Abstract
The cytokine IL-1 mediates diverse forms of neurodegeneration, but its mechanism of action is unknown. We have demonstrated previously that exogenous and endogenous IL-1 acts specifically in the rat striatum to dramatically enhance ischemic and excitotoxic brain damage and cause extensive cortical injury. Here we tested the hypothesis that this distant effect of IL-1 is mediated through polysynaptic striatal outputs to the cortex via the hypothalamus. We show that IL-1β injected into the rat striatum with the excitotoxin α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (S-AMPA) caused increased expression of IL-1β (mRNA and protein) mainly in the cortex where maximum injury occurs. Marked increases in IL-1β mRNA and protein were also observed in the hypothalamus. S-AMPA, injected alone into the striatum, caused only localized damage, but administration of IL-1β into either the striatum or the lateral hypothalamus immediately after striatal S-AMPA resulted in widespread cell loss throughout the ipsilateral cortex. Finally we showed that the cortical cell death produced by striatal coinjection of S-AMPA and IL-1β was significantly reduced by administration of the IL-1 receptor antagonist into the lateral hypothalamus. These data suggest that IL-1β can act in the hypothalamus to modify cell viability in the cortex. We conclude that IL-1-dependent pathways project from the striatum to the cortex via the hypothalamus and lead to cortical injury, and that these may contribute to a number of human neurological conditions including stroke and head trauma.
Excessive activation of glutamate receptors (excitotoxicity) contributes to a number of neurodegenerative disorders (1). Thus direct injection of glutamatergic agonists into the brains of experimental animals has proved a useful model to study, in vivo, the underlying mechanisms of cell death in such acute conditions. There is increasing interest in the role of inflammatory mediators such as IL-1 in cell death in this and other forms of neurodegeneration, such as cerebral ischemia (2).
The cytokine IL-1 has diverse actions in the brain and mediates acute experimental neurodegeneration (3). Injection or overexpression of IL-1 receptor antagonist (IL-1ra) significantly inhibits neuronal damage induced in rodents by focal cerebral ischemia, traumatic brain injury, or excitotoxin administration (see ref. 2). Administration of exogenous IL-1β fails to cause neuronal death in normal rat brain but markedly exacerbates ischemic, traumatic, or excitotoxic brain injury (4–6). When IL-1β is coinjected with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (S-AMPA) in the rat striatum, extensive cell death occurs throughout the ipsilateral cortex, which is not observed after S-AMPA alone (6). The mechanism of this potent effect of IL-1β is not known, although in focal cerebral ischemia, exacerbation by IL-1β and neuroprotective actions of IL-1ra are also mediated through actions in the striatum that influence cortical damage (7). Because excitotoxicity contributes to many forms of neurodegeneration, understanding the interactions between IL-1β and S-AMPA that lead to cortical death could be important for a number of acute conditions.
Effects of IL-1β on AMPA-receptor-mediated cell death in the striatum appear to be “all or nothing.” We have demonstrated that certain regions are more responsive, i.e., a greater frequency of cortical damage is seen after S-AMPA plus IL-1β treatment. These positive sites include the ventral striatum and shell of the nucleus accumbens (R. I. Grundy, N.J.R., and S.M.A., unpublished data), which show quite distinct neuronal projections to other brain areas when compared with other striatal sites of injection where IL-1β is relatively ineffective. Both regions have connections with the limbic system (8) and the hypothalamus (9). The hypothalamus is a known site of IL-1 action on fever, food intake, and various endocrine responses (3, 10), so connections to this brain area from the ventral striatum and nucleus accumbens could contribute to the observed effects of IL-1β on cell death. The hypothalamus has afferent connections with the entire cortical mantle, either directly, or indirectly via the basal ganglia and related structures such as the thalamus and amygdala (9, 11) and could thus influence cortical cell excitability and survival.
The aim of this study was to test the hypothesis that effects of IL-1β on excitotoxic cell death in the striatum are mediated through polysynaptic striatal outputs via the hypothalamus. To do this, we measured expression of IL-1β (mRNA and protein) in the striatum, cortex, and hypothalamus after striatal injections of S-AMPA alone and together with IL-1β. The functional importance of hypothalamic IL-1β was investigated by defining whether direct hypothalamic injection of IL-1β or IL-1ra affected the damage produced by striatal administration of S-AMPA alone or with IL-1β.
Materials and Methods
Experimental Treatments.
The excitotoxin S-AMPA (Tocris Neuramin, Bristol, U.K.) was dissolved in sterile PBS (0.1 M) to the required concentration (15 mM). Human recombinant IL-1β (DuPont) and human recombinant IL-1ra (PeproTech, Rocky Hill, NJ) were dissolved in sterile 0.9% saline containing 0.1% BSA (Sigma). The concentration of S-AMPA used was based on dose-response studies (data not shown) and produces a submaximal and reproducible lesion within the striatum and, together with hrIL-1β, consistently results in cortical damage after striatal coinjection. Doses of hrIL-1β are based on previous studies demonstrating the effectiveness of this concentration on AMPA-receptor-mediated responses (6, 12) and induction of fever (13).
Brain Injections.
Male Sprague–Dawley rats (250–350 g, Charles River Breeding Laboratories) were anaesthetized with halothane (2.5% in 100% O2; AstraZeneca, Macclesfield, U.K.) and placed in a stereotaxic frame (Stoelting). Stereotaxic injections were performed as described previously (6) into the striatum [coordinates relative to bregma (mm): anterior–posterior (AP) +0.7, medial–lateral (ML) −2.7, dorsal–ventral (DV) −5.5], or lateral hypothalamus (AP −3,6, ML −1.8, DV −8.4) (14). All injections were performed at a rate of 0.5 μl/min with the needle left in place for a further 4–5 min to allow for diffusion of the injected solution. The striatal site used in this study was based on that used in previous reports on the effect of S-AMPA and IL-1β coinjections in the striatum (6).
Reverse Transcription–PCR.
Expression of IL-1β mRNA was studied within the striatum, cortex, and hypothalamus in animals injected with 0.1%BSA/saline + PBS (vehicle), S-AMPA (7.5 nmol) + 0.1% BSA/saline, hrIL-1β (10 ng) + PBS, or hrIL-1β (10 ng) + S-AMPA (7.5 nmol) in the striatum or subjected to sham operation (insertion of needle alone). Animals were injected with an overdose of sodium pentobarbitone (Sagatal; Rhoné Meriex, U.K.) 3 or 8 h after treatment and transcardially perfused with ice-cold saline (diethyl pyrocarbonate treated) for 10 min. Appropriate brain regions were dissected free and snap frozen in isopentane (−35°C) on dry ice by using RNase-free conditions. Total RNA was extracted from the tissue by using the TRIzol extraction kit (GIBCO/BRL). RNA concentration was determined by spectrophotometry at 260 nm. RNA (1 μg) was reverse transcribed by using the First Strand cDNA Synthesis kit (Amersham Pharmacia). PCR amplification of an aliquot (3 μl) of cDNA was undertaken by the addition of 250 ng each of the IL-1β-specific primers βFOR and βREV (Table 1), 2.0 units of Taq DNA polymerase (Perkin–Elmer), 10 mM of each dNTP (Boehringer Mannheim), and PCR-compatible buffer in a total volume of 50 μl. PCR cycling conditions for IL-1β were: an initial hot start (3 min at 95°C) followed by denaturation at 95°C for 30 sec, annealing at 56°C, extension at 72°C for 1 min for 36 cycles, and a 10-min final extension period at 72°C. A competitive PCR standard was synthesized according to the method of Celi et al. (15). Briefly, the 335-bp PCR product was used as a starting template in an additional PCR reaction driven by primers βREV and βST (Table 1) giving a PCR product 308 bp in length, which is shorter than the conventional product but contains sites that allow it to be amplified by the original primers. The 308-bp product was purified and cRNA copies (βst) produced (16) and diluted to 10 ng/μl. Quantification was carried out with 10–1,000 pg of standard cRNA. The results, determined by comparison with the competitive standard, were analyzed by densitometry from negatives by using image analysis software (Scion, Frederick, MD) and expressed as picograms/micrograms of total RNA.
Table 1.
Primer sequences for PCR
| Primer | Sequence |
|---|---|
| βFOR | 5′-GACAGAACATAAGCCAACAAG-3′ |
| βREV | 5′-GTCAACTATGTCCCGACCATT-3′ |
| βST | 5′-GACAGAACATAAGCCAACAAGTCAAGGGGTTGAATCTATAC-3′ |
ELISA.
Immunoreactive IL-1β levels were determined by rat-specific ELISA in animals treated as described above. At specific time points (3 and 8 h) after injection, animals were killed by an overdose of halothane anesthesia followed by cervical dislocation. Appropriate brain regions were dissected free and snap frozen in isopentane (−35°C) on dry ice.
Brain samples were placed in sterile PBS containing a protease inhibitor mixture [0.2 mM 4-(2-aminoethyl)benzenesulfonyl fluoride/1 μg/ml aprotinin, 1 mM benzamidine/1 mM EDTA/10 μg/ml leupeptin/10 μg/ml pepstatin], homogenized, centrifuged (11,000 rpm, 20 min, 4°C), (Heraeus Biofuge Stratos) and the supernatant removed. The supernatant was diluted in high-performance buffer (HPE; Central Laboratory for Blood Transfusion, Amsterdam) and assayed for immunoreactive IL-1β by rat-specific sandwich ELISA (17). Protein concentrations of all samples were measured by the Bradford method and cytokine levels expressed as picograms/micrograms of protein. The average detection limit in brain homogenate was 70 pg/ml, and the inter- and intra-assay coefficients of variation were 19% and 5%, respectively.
Histological Analysis.
Animals were injected either with S-AMPA (7.5 nmol) in the striatum, followed by 0.1% BSA/saline or hrIL-1β (0.1, 1 or 10 ng) in the lateral hypothalamus (LH), or with hrIL-1β (10 ng) plus S-AMPA (7.5 nmol) in the striatum, followed by 0.1% BSA/saline or hrIL-1ra (0.1, 1, or 10 μg) in the LH. Animals were killed by an overdose of halothane anesthesia followed by cervical dislocation 48 h after injection, and the brains were removed and snap frozen in isopentane (−35°C) on dry ice. The total lesion volume was determined from coronal cryostat sections stained with cresyl fast violet as described previously (6). The lesion volume (a measure of gross infarction) was used to assess cell death after treatments. Cortical lesions were assessed over an anterior–posterior distance of approximately 8 mm, i.e., from sections corresponding to coronal levels from + 2.7 mm to −4.8 mm (14).
Statistical Analysis.
All data are expressed as mean ± SEM for the number of animals given. Significant differences between two groups of animals were assessed by a Student's t test for unpaired data (with Welch's correction when the variances between groups were significantly different), whereas differences between more than two groups were assessed by analysis of variance followed by a Tukey–Kramer multicomparisons test. Statistical significance assumed a probability of less than 5%.
Results
Striatal injection of vehicle, hrIL-1β, or S-AMPA + hrIL-1β produced a significant increase in IL-1β mRNA in the striatum 3 h after injection, when compared with the S-AMPA-treated animals (Table 2). In contrast, 8 h after injection, no marked differences in striatal IL-1β mRNA expression were observed between any of the treatments (Table 2). Sham-operated animals expressed similar IL-1β mRNA levels to the treated-groups, and in all cases these were significantly greater after both 3 h and 8 h than constitutive IL-1β mRNA expression in naïve animals (data not shown). This increase in sham-operated rats was most likely because of a local inflammatory reaction in response to placement of the injection needle within the striatum.
Table 2.
IL-1β mRNA expression
| Treatment | Striatum | Cortex | Hypothalamus |
|---|---|---|---|
| After 3 h | |||
| Sham | 80 ± 6 | 77 ± 1 | 10 ± 1 |
| Vehicle | 100 ± 2***,†† | 86 ± 1 | 13 ± 1 |
| S-AMPA | 75 ± 2 | 84 ± 3 | 11 ± 1 |
| hrIL-1β | 101 ± 5***,†† | 98 ± 3**,†††,‡ | 91 ± 4***,†††,‡‡‡ |
| S-AMPA + hrIL-1β | 92 ± 1** | 102 ± 2**,†††,‡‡ | 97 ± 2***,†††,‡‡‡ |
| After 8 h | |||
| Sham | 80 ± 4 | 11 ± 0§§§ | 8 ± 0 |
| Vehicle | 81 ± 7 | 10 ± 1§§§ | 7 ± 2§ |
| S-AMPA | 96 ± 7 | 108 ± 22††,‡ | 10 ± 1 |
| hrIL-1β | 105 ± 1† | 103 ± 2††,‡ | 93 ± 2***,†††,‡‡‡ |
| S-AMPA + hrIL-1β | 100 ± 6 | 910 ± 38***,†††,‡‡‡,§§,∥∥∥ | 89 ± 7***,†††,‡‡‡ |
Expression of IL-1β mRNA (pg/μg total RNA) in different regions of the rat brain 3 h and 8 h after intrastriatal injection (n = 3–4) of vehicle, S-AMPA (7.5 nmol), hrIL-1β (10 ng) or S-AMPA (7.5 nmol) + hrIL-1β (10 ng). ∗∗, P < 0.01; ∗∗∗, P < 0.001 vs. S-AMPA. †, P < 0.05; ††, P < 0.01; †††, P < 0.001 vs. sham. ‡, P < 0.05; ‡‡, P < 0.01; ‡‡‡, P < 0.001 vs. vehicle. §, P < 0.05; §§, P < 0.01; §§§, P < 0.001 vs. 3 h. ∥∥∥, P < 0.001 vs. hrIL-1β.
In contrast, in the ipsilateral cortex, IL-1β mRNA expression 3 h after injection of either hrIL-1β or hrIL-1β + S-AMPA was significantly higher than in vehicle or S-AMPA-injected rats (Table 2). In the ipsilateral cortex 8 h after striatal injection, animals treated with hrIL-1β + S-AMPA exhibited 100-fold increases (P < 0.001) in IL-1β mRNA compared with vehicle animals and 10-fold greater amounts (P < 0.001) than those injected with S-AMPA or hrIL-1β (Table 2). IL-1β mRNA levels at 8 h in the hrIL-1β + S-AMPA group were significantly higher than at 3 h, whereas sham and vehicle groups had lower levels (Table 2).
Intrastriatal injection of hrIL-1β or hrIL-1β + S-AMPA also induced significantly higher levels (10-fold; P < 0.001) of IL-1β mRNA in the hypothalamus at both 3 h and 8 h after treatment when compared with vehicle or S-AMPA groups (Table 2).
Striatal levels of immunoreactive rat IL-1β measured after 3 h were no different between animals that received intrastriatal injection of vehicle, S-AMPA, hrIL-1β, or hrIL-1β + S-AMPA (Table 3). In contrast, after 8 h, the IL-1β immunoreactivity in the striatum of hrIL-1β- or hrIL-1β + S-AMPA-treated animals was significantly greater (>5-fold) than that observed in animals injected with S-AMPA alone. However, there was no significant difference between the hrIL-1β- or hrIL-1β + S-AMPA-treated groups and those animals injected with vehicle, although in all these groups a significant increase over levels at 3 h was found (Table 3).
Table 3.
Immunoreactive IL-1β levels
| Treatment | Striatum | Cortex | Hypothalamus |
|---|---|---|---|
| After 3 h | |||
| Sham | ND | ND | ND |
| Vehicle | 32 ± 4 | 28 ± 1 | 20 ± 3 |
| S-AMPA | 28 ± 6 | 86 ± 23 | 26 ± 7 |
| hrIL-1β | 29 ± 3 | 56 ± 20 | 41 ± 4 |
| S-AMPA + hrIL-1β | 47 ± 9 | 128 ± 27* | 56 ± 9**,† |
| After 8 h | |||
| Sham | ND | ND | ND |
| Vehicle | 157 ± 46‡ | 65 ± 20 | 18 ± 3 |
| S-AMPA | 48 ± 7 | 72 ± 10 | 35 ± 7 |
| hrIL-1β | 304 ± 70††,‡ | 147 ± 41 | 128 ± 30**,†,‡ |
| S-AMPA + hrIL-1β | 241 ± 34†,‡‡ | 870 ± 186***,†††,‡,§§§ | 186 ± 27***,†††,‡ |
Expression of immunoreactive rat IL-1β (pg IL-1β/mg protein) in different regions of the rat brain 3 h and 8 h after intrastriatal injection (n = 4–6) of vehicle, S-AMPA (7.5 nmol), hrIL-1β (10 ng) or S-AMPA (7.5 nmol) + hrIL-1β (10 ng). ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 vs. vehicle. †, P < 0.05, ††, P < 0.01, †††, P < 0.001 vs. S-AMPA. ‡, P < 0.05; ‡‡, P < 0.01 vs. 3 h; §§§, P < 0.001 vs. hrIL-1β. ND, not detectable.
Three hours after intrastriatal injection of hrIL-1β + S-AMPA, immunoreactive rat IL-1β levels in the ipsilateral cortex were significantly higher (×5) than those in animals injected with vehicle (Table 3). No significant differences were observed between hrIL-1β + S-AMPA-treated animals and those injected with either S-AMPA or hrIL-1β alone (Table 3). In the ipsilateral cortex 8 h after striatal injection, animals treated with hrIL-1β + S-AMPA showed at least 5-fold higher levels of immunoreactive IL-1β when compared with animals injected with vehicle, S-AMPA or hrIL-1β, or the corresponding 3-h value (Table 3). Within the contralateral hemisphere, there was a significant increase in immunoreactive IL-1β between the vehicle and hrIL-1β + S-AMPA group (data not shown). However, contralateral levels of immunoreactive IL-1β 8 h after S-AMPA or hrIL-1β + S-AMPA treatment were significantly lower than the ipsilateral values (data not shown).
Intrastriatal injection of hrIL-1β + S-AMPA produced a 2-fold increase in the levels of immunoreactive rat IL-1β in the hypothalamus 3 h after treatment, compared with striatal injection of vehicle or S-AMPA (Table 3). No significant difference was observed in immunoreactive rat IL-1β levels between the vehicle- or S-AMPA-treated groups and animals injected with hrIL-1β alone (Table 3). Eight hours after intrastriatal injection, immunoreactive rat IL-1β levels in the hypothalamus were significantly higher (>5-fold) in animals treated with hrIL-1β alone or together with S-AMPA than in animals injected with vehicle or S-AMPA separately (Table 3). In addition, the amount of immunoreactive IL-1β was significantly greater in the hrIL-1β and hrIL-1β + S-AMPA groups when compared with the 3-h levels.
Given that both IL-1β mRNA and immunoreactive IL-1β increased in the hypothalamus after striatal coinjection of S-AMPA and hrIL-1β, we studied whether direct injection of IL-1β itself in this region could affect cortical cell death in response to striatal S-AMPA.
Administration of hrIL-1β alone caused no damage either at the site of injection or in distant sites (data not shown). Injection of hrIL-1β (10 ng) in the LH immediately after intrastriatal S-AMPA resulted in extensive cortical cell death compared with the modest and localized (piriform) cortical injury caused by striatal injection of S-AMPA and intrahypothalamic vehicle injection (Fig. 1 and Table 4). The volume of striatal damage resulting from intrastriatal injection of S-AMPA was no different between rats treated with vehicle or hrIL-1β in the LH (Table 4). This effect appeared to be of an “all-or-nothing” nature, with 13/19 animals injected with hrIL-1β (10 ng) displaying extensive cortical damage that incorporated both the parietal and piriform cortices, whereas in the others, damage was confined to the piriform region. Lower doses of hrIL-1β (0.1 or 1 ng) caused parietal cortex damage only in a single animal (Table 4).
Figure 1.
Representative coronal brain sections at a single level illustrating the cell death (pale areas) observed after stereotaxic injection of S-AMPA (7.5 nmol) in the rat striatum (str), immediately followed by vehicle (A) or hrIL-1β (10 ng; B) in the LH.
Table 4.
Lesion volumes
| Treatment | LV mm3, striatal | LV mm3, cortical | N |
|---|---|---|---|
| IL-1β | |||
| S-AMPA (ST) + vehicle (LH) | 25.0 ± 1.4 | 24.9 ± 3.3 | 0/23 |
| S-AMPA (ST) + hrIL-1β (0.1 ng; LH) | 28.6 ± 2.8 | 56.1 ± 20.0 | 1/4 |
| S-AMPA (ST) + hrIL-1β (1 ng; LH) | 30.1 ± 4.3 | 46.7 ± 3.0 | 0/4 |
| S-AMPA (ST) + hrIL-1β (10 ng; LH) | 24.5 ± 2.0 | 110.6 ± 14.5***,† | 13/19 |
| IL-1ra | |||
| S-AMPA + hrIL-1β (ST) + vehicle (LH) | 33.2 ± 2.5 | 155.9 ± 13.4 | 15/18 |
| S-AMPA + hrIL-1β (ST) + hrIL-1ra (0.1 μg; LH) | 31.6 ± 5.7 | 145.3 ± 30.2 | 6/8 |
| S-AMPA + hrIL-1β (ST) + hrIL-1ra (1 μg; LH) | 24.8 ± 5.5 | 100.7 ± 20.1 | 2/6 |
| S-AMPA + hrIL-1β (ST) + hrIL-1ra (10 μg; LH) | 30.8 ± 1.8 | 84.8 ± 13.7** | 6/15 |
The upper part of the table describes the effect of hypothalamic IL-1β treatment on the lesion volume (LV) produced by intrastriatal injection of S-AMPA in the rat brain. Animals were injected in the striatum (ST) with S-AMPA (7.5 nmol), followed by injection of hrIL-1β or vehicle in the LH. The lower part of the table describes the effect of hypothalamic IL-1ra treatment on the lesion volume (LV) produced by intrastriatal coinjection of IL-1β plus S-AMPA in the rat brain. Animals were injected in the striatum (ST) with S-AMPA (7.5 nmol) + hrIL-1β (10 ng) then hrIL-1ra or vehicle in the LH. Data represented as mean lesion volume (±SEM). The number of animals displaying distinct cell death in the parietal cortical region compared to the number injected is also indicated (N).
, P < 0.01; ∗∗∗, P < 0.001 vs. vehicle treatment; †, P < 0.05 vs. hrIL-1β (1 ng) treatment.
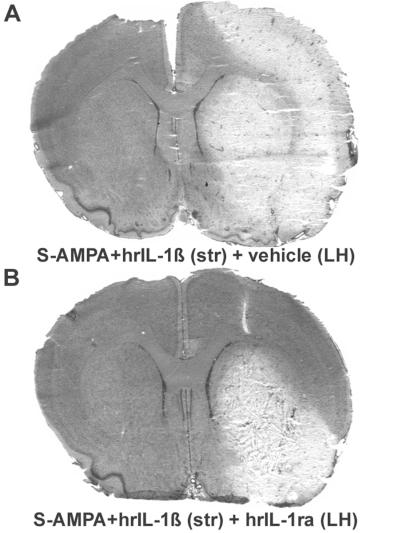
IL-1ra was injected in the LH to determine whether the increase in endogenous IL-1β in this region mediated the cortical damage resulting from striatal injection of S-AMPA plus hrIL-1β. Intrastriatal coinjection of hrIL-1β plus S-AMPA resulted in an extensive lesion throughout the ipsilateral cortex (Fig. 2) as described previously (6). Injection of hrIL-1ra (10 μg) into the LH dose dependently reduced the lesion volume in the cortex when compared with vehicle-treated animals (Fig. 2 and Table 4). The striatal lesion volume was unaffected by hypothalamic IL-1ra treatment (Table 4).
Figure 2.
Representative coronal brain sections at a single level illustrating the cell death (pale areas) observed after stereotaxic coinjection of hrIL-1β (10 ng) and S-AMPA (7.5 nmol) in the rat striatum (str), followed by vehicle (A) or hrIL-1ra (10 μg; B) in the LH.
Discussion
To our knowledge, this study demonstrates for the first time that hypothalamic IL-1 can contribute to cortical neurodegeneration. Injection of hrIL-1β + S-AMPA into the striatum induced expression of IL-1β mRNA and protein at the sites of injection (striatum) and distant injury (cortex), and within the hypothalamus, where no injury was detected. Subsequently, it was demonstrated that blocking the actions of endogenous IL-1β by exogenous application of IL-1ra directly into the hypothalamus reduced the cortical lesion volume produced by striatal coadministration of IL-1β and S-AMPA, whereas application of exogenous IL-1β in the LH with S-AMPA in the striatum caused extensive cortical damage. It appears, therefore, that the hypothalamus is a primary site of action of IL-1, which directly influences cortical cell viability after activation of striatal AMPA receptors. In separate studies, we have shown that cortical cell death produced by intrastriatal injection of IL-1β and S-AMPA is mediated via activation of NMDA receptors (6), which suggests the involvement of a glutamatergic pathway(s) from the striatum to the cortex. Detailed neuronal loss, which may have provided a clearer indication of discrete changes or more diffuse cell loss either locally or in distant regions, was not quantified.
IL-1 has diverse actions in the hypothalamus (3, 10) through effects at a number of sites (18, 19). We targeted the LH because of its known connections with the cortex, ventral striatum (including the nucleus accumbens), and other major hypothalamic nuclei (9, 20). However, other brain areas may contribute to the effects of IL-1β, and our preliminary data indicate that injection of hrIL-1β into the anterior hypothalamus produces extensive cortical cell death when injected after striatal S-AMPA (data not shown). Injection of hrIL-1β immediately after intrastriatal S-AMPA into sites adjacent (<0.7 mm distant) to the LH site described here produce damage in only a minority of animals, e.g., 1/8 rats injected rostromedial to the LH have cortical damage, whereas injections either dorsomedial or dorsolateral are more likely to produce cortical death (6/7 and 4/7 respectively; data not shown). The reason for this apparent site specificity is not clear.
Hypothalamic actions of IL-1 include the induction of fever (21), and increases in temperature have been shown to exacerbate neuronal damage (22, 23) so it is possible that IL-1-induced changes in body and/or brain temperature could be responsible for the observed effect of IL-1 on excitotoxic damage. However, our earlier studies indicate increases in core body temperature are not involved in actions of IL-1β on neuronal damage (12), although local changes in brain temperature cannot be fully excluded (24, 25).
The dramatic increase in expression of IL-1β in the cortex of animals injected with hrIL-1β + S-AMPA in the striatum was most notable 8 h after injection when injury is emerging (Tables 2 and 3). This change is consistent with direct involvement of IL-1β, synthesized locally, in cortical cell death induced by striatal insults. Indeed, preliminary data indicate that an increase in cortical IL-1β levels is necessary for cortical cell death to occur after striatal administration of S-AMPA and hrIL-1β, because direct cortical injection of IL-1ra immediately after the striatal coinjection markedly reduces the amount of cortical injury (data not shown).
Injection of hrIL-1β alone into the striatum caused a significant increase in both mRNA and immunoreactivity for IL-1β in the hypothalamus (Tables 2 and 3), although the latter was observed only after 8 h. However, in these animals there was no obvious cortical cell death, and thus we speculate that increases of IL-1β in the hypothalamus alone are necessary, but not sufficient, to produce remote cell death in the cortex. It therefore seems that an additional “priming” stimulus is required, such as striatal activation of AMPA receptors, which results in the activation of a number of neuronal pathways, particularly those associated with seizures and activation of the limbic system (26, 27). The distant propagation of neural signals has been described by Goto et al. (28). This study measured local cerebral blood flow after hippocampal injection of kainic acid and found a gradual spreading of activity to a number of structures including the amygdala, striatum, thalamus, and cortex (sensorimotor and parietal). However, no correlation was made between the increased blood flow and cell death. We find that S-AMPA alone in the striatum does not produce cell death in the parietal cortex, although distant lesions are seen in localized areas of the piriform cortex, amygdala, and occasionally the thalamus. In contrast, striatal activation of AMPA receptors together with increased IL-1β in the hypothalamus can result in activation of cortical neurones, leading ultimately to extensive cell death throughout the cortex.
The change in IL-1β expression (both mRNA and protein) after hrIL-1β + S-AMPA treatment was much greater in the cortex than in the hypothalamus, but this results from the extensive tissue insult in this region that stimulates IL-1β expression directly. However, cells also die in the striatum, yet IL-1β expression was lower here than in the cortex, and it may be that the form of cell death is different between the two regions. In addition, the absolute levels of IL-1β in each area may not be of functional significance, as it is well documented that very few IL-1 receptors need be occupied to produce a biological action (10).
IL-1β levels (mRNA and protein) were measured 3 h and 8 h after treatment because previous work has demonstrated that cell death in the striatum and cortex is apparent between 8 h and 12 h after injection and is complete by 24 h (S.M.A., R. I. Grundy, & N.J.R., unpublished data). The anti-rat antibody used in the ELISA does not show crossreactivity with hrIL-1β (29), so this could not account for the observed increase in immunoreactive IL-1β found in both the hrIL-1β- and hrIL-1β + S-AMPA-treated groups. Indeed if the injected hrIL-1β had been detected by the ELISA, this should have been most apparent at early time points after injection, which was clearly not the case (Table 3).
Increased local expression of immunoreactive IL-1β after hippocampal kainic acid injection has been reported recently (30). These authors did not study any distant changes in IL-1β expression but found that IL-1β can prolong seizure activity and proposed that IL-1β can enhance glutamatergic neurotransmission. This appears to contradict other reports showing that IL-1β inhibits synaptic transmission (31) and long-term potentiation (32, 33) and augments inhibitory neurotransmission (34). However, the specific effects of IL-1β may depend on a number of factors (3), including the brain area involved (6, 7, 19).
Several studies have reported increased expression of IL-1β in response to brain insults, including peripheral kainic acid (35), methamphetamine (36), intracerebral administration of N-methyl-d-aspartate (NMDA) agonists (37, 38), physical injury (39), and cerebral ischemia (40–42). Microglia are the major early source of IL-1β in response to kainic acid, NMDA agonists (30, 35, 38, 43), or cerebral ischemia (42), although delayed expression has been reported by astrocytes (38, 42). It is probable that the increased levels of IL-1β (mRNA and protein) observed in the present study would show similar patterns of cellular expression. Interestingly, peripheral administration of methamphetamine in the rat induces a specific increase in IL-1β mRNA in the hypothalamus (36), but the relevance of this to neuronal injury was not considered. Likewise, audiogenic seizures have been demonstrated to increase IL-1α mRNA specifically in the hypothalamus and not in other regions such as the hippocampus or cortex (44).
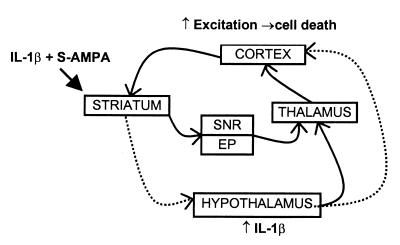
In summary, IL-1β has site-specific actions within the striatum and is most likely to produce remote cortical death when coinjected with S-AMPA in the ventral striatum or shell of the nucleus accumbens (R. I. Grundy, N.J.R. & S.M.A., unpublished data). Such regions connect directly with the hypothalamus and other brain regions that project back to the cortex (9, 11). We propose that intrastriatal IL-1β and S-AMPA can induce extensive distant injury in the cortex by activation of these complex neuronal circuits, which increase local expression of IL-1β in the hypothalamus (Fig. 3). Exactly how IL-1 produces its effects in the hypothalamus is not known, although it could be due to the release of other known mediators of IL-1 action and neurodegeneration such as the hypothalamic neuropeptide corticotrophin-releasing factor (2). Similarly, the outputs from the hypothalamus to the cortex remain to be described in detail. We propose that hypothalamic IL-1 may contribute to other forms of neurodegeneration. We have already shown some site-specific effects of IL-1 and IL-1ra in the striatum on ischemic brain damage (7, 45). Thus, regulation of IL-1 expression or action in the hypothalamus may yield new targets for therapeutic intervention in neurodegenerative disease.
Figure 3.
Simplified schematic diagram illustrating putative pathways (dashed lines) involved in the effects of IL-1β on S-AMPA-mediated excitotoxic cell death in the rat brain. It is proposed that IL-1β can act via the hypothalamus (and possibly other areas) to exacerbate the effects of striatal S-AMPA and produce cell death in the cortex. SNR, substantia nigra pars reticulata; EP, endopeduncular nucleus.
Acknowledgments
The authors express their gratitude to Drs. A. Bristow, S. Poole, and G. Rees (National Institute for Biological Standards and Control, Potters Bar, U.K.) for providing the ELISA reagents as part of the European Union Concerted Action Program (BIOMED 2, CYBRAINET BMH4-CT97–2492). IL-1β was a generous gift from DuPont. Thanks also to Mr. D. Rushforth for his invaluable technical assistance. This work was supported by the Medical Research Council.
Abbreviations
- IL-1ra
IL-1 receptor antagonist
- S-AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- LH
lateral hypothalamus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.090464197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.090464197
References
- 1.Doble A. Pharmacol Ther. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell N J. J Physiol. 1999;514.1:3–17. doi: 10.1111/j.1469-7793.1999.003af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothwell N J, Hopkins S J. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- 4.Loddick S A, Rothwell N J. J Cereb Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Stroke. 1995;26:676–681. doi: 10.1161/01.str.26.4.676. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence C B, Allan S M, Rothwell N J. Eur J Neurosci. 1998;10:1188–1195. doi: 10.1046/j.1460-9568.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- 7.Stroemer R P, Rothwell N J. J Cereb Blood Flow Metab. 1998;18:833–839. doi: 10.1097/00004647-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Heimer L, Alheid G F, de Olmos J S, Groenewegen H J, Haber S N, Harlan R E, Zahm D S. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- 9.Risold P Y, Thompson R H, Swanson L W. Brain Res Rev. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello C A. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 11.Saper C B. J Comp Neurol. 1985;237:21–46. doi: 10.1002/cne.902370103. [DOI] [PubMed] [Google Scholar]
- 12.Grundy R I, Rothwell N J, Allan S M. Brain Res. 1999;830:32–37. doi: 10.1016/s0006-8993(99)01377-3. [DOI] [PubMed] [Google Scholar]
- 13.Anforth H, Bluthe R, Bristow A, Hopkins S, Lenczowski M, Luheshi G, Lundkvist J, Michaud B, Mistry Y, Van Dam A, et al. Eur Cytokine Network. 1998;9:279–288. [PubMed] [Google Scholar]
- 14.Paxinos G, Watson C. The Rat Brain in Stereotaxic Co-ordinates. San Diego: Academic; 1986. [Google Scholar]
- 15.Celi F S, Zenilman M E, Shuldiner A R. Nucleic Acids Res. 1993;21:1047. doi: 10.1093/nar/21.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts D A, Balderson D, Pickering-Brown S M, Deakin J F, Owen F. Schizophr Res. 1996;20:171–174. doi: 10.1016/0920-9964(96)88526-4. [DOI] [PubMed] [Google Scholar]
- 17.Cartmell T, Southgate T, Rees G S, Castro M G, Lowenstein P R, Luheshi G N. J Neurosci. 1999;19:1517–1523. doi: 10.1523/JNEUROSCI.19-04-01517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shintani F, Kanba S, Nakaki T, Nibuya M, Kinoshita N, Suzuki E, Yagi G, Kato R, Asai M. J Neurosci. 1993;13:3574–3581. doi: 10.1523/JNEUROSCI.13-08-03574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sellami S, De Beaurepaire R. Brain Res. 1995;694:69–77. doi: 10.1016/0006-8993(95)00763-g. [DOI] [PubMed] [Google Scholar]
- 20.Bernardis L L, Bellinger L L. Neurosci Biobehav Rev. 1993;17:141–193. doi: 10.1016/s0149-7634(05)80149-6. [DOI] [PubMed] [Google Scholar]
- 21.Kluger M J. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busto R, Dietrich W D, Globus M Y T, Valdés I, Scheinberg P, Ginsberg M D. J Cereb Blood Flow Metab. 1987;7:729–738. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- 23.Malberg J, Seiden L. J Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hara S, Mukai T, Kuriiwa F, Iwata N, Yanase T, Kano S, Endo T. Brain Res. 1997;756:301–304. doi: 10.1016/s0006-8993(97)00301-6. [DOI] [PubMed] [Google Scholar]
- 25.McDonald J W, Chen C, Trescher M, Johnston M. Neurosci Lett. 1991;126:83–86. doi: 10.1016/0304-3940(91)90377-6. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Ari Y. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Ari Y, Tremblay E, Ottersen O P, Meldrum B S. Brain Res. 1980;191:79–97. doi: 10.1016/0006-8993(80)90316-9. [DOI] [PubMed] [Google Scholar]
- 28.Goto Y, Araki T, Kato M, Fukui M. Brain Res. 1994;634:203–213. doi: 10.1016/0006-8993(94)91923-2. [DOI] [PubMed] [Google Scholar]
- 29.Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf C J. Br J Pharmacol. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vezzani A, Conti N, De Luigi A, Ravizza T, Moneta D, Marchesi F, De Simoni M G. J Neurosci. 1999;19:5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu B, Shinnick-Gallagher P. J Pharmacol Exp Ther. 1994;271:590–600. [PubMed] [Google Scholar]
- 32.Cunningham A J, Murray C A, O'Neill L A J, Lynch M A, O'Connor J J. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- 33.Coogan A, O'Connor J J. NeuroReport. 1997;8:2107–2110. doi: 10.1097/00001756-199707070-00004. [DOI] [PubMed] [Google Scholar]
- 34.Miller L G, Galpern W R, Dunlap K, Dinarello C A, Turner T J. Mol Pharmacol. 1991;39:105–108. [PubMed] [Google Scholar]
- 35.Yabuuchi K, Minami M, Katsumata S, Satoh M. Mol Brain Res. 1993;20:153–161. doi: 10.1016/0169-328x(93)90121-5. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi T, Kuraishi Y, Minami M, Nakai S, Hirai Y, Satoh M. Neurosci Lett. 1991;128:90–92. doi: 10.1016/0304-3940(91)90766-m. [DOI] [PubMed] [Google Scholar]
- 37.Hagan P, Poole S, Bristow A F, Tilders F, Silverstein F S. J Neurochem. 1996;67:2215–2218. doi: 10.1046/j.1471-4159.1996.67052215.x. [DOI] [PubMed] [Google Scholar]
- 38.Pearson V L, Rothwell N J, Toulmond S. Glia. 1999;25:311–323. [PubMed] [Google Scholar]
- 39.Gabellec M M, Crumeyrolle-Arias M, Le Saux F, Auriou N, Jacque C, Haour F. Neurosci Res. 1999;33:251–260. doi: 10.1016/s0168-0102(99)00014-0. [DOI] [PubMed] [Google Scholar]
- 40.Yabuuchi K, Minami M, Katsumata S, Yamazaki A, Satoh M. Mol Brain Res. 1994;26:135–142. doi: 10.1016/0169-328x(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 41.Sairanen T R, Lindsberg P J, Brenner M, Siren A-L. J Cereb Blood Flow Metab. 1997;17:1107–1120. doi: 10.1097/00004647-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Davies C A, Loddick S A, Toulmond S, Stroemer R P, Hunt J, Rothwell N J. J Cereb Blood Flow Metab. 1999;19:87–98. doi: 10.1097/00004647-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Eriksson C, Van Dam A M, Lucassen P J, Bol J G, Winblad B, Schultzberg M. Neuroscience. 1999;93:915–930. doi: 10.1016/s0306-4522(99)00178-5. [DOI] [PubMed] [Google Scholar]
- 44.Gahring L C, White H S, Skradski S L, Carlson N G, Rogers S W. Neurobiol Dis. 1997;3:263–269. doi: 10.1006/nbdi.1996.0123. [DOI] [PubMed] [Google Scholar]
- 45.Stroemer R P, Rothwell N J. J Cereb Blood Flow Metab. 1997;17:597–604. doi: 10.1097/00004647-199706000-00001. [DOI] [PubMed] [Google Scholar]