Abstract
A highly stereoselective synthesis of chiral α-amino-β-lactam through an ynamide-Kinugasa reaction is described. In addition, a mechanistic model is illustrated here to rationalize the observed diastereoselectivity, which depends on both the initial [3 + 2] cycloaddition step and the subsequent protonation for which both are highly selective.
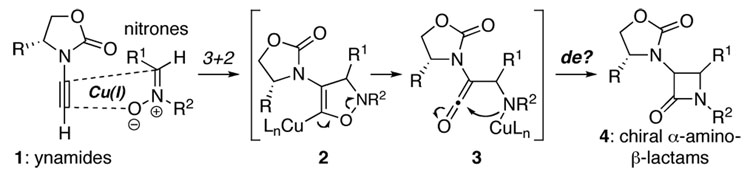
Since Staudinger’s first preparation,1 β-l actams have captured the attention of synthetic and medicinal communities for nearly a century.2–6 Rendered famous by penicillin, those substituted with α-amino groups are among the most sought after β-lact ams. Consequently, an impressive array of stereoselective approaches toward chiral α-amino-β-lactams has been reported.4–6 While the Kinugasa reaction7,8 represents an elegant approach toward β-lactams, it has remained relatively unexplored until recently, and this is particularly true in the development of enantioselective protocols.9–11 With such immense significance, we recognized the unique potential of an ynamide-Kinugas a reaction. As shown in Scheme 1, reactions of chiral ynamides 112–13 with nitrones in a Kinugasa manner would not only lead to a stereoselective manifold for constructing β-l actams, but also more importantly, provide a direct synthesis of chiral α-amino-β-lactams [see 4]. We report here a highly stereoselective ynamide-Kinugas a reaction.
Scheme 1.
An Ynamide-Kinugasa Reaction
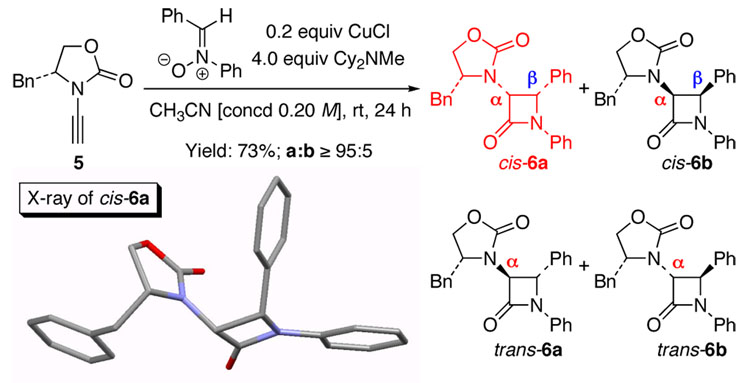
The feasibility of an ynamide-Kinugasa reaction was readily established employing ynamide 5 [Scheme 2]. With 0.2 equiv CuCl and 4.0 equiv Cy2NMe, the reaction of 5 with N-benzylidene-N-phenyl nitrone proceeded effectively in CH3CN at rt to give β-lactam cis-6a14 in 73% yield as the major isomer. X-Ray structural analysis unambiguously revealed that the relative stereochemistry between the α- and β-carbons is cis. This suggests that the minor isomer(s) could be cis-6b and/or trans-6a/6b with a/b isomers differing at the β-carbon stereochemistry.
Scheme 2.
Establishing the Feasibility and Stereochemistry.
The scope of this reaction is distinctly diverse. As shown in Table 1, we found several interesting features: (1) Sterically more encumbered auxiliaries retard the reaction rate [entries 2 and 3 versus 1]; (2) CuI is also feasible as a catalyst and can be more effective than CuCl [entries 3, 6, 8, and 13]; and (3) the minor isomer b was assigned as trans initially based on proton coupling constants15 [entries 5–7, 11, and 13] and was confirmed later via nOe experiments [vide infra].
Table 1.
Scope of Ynamide-Kinugasa Reaction
| entry | ynamides | α-amino-β-lactams | yield [%]a | dr: [a:b]b | ||
|---|---|---|---|---|---|---|
| 1 | 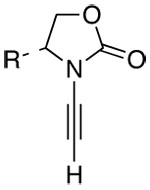 |
7 | 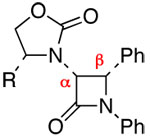 |
12: R = Ph | 80 | ≥95:5 |
| 2 | 8 | 13: R = i-Pr | 36 | ≥ 95:5 | ||
| 3 | 9 | 14: R = CHPh2 | 28c,d | nde | ||
| 4 | 7 | 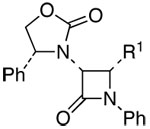 |
15: R1 = 4-Br-Ph | 77 | ≥95:5 | |
| 5 | 7 | 16: R1 = 1Naph | 71 | 90:10 | ||
| 6 | 7 | 17: R1 = styryl | 72c,f | 93:7 | ||
| 7 | 7 | 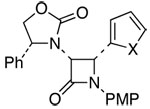 |
18: X = O | 61c | 82:18 | |
| 8 | 7 | 19: X = S | 60c,g | ≥95:5 | ||
| 9 | 7 | 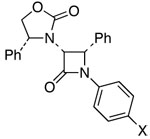 |
20: X = Cl | 65 | ≥95:5 | |
| 10 | 7 | 21: X = CO2Et | 63 | ≥95:5 | ||
| 11 | 5 | 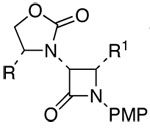 |
22: R = Bn; R1 = Ph | 59h | 91:9 | |
| 12 | 7 | 23: R = Ph; R1 = Ph | 60h | ≥95:5 | ||
| 13 | 7 | 24: R = Ph; R1 = c-hex | 60c,i,h | 92:8 | ||
| 14 | 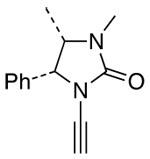 |
10 | 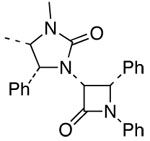 |
25 | 61 | ≥95:5 |
| 15 | 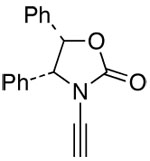 |
11 | 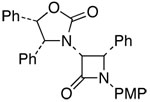 |
26 | 60c,h | ≥95:5 |
Reaction conditions are as shown in Scheme 2 unless otherwise indicated. All are isolated yields.
dr is determined using 1H NMR. All isomers-a are cis. The minor isomer-b is trans.
0.2 equiv of CuI was used, and the reaction was run at 0 °C to rt.
With 0.2 equiv of CuCl, the yield was 13%.
nd: Not determined.
With 0.2 equiv of CuCl, yield was 71% and dr = 91:9.
With 0.2 equiv of CuCl, yield was 48% and dr = 86:14.
PMP: para-methoxyphenyl.
With 0.2 equiv of CuCl and 4.8 equiv of Hünig's base, yield was 54% and dr = 87:13.
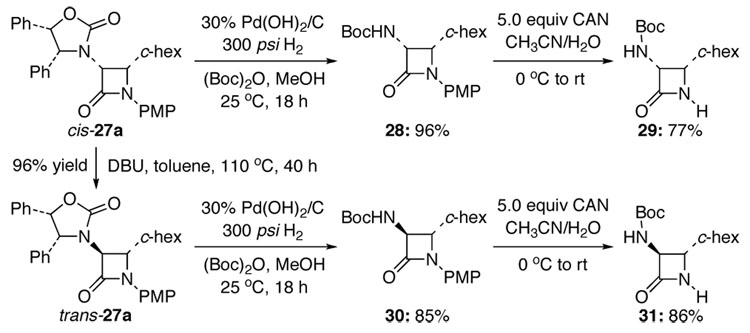
An immediate application of this reaction is the preparation of chiral α-amino-β-lactams [Scheme 3]. Toward this goal, we prepared cis-27a in 44–62% yield from 11 with an a:b ratio of in the range of 10:1–19:1. Hydrogenation with Boc-protection followed by oxidative removal of the PMP group in cis-27a using CAN provided chiral α-amino-β-lactam 29. An α-epimerization of cis-27a via refluxing in toluene in the presence of DBU for 40 h afforded trans-27a, which could be converted to the isomeric α-amino-β-lactam 31 through the same sequence used for cis-27a.
Scheme 3.
Synthesis of Chiral α-Amino-β-Lactams.
During the isolation of cis-27a, we were able to attain a clean sample of the minor isomer trans-27b and confirmed its relative stereochemistry between the α-and β-carbons using nOe experiments.14 We also isolated a small sample of cis-27b and spectroscopically observed a trace amount of trans-27a. Neither had been seen in other reactions. The assignment of cis-27b was confirmed through α-epimerization to trans-27b using DBU.14 With these assignments, this ynamide-Kinugasa reaction became very intriguing from a stereochemical perspective. A unified mechanistic model is proposed in Scheme 4.
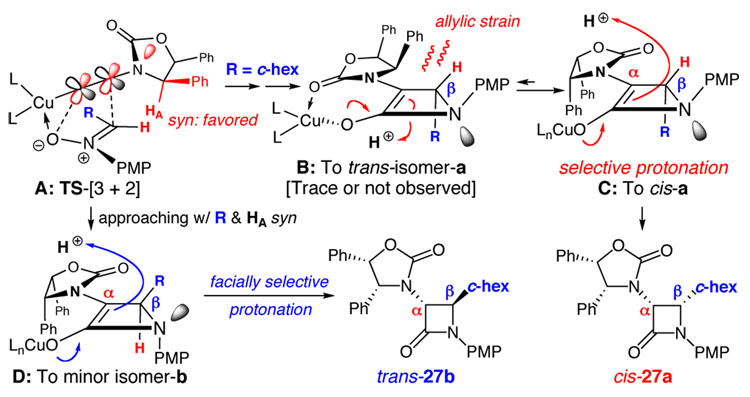
Scheme 4.
A Proposed Mechanistic Model.
Based on the assumption that the more reactive of the two π-bonds is the one conjugated with the nitrogen lone-pair [all in red], the Cu(I)-promoted nitrone-[3 + 2] cycloaddition via intermediate A could diverge into two pathways that would determine the β-carbon stereochemistry. The preferred pathway would involve the approaching nitrone with its vinyl hydrogen [in red] being syn to HA on the chiral auxiliary and the larger R group [chex in blue] anti to HA to minimize steric interactions. This pathway would lead to intermediate B [skipping respective intermediates 2 and 3 shown in Scheme 1], and while B could undergo protonation at the more open bottom face away from the phenyl rings, it would lead to the trans-isomer-a that was not observed from most of these reactions. Therefore, we reason that a facially selective protonation takes place instead via intermediate C on the top face to give cis-27a because C is more stable than B given the presence of allylic strain.
On the other hand, the less favorable cycloaddition pathway would involve the larger R group approaching syn relative to HA on the auxiliary, and should lead to minor isomers b via related intermediate D. We believe a facially selective protonation also occurs here in D to provide trans-27b as the most dominant minor isomer. Intriguingly, B3LYP-6-31G* calculations reveal that trans-27a is ~ 2.50 kcal mol-1 more stable than cis-27a, and trans-27b is ~ 4.86 kcal mol-1 more stable than cis-27b. This implies that for the major reaction pathway, a facially selective protonation gives the kinetic product cis-27a, whereas a selective protonation in the minor reaction pathway gave the more stable trans-27b. Re-subjecting cis-27b to the same reaction conditions did not lead to any α-epimerization or observation of trans-27b. Therefore, despite being more stable, trans isomers are not likely derived from α-epimerizations of their respective cis isomers.
We have described here a highly stereoselective ynamide-Kinugas a reaction and featured its application as a stereoselective manifold for constructing chiral α-amino-β-lactam. A proposed model reveals that the observed selectivity requires both the initial cycloaddition and subsequent protonation to be stereoselective.
Supplementary Material
Acknowledgement
Authors thank NIH [GM066055] for support and Dr. Victor Young and Ben Kucera [University of Minnesota] for X-ray analysis.
Footnotes
Supporting Information Available: Experimental procedures as well as 1H NMR spectral and characterizations are available for all new compounds and free of charge via Internet http://pubs.acs.org.
References
- 1.Staudinger H. Liebigs Ann. Chem. 1907;356:51. [Google Scholar]
- 2.(a) Page MI. The Chemistry of β-Lactams. New York: Blackie Academic and Professional; 1992. [Google Scholar]; (b) G I, editor. The Organic Chemistry of β-Lactams Georg. New York: VCH; 1993. [Google Scholar]
- 3.(a) Masaretti OA, Boschetti CE, Danelon GO, Mata EG, Roveri OA. Curr. Med. Chem. 1995;1:441. [Google Scholar]; (b) Neuhaus FC, Georgopadaku NH. In: Emerging Targets in Antibacterial and Antifungal Chemotherapies; Sutcliffe, J. Georgopadaku NH, editor. new York: Chapman and hall; 1992. [Google Scholar]; (c) Morin RB, Gorman M, editors. The Chemistry and Biology of β-Lactams Antibiotics. New York: Academic Press; 1982. [Google Scholar]
- 4.For reviews on synthetic approaches, see: Miller M. J Acc. Chem. Soc. 1986;19:49. Hart DJ, Ha DC. Chem. Rev. 1989;89:1447. Ghosez L, Marchand-Brynaert J. In: Comp. Org. Syn. Trost B, Flemming I, editors. Vol 5. Oxford: Pergamon; 1991. p. p85. Van der Steen FH, Van Koten G. Tetrahedron. 1991;47:7503. Hegedus LS. Acc. Chem. Soc. 1995;28:299. Ojima I. Acc. Chem. Soc. 1995;28:383. Ojima I, Delaloge F. Chem. Soc. Rev. 1997;26:377. DeKimpe N. In: Comp. Heterocycl. Chem. 1B. Katritzky AR, Rees CW, Scriven EFV, Padwa A, editors. II Vol 1B. Oxford: Pergamon; 1996. Palomo C, Aizpurua JM, Inaki G, Oiarbide M. Eur. J. Org. Chem. 1999:3223. Bruggink A, editor. Synthesis of β-Lactams Antibiotics. Chemistry, Biocatalysis and Process Integration. Netherlands: Kluwer, Dordrecht; 2001. Taggi AE, Hafez AM, Lectka T. Acc. Chem. Res. 2003;36(10) doi: 10.1021/ar020137p. France S, Weatherwax A, Taggi AE, Lectka T. Acc. Chem. Res. 2004;37:592. doi: 10.1021/ar030055g.
- 5.For Rh-catalyzed asymmetric manifolds, see: Doyle MP, Pieters RJ, Tauton J, Pho HQ, Padwa A, Hertzog DL, Precedo L. J. Org. Chem. 1991;56:820. Anada M, Watanabe N, Hashimoto S. Chem Commun. 1998:1517. Davioli P, Moretti I, Prati F, Alper H. J. Org. Chem. 1999;64:518.
- 6.For asymmetric Staudinger reactions, see: Taggi AE, Hafez AM, Wack H, Young B, Ferraris D, Lectka T. J. Am. Chem. Soc. 2002:6626. doi: 10.1021/ja0258226. Hafez AM, Dudding T, Wagerle TR, Shah MH, Taggi AE, Lectka T. J. Org. Chem. 2003;68:5819. doi: 10.1021/jo034150e. Hodous BL, Fu GC. J. Am. Chem. Soc. 2002;124:1578. doi: 10.1021/ja012427r. Also see: Tomioka K, Fujieda H, Hayashi S, Hussein MA, Kambara T, Nomura Y, Kanai M, Koga K. Chem. Commun. 1999:715.
- 7.Kinugasa M, Hashimoto S. J. Chem. Soc. Chem. Commun. 1972:466. [Google Scholar]
- 8.For reviews, see: Evans DA, Kleinbeck F, Rüping M. In: Asymmetric Synthesis – The Essentials. Christmann M, Bräse S, editors. Weinheim: Wiley-VCH, Verlag GmbH & Co. KGaA; 2007. p. 72. Pal, Ghosh SC, Chandra K, BVasak A. Synlett. 2007:2321. Marco-Contelles J. Angew. Chem. Int. Ed. 2004;42:2198. doi: 10.1002/anie.200301730. Also see: Okuro K, Enna M, Miura M, Nomura M. J. Chem. Soc. Chem. Commun. 1993:1107. Ding LK, Irwin WJ. J. Chem. Soc. Perkin Trans. 1976;1:2382.
- 9.For recent asymmetric accounts, see: Lo MM-C, Fu GCJ. Am. Chem. Soc. 2002;124:4572. doi: 10.1021/ja025833z. Shintani R, Fu GC. Angew. Chem. Int. Ed. 2003;115:4216. Ye M-C, Zhou J, Huang Z-Z, Tang Y. Chem. Commun. 2003:2554. doi: 10.1039/b306653c. Ye M-C, Zhou J, Tang Y. J. Org. Chem. 2006;71:3576. doi: 10.1021/jo0602874. Coyne AG, Muller-Bunz H, Guiry PJ. Tetrahedron: Asymmetry. 2007;18:199.
- 10.For an earliest account, see:Miura M, Enna M, Okuro K, Nomura M. J. Org. Chem. 1995;60:4999.
- 11.For stereoselective approaches, see:Pal R, Basak A. Synlett. 2007:1585. Pal R, Basak A. Chem. Commun. 2006:2992. doi: 10.1039/b605743h. Basak A, Ghosh SC, Bhowmick T, Das AK, Bertolasi V. Tetrahedron Lett. 2002;43:5499. Basak A, Mahato T, Bhattacharya G, Mukherjee B. Tetrahedron Lett. 1997;38:643.
- 12.For reviews on ynamides, see:Zificsak CA, Mulder JA, Hsung RP, Rameshkumar C, Wei L-L. Tetrahedron. 2001;57:7575. Mulder JA, Kurtz KCM, Hsung RP. Synlett. 2003:1379. Katritzky AR, Jiang R, Singh SK. Heterocycles. 2004;63:1455.
- 13.For recent references on chemistry of ynamides, see:Oppenheimer J, Johnson WL, Tracey MR, Hsung RP, Yao P-Y, Liu R, Zhao K. Org. Lett. 2007;9:2361. doi: 10.1021/ol0707362. You L, Al-Rashid ZF, Figueroa R, Ghosh SK, Li G, Lu T, Hsung RP. Synlett. 2007:1656. Hashimi ASK, Salathe R, Frey W. Synlett. 2007:1763. For a special issue dedicated to the chemistry of ynamides, see: Tetrahedron-Symposium-In-Print: Chemistry of Electron-Deficient Y namines and Y namides. Tetrahedron. 2006;62(Issue No16)
- 14.See Supporting Information
- 15.The range of proton couple constants for our cis-β-lactams is 5.0–5.6 Hz, and it is 2.0–2.4 Hz for trans-β-lactams
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.