Abstract
Toll-like receptor (TLR) ligands and other allergen nonspecific immunostimulatory molecules are ubiquitous in ambient air and have profound modulatory activities in animal models of allergic asthma. However, several of these molecules have been shown to promote exaggerated Th2 biased airway hypersensitivity responses (AHRs), while others attenuate the asthmatic phenotype. Therefore, it has proven difficult to extrapolate experimental results with purified molecules towards a more general understanding of the allergen nonspecific immunomodulatory influence of living environments on the natural history of allergic asthma. These investigations determined how regular and intermittent airway exposures to an unpurified but sterile house dust extract standard (HDEst) affected the ovalbumin (OVA) specific AHR and immune status of previously Th2 sensitized mice. Low dose daily and high dose intermittent HDEst exposures modulated ongoing airway hypersensitivity responses considerably, reducing eosinophil recruitment and methacholine responsiveness, while increasing neutrophilic inflammation. However, only daily airway delivery of low dose HDEst attenuated OVA specific Th2 cytokine production and Th2 biased AHRs to allergen challenge a month later. Finally, while LPS mimicked many of the immunomodulatory characteristics of HDEst in this murine asthma model, daily airway HDEst delivery was highly effective in attenuating the AHR of OVA/alum sensitized TLR4 deficient mice. Taken together, these investigations provide direct evidence that living environments contain allergen nonspecific immunostimulatory molecules that influence the airway hypersensitivity status of allergen-sensitized mice by TLR4 dependent and independent mechanisms.
Keywords: Allergy, Lung, T cells, Rodent
Introduction
Children raised in industrialized countries are far more likely to develop asthma and other atopic diseases than children living in underdeveloped parts of the world. Moreover, prevalence rates have increased dramatically over the last half-century in affected countries, a time span too brief to be accounted for by genetic drift alone (1-3). Therefore, while unproven, it is generally believed that environmental changes associated with the “modern life style” increase a child's risk of becoming allergic (4-6). Consistent with this view, the hygiene hypothesis proposes that children of affluent countries suffer from a deficiency in environmental contact with microbes due to “modern” public health practices (i.e. clean water supplies, sterilized and processed foods, the routine use of antibiotics and vaccines) rendering them at increased risk for dysregulated immunity to allergens ubiquitous in their living environments (3, 7, 8).
Microbes produce a wide variety of molecules that directly activate receptors expressed on cellular constituents of the innate immune system (9). These microbe associated molecular patterns include toll-like receptor (TLR)3 ligands, which can dramatically influence antigen specific immunity. Mice and humans immunized with antigen and immunostimulatory sequence oligodeoxynucleotide (ISS-ODN, TLR9) develop robust Th1 biased adaptive responses and are protected from Th2 biased airway hypersensitivities (3). In contrast, several laboratories have found that mice immunized with antigen and TLR2 ligands, develop Th2 biased adaptive responses and experimental asthma upon antigen challenge (10, 11). Likewise, mice intranasally (i.n.) immunized weekly with antigen and appropriate doses of lipopolysacchride (LPS; TLR4) develop Th2 biased airway hypersensitivities (12, 13). However, if antigen delivery remains weekly while LPS is delivered daily, at 1/7th the adjuvant dose, mice develop short term (13) and long term antigen specific tolerance (manuscript in preparation).
In addition to their study in allergen naïve mice, TLR ligands have been investigated as immunomodulatory agents given to previously Th2 sensitized mice at the time of airway allergen challenge. A single dose of ISS-ODN delivered within 24 hours of allergen challenge has been shown to protect mice from developing Th2 biased hypersensitivity responses in murine models of asthma, allergic rhinitis, and allergic conjunctivitis (14-16). In contrast, several laboratories have found that peptidoglycan (TLR2) and LPS exacerbate experimental asthma when co-delivered to sensitized mice during the allergen challenge period (17-20). However, study results have not always been consistent. For example, Dr Velasco and colleagues reported that airway peptidoglycan or Lipid A (TLR4) administration to Th2 sensitized mice reduced the percentage of eosinophils seen in their airways after allergen challenge (21). Such inconsistencies in the reported effects of TLR2 and TLR4 ligands on the AHR have yet to be reconciled.
The modulatory influence of repeated TLR ligand exposures on ongoing AHRs has not been adequately assessed. However, one study with endotoxin contaminated OVA addressed this issue indirectly (22). In this model, OVA/alum sensitized mice received daily airway challenges with purified (endotoxin free) or commercial (endotoxin contaminated) OVA for 9 days before AHRs were assessed. The investigators found that Th2 sensitized mice airway exposed to purified OVA developed exaggerated Th2 biased AHRs, compared to mice challenged with endotoxin contaminated OVA. This observation was unexpected, as endotoxin exposures are known to induce airway inflammation.
The fact that TLR2, TLR4, and TLR9 ligands can readily modify the allergic phenotype in experimental animals has clinical relevance, as these molecules are ubiquitous in homes and ambient air (23-26). Nonetheless, available evidence suggests that the multitude of TLR dependent and independent immunostimulatory factors to which infants are daily exposed have both synergistic and antagonistic immunomodulatory effects on atopic status. This is a major impediment to extrapolating results of laboratory studies with purified molecules towards a more unifying and predictive model of how relevant ambient exposures influence the development and persistence of allergic respiratory diseases.
Recognizing the molecular complexity of the world in which we live and that ambient immunostimulatory particulates of clinical relevance collect in house dust, we have begun to characterize the immunostimulatory activities of unpurified but sterile house dust extracts (HDEs) (13, 27, 28). Previous studies have found that HDE activation of bone marrow derived dendritic cells is partially dependent on TLR2, TLR4, and TLR9, and almost completely dependent on MyD88, a molecule involved in signaling through all TLRs except TLR3 (28). Moreover, as with LPS, weekly i.n. ovalbumin (OVA) vaccinations with HDEs derived from 10 homes consistently primed mice to develop Th2 biased hypersensitivities, while daily HDE delivery at 1/7th the adjuvant dose, rendered mice resistant to OVA sensitization (13). These studies suggest that airway exposures to allergen nonspecific immunostimulants ubiquitous in living environments have the potential to either promote the development of Th2 biased hypersensitivities or tolerance to ambient aeroallergens, depending on the level and frequency of exposures. Furthermore, as LPS and HDEs were found to have similar Th2 adjuvant and tolerogenic activities, these investigations left open the possibility that LPS was responsible for a majority of immunomodulatory activities associated with HDEs and their homes of origin.
While children with allergic asthma continually inspire air containing the allergen nonspecific immunostimulatory constituents of HDEs, it remains to be determined whether these exposures modify pre-existing aeroallergen hypersensitivities and pulmonary responses to aeroallergen encounter. The current investigations characterized how intermittent and daily i.n. delivery of a HDEst affected the AHRs and immune profiles of previously Th2 sensitized mice. Treatment with i.n. HDEst was found to have both rapid and long-lasting effects on pulmonary responses to allergen challenge. As in previous studies, dose and dosing intervals proved important variables in determining the modulatory influence of HDEst on pre-existing Th2 biased airway hypersensitivities. Finally, experiments with TLR4 deficient mice established that daily i.n. HDEst exposures attenuated AHRs, at least in part, by TLR4 independent mechanisms.
Materials and Methods
Mice, OVA, and purified LPS
Investigations received prior approval from our institution's animal welfare committee. Female mice aged 4-6 weeks were used for all studies. BALB/c and C57BL/6 mice were purchased from Harlan Sprague Dawley (Indianapolis, IN) and TLR4 knock out (ko) mice were bred in our animal facility. Except for experiments with TLR4 ko mice (C57BL/6 background), BALB/c mice were used in all investigations. OVA (Grade VI; Sigma, St Louis, MO) and E. coli 026-B6 LPS (Sigma; 10EU = 1ng) were purchased from commercial vendors.
Preparation of individual HDEs and the HDE standard
With approval from our institution's human subjects committee, dust samples were obtained by vacuuming a single carpeted bedroom in each of fifteen suburban homes in San Diego County, California. Methods used for the collection and processing of house dust have been described in detail previously (28). Briefly, study bedrooms were left un-vacuumed for 1 week before exposed carpeting was vacuumed with a Quick Broom™. (Hoover, Canton, OH) for five minutes. Collected house dust was then run through a course sieve to remove large particulate matter and suspended in sterile PBS at 100mg/ml. House dust suspensions were then placed on a rotor at room temperature for 18 hours, filtered through glass wool, and finally through 0.22μm Steriflip™ filters (Millipore, Bedford, MA) to obtain sterile HDEs. In previously published studies we compared the relative bioactivities of these HDEs (28). For the current studies, eight of the HDEs found to be highly bioactive were combined in order to prepare a large volume of high bioactivity HDEst that could be used for all experiments presented in this paper. The endotoxin concentration of the HDEst discussed herein was determined with the QCL-1000 kit (Bio-Whittaker, Walkersville, MD) according to the manufacturer's instructions.
Ova sensitization and airway challenge
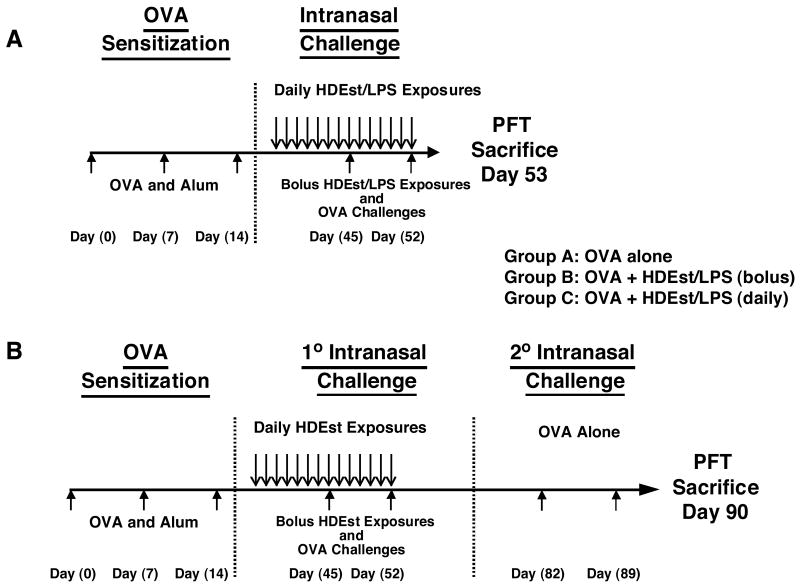
Mice (n=4/group) were initially Th2 sensitized by three weekly i.p. injections of OVA (100μg) and alum (1mg) in a volume of 500μl PBS, as outlined in Figure 1. One month after the final injection, mice received two i.n. OVA (10μg) challenges, delivered seven days apart, in a volume 30μl PBS, divided equally and delivered bilaterally to each nare. In each experiment, one group of mice additionally received 21μl of HDEst or 70ng of LPS (700EU) with each OVA challenge. Another group of mice began receiving i.n. HDEst (3μl) or LPS (10ng or 70EU) on a daily basis, beginning 7 days before the first and ending with the last OVA challenge. A third control group of OVA challenged HDEst/LPS naïve mice was included in each experiment. Mice were lightly anesthetized (isoflurane, Abbott Laboratories, North Chicago, IL) before i.n. delivery of all reagents. For experiments described in Figures 2, 4, and 5, outcome parameters were assessed 24 hours after the second airway OVA challenge (Figure 1A). For experiments presented in Figure 3, mice were observed for an additional month before receiving a second round of OVA challenges without HDEst co-administration (Figure 1B).
Figure 1. Th2 sensitization, allergen challenge, and HDEst/LPS delivery schedules.
Naïve mice (n=4 per group) were Th2 sensitized by three weekly i.p. injections of OVA and alum. Airway OVA challenges were initiated one month after the final OVA/alum injection. Seven days before the first of two weekly i.n. OVA challenges, one group of mice began receiving low dose i.n. HDEst (3μl) or LPS (10ng) on a daily basis, the last dose being delivered with the final OVA challenge. Another group of Th2 sensitized mice received a high dose HDEst (21μl) or LPS (70ng) bolus concurrently with each i.n. OVA challenge. A) For experiments presented in Figures 2, 4, and 5, airway hypersensitivity and BLN cytokine responses were assessed 24 hours after the second OVA challenge. B) For experiments described in Figure 3, mice were observed for an additional month before receiving a second series of OVA challenges (2° challenge), and outcome parameters were assess 24 hours after the final i.n. OVA challenge.
Figure 2. Concurrent airway HDEst exposures modify allergen induced AHRs.
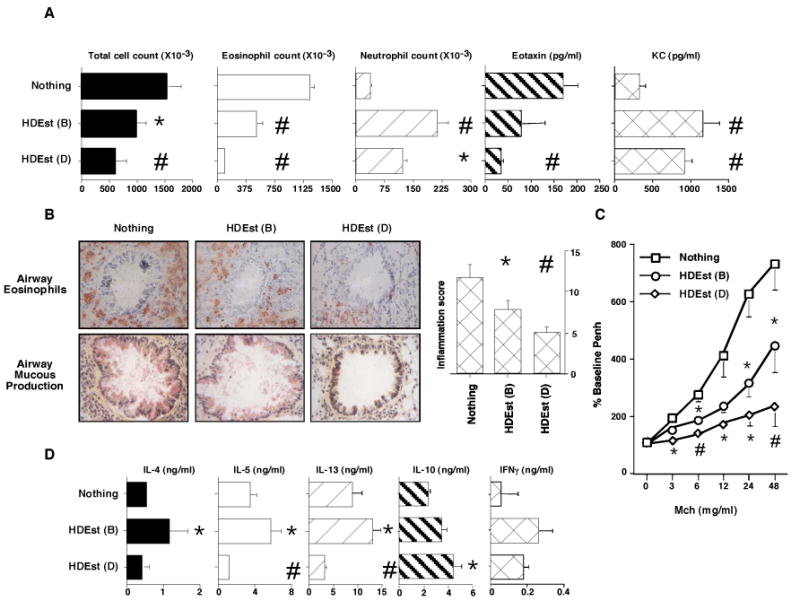
OVA/alum sensitized BALB/c mice (n=4 per group) were i.n. allergen challenged. Select groups of mice received i.n. HDEst during the OVA challenge period in accord with the bolus (B) and daily (D) delivery schedules described in Figure 1A. AHRs and OVA induced BLN cytokine responses were assessed 24 hours later. Reported results were reproduced in three independent experiments and bar and line graph data points present mean values with standard errors. * =P≤0.05 and # =P≤0.01 for HDEst treated versus HDEst untreated mice. A) BALF total cell, eosinophil, neutrophil, counts, and eotaxin and KC levels. B) Lung histology and inflammation scores. C) Airway Mch sensitivity. D) OVA induced BLN cell cytokine production.
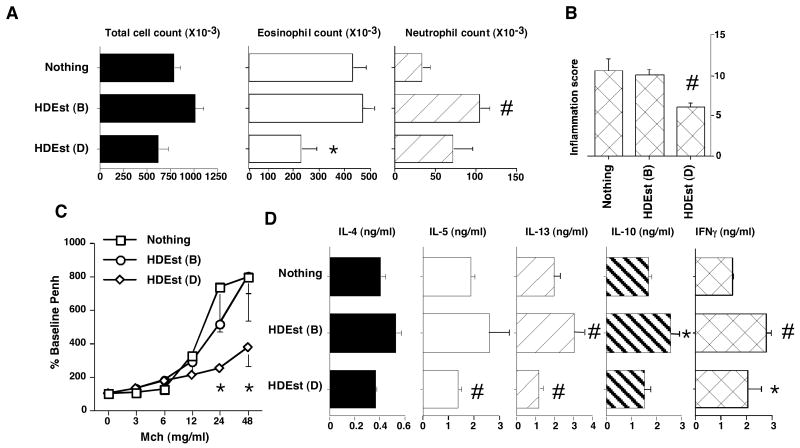
Figure 4. Airway LPS exposures modify allergen induced AHRs.
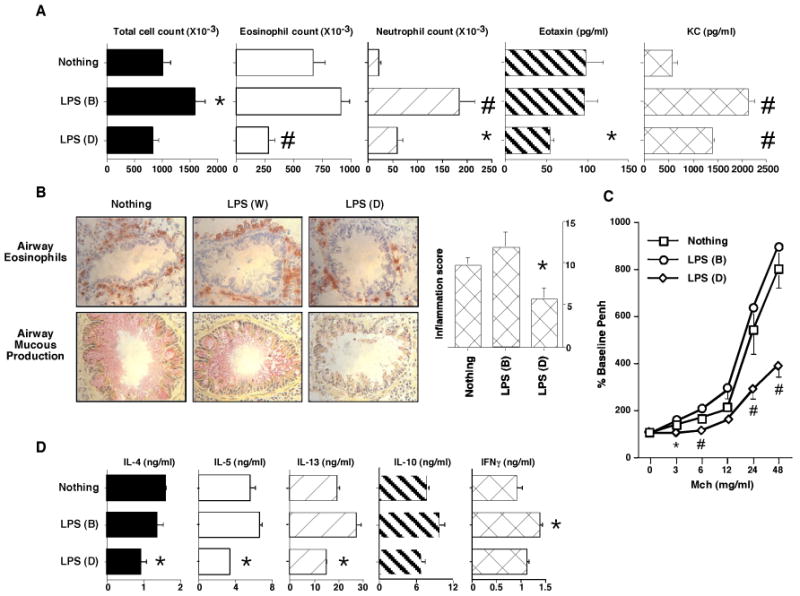
OVA/alum sensitized BALB/c mice (n=4 per group) were i.n. allergen challenged. Select groups of mice received i.n. LPS during the OVA challenge period in accord with the bolus (B) and daily (D) delivery schedules described in Figure 1A. AHRs and OVA induced BLN cytokine responses were assessed 24 hours later. Reported results were reproduced in two independent experiments and bar and line graph data points present mean values with standard errors. * =P≤0.05 and # =P≤0.01 for LPS treated versus LPS untreated mice. A) BALF total cell, eosinophil, neutrophil, counts, and eotaxin and KC levels. B) Lung histology and inflammation scores. C) Airway Mch sensitivity. D) OVA induced BLN cell cytokine production.
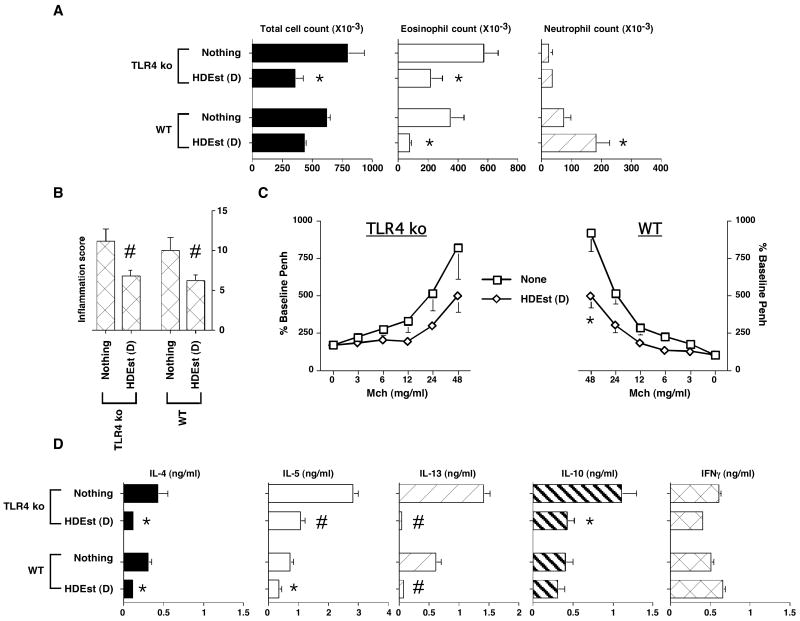
Figure 5. Daily airway HDEst exposures modify allergen induced AHRs in TLR4 deficient mice.
OVA/alum sensitized WT (C57BL/6) and TLR4 deficient (C57BL/6 background) mice (n=4 per group) were i.n. allergen challenged. Select mouse groups received i.n. HDEst during the OVA challenge period in accord with the bolus (B) and daily (D) delivery schedules described in Figure 1A. AHRs and OVA induced BLN cytokine responses were assessed 24 hours later. Reported results were reproduced in a second experiment. Bar and line graph data points present mean values with standard errors. * =P≤0.05 and # =P≤0.01 for HDEst treated versus HDEst untreated mice. A) BALF total cell, eosinophil, neutrophil, counts. B) Lung inflammation scores. C) Airway Mch sensitivity. D) OVA induced BLN cell cytokine production.
Figure 3. Intranasal HDEst exposures during primary AHRs lead to long-lived changes in allergen responsiveness.
OVA/alum sensitized BALB/c mice (n=4 per group) received primary i.n. allergen challenges and select mouse groups received i.n. HDEst in accord with the bolus (B) and daily (D) delivery schedules described in Figure 1B. Mice had secondary i.n. OVA challenges delivered 30 days later and responses were assessed 24 hours after the last. Reported results were reproduced in a second experiment. Bar and line graph data points present mean values with standard errors. * =P≤0.05 and # =P≤0.01 for HDEst treated versus HDEst untreated mice. A) BALF total cell, eosinophil, neutrophil, counts. B) Lung inflammation scores. C) Airway Mch sensitivity. D) OVA induced BLN cell cytokine production.
Assessment of AHRs
Airway responsiveness to methacholine (Mch) was assessed with a single-chamber whole-body plethysmograph from Buxco (Troy, NY). Mice were exposed to increasing concentrations of nebulized Mch (Sigma; 3–48 mg/ml) by Aerosonic ultrasonic nebulizer (DeVilbiss) and the percent increase in Penh from baseline for each Mch challenge dose was determined. After Mch challenge, mice were sacrificed, lungs were lavaged with 800μl of PBS, and BALF collected and centrifuged. Supernatants were saved for chemokine ELISA. Cell pellets were resuspended in 1ml of PBS and total BALF cell counts determined with a hemocytometer. In addition, BALF cytospins were prepared, slides fixed in acetone, and then Wright-Giemsa stained. A blinded observer determined the percentage of eosinophils, neutrophils, and mononuclear cells on each slide by counting a minimum of 200 cells in random high-power fields with a light microscope. Lung tissue was flash frozen, cryo-sectioned, acetone-fixed onto poly-L-lysine-coated slides, and stained with hematoxylin-eosin, peroxidase/DAB, and PAS, by standardized techniques. To quantitate peribronchial inflammation, eosinophil infiltration, and airway mucous production, a scoring system (0-5) was devised in which a blinded observer scored 4-8 airways per mouse for each of these parameters. Mean inflammation scores were determined by averaging the total cellular infiltration, eosinophil infiltration, and airway mucous production scores for each mouse group and combining them to generate a total score (0-15). Experimental techniques used for these analyses are further described in our previous publications (10, 29).
BALF chemokine and OVA specific cytokine responses
BALF KC and eotaxin levels were determined with R and D systems reagents (Minneapolis, MN) according to the manufacturer's instructions. OVA specific bronchial lymph node (BLN) cytokine responses were assessed by previously published methods (10, 29). Briefly, BLNs harvested from each group of experimental mice were pooled and single cell suspensions prepared by enzymatic digestion with Collagenase VIII (300U/ml Sigma) and DNase-I (1.5 μg/ml; Sigma). BLN cells were cultured in triplicate at 1 X106 cells/ml in media with or without OVA (50μg/ml) for 72 hours prior to harvesting supernatants. Interleukin-4, -5, -10, -13, and IFNγ levels in culture supernatants were determined by ELISA using Pharmingen reagents (San Diego, CA), according to the manufacturer's recommendations. BLN cytokine responses were calculated by subtracting background cytokine production from responses of BLN cells cultured with OVA.
Statistical considerations
Statistical analyses were conducted using Statview™ software. Two tailed unpaired Student's t tests were used to analyze all data. Outcome measures for mice receiving HDEst/LPS by the daily or intermittent delivery schedules were compared to those of mice that remained HDEst/LPS naïve, The Bonferroni correction factor was included in the calculation of p values, to account for the increased probability of type-I errors when multiple groups are statistically compared. Results were considered statistically significant if p values were ≤ 0.05 (*) or ≤ 0.01 (#).
Results
Intranasal HDEst exposures during allergen challenge modify the AHR
To assess the immunomodulatory influence of intermittent and daily airway HDEst exposures on allergen induced AHRs, experiments were conducted in accord with a schedule outlined in Figure 1A. Mice were first Th2 sensitized to OVA. Airway OVA challenges were initiated a month after the final sensitization. One group of mice received a high dose HDEst bolus concurrently with each i.n. OVA challenge. Another group of mice received low dose i.n. HDEst (1/7th bolus dose) on a daily basis beginning seven days before the first and ending with the final OVA challenge. Bolus and daily delivery schedules were standardized to provide the same total amount of HDEst to mice over the course of the experiment.
In one representative experiment, mice receiving daily i.n. HDEst during the airway allergen challenge period had mean reductions of approximately 58% and 92% in their BALF total cell and eosinophil counts and a 233% average increase in BALF neutrophil counts, compared to sensitized mice challenged with OVA alone (Figure 2A). Consistent with these findings, levels of eotaxin, a eosinophil specific chemokine, were reduced 79%, and levels of KC, a neutrophil specific chemokine, were increased 180% in BALF recovered from daily HDEst treated versus HDE naïve mice. Evaluation of lung histology confirmed that daily i.n. HDEst delivery during the OVA challenge period reduced total cellular accumulation, eosinophilic inflammation, mucous secretion, and goblet cell hyperplasia in and around the airways, with a 57% mean reduction in inflammation scores, compared to those of HDEst non-treated mice (Figure 2B). Although less sensitive and specific than invasive measures of bronchial hyper-responsiveness, which were unavailable at the time of these investigations, Penh measurements further demonstrated that daily HDEst treated mice were less responsive to inhaled Mch than HDEst unexposed mice (Figure 2C).
Compared to HDEst delivery by the daily low dose schedule, intermittent high dose HDEst delivery was less effective, but also attenuated features of the Th2 biased AHR. BALF analyses demonstrated 34% and 59% average reductions in total cell and eosinophil counts compared to BALF from HDEst unexposed mice, while neutrophil counts rose 476%. In line with these findings, mean BALF eotaxin and KC levels for mice treated with intermittent high dose HDEst were 53% lower and 225% higher, respectively, than for control mice. Lung sections from mice receiving HDEst only on airway OVA challenge days also displayed reductions in airway inflammation compared to those of HDEst unexposed mice (average inflammation score reduced 34%) and their airways were less sensitive to Mch inhalation.
Consistent with changes noted in their AHRs, BLN cells from mice receiving daily i.n. HDEst during the airway allergen challenge period produced lower levels of the pro-asthmatic cytokines, IL-4 (19%), IL-5 (65%), and IL-13 (68%) and higher levels of cytokines antagonistic to the Th2 biased AHR, i.e. IL-10 (77%) and IFNγ (220%), than BLN cells from control mice (Figure 2D). In contrast, BLN cells from mice receiving intermittent high dose HDEst with each challenge dose of OVA produced higher levels of IL-4 (104%), IL-5 (62%), IL-13 (49%), IL-10 (38%), and IFNγ (370%) than BLN cells from HDEst unexposed mice. Interestingly, despite reproducible and significant differences in cytokine production, at sacrifice, the OVA specific serum IgE, IgG1, and IgG2a levels of mice from experimental and control groups were similar.
Intranasal HDEst delivery during primary allergen challenges leads to persistent changes in airway allergen responsiveness
To determine if the modulatory effects of daily and bolus i.n. HDEst exposures on AHRs were long-lived, mice described in Figure 2 were not sacrificed after the primary airway allergen challenge period, but instead, were observed for an additional month before receiving a second round of OVA challenges without HDEst, as depicted in Figure 1B. While effects on primary challenge responses were more dramatic, after secondary allergen challenge, BALF from mice receiving daily i.n. HDEst continued to display reductions in total cell (22%) and eosinophil (50%) counts and increases in neutrophil (115%) counts compared to HDEst untreated mice (Figure 3A). Likewise, lung sections from daily HDEst treated mice had quantifiable reductions in airway inflammation (Figure 3B; mean inflammation score reduced 43%) and their airway responses to Mch inhalation were attenuated (Figure 3C) compared to HDEst untreated mice. In contrast, mice receiving intermittent i.n. HDEst during the primary OVA challenge period had increased BALF total cell (22%) and eosinophil (10%) counts, and substantially increased neutrophil (215%) counts compared to control mice. However, little difference was appreciated in the lung section inflammation scores and Mch sensitivity of bolus HDEst treated and HDEst untreated mice.
In addition to long-lived modifications in their AHRs, OVA stimulated BLN cells from Th2 sensitized mice receiving daily low dose HDEst during the primary allergen challenge period produced 10-40% less IL-4, IL-5, IL-13, and IL-10 than BLN cells from HDEst unexposed mice, while IFNγ production was 42% higher. In contrast, BLN cells from Th2 sensitized mice exposed to high dose HDEst intermittently during the primary OVA challenge period produced 33-92% higher levels of all cytokines measured than BLN cells from HDEst naïve mice.
Bolus and daily intranasal LPS exposures attenuate and augment AHRs, respectively
Additional investigations determined whether the modulatory influence of HDEst on experimental asthma could be replicated with purified LPS. The endotoxin content of the HDEst was first determined by limulus lysate assay. Bolus (21μl) and daily (3μl) HDEst delivery doses used in Figure 2 experiments were found to contain the equivalent of approximately 700EU and 100EU (70ng and 10ng) of LPS, respectively. Therefore, these LPS doses were used to conduct studies analogous to those executed with HDEst (Figure 1A and 2). In one representative experiment, BALF samples from mice receiving daily i.n. LPS during the airway allergen challenge period had 17%, 58%, and 44% mean reductions in total cell and eosinophil counts and eotaxin levels, while neutrophil counts and KC levels increased by 163% and 141% compared to LPS untreated mice, respectively (Figure 4A). Histological evaluation of lung sections confirmed that daily LPS treated mice had fewer inflammatory changes than LPS naïve mice (Figure 4B; average inflammation score reduced 43%), and pulmonary function testing demonstrated reductions in Mch sensitivity (Figure 4C). Unlike daily LPS delivery, intermittent LPS delivery only on OVA challenge days consistently provoked increased BALF total cell (60%) and eosinophil counts (36%), and lung inflammation scores (23%), while BALF eotaxin levels and airway responses to inhaled Mch were similar to those of LPS unexposed mice. Likewise, intermittent LPS delivery led to dramatic increases in allergen challenge induced BALF neutrophil (700%) counts and KC (269%) levels, compared to those of control mice,
Along with attenuating characteristic features of the Th2 biased AHR, daily LPS delivery was found to inhibit pro-inflammatory Th2 cytokine production by OVA stimulated BLN cells harvested from experimental mice (IL-4, IL-5, and IL-13 responses reduced 45%, 50%, and 25%, respectively), while IL-10 production was relatively preserved and IFNγ production increased 18%. (Figure 4D). In juxtaposition, with the exception of IL-4 (production reduced 17%), BLN cells from mice, treated with LPS only on OVA challenge days produced modestly increased amounts of IL-5, IL-13, IL-10, and IFNγ, (15%, 42%, 28%, and 52% respectively) compared to BLN cells from LPS naive mice.
Daily intranasal HDEst exposures modify allergen induced AHRs by TLR4 deficient mice
Despite determining the endotoxin content of HDEst and using LPS at equivalent doses, HDEst proved more effective than LPS at protecting against allergen induced Th2 hypersensitivity responses, by both the daily and intermittent delivery schedules (Figures 2 versus 4). These observations led us to speculate that aside from LPS, HDEst might contain additional immunostimulatory molecules that contributed to its protective influence on Th2 biased airway hypersensitivities. To test this hypothesis, wild type (WT; C57BL/6) and TLR4 knock out (ko; C57BL/6 background) mice were Th2 sensitized and challenged with OVA, as outlined in Figure 1A, with experimental groups receiving, daily low dose or no HDEst during the airway challenge period, as in Figure 2 experiments.
As seen with BALB/c mice, daily i.n. HDEst delivery attenuated the AHR of OVA sensitized C57BL/6 mice undergoing airway OVA challenge (Figure 5). Moreover, TLR4 deficient and competent mice treated with daily i.n. HDEst delivery had similar reductions in BALF total cell (56% versus 30%) and eosinophil (62% versus 80%) counts compared to their corresponding controls (Figure 5A). However, daily HDEst exposures during the allergen challenge period elicited far smaller increases in BALF neutrophil counts in TLR4 ko than in WT mice (54% versus 148%). As in experiments presented earlier, histological analyses (Figure 5B) and pulmonary function testing (Figure 5C) confirmed that daily i.n. HDEst delivery reduced airway inflammation and bronchial hyper-responsiveness to Mch in both TLR4 ko and WT mice. Moreover, BLN cells from TLR4 ko and WT mice treated by daily i.n. HDEst delivery demonstrated similar changes in OVA induced BLN cell cytokine production compared to their respective controls (Figure 5D).
Discussion
These investigations considered how airway exposures to ambient allergen nonspecific immunostimulants modify allergen induced AHRs of previously Th2 sensitized mice. Study results established that local delivery of the immunostimulatory contents of living environments, in the form of HDEst, had a long-lived effect on airway responses to allergen challenge and allergen specific immunity. Delivery schedule and dose proved important variables in determining how HDEst exposures impacted on relevant outcome variables. Further experiments demonstrated that in addition to LPS, HDEst contained immunostimulatory molecules that modified allergen induced AHRs by TLR4 independent mechanisms.
Experiments presented in Figure 2 established that concurrent airway HDEst exposures during allergen challenge attenuated outcome measures of the Th2 biased AHR, while increasing airway neutrophilia. Recognizing that human airways are regularly exposed to the contents of HDEst, these findings may help explain why airway neutrophilia is far more prominent in asthma patients than in mouse models that depend on allergen/alum sensitization and subsequent airway challenges with allergen alone (30, 31). Furthermore, these experimental results highlight the impact that allergen non-specific immunostimulants ubiquitous in living environments can have on the functionality of cells that contribute to allergic asthma.
Another finding presented in Figure 2 is that the same total dose of HDEst divided into two (bolus) or fourteen (daily) treatments had qualitatively different modulatory effects on outcome measures in this murine experimental asthma model. For example, i.n. HDEst delivery by the daily schedule was consistently more effective than by the bolus schedule in attenuating all outcome measures associated with the Th2 biased AHR, while bolus delivery was more effective at inducing airway neutrophilia. Likewise, only daily HDEst delivery led to decreases in BLN Th2 cytokine production, while bolus HDEst delivery led to increased BLN cell production of all cytokines measured. These experimental results are of potential clinical relevance, as air-sampling studies demonstrate that while the content of endotoxin and other allergen nonspecific immunostimulants in ambient air can vary by 5 logs or more, human airways are generally exposed to relatively low levels of ambient immunostimulants on a continuous basis (23, 32).
The cellular and molecular mechanisms by which HDEst exposures modify responses of Th2 sensitized mice undergoing concurrent airway allergen challenges require additional characterization. Nonetheless, we have observed that within hours of i.n. HDE delivery, a “cytokine storm” develops in the airways of allergen naïve mice. Cytokines released include IL-12 (13), IL-10, IL-17, and IL-23 (unpublished), all of which have the potential to inhibit features of the Th2 biased AHR and/or promote neutrophil recruitment (33-35). Therefore, the local cytokine milieu created by airway HDE delivery may temporarily inhibit airway responses to allergen challenge. In support of this view, a previous report found that ISS-ODN exposures inhibited allergen induced conjunctivitis by an IL-12 dependent mechanism (16). Airway ISS-ODN delivery during allergen challenge has also been shown to compromise the ability of resident dendritic cells to present antigen and support Th2 effector cell responses (36). Given that dendritic cell activation by HDEs is largely TLR dependent (28), it is reasonable to suggest that airway HDE exposures may also inhibit Th2 biased AHRs by modifying the functional characteristics of dendritic cells and other antigen presenting cells within the lungs and their draining lymph nodes. Alternatively, a growing body of literature suggests that CD4 cells and in particular, T regulatory cells and activated effector CD4 cells, express TLRs and can respond directly to TLR ligands(37-39). Therefore, the capacity of HDEs to modify the allergen induced AHR, could in part be due to direct effects on TLR expressing CD4 cells. These considerations are the focus of ongoing and planned investigations.
Additional experiments demonstrated that compared to HDEst unexposed mice, Th2 sensitized mice receiving daily i.n. HDEst during a primary series of airway allergen challenges continued to display modest reductions in all outcome measures of the Th2 AHR and elevated BALF neutrophil counts when challenged with allergen alone, a month later. In contrast, secondary airway allergen challenge outcome measures of mice receiving bolus HDEst during the initial challenge period were equivalent to or greater then those of control mice. Consistent with these findings, BLN cells from daily and bolus HDEst exposed mice continued to have attenuated and augmented Th2 cytokine responses after secondary airway allergen challenge, respectively. Design considerations limited the number of OVA/HDEst co-exposures given to mice in these experiments. Nonetheless, we speculate that immunological themes identified in these studies could have a far more profound influence on clinical manifestations of allergic asthma for patients exposed to ubiquitous aeroallergens and allergen non-specific immunostimulants on a semi-continuous basis.
Despite mimicking the anti-asthmatic activities of HDEst, when delivered at endotoxin dose equivalence , LPS was found to be generally less effective at attenuating outcome measures of the Th2 biased AHR (Figure 4 versus Figure 2). This suggested HDEst might contain molecules capable of modifying the AHR by TLR4 independent mechanisms. The impression was confirmed in a final series of experiments in which daily HDEst delivery was observed to be highly effective in attenuating outcome measures of experimental asthma and the BLN cell Th2 cytokine responses of TLR4 ko mice. However, BALF neutrophil increases associated with daily HDEst delivery in WT mice, were greatly attenuated in TLR4 ko mice. These results established that LPS is not the only immunostimulatory molecule within HDEst responsible for its protective influence on the Th2 biased AHR but that it had a major role in recruiting neutrophils to the airways of mice treated with HDEst.
Experimental results presented in this paper demonstrate that airway exposures to allergen nonspecific immunostimulants contained in HDEst and ubiquitous in living environments modify the allergen induced AHR of Th2 sensitized mice for a month or more. We previously published that dendritic cells respond to HDEs by mechanisms that are partially TLR2, TLR4, and TLR9 and largely MyD88 dependent (28). Additional studies demonstrated that weekly airway HDE delivery provided MyD88 dependent Th2 adjuvant activity in naïve mice receiving concurrent i.n. OVA vaccinations, while daily HDE delivery promoted both short-term (13) and long-term (unpublished) OVA specific tolerance. These observations are consistent with results described herein and lead us to suggest that the immunomodulatory potential of living environments is a sword that cuts both ways in the natural history of allergic respiratory diseases. Our ongoing investigations suggest that the absolute level and frequency of airway exposures to ambient TLR ligands and potentially other allergen non-specific immunostimulants will prove critically important variables in determining their net influence on the genesis and duration of aeroallergen driven diseases.
Acknowledgments
We thank Drs Shizuo Akira and Maripat Corr for the generation and breeding of TLR4 deficient mice used in these experiments, respectively.
Footnotes
This work was supported with grant AI061772 from the National Institutes of Health
Abbreviations used in this paper: TLR, toll-like receptor; AHR, airway hypersensitivity response; i.n., intranasal; ISS-ODN, immunostimulatory sequence oligodeoxynucleotide; LPS. lipopolysacchride; HDE, house dust extract; OVA, ovalbumin; HDEst, house dust extract standard; BLN, bronchial lymph node; WT, wild type; knock out, ko
References
- 1.Linneberg A, Gislum M, Johansen N, Husemoen LL, Jorgensen T. Temporal trends of aeroallergen sensitization over twenty-five years. Clin Exp Allergy. 2007;37:1137–1142. doi: 10.1111/j.1365-2222.2007.02760.x. [DOI] [PubMed] [Google Scholar]
- 2.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbami LJ. National surveillance for asthma--United States, 1980-2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 3.Horner AA. Toll-like receptor ligands and atopy: a coin with at least two sides. J Allergy Clin Immunol. 2006;117:1133–1140. doi: 10.1016/j.jaci.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 4.Tse K, Horner AA. Defining a role for ambient TLR ligand exposures in the genesis and prevention of allergic diseases. Semin Immunopathol. 2008;30:53–62. doi: 10.1007/s00281-007-0098-8. [DOI] [PubMed] [Google Scholar]
- 5.Zeldin DC, Eggleston P, Chapman M, Piedimonte G, Renz H, Peden D. How exposures to biologics influence the induction and incidence of asthma. Environ Health Perspect. 2006;114:620–626. doi: 10.1289/ehp.8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Mutius E. Environmental factors influencing the development and progression of pediatric asthma. J Allergy Clin Immunol. 2002;109:S525–532. doi: 10.1067/mai.2002.124565. [DOI] [PubMed] [Google Scholar]
- 7.Janson C, Asbjornsdottir H, Birgisdottir A, Sigurjonsdottir RB, Gunnbjornsdottir M, Gislason D, Olafsson I, Cook E, Jogi R, Gislason T, Thjodleifsson B. The effect of infectious burden on the prevalence of atopy and respiratory allergies in Iceland, Estonia, and Sweden. J Allergy Clin Immunol. 2007;120:673–679. doi: 10.1016/j.jaci.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Floistrup H, Swartz J, Bergstrom A, Alm JS, Scheynius A, van Hage M, Waser M, Braun-Fahrlander C, Schram-Bijkerk D, Huber M, Zutavern A, von Mutius E, Ublagger E, Riedler J, Michaels KB, Pershagen G, The Parsifal Study Group Allergic disease and sensitization in Steiner school children. J Allergy Clin Immunol. 2006;117:59–66. doi: 10.1016/j.jaci.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Chisholm D, Libet L, Hayashi T, Horner AA. Airway peptidoglycan and immunostimulatory DNA exposures have divergent effects on the development of airway allergen hypersensitivities. J Allergy Clin Immunol. 2004;113:448–454. doi: 10.1016/j.jaci.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, Maliszewski C, Akira S, Pulendran B, Agrawal S, Doughty B, Gerwitz A, Blenis J. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J Immunol. 2004;172:4733–4743. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 12.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng N, Lam D, Paulus P, Batzer G, Horner AA. House dust extracts have both Th2 adjuvant and tolerogenic activities. J Allergy Clin Immunol. 2006;117:1074–1081. doi: 10.1016/j.jaci.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Broide D, Schwarze J, Tighe H, Gifford T, Nguyen MD, Malek S, Van Uden J, Martin-Orozco E, Gelfand EW, Raz E. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol. 1998;161:7054–7062. [PubMed] [Google Scholar]
- 15.Rhee CS, Libet L, Chisholm D, Takabayashi K, Baird S, Bigby TD, Lee CH, Horner AA, Raz E. Allergen-independent immunostimulatory sequence oligodeoxynucleotide therapy attenuates experimental allergic rhinitis. Immunology. 2004;113:106–113. doi: 10.1111/j.1365-2567.2004.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magone MT, Chan CC, Beck L, Whitcup SM, Raz E. Systemic or mucosal administration of immunostimulatory DNA inhibits early and late phases of murine allergic conjunctivitis. Eur J Immunol. 2000;30:1841–1850. doi: 10.1002/1521-4141(200007)30:7<1841::AID-IMMU1841>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto K, Kawamura I, Ito J, Mitsuyama M. Modification of allergic inflammation in murine model of rhinitis by different bacterial ligands: involvement of mast cells and dendritic cells. Clin, Exp Allergy. 2006;36:760–769. doi: 10.1111/j.1365-2222.2006.02488.x. [DOI] [PubMed] [Google Scholar]
- 18.Murakami D, Yamada H, Yajima T, Masuda A, Komune S, Yoshikai Y. Lipopolysaccharide inhalation exacerbates allergic airway inflammation by activating mast cells and promoting Th2 responses. Clin Exp Allergy. 2007;37:339–347. doi: 10.1111/j.1365-2222.2006.02633.x. [DOI] [PubMed] [Google Scholar]
- 19.Komlosi ZI, Pozsonyi E, Tabi T, Szoko E, Nagy A, Bartos B, Kozma GT, Tamasi L, Orosz M, Magyar P, Losonczy G. Lipopolysaccharide exposure makes allergic airway inflammation and hyper-responsiveness less responsive to dexamethasone and inhibition of iNOS. Clin Exp Allergy. 2006;36:951–959. doi: 10.1111/j.1365-2222.2006.02514.x. [DOI] [PubMed] [Google Scholar]
- 20.Nigo YI, Yamashita M, Hirahara K, Shinnakasu R, Inami M, Kimura M, Hasegawa A, Kohno Y, Nakayama T. Regulation of allergic airway inflammation through Toll-like receptor 4-mediated modification of mast cell function. Proc Natl Acad Sci U S A. 2006;103:2286–2291. doi: 10.1073/pnas.0510685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velasco G, Campo M, Manrique OJ, Bellou A, He H, Arestides RS, Schaub B, Perkins DL, Finn PW. Toll-like receptor 4 or 2 agonists decrease allergic inflammation. Am J Respir Cell Mol Biol. 2005;32:218–224. doi: 10.1165/rcmb.2003-0435OC. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe J, Miyazaki Y, Zimmerman GA, Albertine KH, McIntyre TM. Endotoxin contamination of ovalbumin suppresses murine immunologic responses and development of airway hyper-reactivity. J Biol Chem. 2003;278:42361–42368. doi: 10.1074/jbc.M307752200. [DOI] [PubMed] [Google Scholar]
- 23.Rabinovitch N, Liu AH, Zhang L, Rodes CE, Foarde K, Dutton SJ, Murphy JR, Gelfand EW. Importance of the personal endotoxin cloud in school-age children with asthma. J Allergy Clin Immunol. 2005;116:1053–1057. doi: 10.1016/j.jaci.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 24.Roy SR, Schiltz AM, Marotta A, Shen Y, Liu AH. Bacterial DNA in house and farm barn dust. J Allergy Clin Immunol. 2003;112:571–578. doi: 10.1016/s0091-6749(03)01863-3. [DOI] [PubMed] [Google Scholar]
- 25.Gehring U, Heinrich J, Hoek G, Giovannangelo M, Nordling E, Bellander T, Gerritsen J, de Jongste JC, Smit HA, Wichmann HE, Wickman M, Brunekreef B. Bacteria and mould components in house dust and children's allergic sensitisation. Eur Respir J. 2007;29:1144–1153. doi: 10.1183/09031936.00118806. [DOI] [PubMed] [Google Scholar]
- 26.van Strien RT, Engel R, Holst O, Bufe A, Eder W, Waser M, Braun-Fahrlander C, Riedler J, Nowak D, von Mutius E. Microbial exposure of rural school children, as assessed by levels of N-acetyl-muramic acid in mattress dust, and its association with respiratory health. J Allergy Clin Immunol. 2004;113:860–867. doi: 10.1016/j.jaci.2004.01.783. [DOI] [PubMed] [Google Scholar]
- 27.Batzer G, Lam DP, Paulus P, Boasen J, Ng N, Horner AA. Using house dust extracts to understand the immunostimulatory activities of living environments. Immunobiology. 2007;212:491–498. doi: 10.1016/j.imbio.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boasen J, Chisholm D, Lebet L, Akira S, Horner AA. House dust extracts elicit Toll-like receptor-dependent dendritic cell responses. J Allergy Clin Immunol. 2005;116:185–191. doi: 10.1016/j.jaci.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Takabayashi K, Libet L, Chisholm D, Zubeldia J, Horner AA. Intranasal immunotherapy is more effective than intradermal immunotherapy for the induction of airway allergen tolerance in th2-sensitized mice. J Immunol. 2003;170:3898–3905. doi: 10.4049/jimmunol.170.7.3898. [DOI] [PubMed] [Google Scholar]
- 30.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 31.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma : evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119:1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 32.Becker S, Fenton MJ, Soukup JM. Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am J Respir Cell Mol Biol. 2002;27:611–618. doi: 10.1165/rcmb.4868. [DOI] [PubMed] [Google Scholar]
- 33.Wills-Karp M. IL-12/IL-13 axis in allergic asthma. J Allergy Clin Immunol. 2001;107:9–18. doi: 10.1067/mai.2001.112265. [DOI] [PubMed] [Google Scholar]
- 34.Sel S, Wegmann M, Bauer S, Garn H, Alber G, Renz H. Immunomodulatory effects of viral TLR ligands on experimental asthma depend on the additive effects of IL-12 and IL-10. J Immunol. 2007;178:7805–7813. doi: 10.4049/jimmunol.178.12.7805. [DOI] [PubMed] [Google Scholar]
- 35.Kawaguchi M, Adachi M, Oda N, Kokubu F, Huang SK. IL-17 cytokine family. J Allergy Clin Immunol. 2004;114:1265–1273. doi: 10.1016/j.jaci.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 36.Hessel EM, Chu M, Lizcano JO, Chang B, Herman N, Kell SA, Wills-Karp M, Coffman RL. Immunostimulatory oligonucleotides block allergic airway inflammation by inhibiting Th2 cell activation and IgE-mediated cytokine induction. J Exp Med. 2005;202:1563–1573. doi: 10.1084/jem.20050631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 39.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172:6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]