Abstract
Oxidative injury to hepatocytes occurs as a result of HCV infection and replication. Modulation of host cell antioxidant enzymes such as heme oxygenase-1 (HO-1) may be useful therapeutically to minimize cellular injury, reduce viral replication, and attenuate liver disease. In this report, we evaluated the effects of HO-1 overexpression on HCV replication and hepatocellular injury. Full length (FL) (Con1) or Non-structural (NS) replicons (I 389 NS3-3′) were transfected with complete human HO-1 sequences or empty vector for control. Cell lines overexpressing HO-1 (2-6 fold above basal values) or empty vector were isolated and their HCV RNA synthesis, pro-oxidant levels, and resistance to oxidative injury were assessed. HO-1 overexpression decreased HCV RNA replication in both FL and NS replicons without affecting cellular growth or DNA synthesis. The attenuation of HCV replication was significantly reversed in both replicon systems with HO-1 siRNA knockdown. Both FL and NS replicons that overexpress HO-1 showed reduced prooxidant levels at baseline and increased resistance to oxidant-induced cytotoxicity. HO-1 induction with hemin also markedly decreased HCV replication in both parental FL and NS replicon cell lines. On the other hand, knock-down of HO-1 mRNA by siRNA in parental FL or NS replicons did not significantly affect HCV replication suggesting that less than basal levels of HO-1 had minimal affect on HCV replication.
Conclusion
Overexpression or induction of HO-1 results in decreased HCV replication as well as protection from oxidative damage. These findings suggest a potential role for HO-1 in antiviral therapy and therapeutic protection against hepatocellular injury in HCV infection.
Keywords: Anti-oxidative enzymes, oxidative stress, viral replication, replicons, Huh7 cells
Introduction
Chronic HCV infection is a worldwide health problem which can lead to chronic hepatitis, cirrhosis, and end stage liver disease (1). The virus has a plus-stranded RNA genome with a single long open-reading frame containing 5′ and 3′ flanking non-translated nucleotide regions that are important for translation and replication. Although wild type virus grows poorly in cell culture (2) the development of subgenomic and full length HCV replicons that stably replicate HCV RNA in permissive human hepatoma cells has provided the opportunity for detailed investigation of HCV cellular activities (3;4).
Standard treatment of patients with chronic HCV includes alpha-interferon and Ribavirin for 24-48 weeks, depending on HCV genotype. Nearly half of all patients treated fail to achieve sustained viral eradication or they relapse after therapy (5). Consequently, new interest has focused on development of additional treatment options (6). A major aim of new management strategies is to prevent chronic liver injury from the virus by interference with the viral life cycle and/or hepatic inflammatory injury at critical points.
An attractive but unexplored strategy for HCV therapy is use of host antioxidant enzymes to reduce chronic inflammation and thus attenuate progressive liver disease. Initial hepatic injury from HCV is likely oxidant-mediated and appears to result from immune mechanisms (7;8). In addition, prooxidant production is also increased by viral proteins such as core and NS5A in host cells (9-15). Collectively, these observations suggest that antioxidant enzymes could be used therapeutically to reduce oxidative injury during chronic HCV infection.
Heme oxygenase (HO) catalyzes the first and rate-limiting step in the catabolism of heme which produces equimolar amounts of biliverdin, carbon monoxide (CO) and free iron. Of the three HO isoenzymes (16), HO-1 has been shown to inhibit inflammation and protect against oxidative damage (17;18). HO-1 is highly inducible by hemin and various stressors while HO-2 and HO-3 are mainly expressed constitutively (19) Recently, induction or overexpression of HO-1 was shown to inhibit full length replication of viruses such as HIV and influenza (20;21) emphasizing the potential importance of the enzyme as a virucidal agent.
We have previously described effects of HCV on the expression of HO-1 in hepatocytes. In liver samples from patients infected with HCV, HO-1 mRNA and protein are decreased (9). The downregulation of HO-1 expression by HCV is reproduced in hepatocyte cell lines that express HCV core protein, but not its envelope or non-structural proteins (10). Furthermore, the induction of HO-1 in response to cytotoxins is impaired in core-expressing hepatocytes (22), suggesting that a diminished HO-1 response to stressors may be an additional source of injury in HCV infection. While these data establish that HCV proteins modulate expression of HO-1, it is unknown whether the enzyme impacts viral replication and hepatocellular injury. The focus of the present work was to evaluate these possibilities by examining the effects of HO-1 overexpression and induction on HCV replication and cellular injury.
Materials and Methods
Materials
Taq DNA polymerase (Perkin-Elmer Cetus, Norwalk, CT), and Maloney murine leukemia virus (MMLV) reverse transcriptase (Gibco/BRL Life Technologies, Gaithersburg, MD) were used in these studies. Tert-butyl-hydroperoxide (tBOOH) was obtained from Sigma Chemical Co. (St. Louis, MO). 2′7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) and 5-carboxyl-2′7′- dichlorodihydrofluorescein diacetate (carboxy-DCFH-DA) were obtained from Molecular Probes, Eugene, OR (catalogue numbers C-399 and C-369, respectively).
Antibodies
Anti-heme oxygenase-1 antibody was obtained from Stressgen (MI) and specificity characterized as described previously (22). Other antibodies included anti-catalase, anti- MnSOD, and anti-CuZnSOD, (kind gifts from Dr. Larry Oberley, University of Iowa), and antiactin (Sigma, MO). Secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell lines and cell culture
The human hepatoma cell line (Huh-7) containing stable replication of sub-genomic selectable HCV RNAs (4) was a kind gift of Dr. Volker Lohmann (Institute for Virology, Johannes- Gutenberg University, Mainz, Germany). We used I389/NS3-3′ (clone 5-15) (Huh5-15NS) and wild type Huh-7 cells as control cells. Clone 5-15 and Huh7 cells were maintained in Dulbecco's modified Eagle medium (DMEM, Gibco-BRL), supplemented with 10% heat-inactivated fetal bovine serum and selection antibiotics as described Lohmann et al (4). Huh7.5 cells harboring full length (Huh7.5FL) Con1 replicons as described by Blight et al (23) were a kind gift of Dr. Charles Rice (Rockefeller University, New York, NY). These cells were passed as recommended in their laboratory of origin (23).
Plasmid construction and transfection
Full length human HO-1 cDNA was PCR amplified from POTB7 plasmid containing HO-1 sequences (InVitrogen, CA) with inserted 5′ BamH1 and 3′ NotI restriction sequences. The HO- 1 cDNA was then ligated into pcDNA3.1/Zeo (Invitrogen) together with Kozak sequences and start and stop codons. The constructs of pcDNA3.1/Zeo/HO-1 or empty vector control, pcDNA3.1/Zeo, were then transfected into Huh5-15NS and Huh7.5FL replicon cells by Lipofectamine TM 2000 (Invitrogen Co., CA) according to the manufacture's protocol. Antibiotic selection medium contained 20 ug/ml Zeocin. The surviving colonies were then subcloned by limiting dilution and amplified to establish sublines overexpressing HO-1 or empty vector controls.
Quantitative Real-time RT-PCR
Total RNA was extracted from cells using Trizol reagent (Invitrogen) and treated with Turbo RNase free DNase (Ambion, TX.). cDNA was synthesized using MMLV-reverse transcriptase according to the manufacturer's protocol. To quantify replication, the primers and probe designed by Takeuchi et al (24) using sequences located in 5'UTR (nt130-290) were employed using conditions described for each replicon line (23;25). Real-time detection RT-PCR was performed on aliquots of the cDNA by use of Taq DNA polymerase with the TaqMan Universal PCR Master Mix Protocol (Perkin Elmer Applied Biosystems). Quantitation was performed using the comparative cycle threshold (CT) method using separate reaction tubes for target (HCV) and reference (18S) RNAs as we described previously (9). Preliminary validation experiments demonstrated that the efficiencies of target and reference signal amplifications were approximately equal with plots of log input RNA versus ΔCt with slopes < 0.1 as recommended (26).
Western blot analysis
Western blot analysis was performed as previously described using enhanced chemiluminescence for signal detection (ECLTM, Amersham) (10). Signal intensities were quantified by densitometry using Image J software (NIH).
Cell proliferation assays
Cell numbers were assessed using the colorimetric MTT assay (Promega, Madison, WI) or [3H]- thymidine incorporation. Cells were plated (2000 cells/well) in 96-well culture plates and cell number assayed daily according to the manufacturer's instructions for MTT assay. For [3H]- thymidine uptake, cells were incubated with [3H]-thymidine (Amersham) (1.0 μCi/ml DMEM) for two hours, washed, then treated with ice cold 10% TCA. The amount of incorporated [3H] was determined by scintillation counting.
HO-1 knockdown
HO-1 siRNA or corresponding scrambled siRNA (Invitrogen) were transfected according to manufacture's protocol. The efficiency of HO-1 knockdown was determined by Western blot analysis and semiquantitative densitometry.
Cytotoxicity assay
Cytotoxicity following treatment with tBOOH was as described (22). Cells (1×104 per well) were seeded in 96-well culture plates and allowed to attach overnight. Cells were then shocked overnight with serum-free medium and incubated with various concentrations of tBOOH for 3 hrs. The MTT assay was then performed as recommended by manufacturer.
Intracellular prooxidant levels
Prooxidant levels were determined using 2′, 7′-dichlorodihydrofluorescein diacetate (DCFH-DA.) as previously described (15). Replicate wells were also treated with carboxy-DCFH-DA, the oxidation-insensitive analog of DCFH-DA, to confirm that differences in the fluorescence of DCFH-DA were related to oxidation of the probe and not the result of differences in uptake, cleavage or efflux of the probe.
Statistical analysis
Data from individual experiments as well as combined data from separate experiments were expressed as mean + standard error of the mean. The significance between means was determined using Student's t-test with ANOVA. P values less than 0.05 were considered significant. All experiments were repeated at least three times.
Results
HO-1 overexpression in transfected hepatoma cells
We first compared antioxidant enzyme expression in parental lines of Huh7, including Huh7 wild type (Huh7), Huh7.5, Huh7.5 vector only controls and Huh5-15NS and Huh7.5FL replicons (Fig. 1A). Interestingly, basal levels of HO-1 were higher in clonal cell lines either harboring replicons or permissive for HCV replication such as Huh7.5 (23). These results suggested that HO-1 may modulate cellular activities that are important for the virus. As reported previously, MnSOD was enhanced in the Huh5-15NS replicons as compared to Huh7 wild type (10;14) which also proved to be the case for the Huh7.5 lines. However, catalase and CuZnSOD were expressed at near equivalent levels regardless of clonal cell line.
Figure 1. Western blots of antioxidative enzymes in replicon parental lines and subclones overexpressing HO-1 or vector only controls.
Whole cell lysates were electrophoresed on SDS gels and immunoblots performed using appropriate antibodies.
A. Analysis of antioxidant enzymes in Huh5-15NS and Huh7.5FL replicon and parental cell lines. Lanes for HO-1 detection contained 15ug of cellular protein, while other lanes contained 30ug of cellular protein.
B. Huh5-15NS subclones overexpressing HO-1 (515ZH7, 8, 15 and 16) vs vector only controls (515Z7 and 8). All lanes contained 30ug of cellular protein.
C. Huh7.5FL subclones overexpressing HO-1 (Huh75ZH2, 3 and 4) vs vector only controls (Huh75Z1 and 2). All lanes contained 30ug of cellular protein.
Plasmids containing full-length sequences for HO-1 were transfected into Huh5-15NS and Huh7.5FL replicon cells. Four stable subclones of Huh5-15NS and 3 subclones of Huh7.5FL were isolated by antibiotic selection and designated 515ZH7, 515ZH8, 515ZH15, 515ZH16 and 75ZH2, 75ZH3, 75ZH4, respectively. Two control subclones expressing empty vector were isolated from each of the parental cell lines (515Z7, 515Z8 and 75Z1, 75Z2 respectively). Western blots demonstrated that levels of immunoreactive HO-1 were increased more than 5-fold in the Huh5-15NS subclones and more than 2-fold in Huh7.5FL subclones, compared to their respective empty vector controls (Figures 1B and C respectively). However, HO-1 overexpression did not appreciably affect expression of other major antioxidant enzymes since levels of catalase, CuZnSOD and MnSOD were comparable regardless of replicon line.
HO-1 inhibition of HCV replication
We next determined the effect of HO-1 overexpression on replication of HCV using Realtime RT-PCR and the comparative CT method (26). For this procedure, the amount of signal in each HO-1 overexpressing clone was compared to control clones expressing the empty vector, (Fig. 2). All four subclones of Huh5-15NS cells overexpressing HO-1 showed a marked decrease in HCV RNA compared to vector controls (Fig. 2A). The reduction in HCV RNA ranged from 2.5- to over 6-fold (average 3.8-fold, p<0.01), despite similar levels of HO-1 in these subclones (Fig. 1A). Likewise, HCV RNA was significantly decreased in the HO-1- overexpressing Huh7.5FL subclones (average 1.8-fold versus controls, p<0.05) (Fig. 2B). Notably, HO-1 levels were more variable in the Huh7.5 FL subclones than in the Huh5-15NSderived cell lines (Fig. 1B). Although no strict linear correlation was observed between levels of HO-1 and HCV RNA, it should be pointed out that 75ZH4, the Huh7.5FL subclone with the most modest increase in HO-1 expression (Figure 1C), also showed the least effect on HCV RNA (1.3-fold decrease) compared to controls (Fig. 2B).
Figure 2. Relative quantification of HCV replication in Huh5-15NS and Huh7.5FL replicons that overexpress HO-1 or vector only.
A and B. HCV RNA was measured in HO-1 overexpressing or control subclones of Huh5-15NS (A) or Huh7.5FL (B) replicons using Real Time RT-PCR and comparative cycle threshold methodology as described in Methods. ANOVA was used to assess differences between the amount of HCV RNA in replicons overexpressing HO-1 as compared to empty vector. Each point is the mean of 9 determinations and plotted as +/- SEM of the group mean.
Huh5-15NS vector only vs HO-1 overexpressing (* p < 0.01).
Huh7.5FL vector only vs HO-1 overexpressing (** p < 0.05).
C and D. HCV RNA was quantified using Real Time RT-PCR after HO-1 RNA knockdown in replicon cells as described in the Methods. Results shown are for HO-1 overexpressing Huh5-15NS (C) or Huh7.5FL (D) subclones after 72hr or 72-120 hr knockdown respectively. The amount of HCV RNA after knockdown (HO-1 siRNA or scramble RNA) in the HO-1 overexpressing subclones was quantified relatively to the amount of HCV RNA in replicon subclones expressing empty vector using the comparative cycle threshold method as described in Methods. ANOVA was used to assess differences between HO-1 siRNA and scramble RNA. Each point is the mean of 9 determinations and plotted as +/− SEM of the group mean.
C and D Lower panels. Immunoblots of HO-1 protein in lysates of subclonal lines following HO-1 siRNA or scramble RNA knockdown.
To further confirm the relationship between HO-1 and HCV replication, we measured replication in HO-1 overexpressing NS and FL subclones after HO-1 knockdown with specific siRNA. Seventy-two hr of HO-1 knockdown in NS subclones led to complete reversal of HCV replication (Fig. 2C). In contrast, FL subclones showed a significant rebound of replication at 72hr, however, complete reversal of the effect of HO-1 overexpression was not achieved until 120 hr of knockdown (Fig. 2D). A shorter knockdown time of 24 hr was without effect in either subclone (not shown). The fact that HCV replication in FL subclones was both harder to inhibit as well as reverse, suggests that there are significant effects of the structural proteins that influence these processes. Furthermore, the fact that extended knockdown times were required to reverse the inhibition of replication suggest a strong inhibitory interaction between HO-1 reaction products on viral replication.
Effects of HO-1 induction and HCV replication
Induction of HO-1 by hemin, a classical inducer (19), also confirmed the results of the HO-1 overexpression experiments. Overnight incubation of either parental replicon cell line with hemin resulted in a greater than 20 fold induction of HO-1 as demonstrated by Western blots that was paralleled by a reduction in HCV RNA and modest decrease in NS5A (50%) and core proteins (90%) (Fig. 3A).
Figure 3. Effects of HO-1 induction (A) with hemin or HO-1 siRNA knockdown (B) on HCV replication.
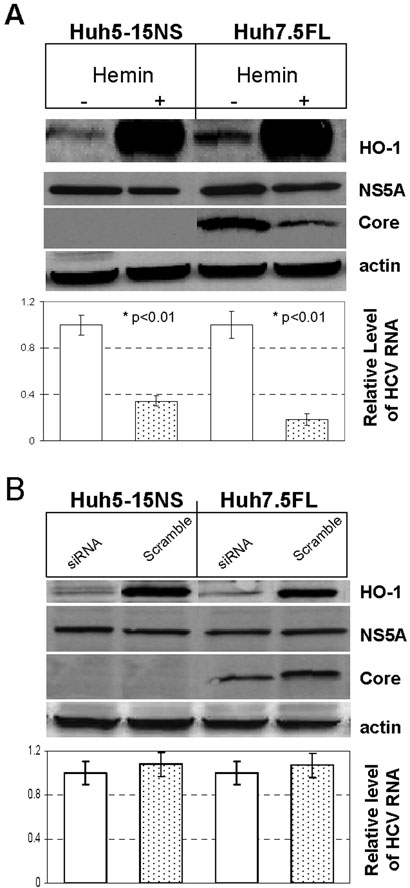
A. HO-1 mRNA was quantified using CT methodology in replicon cells after incubation with control vehicle or 25 uM hemin overnight. B. HCV RNA was quantified similarly after 48 hr of HO-1 mRNA knockdown in replicon cells. The relative quantity of HCV RNA in either un-induced vs hemin treated cells (A) or siRNA vs scramble RNA knockdown (B) were compared. Each point is the mean of 9 determinations and plotted as +/− SEM of the group mean.After either treatment, cells were also assayed for HO-1, core protein, or NS5A protein by Western blots.
Because overexpression or induction of HO-1 reduced HCV replication it was important to determine whether reduced basal levels of HO-1 would also impact HCV replication. Although siRNA knockdown of HO-1 mRNA led to over 80% reduction in HO-1 protein, we observed no effect on HCV replication or viral protein expression in either replicon cell line after 48 hr (Fig. 3B) or longer knockdown times (not shown). However, as noted above, Huh7 clonal lines that either harbor replicons or are permissive for replication showed higher basal values of HO-1 (Fig. 1A). While the reasons for these findings are unclear, they may indicate that increased basal levels of HO-1 are necessary for permissive cells to support other viral activities.
Effect of HO-1 expression on cell proliferation
HCV replication is critically dependent on hepatocyte growth (27) and HO-1 is a known modulator of growth in various cell lines (28;29). Consequently, it was important to determine whether the decrease in HCV replication in the subclones overexpressing HO-1 was an indirect effect of HO-1 on cell growth. As shown in Figure 4A-C, the growth rates of the replicon subclones overexpressing HO-1 were not statistically different from those of empty vector controls as measured by either the MTT assay or by thymidine incorporation. Thus, the effects of HO-1 overexpression on HCV replication do not appear to be the result of alterations in growth rates.
Figure 4. Proliferation of Huh5-15NS and Huh7.5FL clonal replicons with HO-1 overexpression or vector only controls.
Clonal lines were seeded into wells and proliferation determined by either MTT dye (A, B) or 3H-thymidine incorporation (C) at various times during growth of cultures. Each point is the mean of 6 determinations and plotted as +/- SEM of the group mean. There was no statistical difference in growth between cells overexpressing HO-1 as compared to vector only control cells [p > 0.05]. Clonal lines of Huh5-15NS overexpressing vector only (5-15Z7 and 5-15Z8) or HO-1 (5-15ZH15 and 5-15ZH18) as well as Huh7.5FL overexpressing vector only (75Z1 and 75Z2) or HO-1 (75ZH2 and 75ZH3) are as described in the text.
Effects of HO-1 on resistance to oxidative stress and intracellular prooxidant status
We have previously shown that hepatoma cell lines that express HCV core protein show diminished resistance to oxidative stress as well as higher basal levels of prooxidants in comparison to NS proteins (10;15;22). Consequently, we determined the effects of HO-1 overexpression on these parameters in replicon cells. Using tBOOH as a model oxidant, HO-1- overexpressing subclones of both replicon cell lines showed enhanced resistance to oxidantmediated cytotoxicity compared with vector-only subclones (Figures 5A-B). The protective effect of HO-1 overexpression was most dramatic in the Huh7.5 FL subclones (Fig. 5B). The empty vector controls of this cell line were extremely sensitive to tBOOH, with complete loss of viability at 200 µM tBOOH. In contrast, the HO-1-overexpressing subclones remained 70-90% viable under these conditions. The HO-1-overexpressing subclones of Huh5-15NS were also significantly resistant to this concentration of tBOOH.
Figure 5. Susceptibility of Huh5-15NS or Huh7.5FL replicons with or without HO-1 overexpression to peroxide injury.
Semiconfluent cultures of Huh5-15NS (A), or Huh7.5FL(B) replicons with or without HO-1 overexpression were treated with varying concentrations of tBOOH for three hours, then viability determined with MTT dye assay. Toxicity was compared in Huh5-15NS replicons overexpressing HO-1 or vector only controls (5-15ZH15 and 16 vs 5-15Z7 and 8) and Huh7.5FL replicons overexpressing HO-1 or vector only controls (75ZH2 and 3 vs 75Z1 and 2). Data are plotted as the group mean of 9 determinations per point +/− SEM. Differences between groups were determined with ANOVA and t-test.
* 515ZH16 vs 515Z7 p < 0.05
** 75ZH2 or 75ZH3 vs 75Z1 or 75Z2 p < 0.01
We next evaluated the resistance of the parental replicon cell lines to oxidant-mediated cytotoxicity from tBOOH after HO-1 knockdown using siRNA as described above. As expected, both Huh5-15NS and Huh7.5FL replicon lines after HO-1 knockdown showed enhanced sensitivity to tBOOH cytotoxicity (p<0.01). Here again, the effect was greater for Huh7.5FL than Huh5.15NS replicons (p<.01) (Figures 6A-B). Additionally, induction of HO-1 with hemin (25 uM) also elicited resistance to tBOOH toxicity in either parenteral line as compared to controls (data not shown).
Figure 6. Effects of HO-1 knockdown on susceptibility to peroxide cytotoxicity.
HO-1 knockdown was assessed after transfection of HO-1 siRNA or scrambled control RNA into parental Huh5-15NS and Huh7.5FL replicons. 48 hours after transfection, cells were assayed for HO-1 expression with either Western blots (A) or treated with tBOOH (400 uM) for three hours, then cytotoxicity assessed with MTT assay (B) as described in Methods. The percentage differences were calculated with respect to control incubations that received vehicle only. Each value is the mean of 9 determinations +/− SEM normalized to a group coefficient of variation which is plotted. Differences between groups were determined with ANOVA and t-test.
*Increased susceptibility to peroxide toxicity with siRNA knockdown of HO-1 vs scrambled RNA in Huh 5-15NS or Huh 7.5FL replicons [p < 0.05 and p < 0.01 respectively].
**Increased tolerability of Huh5-15NS replicons to HO-1 knockdown and peroxide toxicity as compared to Huh7.5FL: [p < 0.01].
Finally, levels of prooxidants were measured in the Huh5-15NS and Huh7.5FL subclones using DCFH-DA (30). Prooxidant levels in HO-1-overexpressing subclones derived from Huh5-15NS were significantly reduced compared to their respective empty-vector controls (average 1.7-fold decrease; p<0.01) (Fig. 7A). A similar effect of HO-1 overexpression was observed in Huh7.5FL subclones (average 1.5-fold decrease; p<0.01) (Figure 7B). The higher baseline level of prooxidants in the Huh7.5FL cells compared to Huh5-15NS might be expected considering the increased sensitivity to injury of core-expressing constructs reported previously (22). Experiments using the oxidative-insensitive analog of DCFH-DA showed no differences in fluorescence between HO-1 overexpressing and empty-vector cells, indicating that the changes in fluorescence of DCFH-DA reflect oxidation of the probe and not differential uptake (data not shown).
Figure 7. Intracellular prooxidant production in HO-1 overexpression vs control cells.
Replicons either with HO-1 overexpression or vector only control were assayed for prooxidant activity using DCF-DA methodology as described in Methods. Data are plotted as the mean of 9 determinations per point +/− SEM. Differences between groups were determined with ANOVA and t-test.
**Significant reduction of DCF activity in HO-1 transfected Huh5-15NS or Huh7.5FL cells as compared with vector only control cells (** p<0.01).
Discussion
New strategies to treat HCV infection and prevent chronic liver disease are urgently needed since nearly half of all treated patients either do not respond or relapse after therapy (5). Because HCV elicits oxidant-mediated liver injury, therapeutic use of antioxidative enzymes represents a plausible, yet unexplored treatment option.
HO-1, also known as heat shock protein 32, is a cellular defense protein that is induced in response to hypoxia, heat shock, UV light, and cytotoxic oxidants. The enzyme has been shown to protect against cellular injury through antioxidative, antiinflammatory, and antiapoptotic activities (19;31;32). While it is not clear whether HO-1 plays a beneficial role in chronic HCV infection, it is becoming apparent that the virus has multifaceted capabilities to regulate the enzyme's expression. We have previously shown that HO-1 is decreased in liver biopsies from HCV-infected patients and in human hepatoma cell lines that express HCV core protein. Furthermore, core protein-expressing cells showed impaired up-regulation of HO-1 and increased injury from cytotoxins as compared to cells expressing NS proteins which exhibited normal HO-1 activity (9;10;22). Other investigators have reported induction of HO-1 in hepatocytes transfected with a plasmid containing non-structural and structural proteins (33). These studies clearly demonstrate the ability of HCV to modulate levels of HO-1 in hepatocytes; however, the importance of HO-1 regulation for both the virus and the host cell has not been extensively addressed.
Herein, we used two well-characterized HCV replicon cell lines to evaluate the effects of HO-1 overexpression and induction on HCV replication, resistance to oxidant-mediated injury, and prooxidant production. These experiments revealed major effects of HO-1 overexpression or induction in HCV replicon cells. First, HO-1 decreased viral replication in both NS and FL replicon systems independent of effects on cellular growth. Second, HO-1 overexpression increased resistance to oxidant-mediated cytotoxicity and reduced basal prooxidant levels. To our knowledge, this is the first demonstration that full length HCV replication is inhibited by HO-1 induction. However, while this manuscript was in preparation, Shan et al (34) reported that HCV RNA synthesis in another NS replicon cell line, (9-13) was attenuated by HO-1 induction. Induction of HO-1 with hemin has been reported to decrease replication of both HIV (20) and HBV (35), while overexpression of HO-1 with an adenoviral vector decreased influenza inflammatory changes in a mouse model (21). Consequently, there appears to be an emerging role for HO-1 in the control of viral infections, although it is not known whether HO-1 is working through similar pathways in these diverse systems.
HO-1 overexpression decreased HCV replication at the same time as it reduced prooxidant production of replicon cells and increased resistance to oxidative injury, strongly suggesting a potential therapeutic role for HO-1. The HO-1 enzymatic products include carbon monoxide,(CO), iron, and biliverdin. Carbon monoxide and biliverdin have chiefly antioxidative and antiinflammatory activities while iron is usually considered a potential cytotoxin (36). Nevertheless, iron was recently shown to inhibit the activity of HCV RNA dependent RNA polymerase (RdRP) as well as reducing replication in NS replicons (37). Our data clearly support these results, however, a potential antiviral role for iron must be considered in the context of clinical data showing that mild increases in storage iron correlate with progressive HCV liver disease and hepatic decompensation (38;39). Furthermore, a potential role for biliverdin and CO as inhibitors of viral replication through antioxidative behavior requires further study. Although not presented here, we have found that nonspecific antioxidants such as Nacetylcysteine inhibit viral replication, however they also severely inhibit cellular growth (Wen et al, unpublished). Thus, these findings cannot be used as unequivocal evidence that nonspecific antioxidants reduce viral replication per se, since the process is closely dependent on cellular growth and available host co-factors (27).
HCV replication has been reported to generate oxidants (14) in spite of the fact that low, non-toxic levels of peroxides (less than the toxic levels used here) were found to inhibit replication (40). In contrast, subgenomic replicons were shown to generate prooxidant conditions leading to the up-regulation of MnSOD and transcriptional activation of stress pathways through regulation of cellular kinases such as p38 mitogen-activated protein kinase, JNK and JAK-2, and enhanced AP-1 DNA binding (14;41). Overall, these studies suggest the importance of oxidants as both primary signaling moieties for replication as well as injury in the infected hepatocyte. In other viral systems, reactive oxygen species are important signaling agents necessary for replication (42;43).
Because HO-1 has marked effects on viral replication, it may be supposed that HCV must control HO-1 induction to optimize replication. We hypothesize that the virus may accomplish this through transcriptional regulation of stress pathways such as signal transduction and activators of transcription (STAT). These pathways are not only important for HO-1 induction (44;45) but are also modulated by HCV in vitro and in vivo (41;46). A variety of cellular stressors channel through an HO-1 “therapeutic amplification funnel ” (32) and our data suggest that the virus has a need to control these kinds of responses for viral activities such as replication. The viral transcriptional activities are likely mediated in part by core and NS5A proteins (47;48).
While NS replicons appeared to be more effectively attenuated by HO-1 overexpression than FL replicons, there were quantitative differences in the amount of HO-1 overexpression actually achieved in the replicon lines (5 fold vs 2 fold respectively). Nevertheless, HO-1 induction with hemin was equally effective in decreasing replication in either replicon system. These findings suggest that compounds such as heme, which not only increase HO-1 levels, but also efficiently deliver iron intracellularly to inhibit the RdRP (37) may prove to be useful as virucidal agents for HCV. Potentially, they may also enhance the antiviral activity of the interferons through the common signaling pathways discussed above.
Comparison of the responses of the two replicon systems to HO-1 overexpression and peroxide toxicity also supports our past work showing that structural and nonstructural proteins influence prooxidant conditions and injury quite differently. In general, NS replicons showed greater stability to HO-1 knockdown and resistance to peroxide damage as compared to FL replicons. This may be due to the fact that NS replicons induce increased expression of MnSOD and enhance generation of reduced glutathione which does not occur in core protein expressing cells (10;15). Furthermore, NS replicons respond to injury appropriately with HO-1 up-regulation (10;33), in contrast to cells expressing core protein which have an attenuated HO- 1 induction (22). Collectively, these findings indicate that cells expressing NS proteins are able to better compensate for oxidative imbalances by up-regulating antioxidant defenses than coreexpressing cells although additional support for this concept is necessary. Finally, the increased susceptibility of HCV FL replicons to peroxide cytotoxicity shown here suggests that core protein influences the stability and oxidative balance of cells, even when core gene is initially expressed in a full length viral transcript.
In conclusion, our findings document that increased expression of HO-1 suppresses HCV replication and also increases the resistance of hepatocytes to oxidative injury. These results suggest that HO-1 overexpression or selective induction may potentially be a useful adjunctive antiviral therapy to treat chronic infection and prevent chronic liver disease.
Acknowledgements
We thank Drs. Rice and Lohmann for the kind gifts of the Huh7.5FL and the Huh5-15NS replicon cells, respectively.
Research Support Supported in part by Merit Review grants from the Veterans Administration (KEB), the NIH R21 DK068453-01A1 (WNS), RO1 DK058597-4 (BAL), and the University of Iowa Carver Trust Foundation (WNS).
List of abbreviations
- HCV
hepatitis C virus
- HO-1
heme oxygenase-1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- RT-PCR
reverse transcriptase-polymerase chain reaction
- TBOOH
tert-butyl-hydroxide
- DMEM
Dulbecco's modified minimal essential medium
- FBS
fetal bovine serum
- CV
coefficient of variation
- ANOVA
analysis of variance
- MnSOD
manganese superoxide dismutase
- CuZnSOD
copper–zinc superoxide dismutase
Footnotes
Conflict of Interest. None of the authors who participated in this study have commercial or other associations that might pose a conflict of interest.
Presented in part at American Association for the Study of Liver Diseases, th Annual Meeting, Boston, MA, abstract # 460, 2007.
References
- 1.Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: A perspective on long-term outcome. Semin Liver Dis. 2000;20(1):17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 2.Bartenschlager R, Lohmann V. Replication of hepatitis C virus. J Gen Virol. 2000;81:1631–1648. doi: 10.1099/0022-1317-81-7-1631. [DOI] [PubMed] [Google Scholar]
- 3.Blight KJ, Kolykhalov AA, Rice CM. Efficient initiation of HCV RNA replication in cell culture. Science. 2000;290(5498):1972–1974. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- 4.Lohmann V, Korner F, Koch JO, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285(5424):110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 5.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: Efficacy, side effects, and complications. Gut. 2006;55(9):1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Firpi RJ, Nelson DR. Current and future hepatitis C therapies. Arch Med Res. 2007;38(6):678–690. doi: 10.1016/j.arcmed.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Cerny A, Chisari FV. Immunological aspects of HCV infection. Intervirology. 1994;37(2):119–125. doi: 10.1159/000150366. [DOI] [PubMed] [Google Scholar]
- 8.Liaw YF, Lee CS, Tsai SL, Liaw BW, Chen TC, Sheen IS, et al. T-cell—mediated autologous hepatocytotoxicity in patients with chronic hepatitis C virus infection. Hepatology. 1995;22(5):1368–1373. [PubMed] [Google Scholar]
- 9.Abdalla MY, Britigan BE, Wen F, Icardi M, McCormick ML, LaBrecque DR, et al. Downregulation of heme oxygenase-1 by hepatitis C virus infection in vivo and by the in vitro expression of hepatitis C core protein. J Infect Dis. 2004;190(6):1109–1118. doi: 10.1086/423488. [DOI] [PubMed] [Google Scholar]
- 10.Abdalla MY, Ahmad IM, Spitz DR, Schmidt WN, Britigan BE. Hepatitis C virus-core and non structural proteins lead to different effects on cellular antioxidant defenses. Med Virol. 2005;76(4):489–497. doi: 10.1002/jmv.20388. [DOI] [PubMed] [Google Scholar]
- 11.Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kB. Proc Natl Acad Sci USA. 2001;98:9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li K, Prow T, Lemon SM, Beard MR. Cellular response to conditional expression of hepatitis C virus core protein in Huh7 cultured human hepatoma cells. Hepatology. 2002;35(5):1237–1246. doi: 10.1053/jhep.2002.32968. [DOI] [PubMed] [Google Scholar]
- 13.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 14.Qadri I, Iwahashi M, Capasso JM, Hopken MW, Flores S, Schaack J, et al. Induced oxidative stress and activated expression of manganese superoxide dismutase during hepatitis C virus replication: role of JNK, p38 MAR and AP-1. Biochem J. 2004;378:919–928. doi: 10.1042/BJ20031587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen F, Abdalla MY, Aloman C, Xiang JH, Ahmad IM, Walewski J, et al. Increased prooxidant production and enhanced susceptibility to glutathione depletion in HepG2 cells co-expressing HCV core protein and CYP2E1. J Med Virol. 2004;72(2):230–240. doi: 10.1002/jmv.10567. [DOI] [PubMed] [Google Scholar]
- 16.Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol. 2006;39(5):479–491. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- 17.Otterbein LE, Choi AMK. Heme oxygenase: Colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279:L 1029–L 1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T, Morita K, Akagi R, Sassa S. Heme oxygenase-1: A novel therapeutic target in oxidative tissue injuries. Curr Med Chem. 2004;11(12):1545–1561. doi: 10.2174/0929867043365080. [DOI] [PubMed] [Google Scholar]
- 19.Immenschuh S, Ramadori G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem Pharmacol. 2000;60(8):1121–1128. doi: 10.1016/s0006-2952(00)00443-3. [DOI] [PubMed] [Google Scholar]
- 20.Devadas K. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J Immunol. 2006;176(7):4252–4257. doi: 10.4049/jimmunol.176.7.4252. [DOI] [PubMed] [Google Scholar]
- 21.Hashiba T, Suzuki M, Nagashima Y, Suzuki S, Inoue S, Tsubarai S, et al. Adenovirusmediated transfer of heme oxygenase-1 cDNA attenuates severe lung injury induced by the influenza virus in mice. Gene Ther. 2001;8(19):1499–1507. doi: 10.1038/sj.gt.3301540. [DOI] [PubMed] [Google Scholar]
- 22.Wen F, Brown KE, Britigan BE, Schmidt WN. Hepatitis C core protein inhibits induction of heme oxygenase-1 and ensitizes hepatocytes to cytotoxicity. Cell Biol Toxicol. 2008;24(2):175–188. doi: 10.1007/s10565-007-9027-9. [DOI] [PubMed] [Google Scholar]
- 23.Blight KJ, McKeating JA, Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol. 2002;76(24):13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi T, Katsume A, Tanaka T, Abe A, Inoue K, Tsukiyama-Kohara K, et al. Real-time detection system for quantification of hepatitis C virus genome. Gastroenterology. 1999;116(3):636–642. doi: 10.1016/s0016-5085(99)70185-x. [DOI] [PubMed] [Google Scholar]
- 25.Lohmann V, Hoffmann S, Herian U, Penin F, Bartenschlager R. Viral and cellular determinants of hepatitis C virus RNA replication in cell culture. J Virol. 2003;77(5):3007–3019. doi: 10.1128/JVI.77.5.3007-3019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.User Bulletin. ABI Prism 77000 Sequence Detection System. Applied Biosystems 2. 2001:11–15. [Google Scholar]
- 27.Pietschmann T, Lohmann V, Rutter G, Kurpanek K, Bartenschlager R. Characterization of cell lines carrying self-replicating hepatitis C virus NAs. J Virol. 2001;75(4):1252–1264. doi: 10.1128/JVI.75.3.1252-1264.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aizawa T, Ishizaka N, Kurokawa K, Nagai R, Nakajima H, Taguchi JI, et al. Different effects of angiotensin II and catecholamine on renal cell apoptosis and proliferation in rats. Kidney Int. 2001;59(2):645–653. doi: 10.1046/j.1523-1755.2001.059002645.x. [DOI] [PubMed] [Google Scholar]
- 29.Durante W. Heme oxygenase-1 in growth control and its clinical application to vascular disease. J Cell Physiol. 2003;195(3):373–382. doi: 10.1002/jcp.10274. [DOI] [PubMed] [Google Scholar]
- 30.Hempel SL, Buettner GR, O'Malley YQ, Wessels DA, Flaherty DM. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: Comparison with 2 ′,7 ′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2 ′,7 ′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med. 1999;27(1-2):146–159. doi: 10.1016/s0891-5849(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 31.Guo X, Shin VY, Cho CH. Modulation of heme oxygenase in tissue injury and its implication in protection against gastrointestinal diseases. Life Sci. 2001;69:3113–3119. doi: 10.1016/s0024-3205(01)01417-5. [DOI] [PubMed] [Google Scholar]
- 32.Bach FH. Heme oxygenase-1: a therapeutic amplification funnel. FASEB J. 2005;19(10):1216–1219. doi: 10.1096/fj.04-3485cmt. [DOI] [PubMed] [Google Scholar]
- 33.Ghaziani T, Shan Y, Lambrecht RW, Donohue SE, Pietschmann T, Bartenschlager R, et al. HCV proteins increase expression of heme oxygenase-1 (HO-1) and decrease expression of Bach1 in human hepatoma cells. J Hepatol. 2006;45(1):5–12. doi: 10.1016/j.jhep.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 34.Shan Y, Zheng JY, Moschenross D, Lambrecht RW, Bonkovsky HL. An antagomir of mir-122 down-regulates hepatitis c virus infection and up-regulates herne oxygenase-1 expression in human hepatocytes. Gastroenterology. 2007;132(4):A824. [Google Scholar]
- 35.Protzer U, Seyfried S, Quasdorff M, Sass G, Svorcova M, Webb D, et al. Antiviral activity and hepatoprotection by heme oxygenase-1 in hepatitis B virus infection. Gastroenterology. 2007;133(4):1156–1165. doi: 10.1053/j.gastro.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24(8):449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 37.Fillebeen C, Rivas-Estilla AM, Bisaillon M, Ponka P, Muckenthaler M, Hentze MW, et al. Iron inactivates the RNA polymerase NS5B and suppresses subgenomic replication of hepatitis C virus. J Biol Chem. 2005;280(10):9049–9057. doi: 10.1074/jbc.M412687200. [DOI] [PubMed] [Google Scholar]
- 38.Beinker NK, Viogt MD, Arendse M, Smit J, Stander IA, Kirsch RE. Threshold effect of liver iron content on hepatic inflammation and fibrosis in hepatitis B and C. J Hepatol. 1996;25(5):633–638. doi: 10.1016/s0168-8278(96)80231-5. [DOI] [PubMed] [Google Scholar]
- 39.Kayali Z, Ranguelov R, Mitros F, Shufelt C, Elmi F, Rayhill SC, et al. Hemosiderosis is associated with accelerated decompensation and decreased survival in patients with cirrhosis. Liver Int. 2005;25(1):41–48. doi: 10.1111/j.1478-3231.2005.01022.x. [DOI] [PubMed] [Google Scholar]
- 40.Choi JN, Lee KJ, Zheng YY, Yamaga AK, Lai MMC, Ou JH. Reactive oxygen species suppress hepatitis C virus RNA replication in human hepatoma cells. Hepatology. 2004;39(1):81–89. doi: 10.1002/hep.20001. [DOI] [PubMed] [Google Scholar]
- 41.Waris G, Turkson J, Hassanein T, Siddiqui A. Hepatitis C virus (HCV) constitutively activates STAT-3 via oxidative stress: Role of STAT-3 in HCV replication. J Virol. 2005;79(3):1569–1580. doi: 10.1128/JVI.79.3.1569-1580.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 43.Peterhans E. Reactive oxygen species and nitric oxide in viral diseases. Biol Trace Elem Res. 1997;56(1):107–116. doi: 10.1007/BF02778986. [DOI] [PubMed] [Google Scholar]
- 44.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8(3):240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 45.Tron K, Samoylenko A, Musikowski G, Kobe F, Immenschuh S, Schaper F, et al. Regulation of rat heme oxygenase-1 expression by interleukin-6 via the Jak/STAT pathway in hepatocytes. J Hepatol. 2006;45(1):72–80. doi: 10.1016/j.jhep.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 46.Larrea E, Aldabe R, Molano E, Fernandez-Rodriguez CM, Ametzazurra A, Civeira MP, et al. Altered expression and activation of signal transducers and activators of transcription (STATs) in hepatitis C virus infection: in vivo and in vitro studies. Gut. 2006;55(8):1188–1196. doi: 10.1136/gut.2005.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macdonald A, Harris M. Hepatitis C virus NS5A: tales of a promiscuous protein. J Gen Virol. 2004;85:2485–2502. doi: 10.1099/vir.0.80204-0. [DOI] [PubMed] [Google Scholar]
- 48.Ray RB, Lagging LM, Meyer K, Steele R, Ray R. Transcriptional regulation of cellular and viral promoters by the hepatitis C virus core protein. Virus Res. 1995;37(3):209–220. doi: 10.1016/0168-1702(95)00034-n. [DOI] [PubMed] [Google Scholar]








