung, colon, breast, ovarian, endometrial, pancreatic and head and neck (75, 76).
Src kinases have been implicated in many normal cellular functions, including cell adhesion, migration, proliferation, survival, angiogenesis, differentiation, that when inappropriately regulated contribute to tumorigenesis, tumor progression, and/or metastases (71, 76). In HNSCC, Src kinases are activated following EGFR stimulation (77), associate with EGFR (77), and exhibit reduced activity following treatment with EGFR inhibitors in vitro (78). No Src inhibitor has been FDA approved for the treatment of solid tumors including HNSCC. However, several Src inhibitors have been evaluated using preclinical models, including dasatinib (Sprycel; Bristol-Myers Squibb, New York, New York), which is a small molecule, ATP-competitive inhibitor of Src family kinases, BCR-Abl, c-Kit, and PDGFR (79). Dasatinib has been FDA approved for chronic myeloid leukemia (80) and is currently being tested in HNSCC clinical trials (Table 2). Dasatinib has been reported to reduce HNSCC migration and invasion in vitro concomitant with the inhibition of Src and downstream mediators of cell adhesion including focal adhesion kinase (FAK) (81).
Src kinases in other solid tumor malignancies
Further insights into Src function in HNSCC can be gleaned from studies in other solid tumor types where Src has been more extensively studied and Src activation or overexpression has been found to contribute to angiogenesis, proliferation and growth as well as motility and invasion (76). Several studies using various solid tumor models have reported the reduced expression of the angiogenic factor, vascular endothelial growth factor (VEGF), upon c-Src inhibition or down-regulation of c-Src expression (82–84). These studies also reported reduced in vivo angiogenesis following treatment to inhibit Src activity, and therefore indicate an important role for c-Src in this process.
Mechanisms of Src regulation
Src activity is regulated by intra- and intermolecular interactions and phosphorylation events. Src kinases contain the following domains: 1) Src homology (SH) 4 domain, 15 amino acids containing signals directing lipid modification that facilitate membrane association (85), 2) unique region, 3) SH3 domain, a 50 amino acid domain involved in intra- and inter-protein interactions that recognizes a core consensus amino acid sequence of P-X-X-P, 4) SH2 domain, a modular domain recognizing predominantly phosphotyrosine containing peptides, 5) SH1, the catalytic domain and region containing the autophosphorylation site, and 6) a carboxyl-terminal negative regulatory tail (71). Src family kinases share extensive sequence homology except within the unique region. The autophosphorylation site within the SH1 domain is needed for full kinase activity. The negative regulatory tail, SH2 and SH3 domains are involved in modulating Src kinase activity. Phosphorylation of the regulatory tyrosine within the regulatory tail results in a closed conformational state with intramolecular interactions between the phosphorylated regulatory tyrosine and the SH2 domain and between the SH3 domain and a peptide sequence within the SH2-catalytic domain linker that contains the SH3 binding consensus sequence P-X-X-P (86, 87). This closed conformation contributes to the inaccessibility of substrates to the kinase domain. In addition to phosphorylation events, interactions with proteins such as focal adhesion kinases (FAKs) and platelet-derived growth factor receptor (PDGF) also disrupt these Src intramolecular interactions leading to Src activation (88, 89).
C-terminal Src kinase (CSK), which has been shown to be vital to development and maintenance of squamous epithelia in mice (90), and its homolog CHK are known to negatively regulate Src kinases through the phosphorylation of the Src negative regulatory tyrosine (91, 92). Overexpression of exogenous CSK in highly metastatic rodent cell lines was reported to down-regulate Src kinase activity and reduce metastases in vivo compared to the parental cell line in two independent studies (93, 94). Colon cancer and hepatocellular carcinoma correlative studies indicated that downregulation of CSK may be a mechanism by which Src activity is enhanced in cancer cells (95, 96). Removal of the phosphate from the Src regulatory tyrosine is performed in breast cancer cells by protein tyrosine phosphatase-1B (PTP1B) in vitro and in vivo (97). Other protein tyrosine phosphastases (PTPs) are likely also involved in the activation of Src kinases (92). To date, a role for CSK or Src-regulating phosphatases in HNSCC has not been established.
Targeting Src for treatment of HNSCC
Src-targeted compounds have been evaluated in preclinical models and are currently in clinical development for solid tumors including HNSCC, including dasatinib and AZD-0530 (AstraZeneca, Wilmington, Delaware). Both dasatinib and AZD-0530 are dual Src/Abl inhibitors. However, unlike dasatinib, which inhibits a spectrum of kinases (79), AZD-0530 is more selective for c-Src and Abl (Table 2) (98).
Dasatinib has been evaluated in NSCLC and HNSCC preclinical models and was found to inhibit migration and induce apoptosis (81). In lung cancer cell lines, dasatinib treatment was found to induce apoptosis only in cell lines expressing gefitinib-sensitive mutant EGFR (99). AZD0530 preclinical studies in HNSCC models have not been reported. However, our group found reduced activated Src levels and decreased cell proliferation and invasion in HNSCC cell lines treated with AZD0530 compared with vehicle control (unpublished observations). AZD0530 has been evaluated in breast cancer preclinical models where it inhibited cell migration and invasion phenotypes (100, 101) and abrogated anchorage-independent growth of breast cancer-derived cell lines in vitro (102). In in vitro studies employing cell lines derived from lung cancers, basal Src phosphorylation was detected in 4 of 5 cell lines evaluated, and in three lines with basal Src phosphorylation, AZD0530 treatment was found to arrest cell growth (103).
Phase I clinical studies have been completed and/or are ongoing for both Src inhibitors. In a phase I clinical study in healthy male subjects, AZD0530 was tolerated in single doses up to 500 mg and in multiple, once-daily 20 mg doses (104). In a separate study, AZD0530 was found to reduce phosphorylation of the Src substrate FAK in tumors in a phase I study of with 81 evaluable patients (105). Two AZD0530 phase I clinical studies are currently ongoing to evaluate: 1) AZD0530 in combination with gemcitabine for patients with locally advanced or metastatic pancreatic cancer that is not surgically respectable (I.D. NCT00265876, ClinicalTrials.gov) and 2) AZD0530 in combination with AZD2171 (Recentin, AstraZeneca), an inhibitor of vascular endothelial growth factor (VEGF) receptor, in patients with advanced solid tumors (I.D. NCT00475956, ClinicalTrials.gov).
Dasatinib is currently being evaluated in phase I clinical trials for solid tumors. In a phase I dose escalation study of 26 patients, where toxicity was generally low and no maximum tolerated dose was found, no objective responses were observed; however 6 subjects experienced stable disease for 2 to 10 months (106). Several phase I clinical trials are ongoing including two in the U.S. that are evaluating dasatinib in patients with advanced solid tumors. One combines dasatinib with gemcitabine (I.D. NCT0049234, ClinicalTrials.gov), and the second is a University of Pittsburgh study evaluating dasatinib in combination with cetuximab (I.D. NCT00388427).
Combining targeted therapeutics for treatment of HNSCC
Results from studies combining EGFR- and Src-targeted therapeutics for HNSCC have yet to be reported. However, combining the Src inhibitor, AZD0530, with gefitinib was found have additive inhibitory effects on cell growth, migration, and invasion compared to either agent alone in tamoxifen-resistant MCF7 breast cancer cells (107). In addition, dasatinib combined with cetuximab was reported to have synergistic growth inhibitory effects on a colon cancer cell line resistant to dasatinib (108). The rationale for combining EGFR- and Src-targeted therapeutics for treatment of HNSCC is based upon general understanding of signal transduction pathways elucidated in HNSCC and other cancer preclinical models as previously discussed. Ideally, selections of combinations of therapeutics would be based upon molecular characteristics of individual tumor molecular profiles. The concerted efforts of clinical trial correlative studies to collect and molecularly analyze tumor specimens and assess correlations with clinical response to targeted therapeutics are ongoing. We anticipate that our general knowledge of EGFR and Src signaling pathways in HNSCC will be expanded through these correlative studies. In this way, our general understanding of the consequences of inhibition of EGFR and Src signaling will provide a framework for our molecular analysis of clinical specimens and specifications for selections of individualized targeted therapies.
Acknowledgments
Grant support:
R01 CA77308-01
P50 CA097190-01A1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. New England Journal of Medicine. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [see comment] [DOI] [PubMed] [Google Scholar]
- 2.Bell DW, Lynch TJ, Haserlat SM, Harris PL, Okimoto RA, Brannigan BW, Sgroi DC, Muir B, Riemenschneider MJ, Iacona RB, Krebs AD, Johnson DH, Giaccone G, Herbst RS, Manegold C, Fukuoka M, Kris MG, Baselga J, Ochs JS, Haber DA. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. Journal of Clinical Oncology. 2005;23:8081–8092. doi: 10.1200/JCO.2005.02.7078. [DOI] [PubMed] [Google Scholar]
- 3.Lee JW, Soung YH, Kim SY, Nam HK, Park WS, Nam SW, Kim MS, Sun DI, Lee YS, Jang JJ, Lee JY, Yoo NJ, Lee SH. Somatic mutations of EGFR gene in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005;11:2879–2882. doi: 10.1158/1078-0432.CCR-04-2029. [DOI] [PubMed] [Google Scholar]
- 4.Loeffler-Ragg J, Witsch-Baumgartner M, Tzankov A, Hilbe W, Schwentner I, Sprinzl GM, Utermann G, Zwierzina H. Low incidence of mutations in EGFR kinase domain in Caucasian patients with head and neck squamous cell carcinoma. Eur J Cancer. 2006;42:109–111. doi: 10.1016/j.ejca.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 5.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579–3584. [PubMed] [Google Scholar]
- 6.Storkel S, Reichert T, Reiffen KA, Wagner W. EGFR and PCNA expression in oral squamous cell carcinomas--a valuable tool in estimating the patient's prognosis. European Journal of Cancer. Part B, Oral Oncology. 1993;29B:273–277. doi: 10.1016/0964-1955(93)90047-i. [DOI] [PubMed] [Google Scholar]
- 7.Lee CS, Redshaw A, Boag G. Epidermal growth factor receptor immunoreactivity in human laryngeal squamous cell carcinoma. Pathology. 1997;29:251–254. doi: 10.1080/00313029700169005. [DOI] [PubMed] [Google Scholar]
- 8.Grandis JR, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, Drenning SD, Tweardy DJ. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 9.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK, Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 10.Maurizi M, Scambia G, Benedetti Panici P, Ferrandina G, Almadori G, Paludetti G, De Vincenzo R, Distefano M, Brinchi D, Cadoni G. EGF receptor expression in primary laryngeal cancer: correlation with clinico-pathological features and prognostic significance. International Journal of Cancer. 1992;52:862–866. doi: 10.1002/ijc.2910520605. [DOI] [PubMed] [Google Scholar]
- 11.Dassonville O, Formento JL, Francoual M, Ramaioli A, Santini J, Schneider M, Demard F, Milano G. Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer. Journal of Clinical Oncology. 1993;11:1873–1878. doi: 10.1200/JCO.1993.11.10.1873. [DOI] [PubMed] [Google Scholar]
- 12.Almadori G, Cadoni G, Galli J, Ferrandina G, Scambia G, Exarchakos G, Paludetti G, Ottaviani F. Epidermal growth factor receptor expression in primary laryngeal cancer: an independent prognostic factor of neck node relapse. International Journal of Cancer. 1999;84:188–191. doi: 10.1002/(sici)1097-0215(19990420)84:2<188::aid-ijc16>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Chung CH, Ely K, McGavran L, Varella-Garcia M, Parker J, Parker N, Jarrett C, Carter J, Murphy BA, Netterville J, Burkey BB, Sinard R, Cmelak A, Levy S, Yarbrough WG, Slebos RJ, Hirsch FR. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24:4170–4176. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 14.Temam S, Kawaguchi H, El-Naggar AK, Jelinek J, Tang H, Liu DD, Lang W, Issa JP, Lee JJ, Mao L. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J Clin Oncol. 2007;25:2164–2170. doi: 10.1200/JCO.2006.06.6605. [DOI] [PubMed] [Google Scholar]
- 15.Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cellular Signalling. 2005;17:1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. Journal of Clinical Oncology. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 17.Xia W, Lau YK, Zhang HZ, Liu AR, Li L, Kiyokawa N, Clayman GL, Katz RL, Hung MC. Strong correlation between c-erbB-2 overexpression and overall survival of patients with oral squamous cell carcinoma. Clin Cancer Res. 1997;3:3–9. [PubMed] [Google Scholar]
- 18.Khan AJ, King BL, Smith BD, Smith GL, DiGiovanna MP, Carter D, Haffty BG. Characterization of the HER-2/neu oncogene by immunohistochemical and fluorescence in situ hybridization analysis in oral and oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2002;8:540–548. [PubMed] [Google Scholar]
- 19.Cavalot AMT, Roggero N, Brondino G, Pagano M, Cortesina G. Prognostic impact of HER-2/neu expression on squamous head and neck carcinomas. Head and Neck. 2007 doi: 10.1002/hed.20574. In Press: In Press. [DOI] [PubMed] [Google Scholar]
- 20.Xia WLY, Zhang HZ, Xiao FY, Johnston DA, Liu AR, Li L, Katz RL, Hung MC. Combination of EGFR, HER-2/neu, and HER-3 is a stronger predictor for the outcome of oral squamous cell carcinoma than any individual family members. Clinical Cancer Research. 1999;5:4164–4174. [PubMed] [Google Scholar]
- 21.Ekberg T, Nestor M, Engstrom M, Nordgren H, Wester K, Carlsson J, Anniko M. Expression of EGFR, HER2, HER3, and HER4 in metastatic squamous cell carcinomas of the oral cavity and base of tongue. Int J Oncol. 2005;26:1177–1185. doi: 10.3892/ijo.26.5.1177. [DOI] [PubMed] [Google Scholar]
- 22.Udayachander M, Dean CJ, Meenakshi AN, Sivakumar N, Babu PB, Sivakumar J. Anti-tumor activity of monoclonal antibody CIBCNSH3 generated to the human EGF receptor. Hum Antibodies. 1997;8:60–64. [PubMed] [Google Scholar]
- 23.He Y, Zeng Q, Drenning SD, Melhem MF, Tweardy DJ, Huang L, Grandis JR. Inhibition of human squamous cell carcinoma growth in vivo by epidermal growth factor receptor antisense RNA transcribed from the U6 promoter. J Natl Cancer Inst. 1998;90:1080–1087. doi: 10.1093/jnci/90.14.1080. [DOI] [PubMed] [Google Scholar]
- 24.Thomas SM, Zeng Q, Epperly MW, Gooding WE, Pastan I, Wang QC, Greenberger J, Grandis JR. Abrogation of head and neck squamous cell carcinoma growth by epidermal growth factor receptor ligand fused to pseudomonas exotoxin transforming growth factor alpha-PE38. Clin Cancer Res. 2004;10:7079–7087. doi: 10.1158/1078-0432.CCR-04-0587. [DOI] [PubMed] [Google Scholar]
- 25.Egloff AM, Grandis J. Epidermal growth factor receptor-targeted molecular therapeutics for head and neck squamous cell carcinoma. Expert Opin Ther Targets. 2006;10:639–647. doi: 10.1517/14728222.10.5.639. [DOI] [PubMed] [Google Scholar]
- 26.Specenier PM, Vermorken JB. Targeted therapies in head and neck cancer. Targ Oncol. 2007;2:73–88. [Google Scholar]
- 27.FDA approval for cetuximab. Vol. 2007 2006. [Google Scholar]
- 28.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 29.Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 30.Vermorken J, Mesia R, Vega V, Remenar E, Hitt R, Kawecki A, Rottey S, Amellal N, Cupissol D, Licitra L. Cetuximab extends survival of patients with recurrent or metastatis SCCHN when added to first line platinum based therapy--Results of a randomized phase III (Extreme) study. Journal of Clinical Oncology, ASCO Annual Meeting Proceedings. 2007;25:6091. [Google Scholar]
- 31.Herbst RS, Arquette M, Shin DM, Dicke K, Vokes EE, Azarnia N, Hong WK, Kies MS. Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. Journal of Clinical Oncology. 2005;23:5578–5587. doi: 10.1200/JCO.2005.07.120. [see comment] [DOI] [PubMed] [Google Scholar]
- 32.Baselga J, Trigo JM, Bourhis J, Tortochaux J, Cortes-Funes H, Hitt R, Gascon P, Amellal N, Harstrick A, Eckardt A. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. Journal of Clinical Oncology. 2005;23:5568–5577. doi: 10.1200/JCO.2005.07.119. [see comment] [DOI] [PubMed] [Google Scholar]
- 33.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, Knecht R, Amellal N, Schueler A, Baselga J. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. Journal of Clinical Oncology. 2007;25:2171–2177. doi: 10.1200/JCO.2006.06.7447. [see comment] [DOI] [PubMed] [Google Scholar]
- 34.Burtness BGM, Flood W, Mattar B, Forastiere AA, Group ECO. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. Journal of Clinical Oncology. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 35.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, Klimstra DS, Fridman D, Kelsen DP, Saltz LB. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. Journal of Clinical Oncology. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [see comment] [DOI] [PubMed] [Google Scholar]
- 36.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. Journal of Clinical Oncology. 2004;22:1201–1208. doi: 10.1200/JCO.2004.10.182. [see comment] [DOI] [PubMed] [Google Scholar]
- 37.Giusti RM, Shastri KA, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: panitumumab (Vectibix) Oncologist. 2007;12:577–583. doi: 10.1634/theoncologist.12-5-577. [DOI] [PubMed] [Google Scholar]
- 38.Bleeker WK, Lammerts van Bueren JJ, van Ojik HH, Gerritsen AF, Pluyter M, Houtkamp M, Halk E, Goldstein J, Schuurman J, van Dijk MA, van de Winkel JG, Parren PW. Dual mode of action of a human anti-epidermal growth factor receptor monoclonal antibody for cancer therapy. Journal of Immunology. 2004;173:4699–4707. doi: 10.4049/jimmunol.173.7.4699. [DOI] [PubMed] [Google Scholar]
- 39.Crombet T, Osorio M, Cruz T, Roca C, del Castillo R, Mon R, Iznaga-Escobar N, Figueredo R, Koropatnick J, Renginfo E, Fernandez E, Alvarez D, Torres O, Ramos M, Leonard I, Perez R, Lage A. Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. Journal of Clinical Oncology. 2004;22:1646–1654. doi: 10.1200/JCO.2004.03.089. [DOI] [PubMed] [Google Scholar]
- 40.Kirby AM, A'Hern RP, D'Ambrosio C, Tanay M, Syrigos KN, Rogers SJ, Box C, Eccles SA, Nutting CM, Harrington KJ. Gefitinib (ZD1839, Iressa) as palliative treatment in recurrent or metastatic head and neck cancer. British Journal of Cancer. 2006;94:631–636. doi: 10.1038/sj.bjc.6602999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22:77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 42.Cohen EE, Rosen F, Stadler WM, Recant W, Stenson K, Huo D, Vokes EE. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003;21:1980–1987. doi: 10.1200/JCO.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 43.FDA approval for Erlotinib Hydrochloride. Vol. 2007 2007. [Google Scholar]
- 44.Siu LL, Soulieres D, Chen EX, Pond GR, Chin SF, Francis P, Harvey L, Klein M, Zhang W, Dancey J, Eisenhauer EA, Winquist E. Princess Margaret Hospital Phase, I. I. C., and National Cancer Institute of Canada Clinical Trials Group, S. Phase I/II trial of erlotinib and cisplatin in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: a Princess Margaret Hospital phase II consortium and National Cancer Institute of Canada Clinical Trials Group Study. Journal of Clinical Oncology. 2007;25:2178–2183. doi: 10.1200/JCO.2006.07.6547. [see comment] [DOI] [PubMed] [Google Scholar]
- 45.FDA approval for gefitinib. National Cancer Institute; 2007. [Google Scholar]
- 46.Ahmed SM, Cohen EE, Haraf DJ, Stenson KM, Blair E, Brockstein BE, Lin S, Lester E, Dekker A, Williams R, Vokkes EE. Updated results of a phase II trial integrating gefitinib (G) into concurrent chemoradiation (CRT) followed by G adjuvant therapy for locally advanced head and neck cancer (HNC) Journal of Clinical Oncology, ASCO Annual Meeting Proceedings. 2007;25:6028. [Google Scholar]
- 47.Stewart JSW, Cohen EE, Licitra L, Van Herpen CML, Khorprasert C, Soulieres D, Vodvarka P, Rischin D, Garin AM, Ghiorghiu S, Hargreaves L, Armour A, Vokes EE. A Phase III randomized parallel-group study of gefitinib (IRESSA) versus methotrexate (IMEX) in patients with recurrent squamous cell carcinoma of the head and neck. American Association for Cancer Research Annual Meeting: Proceedings 3522; Los Angeles, CA. AACR; 2007. [Google Scholar]
- 48.Niwa H, Wentzel AL, Li M, Gooding WE, Lui VW, Grandis JR. Antitumor effects of epidermal growth factor receptor antisense oligonucleotides in combination with docetaxel in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2003;9:5028–5035. [PubMed] [Google Scholar]
- 49.Li M, Ye C, Feng C, Riedel F, Liu X, Zeng Q, Grandis JR. Enhanced antiangiogenic therapy of squamous cell carcinoma by combined endostatin and epidermal growth factor receptor-antisense therapy. Clin Cancer Res. 2002;8:3570–3578. [PubMed] [Google Scholar]
- 50.FDA approval for lapatinib ditosylate. Vol. 2007 2007. [Google Scholar]
- 51.Rusnak DW, Affleck K, Cockerill SG, Stubberfield C, Harris R, Page M, Smith KJ, Guntrip SB, Carter MC, Shaw RJ, Jowett A, Stables J, Topley P, Wood ER, Brignola PS, Kadwell SH, Reep BR, Mullin RJ, Alligood KJ, Keith BR, Crosby RM, Murray DM, Knight WB, Gilmer TM, Lackey K. The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: potential therapy for cancer. Cancer Res. 2001;61:7196–7203. [PubMed] [Google Scholar]
- 52.Wood ER, Truesdale AT, McDonald OB, Yuan D, Hassell A, Dickerson SH, Ellis B, Pennisi C, Horne E, Lackey K, Alligood KJ, Rusnak DW, Gilmer TM, Shewchuk L. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Research. 2004;64:6652–6659. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 53.Abidoye OO, Cohen EE, Wong SJ, Kozloff MF, Nattam SR, Stenson KM, Blair A, Day S, Dancey JE, Vokes EE. A phase II study of lapatinib ( GW572016) in recurrent/metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN); ASCO Annual Meeting; 2006. [Google Scholar]
- 54.Reid A, Vidal L, Shaw H, de Bono J. Dual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu) European Journal of Cancer. 2007;43:481–489. doi: 10.1016/j.ejca.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 55.Morin MJ. From oncogene to drug: development of small molecule tyrosine kinase inhibitors as anti-tumor and anti-angiogenic agents. Oncogene. 2000;19:6574–6583. doi: 10.1038/sj.onc.1204102. [DOI] [PubMed] [Google Scholar]
- 56.Harari PM. Epidermal growth factor receptor inhibition strategies in oncology. Endocrine-Related Cancer. 2004;11:689–708. doi: 10.1677/erc.1.00600. [DOI] [PubMed] [Google Scholar]
- 57.Maa MC, Leu TH, McCarley DJ, Schatzman RC, Parsons SJ. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc Natl Acad Sci U S A. 1995;92:6981–6985. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1999;96:1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Can Res. 2006;12:5268–5272. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- 60.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 62.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 63.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glysogen symthase kinase-3 beta regulates cyclin D1 proteolysis and subcellular localization. Genes and Development. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes and Development. 2000;14:2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, Pitt AS, Tweardy DJ. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth in vitro. J Clin Invest. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y, Kim JD. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kijima T, Niwa H, Steinman RA, Drenning SD, Gooding WE, Wentzel AL, Xi S, Grandis JR. STAT3 activation abrogates growth factor dependence and contributes to head and neck squamous cell carcinoma tumor growth in vivo. Cell Growth Differ. 2002;13:355–362. [PubMed] [Google Scholar]
- 68.Zhang Q, Thomas SM, Lui VW, Xi S, Siegfried JM, Fan H, Smithgall TE, Mills GB, Grandis JR. Phosphorylation of TNF-alpha converting enzyme by gastrin-releasing peptide induces amphiregulin release and EGF receptor activation. Proc Natl Acad Sci U S A. 2006;103:6901–6906. doi: 10.1073/pnas.0509719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Q, Thomas SM, Xi S, Smithgall TE, Siegfried JM, Kamens J, Gooding WE, Grandis JR. SRC family kinases mediate epidermal growth factor receptor ligand cleavage, proliferation, and invasion of head and neck cancer cells. Cancer Res. 2004;64:6166–6173. doi: 10.1158/0008-5472.CAN-04-0504. [DOI] [PubMed] [Google Scholar]
- 70.Ishizawar RC, Miyake T, Parsons SJ. c-Src modulates ErbB2 and ErbB3 heterocomplex formation and function Oncogene. 2007;36:3503–3510. doi: 10.1038/sj.onc.1210138. [DOI] [PubMed] [Google Scholar]
- 71.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annual Review of Cell & Developmental Biology. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 72.Irby RB, Mao W, Coppola D, Kang J, Loubeau JM, Trudeau W, Karl R, Fujita DJ, Jove R, Yeatman TJ. Activating SRC mutation in a subset of advanced human colon cancers. Nature Genetics. 1999;21:187–190. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- 73.Daigo Y, Furukawa Y, Kawasoe T, Ishiguro H, Fujita M, Sugai S, Nakamori S, Liefers GJ, Tollenaar RA, van de Velde CJ, Nakamura Y. Absence of genetic alteration at codon 531 of the human c-src gene in 479 advanced colorectal cancers from Japanese and Caucasian patients. Cancer Research. 1999;59:4222–4224. [PubMed] [Google Scholar]
- 74.Nilbert M, Fernebro E. Lack of activating c-SRC mutations at codon 531 in rectal cancer. Cancer Genetics & Cytogenetics. 2000;121:94–95. doi: 10.1016/s0165-4608(00)00226-0. [DOI] [PubMed] [Google Scholar]
- 75.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 76.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 77.Xi S, Zhang Q, Dyer KF, Lerner EC, Smithgall TE, Gooding WE, Kamens J, Grandis JR. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003;278:31574–31583. doi: 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- 78.Yang Z, Bagheri-Yarmand R, Wang RA, Adam L, Papadimitrakopoulou VV, Clayman GL, El-Naggar A, Lotan R, Barnes CJ, Hong WK, Kumar R. The epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 (Iressa) suppresses c-Src and Pak1 pathways and invasiveness of human cancer cells. Clin Cancer Res. 2004;10:658–667. doi: 10.1158/1078-0432.ccr-0382-03. [DOI] [PubMed] [Google Scholar]
- 79.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM, Fairchild C, Hunt JT, Inigo I, Johnston K, Kamath A, Kan D, Klei H, Marathe P, Pang S, Peterson R, Pitt S, Schieven GL, Schmidt RJ, Tokarski J, Wen ML, Wityak J, Borzilleri RM. Discovery of N-(2-chloro-6-methyl- phenyl)-2- (6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5- carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. Journal of Medicinal Chemistry. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 80.FDA approves dasatinib (Sprycel) for use in the treatment of adults with chronic phase, accelerated phase, or myeloid or lymphoid phase chronic myeloid leukemia. 2006 [Google Scholar]
- 81.Johnson FM, Saigal B, Talpaz M, Donato NJ. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005;11:6924–6932. doi: 10.1158/1078-0432.CCR-05-0757. [DOI] [PubMed] [Google Scholar]
- 82.Donnini S, Monti M, Castagnini C, Solito R, Botta M, S S, Giachetti A, Ziche M. Pyrazolo-pyrimidine-derived c-Src inhibitor reduces angiogenesis and survival of squamous cell carcinoma cells by suppressing vascular endothelial growth factor production and signaling. Int J Cancer. 2007;120:995–1004. doi: 10.1002/ijc.22410. [DOI] [PubMed] [Google Scholar]
- 83.Summy JM, Trevino JG, Lesslie DP, Baker CH, Shakespeare WC, Wang Y, Sundaramoorthi R, Metcalf CA, Keats JA, Sawyer TK, Gallick GE. AP23846, a novel and highly potent Src family kinase inhibitor, reduces vascular endothelial growth factor and interleukin-8 expression in human solid tumor cell lines and abrogates downstream angiogenic processes. Mol Cancer Ther. 2005;4:1900–1911. doi: 10.1158/1535-7163.MCT-05-0171. [DOI] [PubMed] [Google Scholar]
- 84.Ellis LM, Staley CA, Liu W, Fleming RY, Parikh NU, Bucana CD, Gallick GE. Down-regulation of vascular endothelial growth factor in a human colon carcinoma cell line transfected with an antisense expression vector specific for c-Src. J Biol Chem. 1998;273:1052–1057. doi: 10.1074/jbc.273.2.1052. [DOI] [PubMed] [Google Scholar]
- 85.Resh MD. Interaction of tyrosine kinase oncoproteins with cellular membranes. Biochimica et Biophysica Acta. 1993;1155:307–322. doi: 10.1016/0304-419x(93)90012-2. [DOI] [PubMed] [Google Scholar]
- 86.Sicheri F, Kuriyan J. Structures of Src-family tyrosine kinases. Curr Opin Struct Biol. 1997;7:777–785. doi: 10.1016/s0959-440x(97)80146-7. [DOI] [PubMed] [Google Scholar]
- 87.Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Crystal structures of c- Src reveal features of its autoinhibitory mechanism. Molecular Cell. 1999;3:629–638. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- 88.Thomas JW, Ellis B, Boerner RJ, Knight WB, White GC, 2nd, Schaller MD. SH2- and SH3-mediated interactions between focal adhesion kinase and Src. Journal of Biological Chemistry. 1998;273:577–583. doi: 10.1074/jbc.273.1.577. [DOI] [PubMed] [Google Scholar]
- 89.Alonso G, Koegl M, Mazurenko N, Courtneidge SA. Sequence requirements for binding of Src family tyrosine kinases to activated growth factor receptors. Journal of Biological Chemistry. 1995;270:9840–9848. doi: 10.1074/jbc.270.17.9840. [DOI] [PubMed] [Google Scholar]
- 90.Yagi R, Waguri S, Sumikawa Y, Nada S, Oneyama C, Itami S, Schmedt C, Uchiyama Y, Okada M. C-terminal Src kinase controls development and maintenance of mouse squamous epithelium. Embo J. 2007;26:1234–1244. doi: 10.1038/sj.emboj.7601595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okada M, Nada S, Yamanashi Y, Yamamoto T, Nakagawa H. CSK: a protein kinase involved in regulation of src family kinases. J Biol Chem. 1991;266:24249–24252. [PubMed] [Google Scholar]
- 92.Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 93.Boyer B, Bourgeois Y, Poupon MF. Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene. 2002;21:2347–2356. doi: 10.1038/sj.onc.1205298. [DOI] [PubMed] [Google Scholar]
- 94.Nakagawa T, Tanaka S, Suzuki H, Takayanagi H, Miyazaki T, Nakamura K, Tsuruo T. Overexpression of the csk gene suppresses tumor metastasis in vivo. Int J Cancer. 2000;88:384–391. [PubMed] [Google Scholar]
- 95.Cam WR, Masaki T, Shiratori Y, Kato N, Ikenoue T, Okamoto M, Igarashi K, Sano T, Omata M. Reduced C-terminal Src kinase activity is correlated inversely with pp60(c-src) activity in colorectal carcinoma. Cancer. 2001;92:61–70. doi: 10.1002/1097-0142(20010701)92:1<61::aid-cncr1292>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 96.Masaki T, Okada M, Tokuda M, Shiratori Y, Hatase O, Shirai M, Nishioka M, Omata M. Reduced C-terminal Src kinase (Csk) activities in hepatocellular carcinoma. Hepatology. 1999;29:379–384. doi: 10.1002/hep.510290239. [DOI] [PubMed] [Google Scholar]
- 97.Bjorge JD, Pang A, Fujita DJ. Identification of protein-tyrosine phosphatase 1B as the major tyrosine phosphatase activity capable of dephosphorylating and activating c-Src in several human breast cancer cell lines. Journal of Biological Chemistry. 2000;275:41439–41446. doi: 10.1074/jbc.M004852200. [DOI] [PubMed] [Google Scholar]
- 98.Manley PW, Cowan-Jacob SW, Mestan J. Advances in the structural biology, design and clinical development of Bcr-Abl kinase inhibitors for the treatment of chronic myeloid leukaemia. Biochimica et Biophysica Acta. 2005;1754:3–13. doi: 10.1016/j.bbapap.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 99.Haura EB, Song L, Lee F, Jove R. Effect of dasatinib, a Src kinase inhibitor, on lung cancer cells with defined epidermal growth factor receptor status. Journal of Clinical Oncology, ASCO Meeting Proceedings. 2006:3019. [Google Scholar]
- 100.Hiscox S, Jordan NJ, Morgan L, Green TP, Nicholson RI. Src kinasepromotes adhesion-independent activation of FAK and enhances cellular migration in tamoxifen-resistant breast cancer cells. Clin Exp Metastasis. 2007;24:157–167. doi: 10.1007/s10585-007-9065-y. [DOI] [PubMed] [Google Scholar]
- 101.Hiscox S, Morgan L, Green T, Nicholson RI. Src as a therapeutic target in anti-hormone/anti-growth factor-resistant breast cancer. Endocr Relat Cancer. 2006;13:S53–S59. doi: 10.1677/erc.1.01297. [DOI] [PubMed] [Google Scholar]
- 102.Herynk MH, Beyer AR, Cui Y, Weiss H, Anderson E, Green TP, Fuqua SA. Cooperative action of tamoxifen and c-Src inhibition in preventing the growth of estrogen receptor-positive human breast cancer cells. Mol Cancer Ther. 2006;5:3023–3031. doi: 10.1158/1535-7163.MCT-06-0394. [DOI] [PubMed] [Google Scholar]
- 103.Gautschi O, Purnell P, Evans CP, Yang JC, Holland WS, Bold RJ, Virudachalam S, Lara PN, Gandara DR, Gumerlock PH. Preclinical evaluation of the dual specific Src/Abl kinase inhibitor AZD0530 in lung cancer. Journal of Clinical Oncology, ASCO Annual Meeting Proceedings. 2006;24:13108. [Google Scholar]
- 104.Lockton JA, Smethurst D, Macpherson M, Tootell R, Marshall AL, Clack G, Gallagher NJ. Phase I ascending single and multiple dose studies to assess the safety, tolerability and pharmacokinetics of AZD0530, a highly selective, dual-specific Src-Abl inhibitor. Journal of Clinical Oncology, ASCO Annual Meeting Proceedings. 23:3125. [Google Scholar]
- 105.Tabernero J, Cervanter A, Hoekman K, Hurwitz HI, Jodrell DI, Hamberg P, Stuart M, Green TP, Iacona RB, Baselga J. Phase I study of AZD00530, an oral potent inhibitor of Src kinase: First demonstration of inhibition of Src activity in human cancers. Journal of Clinical Oncology, ASCO Annual Meeting Proceedings. 2007;25:3520. [Google Scholar]
- 106.Johnson FM, Chiappori A, Burris H, Rosen L, McCann B, Luo FR, Mayfield S, Palme H, Platero J, Blackwood-Chirchir A. A phase I study ( CA180021-Segment 2) of dasatinib in patients (pts) with advanced solid tumors. Journal of Clinical Oncology, ASCO Annual Meeting Proceedings. 2007;25:14042. [Google Scholar]
- 107.Hiscox S, Morgan L, Green TP, Barrow D, Gee J, Nicholson RI. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res Treat. 2005:1–12. doi: 10.1007/s10549-005-9120-9. [DOI] [PubMed] [Google Scholar]
- 108.Kopetz S, Wu J, Davies M, Johnson F, Donato N. Synergistic effects of combination therapy with anti-EGFR and anti-Src therapy in vitro in colon cancer; Gastrointestinal Cancer Symposium American Society of Clinical Oncology; 2007. p. 406. [Google Scholar]