Abstract
Background
Histopathologic diagnosis of endometrial biopsies is used to estimate the risk of progression to carcinoma and guide clinical management. Problems with the widely used World Health Organization (WHO) system for classifying endometrial hyperplasia (EH) have prompted the development of an alternative system based on Endometrial Intraepithelial Neoplasia (EIN). We estimated progression risk associated with EIN among endometrial biopsies in a nested case-control study of EH progression.
Methods
Index biopsies with original community pathology diagnoses of disordered proliferative endometrium (DPEM) or EH that were independently confirmed by a panel of pathologists were independently reviewed and assigned EIN classifications (inadequate, benign, EIN, or cancer) by a second panel of pathologists. Cases (N=138) progressed to carcinoma at least 1 year (median, 6 years) after their index biopsy. Controls (N=241) also had EH, did not progress to carcinoma, and were individually matched to cases on age at EH, date of EH, and length of follow-up. Using conditional logistic regression, we estimated relative risks (RRs, with 95% confidence intervals, CIs) for progression to carcinoma for EIN vs. benign.
Results
In the EIN system, 71 cases (56.3%) and 159 controls (67.9%) were classified as benign; 42 cases (33.3%) and 65 controls (27.8%) were classified as EIN. The RR for EIN vs. benign was 7.76 (95% CI, 3.36–17.91). In the WHO system, the RR for AH vs. DPEM-or-SH-or-CH was 9.19 (95% CI, 3.87–21.83).
Conclusions
Among women followed for at least one year after receiving a biopsy-based EH diagnosis, EIN and AH both had similarly increased risks of progression to carcinoma.
Keywords: endometrial hyperplasia, atypical hyperplasia, uterine cancer, cancer precursor, volume percentage stroma
INTRODUCTION
The term endometrial hyperplasia (EH) refers to endometrial abnormalities that range from mild proliferation to incipient carcinoma (1;2). The World Health Organization (WHO) classification scheme combines architectural (simple vs. complex) and cytologic (no atypia vs. atypia) features to classify the severity of EH. Lesions with diffuse and variably sized glands but a normal ratio of glands to stroma are called simple hyperplasia (SH), whereas lesions with architecturally irregular glands and an increased gland-to-stroma ratio are called complex hyperplasia (CH). When cytologic atypia is present in glandular cells, the former becomes simple atypical hyperplasia (SAH) and the latter becomes complex atypical hyperplasia (CAH) (3;4). Because SAH is exceptionally rare, the term atypical hyperplasia (AH) combines SAH and CAH for practical reasons and is often use to describe any EH with atypia (5). Both in definition and practice, precise boundaries for simple vs. complex or non-atypical vs. atypical are lacking.
Nonetheless, these classifications guide clinical management of women with EH (6). Non-atypical EH (i.e., SH or CH) has a low risk of progression to carcinoma (7), but AH often coexists with invasive carcinoma and is considered a precursor (5;8). Recent population-based data demonstrate that women diagnosed with AH are over ten times more likely than women diagnosed with non-atypical EH to subsequently develop carcinoma (7). Yet the WHO system has been criticized (8) because its imprecisely defined criteria can be inconsistently and idiosyncratically applied by practicing pathologists, among whom inter-observer reproducibility of EH is low (9;10). In addition, atypia, the critical determinant of EH behavior, is difficult to reliably identify in endometrial biopsy specimens (11).
The endometrial intraepithelial neoplasia (EIN) classification attempts to better codify the distorted cellular architecture and nuclear characteristics that underlie endometrial neoplasia. EIN was developed by incorporating morphometric criteria derived from computerized image analysis of endometrial biopsies, histopathologic findings with expected characteristics of premalignant lesions (e.g., monoclonal growth and lineage continuity with cancer), and clinical outcomes. A single premalignant category, called EIN, is present when the lesion has a minimum diameter of 1 mm, the area of glands exceeds the area of stroma, the cytology is changed relative to background, and both benign mimics (e.g., polyps, secretory endometrium, and effects of exogenous estrogen) and cancer can be excluded. The EIN classification can be assigned using computerized image analysis, which yields a D-score that quantifies architectural [volume percentage stroma (VPS) and outer surface density of the glands] and cytologic (the standard deviation of the shortest nuclear axis) features (12) or diagnostic criteria that can be applied to standard H&E slides by practicing pathologists (13). Sufficiently large cytologically distinct glandular lesions with gland area exceeding stromal area are called EIN and considered carcinoma precursors that would progress to carcinoma if left untreated (12). In contrast, lesions that do not meet the criteria for EIN are unlikely to clinically progress (14;15).
One pooled analysis of convenience samples from patients at 8 clinical centers reported that patients diagnosed with EIN were 45 times more likely than patients diagnosed with a benign endometrium to progress to carcinoma (16). When the investigators classified participants under the WHO system, patients diagnosed with AH were only 7 times more likely than patients with non-atypical EH to progress (16). Such convenience series typically suffer from selection bias, losses-to-follow-up, suboptimal statistics, and incomplete statistical adjustment for other potential progression factors. We therefore tested whether EIN assigned by microscopic assessment was associated with subsequent risk of endometrial carcinoma in a rigorous epidemiologic study with masked pathology reviews and statistical analyses that accounted for treatment, clinical factors, and follow-up time.
METHODS
We previously described our study design (Lacey et al., submitted) and methods (7).
Study participants
Participants were members of the Kaiser Permanente Northwest (KPNW) prepaid health plan (17) who were originally diagnosed with incident EH at KPNW between 1970 and 2002 and were then diagnosed with endometrial carcinoma at least one year later. Women who were diagnosed with endometrial carcinoma less than one year after their EH diagnosis were considered to have had prevalent carcinoma at presentation and were excluded.
We retrieved original pathology reports and diagnostic slides for all endometrial procedures, including hysterectomy. In consultation with the KPNW Department of Pathology, we translated EH terminology used before 1995 (5) into WHO nomenclature as needed (e.g., “mild” to SH, “moderate” or “adenomatous” to CH, and “severe” to AH). One pathologist (MES) reviewed all slides, assigned a WHO diagnosis, and chose a single representative slide from each accession. Two experienced pathologists (BMR and OBI) then independently reviewed the selected slides and assigned a WHO diagnosis to each specimen. We combined the three pathologists’ data to assign a single, pathology panel WHO diagnosis based on a standard algorithm of an exact match between at least two of the three reviewers (7). When all three reviewers’ classifications differed, the first reviewer’s (MES’s) classification became the panel WHO diagnosis. For analysis, diagnostic categories were negative (i.e., normal), disordered proliferative endometrium (DPEM; which represents equivocal EH (1;5;18)), SH, CH, AH (almost all of which were CAH), and carcinoma.
For simplicity, we hereafter refer to the participants’ first diagnosis of EH as their index biopsy; to the original KPNW diagnoses of the endometrial biopsy specimens as the community WHO diagnoses; and to the pathology panel diagnoses of the selected slides as the panel WHO diagnoses.
We originally identified 229 potential cases based on their community WHO diagnoses: 188 women who had EH and then carcinoma, plus another 41 women who had EH and then received a community diagnosis of CAH at hysterectomy. After reviewing the original pathology reports, we excluded 15 potential cases who were miscoded as EH or carcinoma. We then excluded the 76 potential cases who had a panel WHO diagnosis of negative (N=55) or carcinoma (N=13) or whose slides were unavailable for review (N=8). This left 138 eligible cases who had a panel WHO diagnoses of DPEM (N=33), SH (N=42), CH (N=21), or AH (N=42).
Controls
We selected controls by first individually matching controls to cases on age at EH, date of EH, and length of follow-up, and then counter-matching controls to cases on the severity of the community WHO diagnosis of EH (i.e., SH, CH, or AH). We identified all women at KPNW who had incident EH at the same age (+/− 1 year) and the same date (+/− 1 year) as the cases but who did not progress to carcinoma and remained at-risk (i.e., remained alive with no hysterectomy or diagnosis of uterine cancer) for an interval at least as long as the progression interval of that case. We identified, on average, 43 potential controls for each case.
To choose 3 controls for each case, we used counter-matching (19) to boost statistical power and ensure that the control group included the full spectrum of EH (Lacey et al., submitted). We selected two controls who had different community WHO diagnoses (i.e., SH, CH, or AH) than the case (e.g., if the case had SH, then we chose 1 control with CH and 1 control with AH). We then oversampled AH by always selecting a third control who had AH. This counter-matching yielded a total of 413 potential controls: 129 with SH (31%), 153 with CH (37%), and 131 with AH (32%) as their community WHO diagnoses.
Controls’ slide review
After retrieving and reviewing the controls’ slides, using the same procedures that were used for the cases’ slides, we excluded 172 controls who had a panel WHO diagnosis of negative (N=160) or carcinoma (N=3) or whose slides were unavailable (N=9). This left 241 eligible controls who had a panel WHO diagnosis of DPEM (N=97), SH (N=67), CH (N=43), or AH (N=34). As was done for the cases, each control was selected based on the community WHO diagnosis but then deemed eligible or ineligible based on the panel WHO diagnosis.
Medical record review
We used a standardized abstract form to extract demographic characteristics, height and weight, reproductive and pregnancy history, other health factors, use of exogenous hormones, and treatment for EH. Risk factors were generally assessed at the time of index biopsy. We supplemented medical record data with linked outpatient pharmacy data (available from 1986 onward).
EIN classification
Two pathologists (GLM and MRN) who did not participate in the WHO pathology review independently reviewed the same original H&E slides that were included in the WHO review from eligible cases and controls (i.e., panel WHO diagnosis of DPEM, SH, CH, or AH). Both reviewers were masked to case-control status, community WHO diagnoses, and panel WHO diagnoses. Each reviewer assigned an EIN main classification (benign, EIN, cancer, or inadequate) and subclassification. When the reviewers did not agree on the main EIN classification, they re-reviewed the slides at a multi-headed microscope to establish a consensus EIN classification. Slides were unavailable for 3 cases and 3 controls and were considered inadequate for diagnosis for 9 cases and 4 controls. An EIN classification (benign, EIN, or cancer) was assigned for 126 cases and 234 controls.
Statistical analysis
To assess concordance between the EIN and WHO panels (e.g., AH vs. EIN and benign vs. non-atypical EH), we calculated unweighted Kappa statistics (K) and standard errors (S.E.) using Stata (StataCorp 2007, Release SE 9.2, College Station, TX) for different combinations of WHO and EIN classifications. Agreement was characterized as slight (0.0–0.2), fair (0.21–0.4), moderate (0.41–0.6), substantial (0.61–0.8), or almost perfect (0.81–1.0).
Conditional logistic regression generated rate ratios (RRs) and 95% confidence intervals (CIs) to estimate the risk of developing endometrial carcinoma based on EIN using SAS software (Cary, NC). “Benign” was the reference group. To assess potential confounding, we evaluated whether EIN was associated with medical record data. The final regression models included sampling weights for both the batch-quota and counter-matched sampling, which were included as an offset in standard conditional logistic regression models (20), and were adjusted for age at index biopsy (1-year intervals), date of index biopsy (1-year intervals), and duration of follow-up (in days).
The RRs measure associations between biopsy diagnoses and subsequent cancer, but strong associations (i.e., high RRs) do not mean that biopsy diagnoses can effectively predict which patients will progress to carcinoma (21). We therefore calculated sensitivity and specificity for predicting progression status (i.e., case or control) based on the biopsy classifications.
To assess the degree to which WHO and EIN classifications complemented each other in their association with outcome, we performed post hoc analyses of risk associated with joint classification. This analysis focused on discordant risks (e.g., AH and benign, or SH and EIN) to evaluate whether joint classification was associated with progression.
Human subjects
The KPCHR’s Research Subjects Protection Office and the National Cancer Institute’s Special Studies IRB approved this study.
RESULTS
Table 1 presents the EIN panel, community WHO, and panel WHO diagnoses among the cases and controls with a panel WHO diagnosis of DPEM or EH. Thirteen (9%) of the 138 cases and 10 (4%) of the 241 controls had an index biopsy that was classified as cancer by the EIN panel. Among the remaining 113 cases and 221 controls, 42 cases (37%) and 65 controls (29%) were classified as EIN.
Table 1.
Classification of index biopsies: community WHO vs. EIN and panel WHO
| EIN Classification
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases
|
Controls
|
|||||||||
| Inadequate | Benign | EIN | Cancer | Total | Inadequate | Benign | EIN | Cancer | Total | |
| EH | ||||||||||
| Classification | ||||||||||
| Community WHO | ||||||||||
| DPEM | 1 (4%) | 16 (64%) | 4 (16%) | 3 (12%) | 25 | - | - | - | - | - |
| SH | 5 (10%) | 26 (50%) | 16 (31%) | 5 (10%) | 52 | 3 (5%) | 52 (85%) | 5 (8%) | 1 2%) | 61 |
| CH | 2 (5%) | 22 (50%) | 16 (36%) | 2 (5%) | 44 | 1 (1%) | 63 (63%) | 29 (29%) | 5 (5%) | 100 |
| AH | 1 (6%) | 7 (41%) | 6 (35%) | 3 (18%) | 17 | 0 (0%) | 44 (55%) | 31 (39%) | 4 5%) | 80 |
| Total | 9 (7%) | 71 (51%) | 42 (30%) | 13 (9%) | 138 | 4 (2%) | 159 (66%) | 65 (27%) | 10 (4%) | 241 |
|
| ||||||||||
| Cases
|
Controls
|
|||||||||
| Inadequate | Benign | EIN | Cancer | Total | Inadequate | Benign | EIN | Cancer | Total | |
|
| ||||||||||
| Panel WHO | ||||||||||
| DPEM | 3 (9%) | 29 (88%) | 1 (3%) | 0 (0%) | 33 | 4 (4%) | 84 (87%) | 6 (6%) | 1 (1%) | 95 |
| SH | 2 (5%) | 32 (78%) | 5 (12%) | 2 (5%) | 41 | 0 (0%) | 60 (90%) | 7 (10%) | 0 (0%) | 67 |
| CH | 2 (10%) | 1 (5%) | 15 (71%) | 2 (10%) | 20 | 0 (0%) | 12 (28%) | 23 (53%) | 7 (16%) | 42 |
| AH | 2 (5%) | 9 (21%) | 21 (49%) | 9 (21%) | 41 | 0 (0%) | 3 (9%) | 29 (85%) | 2 (6%) | 34 |
| Total | 9 (7%) | 71 (51%) | 42 (30%) | 13 (9%) | 135 | 4 (2%) | 159 (66%) | 65 (27%) | 10 (4%) | 238 |
DPEM, disordered proliferative endometrium. SH, simple hyperplasia. CH, complex hyperplasia. AH, atypical hyperplasia. EIN, endometrial intraepithelial neoplasia.
Community WHO refers to the original EH diagnoses at KPNW (1970–2003). EIN and Panel WHO were independently assigned by separate panels of gynecologic pathologists.
Cases with community WHO diagnoses of DPEM were eligible for inclusion, but all controls had a community WHO diagnosis of SH, CH, or AH.
Slides from 3 cases and 3 controls were not available for review.
Row percentages might not sum to 100 because of rounding.
There was moderate agreement (K=0.47, SE=0.05) between EIN (benign vs. EIN) and collapsed WHO (DPEM, SH, or CH vs. AH) classifications. Of the 41 cases and 34 controls with a panel WHO diagnosis of AH, 21 (51.2%) and 29 (85.3%), respectively, were classified as EIN. Using either community WHO or panel WHO diagnosis, almost all of the patients called DPEM or SH were called benign by the EIN panel. The correlation between EIN and panel WHO was better than the correlation between EIN and community WHO.
Our matched design generated similar ages and dates at index biopsy and progression intervals for cases and controls. There were no statistically significant case-control differences in number of follow-up biopsies or treatment (data not shown).
EIN status was statistically significantly associated with irregular menses, gravidity, BMI, diabetes, and hormone therapy use at index biopsy and diagnosis or censor date (Table 2). For all of those factors except diabetes, the associations remained statistically significant after excluding the 23 patients (13 cases and 10 controls) whose index biopsies were called cancer by the EIN panel (data not shown). Similar associations were observed for AH vs. DPEM (7).
Table 2.
Associations between selected clinical or reproductive factors and EIN status at index biopsy
| EIN Classification
|
|||||||
|---|---|---|---|---|---|---|---|
| Benign | EIN | Cancer | |||||
| N | % | N | % | N | % | P-value | |
| Menarche (in years) | 0.30 | ||||||
| <12 | 48 | 17% | 12 | 4% | 2 | 1% | |
| 12–13 | 95 | 33% | 53 | 19% | 8 | 3% | |
| 14+ | 45 | 16% | 17 | 6% | 4 | 1% | |
| Ever had irregular menses | 0.02 | ||||||
| Yes | 110 | 56% | 54 | 27% | 8 | 3% | |
| No | 19 | 10% | 6 | 2% | 0 | 0% | |
| Ever pregnant | 0.007 | ||||||
| No | 19 | 5% | 23 | 6% | 5 | 1% | |
| Yes | 211 | 59% | 84 | 23% | 18 | 5% | |
| No. of live births | 0.30 | ||||||
| 0–1 | 40 | 11% | 21 | 6% | 4 | 1% | |
| 2–3 | 115 | 32% | 53 | 15% | 11 | 3% | |
| 4+ | 75 | 21% | 33 | 9% | 8 | 2% | |
| BMI (body mass index; kg/m2) | 0.02 | ||||||
| <25 | 58 | 17% | 26 | 8% | 8 | 2% | |
| 25–29 | 53 | 15% | 17 | 5% | 4 | 1% | |
| 30–34 | 46 | 14% | 10 | 3% | 4 | 1% | |
| 35–39 | 30 | 9% | 13 | 4% | 2 | 1% | |
| 40+ | 26 | 8% | 34 | 10% | 4 | 1% | |
| Menopausal status at index biopsy | 0.87 | ||||||
| Pre/peri | 151 | 43% | 66 | 19% | 8 | 2% | |
| Postmenopausal | 75 | 21% | 36 | 10% | 15 | 4% | |
| Age at menopause (in yrs) | 0.87 | ||||||
| <=45 | 11 | 3% | 7 | 2% | 0 | 0% | |
| 46–52 | 37 | 11% | 16 | 5% | 8 | 2% | |
| 52+ | 23 | 7% | 8 | 2% | 6 | 2% | |
| Ever had diabetes | 0.03 | ||||||
| No | 158 | 52% | 66 | 22% | 15 | 5% | |
| Yes | 39 | 13% | 24 | 9% | 4 | 1% | |
| Smoking status at index biopsy | 0.87 | ||||||
| Never | 134 | 40% | 61 | 18% | 10 | 3% | |
| Former | 51 | 15% | 22 | 7% | 6 | 2% | |
| Current | 26 | 8% | 20 | 6% | 4 | 1% | |
| Oral contraceptive use | 0.08 | ||||||
| Never | 137 | 42% | 61 | 19% | 18 | 6% | |
| Ever | 69 | 21% | 34 | 11% | 4 | 1% | |
| HT use at index biopsy | <0.0001 | ||||||
| Never | 96 | 31% | 44 | 14% | 11 | 4% | |
| Former | 41 | 13% | 21 | 7% | 3 | 1% | |
| Current | 62 | 20% | 27 | 9% | 7 | 2% | |
| HT use at diagnosis/censor | <0.0001 | ||||||
| Never | 28 | 8% | 19 | 6% | 6 | 2% | |
| Former | 99 | 29% | 42 | 12% | 4 | 1% | |
| Current | 92 | 27% | 42 | 12% | 11 | 3% | |
| Type of treatment for EH | 0.48 | ||||||
| None | 28 | 8% | 4 | 1% | 2 | 1% | |
| MPA-based | 202 | 56% | 103 | 29% | 21 | 6% | |
HT, menopausal hormone therapy. MPA, medroxyprogesterone acetate.
P-value <0.05 indicates a statistically significant association between that clinical or reproductive factor and EIN classification.
Table combines 126 cases and 234 controls with EIN classification of benign, EIN, or cancer.
Table 3 shows the progression risks associated with the panel WHO and EIN classifications. As described in our previous publication (7), using a panel diagnosis of DPEM as the reference, non-atypical EH (SH or CH) only slightly increased the risk of subsequent carcinoma (RR=2.24, 95% CI, 1.08–4.46), whereas AH strongly increased the risk of subsequent carcinoma (RR=14.21, 95% CI, 5.32–37.96).
Table 3.
Cancer risks by EIN status and pathology-panel-based WHO classifications
| Cases N | Controls N | RR | 95% CI | |
|---|---|---|---|---|
| Panel WHO classification | ||||
| DPEM | 33 | 97 | 1.00 | Ref. |
| Non-atypical EH (SH or CH) | 62 | 110 | 2.24 | 1.08–4.66 |
| AH | 43 | 34 | 14.21 | 5.32–37.96 |
| Collapsed categories: | ||||
| DPEM or non-atypical EH | 95 | 207 | 1.00 | Ref. |
| AH | 43 | 34 | 9.19 | 3.87–21.83 |
| EIN classification | ||||
| Benign | 71 | 159 | 1.00 | Ref. |
| EIN | 42 | 65 | 7.76 | 3.36–17.91 |
| Cancer | 13 | 10 | 17.11 | 4.18–70.10 |
| Collapsed categories: | ||||
| Benign | 71 | 159 | 1.00 | Ref. |
| EIN or cancer | 55 | 75 | 8.95 | 4.06–19.74 |
RRs are based on conditional logistic regression analysis, weighted based on the sampling methods used to select controls. The RRs represent the increased probability of developing carcinoma associated with that EH or EIN classification compared with the probability of develop carcinoma associated with the reference category (i.e., WHO=DPEM, WHO=SH, EIN=benign, or some combination thereof).
Non-atypical EH = SH or CH.
RRs are adjusted for age at index biopsy, date of index biopsy, interval between EH and carcinoma (for cases) or matched censoring date (for controls), BMI at the time of EH diagnosis, MPA-based treatment for EH, and follow-up biopsies.
For EIN classification, 9 cases and 4 controls were judged to be inadequate for EIN classification (RR=2.19, 95% CI, 0.44–10.82). Another 3 case and 3 control slides were not available for review.
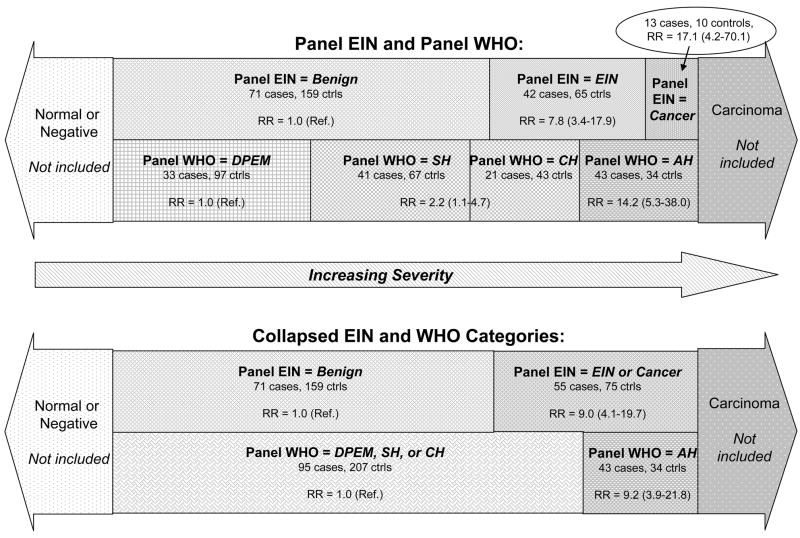
The RR for EIN, compared with benign, was 7.76 (95% CI, 3.36–17.91). The RR for an index biopsy that was called cancer by the EIN panel was 17.11 (95% CI, 4.18–70.10), based on 13 cases and 10 controls. Combining these patients with the EIN group produced an RR of 8.95 (95% CI, 4.06–19.74) for EIN-or-cancer (55 cases, 75 controls), compared with benign. Figure 1 shows the overlap between those combinations and their respective RRs. Excluding these 13 cases and 10 controls from the progression risks based on the panel WHO produced RRs for non-atypical EH (2.25, 95% CI, 1.07–4.73) or AH (10.66, 95% CI, 3.73–30.47) that were similar to the RRs based on 138 cases and 241 controls. Note that the slides from another 13 cases (6.1% of all potential cases) and 3 controls (0.7% of all potential controls) whose index biopsies were called cancer by the WHO panel (7) were not included in the EIN diagnostic review and are not included in any of the analyses presented here.
Figure 1. Schematic comparison of progression risks for EIN and WHO.
RR, relative risk; see table 3. The area of the categories is proportional to the total number of cases and controls in each category, relative to the 138 eligible cases and 241 eligible controls.
Combining diagnoses from both systems did not produce associations that notably differed from the individual associations with EIN or AH. Compared with a reference group of DPEM, the progression risks for index biopsies called AH, EIN, or cancer (64 cases and 71 controls; RR=9.88, 95% CI, 4.65–21.01) or AH and EIN (21 cases and 29 controls; RR=8.29, 95% CI, 53–27.21) were similar to the progression risks for AH or EIN shown in table 3. The progression risk for AH did not differ after further stratification by EIN status, but only 9 cases and 3 controls with a panel WHO diagnosis of AH had an EIN classification of benign (data not shown).
The sensitivity and specificity for AH (vs. DPEM, SH, or CH) were 31% (95% CI, 21%–40%) and 86% (95% CI, 81%–90%), respectively. For EIN vs. benign, sensitivity was 37% (95% CI, 28%–47%) and specificity was 71% (95% CI, 64%–77%). When the 23 cases whose index biopsies were called cancer were included (i.e., EIN-or-cancer vs. benign), sensitivity (44%, 95% CI, 35%–53%) increased but specificity (68%, 95% CI, 62%–74%) declined.
DISCUSSION
By enlisting two panels of gynecologic pathologists to independently review the same original diagnostic slides from women with who clinically progressed to carcinoma and matched controls who did not progress, our analysis provides useful evidence on the association between diagnosis of EIN at endometrial biopsy and subsequent cancer risk. The EIN and WHO systems use different criteria and numbers of categories to classify endometrial lesions, but the progression risk for dichotomous EIN (RR=7.76 for EIN vs. benign) was quantitatively similar to the progression risk for a dichotomous WHO scheme (RR=9.19 for AH vs. DPEM, SH, or CH). Thus, both systems identified women whose endometrial biopsies reflect an almost ten-fold higher risk of progressing to carcinoma. Both review panels generally agreed on what constituted a low-risk lesion; index biopsies called DPEM or SH by our WHO panel were almost always called benign by our EIN panel. This suggests that both systems identify key features (i.e., normal-appearing architecture and nuclei) that characterize low-risk lesions.
AH was more strongly associated with carcinoma when compared with DPEM only (RR=14.21) than with DPEM, SH, and CH together (RR=9.19) because the latter used a broader reference (i.e., comparison) group that included everything from the equivocal DPEM to CH lesions with severe architectural complexity. We previously concluded (7) that the strong association between progression and AH (but not SH or CH) could justify treatment decisions based on a non-atypical EH vs. AH dichotomy. The similar association between EIN and carcinoma (RR=7.76 for EIN vs. benign) could provide support for a similar approach to clinical management for EIN (12). Although the relative risks were nearly identical, the two systems had different strengths when predicting subsequent progression to carcinoma. EIN had a higher sensitivity than AH (37% vs. 31%, respectively) but a lower specificity (71% vs. 86%, respectively). As applied by our pathology panels in this dataset, both systems better predicted which lesions did not progress (i.e., high specificity) than which lesions did progress (i.e., low sensitivity). However, given the sampling errors inherent in endometrial biopsy specimens and the unpredictable natural history of endometrial precursors, it might be unrealistic to expect any classification system to possess both high sensitivity and specificity.
Secretory endometrium, basalis, polyps, or other benign lesions that mimic glandular proliferative lesions can be misclassified under both WHO (i.e., as EH) and EIN (i.e., as EIN) classification systems (12). Because all EIN lesions are also considered EH (12), we excluded patients who had an index biopsy that our WHO panel classified as being less severe than DPEM. This removed many of the benign lesions from the EIN diagnostic review. The overall severity of our benign group is therefore higher than the severity of benign groups in other EIN studies that include patients whose endometrial biopsies would have been classified as less-than-EH. Our results on progression risk associated with EIN should therefore be generalized only to women whose community WHO diagnoses are likely to be considered DPEM or EH and who remain at-risk for at least one year after their index biopsy. Our results are not necessarily generalizable to all women who undergo endometrial biopsy, particularly women whose community WHO diagnoses overestimate their true lesion severity (5) or who have occult carcinoma at the time of their EH is diagnosed (22).
At the other end of the spectrum, EIN is a premalignant lesion distinct from carcinoma. Distinguishing a severe premalignant lesion from carcinoma is especially difficult with biopsy specimens (13), but the EIN panel felt that 13 cases and 10 controls had cancer at their index biopsies. Nine of these 13 cases (69%) had a panel WHO diagnosis of AH; on average, they were diagnosed with carcinoma 4.2 years (range, 1.2–11.1 years) after their index biopsy. For 7 of these 13 cases, at least one of the WHO panelists interpreted the biopsy as mucinous carcinoma, which can be difficult to recognize (23). Two of these 10 controls had a panel WHO diagnosis of AH and 7 had CH. The average disease-free interval after EH for these 10 controls was 10.6 years (range, 2.4–17.1 years). Thresholds for distinguishing precursors (e.g., AH or EIN) from carcinoma in biopsies vary among pathologists and by classification system, but all of these lesions warrant close follow-up. A bigger challenge may be how to evaluate and manage the larger group of women with less severe abnormalities. Variable thresholds across schema will affect how patients are clinically managed, precursors are studied, and performance of classification systems is evaluated.
In our analysis, EIN diagnoses were more common (29% of all index biopsies) than AH diagnoses (20% of all index biopsies), especially among controls (i.e., N=65 EIN vs. N=34 AH). These factors tended to reduce the progression risk and specificity associated with EIN relative to those for AH. The RR of 7.76 for EIN in our study was much lower than the relative risk of 45 for EIN in a study of 477 women who originally received a community diagnosis of EH and were followed for at least one year by Baak et al (16). In contrast to previous studies, our report included a matched control group with equivalent progression risk who received similar treatment and had complete clinical follow-up data available. Our two pathology panels included experienced gynecologic pathologists who independently reviewed the original slides and were masked to case-control status and clinical outcomes. The higher prevalence of EIN than AH among our study’s controls indicates that the true progression risk for EIN relative to benign lesions is much closer to the RR of 10 that we observed, because EIN would need to have nearly perfect predictive power among women who progressed in order to generate the 45-fold increased RR of 45 that has been previously reported (16). Other strengths of our study, such as the ability to control for treatment, repeat biopsies, and other confounders, lend further validity to our findings.
Our study possesses some limitations. Few women with severe EH were followed for long intervals, so statistical power for some regression models was low. The wide confidence intervals for the risk estimates are a result of the conditional logistic regression and careful individual matching on age, length of follow-up, and calendar period. Some of the older slides were in suboptimal condition, and all endometrial biopsies suffer from tissue sampling variability. All of these issues would equally affect both the WHO and EIN classifications. We did not incorporate an intra-observer reproducibility analysis for the EIN classifications. Reported reproducibility is high, however (16). Although we had both community and panel WHO classifications, our study did not include EIN classifications that were assigned by community pathologists.
We designed this analysis to test the association between EIN classification at index biopsy and subsequent risk of carcinoma. For practical reasons, we selected patients using a modified WHO classification system; we combined SAH and CAH because too few women at KPNW had index biopsies that were classified as SAH by the community pathologists or our pathology review panel to consider SAH and CAH as separate entities for analysis or when selecting counter-matched controls. Such modifications commonly occur in clinical practice and are not surprising because assigning a WHO classification requires pathologists to subjectively weight the relative importance of multiple histologic criteria (8). Those challenges might contribute to better correlation between our WHO and EIN panel diagnoses than between either panel and the community WHO diagnoses, although the changing WHO nomenclature used over the 34-year period of our data collection also contributed to those differences (Sherman ME, et al., In press). Our results show that, despite different formal classification criteria and application of those criteria, high-risk lesions within dichotomous WHO (AH vs. non-atypical EH or DPEM) and EIN (EIN vs. benign) classification systems had similar associations with subsequent carcinoma. Dichotomous systems would simplify classification of endometrial biopsies and subsequent clinical management, but simply giving pathologists fewer choices would not eliminate the difficult task of assigning a single classification to lesions that are neither entirely benign nor obviously severe.
Problems with the WHO classification system persist, in part, because of the low reproducibility of EH in both community and research settings (8). Comparable evaluations of EIN reproducibility among community pathologists, plus additional population-based studies of EIN in diverse clinical settings, are needed in order to continue to evaluate the performance of EIN compared with existing application of WHO-based critera. We observed that the progression risk associated with EIN is lower than what has previously been reported and quantitatively similar to the progression risk associated with AH when both systems are applied by experienced pathologists as we have done here. Continued efforts to improve histopathologic diagnosis of endometrial biopsies could potentially lead to improved clinical management.
Acknowledgments
We thank Stella Munuo, MSc, and Ruth Parsons, BA, at IMS, Inc., for data management. We thank J. Danny Carreon, MPH, at the Division of Cancer Epidemiology and Genetics, NCI, for technical assistance.
We thank Kris Bennett, Chris Eddy, BS, Beverly Battaglia, and the rest of the KPCHR staff.
The National Cancer Institute’s intramural research program (Division of Cancer Epidemiology and Genetics) funded this study.
References
- 1.Mazur MT. Endometrial hyperplasia/adenocarcinoma a conventional approach. Ann Diagn Pathol. 2005;9:174–81. doi: 10.1016/j.anndiagpath.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery BE, Daum GS, Dunton CJ. Endometrial hyperplasia: a review. Obstet Gynecol Surv. 2004;59:368–78. doi: 10.1097/00006254-200405000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg SG, Mutter GL, Kurman RJ, Kubik-Huch RA, Nogales F, Tavassoli FA. Tumors of the uterine corpus: epithelial tumors and related lesions. In: Tavassoli FA, Stratton MR, editors. WHO Classification of Tumors: Pathology and Genetics of Tumors of the Breast and Female Genital Organs. Lyon, France: IARC Press; 2003. pp. 221–32. [Google Scholar]
- 4.Wells M. Hyperplasias of the endometrium. In: Gershenon DM, McGuire WP, Gore M, Quinn MA, Thomas G, editors. Gynecologic Cancer: Controversies in Management. Philadelphia, PA: Elsevier Churchill Livingstone; 2004. pp. 249–57. [Google Scholar]
- 5.Silverberg SG. Problems in the differential diagnosis of endometrial hyperplasia and carcinoma. Mod Pathol. 2000;13:309–27. doi: 10.1038/modpathol.3880053. [DOI] [PubMed] [Google Scholar]
- 6.Clark TJ, Neelakantan D, Gupta JK. The management of endometrial hyperplasia: an evaluation of current practice. Eur J Obstet Gynecol Reprod Biol. 2006;125:259–64. doi: 10.1016/j.ejogrb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Lacey JV, Jr, Ioffe OB, Ronnett BM, Rush BB, Richesson DA, Chatterjee N, et al. Endometrial carcinoma risk among women diagnosed with endometrial hyperplasia: the 34-year experience in a large health plan. Br J Cancer. 2007 doi: 10.1038/sj.bjc.6604102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaino RJ. Endometrial hyperplasia: is it time for a quantum leap to a new classification? Int J Gynecol Pathol. 2000;19:314–21. doi: 10.1097/00004347-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Skov BG, Broholm H, Engel U, Franzmann MB, Nielsen AL, Lauritzen AF, et al. Comparison of the reproducibility of the WHO classifications of 1975 and 1994 of endometrial hyperplasia. Int J Gynecol Pathol. 1997;16:33–7. doi: 10.1097/00004347-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Sherman ME, Ioffe OB, Ronnett BM, Richesson D, Rush BB, Glass AG, et al. Reproducibility of biopsy diagnoses of endometrial hyperplasia: evidence supporting a simplified classification. Int J Gyn Path. 2007 doi: 10.1097/PGP.0b013e3181659167. In press. [DOI] [PubMed] [Google Scholar]
- 11.Dietel M. The histological diagnosis of endometrial hyperplasia. Is there a need to simplify? Virchows Arch. 2001;439:604–8. doi: 10.1007/s004280100503. [DOI] [PubMed] [Google Scholar]
- 12.Baak JP, Mutter GL. EIN and WHO94. J Clin Pathol. 2005;58:1–6. doi: 10.1136/jcp.2004.021071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutter GL. Endometrial intraepithelial neoplasia (EIN): will it bring order to chaos? The Endometrial Collaborative Group. Gynecol Oncol. 2000;76:287–90. doi: 10.1006/gyno.1999.5580. [DOI] [PubMed] [Google Scholar]
- 14.Hecht JL, Ince TA, Baak JP, Baker HE, Ogden MW, Mutter GL. Prediction of endometrial carcinoma by subjective endometrial intraepithelial neoplasia diagnosis. Mod Pathol. 2005;18:324–30. doi: 10.1038/modpathol.3800328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutter GL, Zaino RJ, Baak JP, Bentley RC, Robboy SJ. Benign endometrial hyperplasia sequence and endometrial intraepithelial neoplasia. Int J Gynecol Pathol. 2007;26:103–14. doi: 10.1097/PGP.0b013e31802e4696. [DOI] [PubMed] [Google Scholar]
- 16.Baak JP, Mutter GL, Robboy S, Van Diest PJ, Uyterlinde AM, Orbo A, et al. The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer. 2005;103:2304–12. doi: 10.1002/cncr.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner EH, Greene SM, Hart G, Field TS, Fletcher S, Geiger AM, et al. Building a research consortium of large health systems: the Cancer Research Network. J Natl Cancer Inst Monogr. 2005:3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 18.McCluggage WG. My approach to the interpretation of endometrial biopsies and curettings. Journal of Clinical Pathology. 2006;59:801–12. doi: 10.1136/jcp.2005.029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langholz B, Clayton D. Sampling strategies in nested case-control studies. Environ Health Perspect. 1994;102(Suppl 8):47–51. doi: 10.1289/ehp.94102s847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langholz B, Goldstein L. Conditional logistic analysis of case-control studies with complex sampling. Biostatistics. 2001;2:63–84. doi: 10.1093/biostatistics/2.1.63. [DOI] [PubMed] [Google Scholar]
- 21.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–90. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 22.Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812–9. doi: 10.1002/cncr.21650. [DOI] [PubMed] [Google Scholar]
- 23.Nucci MR, Prasad CJ, Crum CP, Mutter GL. Mucinous endometrial epithelial proliferations: a morphologic spectrum of changes with diverse clinical significance. Mod Pathol. 1999;12:1137–42. [PubMed] [Google Scholar]