Abstract
Background
Patient awareness of chronic diseases is low. Unawareness may represent poor understanding of chronic illness and may be associated with poor outcomes in end-stage renal disease (ESRD) patients.
Study Design
Concurrent, prospective national cohort study.
Setting & Participants
Incident hemodialysis and peritoneal dialysis patients enrolled in the Choices for Healthy Outcomes In Caring for ESRD (CHOICE) Study and followed until 2004.
Predictor
Inaccurate patient self-report of eight comorbid diseases as compared to the medical record.
Outcomes & Measurements
All-cause mortality was the primary outcome. Cox proportional hazard models were used to assess the contribution of demographics and clinical measures in the relation of inaccurate self-report to mortality.
Results
In 965 patients, the proportion of inaccurate self-reporters ranged from 3% for diabetes mellitus to 35% for congestive heart failure. Generally, inaccurate self-reporters were older and had more chronic diseases. A higher risk of death was found for inaccurate self-reporters of ischemic heart disease (Hazard Ratio (HR) [95% CI] =1.34 [1.12 –1.59]; p=0.001), coronary intervention (HR=1.46 [1.08 – 1.97]; p=0.01), and chronic obstructive pulmonary disease (COPD) (HR=1.40 [1.14–1.70]; p=0.001). The higher risk of death remained significant for COPD (HR=1.36 [1.11 – 1.66]; p=0.003) after adjustment for age, sex, and race. In patients receiving peritoneal dialysis, a higher risk of death (HR=2.06 [1.34 – 3.15]; p=0.001) was found for inaccurate self-reporters of ischemic heart disease.
Limitations
Includes potential for residual confounding, medical record error, misclassification of patient accuracy of self-report, and low inaccurate self-report of some chronic diseases, reducing the power to measure associations.
Conclusions
Accuracy of self-report depends upon the specific comorbid disease. ESRD patients, especially those receiving peritoneal dialysis, who inaccurately report heart disease may be less aware of their chronic comorbid disease and may be at higher risk of mortality compared to those who accurately report their comorbid disease.
Index words: End-stage renal disease (ESRD), mortality, self-report, awareness, comorbid disease, outcomes
Introduction
Awareness of a disease is the first step for a patient to acquire knowledge about a condition and how it will impact their health. It is likely that awareness of a disease will lead to greater understanding about its evaluation, course, treatment, and complications. Therefore, low awareness and subsequently limited knowledge about a disease may contribute to ineffective application of clinical recommendations and ultimately to poor outcomes. Awareness of chronic diseases, such as diabetes mellitus, hypertension, and chronic kidney disease, is low in both the general population(1–3) and selected populations(4–6).
End-stage renal disease (ESRD) patients experience a high level of chronic disease burden. For incident dialysis patients, the prevalence of diabetes mellitus is as high as 50%, and in those who survive one year after dialysis initiation this proportion increases to 66%.(7) Cardiovascular disease and hypertension are also common, with nearly 40% of dialysis patients with a diagnosis of coronary artery disease(8) and as many as 87–90% with hypertension.(9) The mortality risk for dialysis patients is 10–20 times that of the general population(8) and a likely factor is the high prevalence of these traditional cardiovascular risk factors, such as hypertension and diabetes mellitus. Studies evaluating the association between low awareness of chronic disease and specific clinical outcomes are limited.
We previously compared patients’ self-reports of comorbid disease to the medical record and physician reports in patients at the start of dialysis.(10) The purpose of this study is to describe, in a cohort of incident dialysis patients, the characteristics of patients who are aware of their comorbid disease status compared to those who are unaware (i.e., accurate versus inaccurate self-reporters) and to evaluate the association between inaccurate self-report and all-cause mortality for eight comorbid diseases. We hypothesize that inaccurate self-reporters will have a higher risk of death than accurate self-reporters.
METHODS
Study Design and Population
This was a concurrent, prospective cohort study with participants from the Choices for Healthy Outcomes In Caring for ESRD (CHOICE) Study. CHOICE is a prospective study of incident hemodialysis and peritoneal dialysis patients; complete details of the cohort design are described elsewhere.(11) Briefly, 1041 patients were enrolled from October 1995 to June 1998 from 81 dialysis clinics located in 19 states throughout the United States and associated with Dialysis Clinic Inc. (Nashville, TN), New Haven CAPD (New Haven, CT), or Saint Raphael’s Hospital (New Haven, CT). Eligibility included initiation of chronic outpatient dialysis in the preceding three months prior to enrollment, ability to give informed consent, age 18 years or greater, and English- or Spanish-speaking. Patients were enrolled at a median time of 45 days after initiation of dialysis and 98% of the cohort was enrolled within four months of initiation of dialysis. All patients provided informed consent and the Johns Hopkins University School of Medicine Institutional Review Board and the review boards of each clinical unit approved the CHOICE protocol.
Data Collection
Patient self-report
All patients completed a baseline questionnaire at enrollment that provided demographic information, social history, and medical history. The patient’s self-report of a disease was elicited by the question “Have you ever been told by a doctor that you have (check all that apply)” and listed eight comorbid medical conditions: diabetes, hypertension, congestive heart failure, ischemic heart disease (or heart attack), coronary intervention (angioplasty or coronary artery bypass graft (CABG) surgery), cerebrovascular disease, chronic obstructive pulmonary disease (COPD), or cancer (any location). The question requested a positive response only, which was classified as the patient reporting positive presence of that disease. A non-response was classified as a negative response. In order to minimize misclassification, we excluded patients with less than 85% of the baseline questionnaire completed.
Medical record diagnosis
The medical record diagnosis of comorbid disease was obtained at enrollment using the Index of Co-Existent Disease (ICED).(12, 13) Clinical evidence as appropriate for each condition (Table 1) or report of a condition (past or present) was sufficient for positive coding. Two trained research nurses abstracted the medical records to determine the diagnoses. The ICED includes the Index of Disease Severity, a 116-item chart-based review of 19 medical conditions, and the Index of Physical Impairment, an 11-item form completed by the local nurse or technician. The interobserver reliability of the ICED scoring was assessed by comparing the aforementioned process to the scoring of an experienced nephrologist in the Mortality and Morbidity in Hemodialysis Patients (MMHD) Study and resulted in a kappa statistic of 0.77.(13)
Table 1.
Comorbid chronic disease definitions for self-report and medical record
| Disease | Medical record definition |
|---|---|
| Diabetes | Diagnosis of diabetes (controlled or uncontrolled)
Requires oral medication and/or insulin |
| Hypertension | Diagnosis of hypertension
Requires anti-hypertensive medications |
| Ischemic heart disease | Diagnosis of coronary artery disease
Any history of myocardial infarction |
| Congestive heart failure | Diagnosis of congestive heart failure or pulmonary edema (pre- or post-dialysis) |
| Angioplasty or heart bypass surgery | Coronary angioplasty or Coronary artery bypass graft (surgery) |
| Cerebrovascular disease | Diagnosis of cerebrovascular disease
Multiple transient ischemic attacks in the past year |
| Chronic obstructive pulmonary disease | Diagnosis of chronic obstructive pulmonary disease, or asthma
Requiring pulmonary disease medication |
| Cancer | Any history of malignancy including current malignancy of any location |
Agreement between Self-Report and Medical Record
Agreement between self-report of a disease and the medical record or clinical evaluation represents awareness of disease.(1, 14) Agreement may be a characteristic of the patient that predicts important information about the clinical status of that patient. Here, we defined an accurate self-report as a self-report that agreed with the medical record, and discordance between the self-report and the medical record was defined as an inaccurate self-report.
Covariates and Outcome
Patient characteristics, including age, sex, race, and date of initial dialysis were obtained from the Center for Medicare & Medicaid Services medical evidence form (Form 2728). The CHOICE baseline questionnaire provided information about education level and smoking status. Date of death was determined from clinic report information, the Center for Medicare and Medicaid Services, and the National Death Index. Baseline laboratory data included repeated measures of albumin. The average of the values in the 3 months surrounding enrollment in the study was considered the baseline albumin level. High-sensitivity C-reactive protein was performed on all patients with frozen serum available in the CHOICE specimen bank.
Statistical Analysis
Descriptive statistics of the total study population were performed. For each comorbid disease, the inaccurate and accurate reporters were evaluated by non-parametric Kaplan-Meier estimates to describe cumulative mortality. The log-rank test was used to test for statistically significant differences between these two groups. Unadjusted and adjusted Cox proportional hazards regression models were applied to describe inaccurate self-report and its association with the risk of death. Survival time was calculated from the date of enrollment in the cohort until death or until the last date of available follow-up data (December 31, 2004) when the patient was censored if they were alive. Patients were also censored if they received a kidney transplant. There were no losses to follow-up in this cohort. Adjusted Cox proportional hazards regression analysis included demographic variables known to predict mortality in ESRD patients, including age, sex and race. This was followed by the addition of the number of comorbid conditions representing overall severity of disease.(15) To account for nonindependence of clinical practice, stratification by clinic site was applied in all models.(16) For all Cox proportional hazard analyses, there was no evidence of collinearity among the covariates, and Schoenfeld residual analyses showed the proportionality assumption was not violated (p>0.3 for all) for any comorbid disease except hypertension (p=0.02). Subgroup analyses by dialysis modality were also performed and p-values for interaction were determined to evaluate evidence of effect modification.
All statistical analyses were performed using Stata 8.0 software (Stata Corp., College Station, Texas). P-values of <0.05 were considered statistically significant; additionally, results that were statistically significant after adjustment by the Bonferroni method, to account for possible effects of multiple comparisons, are also presented as sensitivity analyses [threshold p=(0.05/8 comparisons)= 0.006].
Results
Study Population
Of the 1041 patients enrolled in the CHOICE cohort study, 965 (93%) patients completed ≥85% of the baseline questionnaire and were included in this study. The only statistically significant differences between the study population and those patients excluded from the total CHOICE cohort was the proportion of patients receiving peritoneal dialysis, which was higher (>50%) in the excluded patients (p<0.001). Missing data were minimal ranging from 0%–2.7%.
Characteristics of Inaccurate and Accurate Self-Reporters
Overall, the average age was 58 years and a majority of patients were male; two-thirds were white and 28% were African American (Table 2). Seventy percent of the patients completed education at least through high school. Clinical measures showed that the mean number of comorbid chronic diseases was 5.5 at baseline. Hemodialysis was the modality received by three-quarters of patients.
Table 2.
Characteristics of patient accurate and inaccurate self-report by comorbid disease.
| Prevalence % (n) | Agea | % Male | % AA | % Educ ≥ HS | % No Smoke | % Albumin>3.5 mg/dL | No. Comorbida | % ICED>1 | % HD | |
|---|---|---|---|---|---|---|---|---|---|---|
| All Patients (n=965) | 58 (15) | 54 | 28 | 70 (n=958) | 39 (n=964) | 69 (n=939) | 5.5 (2.6) | 65 | 76 | |
|
| ||||||||||
| Diabetes | 54% (526) | |||||||||
| Accurate (n=933; 97%) | 58 (15) | 54 | 28 | 70 | 39 | 69 | 5.4 (2.5) | 64 | 75 | |
| Inaccurate (n=32; 3%) | 62 (14) | 59 | 25 | 75 | 34 | 72 | 6.9 (2.9)† | 78 | 84 | |
|
| ||||||||||
| Hypertension | 96% (924) | |||||||||
| Accurate (n=777; 81%) | 57 (14) | 52 | 30 | 70 | 40 | 69 | 5.5 (2.5) | 65 | 75 | |
| Inaccurate (n=188; 19%) | 61 (14)† | 65† | 19† | 70 | 36 | 70 | 5.6 (2.7) | 63 | 76 | |
|
| ||||||||||
| Ischemic heart disease | 45% (430) | 28 | ||||||||
| Accurate (n=676; 70%) | 55 (15) | 53 | 30 | 72 | 40 | 70 | 4.9 (2.5) | 62 | 74 | |
| Inaccurate (n=289; 30%) | 64 (11)† | 57 | 24 | 67 | 38 | 67 | 6.9 (2.1)† | 71* | 78 | |
|
| ||||||||||
| Congestive heart failure | 46% (446) | |||||||||
| Accurate (n=717; 65%) | 57 (15) | 55 | 25 | 73 | 39 | 68 | 5.2 (2.7) | 60 | 74 | |
| Inaccurate (n=248; 35%) | 61 (14)† | 53 | 36† | 62† | 41 | 71 | 6.3 (1.9) | 69 | 80# | |
|
| ||||||||||
| Coronary intervention | 20% (193) | |||||||||
| Accurate (n=902; 94%) | 57 (15) | 53 | 28 | 71 | 38 | 69 | 5.4 (2.6) | 64 | 75 | |
| Inaccurate (n=62; 6%) | 66 (12) † | 71‡ | 24 | 53† | 39 | 69 | 7.0 (2.0)† | 76* | 79 | |
|
| ||||||||||
| Cerebrovascular disease | 16% (158) | |||||||||
| Accurate (n=867; 90%) | 57 (15) | 54 | 28 | 70 | 39 | 69 | 5.3 (2.5) | 63 | 75 | |
| Inaccurate (n=98; 10%) | 66(12)† | 54 | 24 | 69 | 39 | 65 | 7.3 (2.4)† | 85† | 83# | |
|
| ||||||||||
| COPD | 20% (194) | |||||||||
| Accurate (n=782; 77%) | 57 (15) | 54 | 29 | 71 | 43 | 69 | 5.2 (2.5) | 63 | 74 | |
| Inaccurate (n=183; 23%) | 62 (13)† | 56 | 24 | 67 | 24† | 70 | 6.9 (2.3)† | 72* | 84† | |
|
| ||||||||||
| Cancer | 10% (98) | |||||||||
| Accurate (n=908; 94%) | 58 (15) | 54 | 29 | 70 | 39 | 69 | 5.4 (2.5) | 64 | 75 | |
| Inaccurate (n=57; 6%) | 63 (16)† | 56 | 19 | 79 | 40 | 66 | 6.0 (2.8) | 79† | 79 | |
AA=African American; Educ ≥ HS= Education ≥ High School; ICED=Index of Coexistent disease; HD=Hemodialysis; COPD=Chronic obstructive pulmonary disease
Mean (Standard deviation);
p<0.005;
p<0.01;
p<0.05;
p<0.10
The prevalence of the eight comorbid conditions ranged from 10% for cancer to 96% for hypertension (Table 2). The proportion of inaccurate self-reporters ranged from 3% for diabetes mellitus to 35% for congestive heart failure (Table 2).
For all eight chronic diseases the inaccurate self-reporters were older by 4–7 years than the accurate self-reporters (p<0.005), although this was not statistically significant in patients with diabetes (p=0.1). Similarly, the total number of and severity of baseline comorbid diseases was higher for inaccurate patients compared to accurate patients for all diseases, except hypertension.
Inaccurate self-reporters of congestive heart failure were more likely to be African American and less likely to have at least a high school education. The inaccurate reporters of coronary intervention were significantly more likely to be male and less likely to have at least a high school education.
For congestive heart failure, cerebrovascular disease, and COPD, inaccurate reporters were more likely to be receiving hemodialysis rather than peritoneal dialysis, compared to accurate self-reporters. No significant differences were found for baseline serum albumin measurements for any of the comorbid diseases. With Bonferroni adjustment, the severity of the Index of Coexistent disease (ICED) score was no longer statistically significantly different between accurate and inaccurate reporters for all diseases, except cerebrovascular disease.
Mortality Risk for Inaccurate and Accurate Self-Reporters
Through 2004, there were 603 deaths (62.5%), 207 patients (21.5%) received a kidney transplant and 155 (16%) were administratively censored because of either change of dialysis location to a non-study site or request for withdrawal from the CHOICE study.
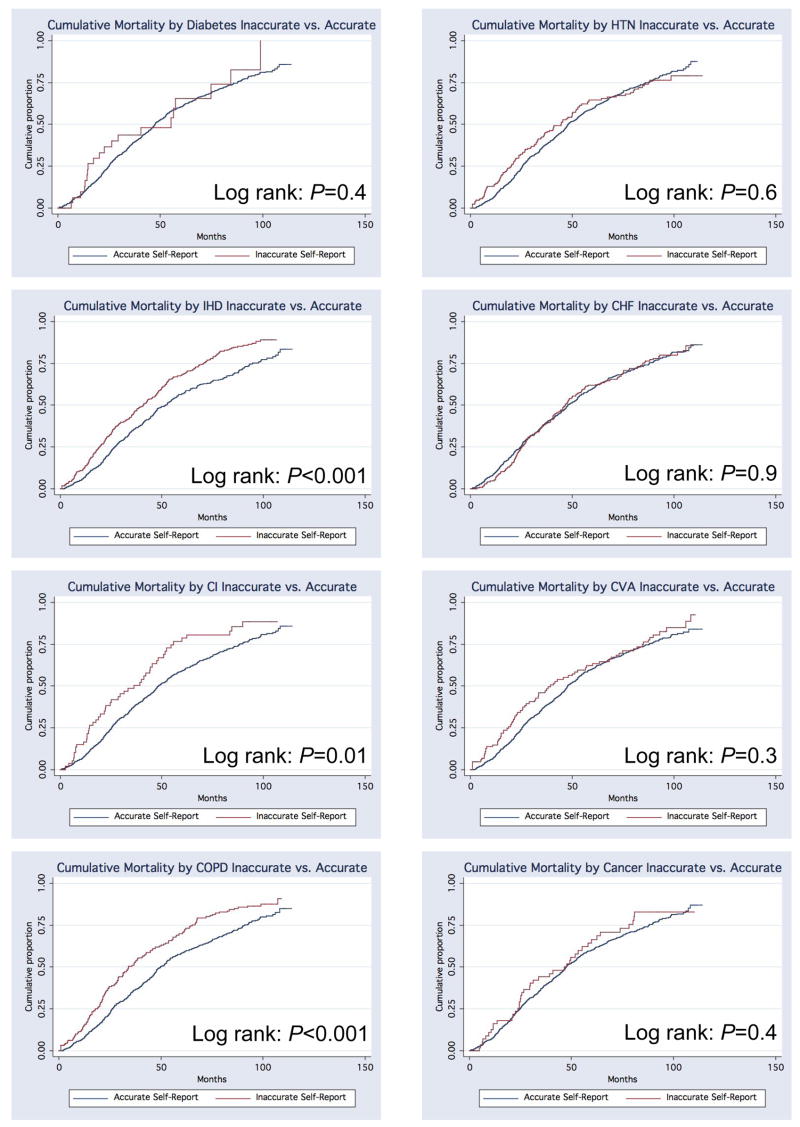
There was no statistically significant difference in the cumulative mortality for inaccurate compared to accurate self-reporters of diabetes, hypertension, cerebrovascular disease, or cancer (Figure 1). For cardiovascular comorbid diseases, estimates of cumulative mortality showed that the risk of death was higher for inaccurate reporters of ischemic heart disease (log-rank p<0.001), coronary intervention (log-rank p=0.01), and chronic obstructive pulmonary disease (log-rank p<0.001) but was not significant for congestive heart failure (Figure 1). Unadjusted Cox proportional hazards models for each of the eight comorbid diseases correspondingly showed statistically significantly higher risks of mortality for inaccurate self-reporters of ischemic heart disease (p<0.005), coronary intervention (p=0.01), and COPD (p<0.005) (Table 3). The hazard ratios for inaccurate reporters of diabetes (Hazard Ratio [HR] =1.30), cerebrovascular disease (HR=1.18), and cancer (HR=1.11) also suggested a higher risk of death; however, these were not statistically significant.
Figure 1. Kaplan-Meier cumulative mortality for inaccurate compared to accurate self-report of eight chronic comorbid diseases in dialysis patients.
HTN= Hypertension
IHD= Ischemic heart disease
CHF=Congestive heart failure
CI=Coronary intervention
CVA=Cerebrovascular disease
COPD=Chronic obstructive pulmonary disease
[Note: Each definition label is placed below its corresponding Kaplan-Meier figure]
Table 3.
Cox proportional hazards models for mortality comparing inaccurate to accurate self-reporters of chronic disease.
| Disease | Unadjusted | Adjusted | |
|---|---|---|---|
| Model | (A) Demographics | (B) + Number of diseases | |
| Hazard Ratio [95% Confidence Interval] | |||
| Diabetes | 1.30 [0.80, 2.09] | 1.08 [0.67, 1.75] | 0.93 [0.57, 1.51] |
| Hypertension | 0.99 [0.80, 1.22] | 0.85 [0.68, 1.05] | 0.86 [0.69, 1.07] |
| Ischemic heart disease | 1.34 [1.12, 1.59]† | 1.06 [0.88, 1.26] | 0.88 [0.74, 1.06] |
| Congestive heart failure | 1.04 [0.86, 1.25] | 1.02 [0.85, 1.24] | 0.90 [0.74, 1.09] |
| Coronary intervention | 1.46 [1.08, 1.97]* | 1.25 [0.92, 1.70] | 1.08 [0.79, 1.47] |
| Cerebrovascular disease | 1.18 [0.91, 1.52] | 1.00 [0.77, 1.29] | 0.82 [0.63, 1.06] |
| Chronic obstructive pulmonary disease | 1.40 [1.14, 1.70]† | 1.36 [1.11, 1.66]† | 1.14 [0.92, 1.41] |
| Cancer | 1.11 [0.80, 1.53] | 1.01 [0.73, 1.41] | 1.01 [0.73, 1.41] |
Models: (A) Demographics (age, sex, race); (B) Model A and number of comorbid diseases.
p<0.005;
p=0.01
After adjustment for age, sex and race, there was still a statistically significant higher risk of death for inaccurate reporters of COPD (HR=1.36; p=0.003). After adjustment for demographic variables and the number of comorbid diseases, there were no significant differences in the mortality risk between inaccurate and accurate reporters for any of the eight comorbid diseases, although the risk for coronary intervention (HR=1.08) and COPD (HR=1.14) remained elevated. Analyses with models incorporating the ICED measure rather than the number or comorbid diseases, baseline serum albumin, baseline C-reactive protein, and educational level yielded similar results (data not shown). With Bonferroni adjustment, the results remained statistically significant except for those for coronary intervention.
Subgroup Analyses
Hemodialysis patients who inaccurately reported ischemic heart disease compared to those who accurately reported their disease had an 18% higher risk of death, although this was not statistically significant (Table 4). However, in peritoneal dialysis patients who inaccurately reported ischemic heart disease there was a 157% higher risk of death (p<0.001); the p-value for interaction was equal to 0.004. Adjusted analysis for age, sex, and race, found that for peritoneal dialysis patients, there was still a higher risk of death for inaccurate reporters compared to accurate reporters (HR=2.06; p=0.001). Similarly, for congestive heart failure, the risk of mortality in peritoneal dialysis inaccurate reporters was high and significantly different from that of inaccurate reporters in hemodialysis patients (p-interaction=0.01). After adjustment for demographic variables there was no longer a significant difference between inaccurate and accurate reporters of congestive heart failure for either type of dialysis modality. With Bonferroni adjustment, the association of awareness of ischemic heart disease with mortality was still statistically significant. The risk of death by accuracy reporting did not differ by dialysis modality for any of the other comorbid diseases.
Table 4.
Risk of death for inaccurate compared to accurate self-report of chronic disease stratified by dialysis modality.
| Comorbid disease | Hazard Ratio [95% CI] | p-Value | Hazard Ratio [95% CI] | p-Value | p-value interaction | ||
|---|---|---|---|---|---|---|---|
| Hemodialysis n=728 | Peritoneal Dialysis n=237 | ||||||
|
| |||||||
| Unadjusted | |||||||
| Diabetes | 1.20 [0.71, 2.03] | 0.5 | 1.67 [0.45, 6.26] | 0.4 | 0.6 | ||
| Hypertension | 1.03 [0.81, 1.30] | 0.8 | 1.03 [0.64, 1.68] | 0.9 | 0.4 | ||
| Ischemic heart disease | 1.18 [0.97, 1.44] | 0.1 | 2.57 [1.71, 3.87] | <0.001 | 0.004 | ||
| Congestive heart failure | 0.92 [0.74, 1.13] | 0.4 | 1.61 [1.01, 2.56] | 0.04 | 0.01 | ||
| Coronary Intervention | 1.43 [1.02, 2.00] | 0.04 | 1.67 [0.81, 3.44] | 0.2 | 0.9 | ||
| Cerebrovascular disease | 1.14 [0.86, 1.51] | 0.4 | 1.46 [0.78, 2.74] | 0.2 | 0.5 | ||
| COPD | 1.36 [1.09, 1.70] | 0.01 | 1.43 [0.85, 2.43] | 0.2 | 0.8 | ||
| Cancer | 1.11 [0.77, 1.59] | 0.6 | 1.32 [0.59, 2.99] | 0.5 | 0.9 | ||
|
| |||||||
| Adjusted† | |||||||
| Diabetes | 1.00 [0.59, 1.70] | 0.9 | 1.63 [0.42, 6.30] | 0.5 | 0.3 | ||
| Hypertension | 0.88 [0.69, 1.13] | 0.3 | 0.86 [0.52, 1.44] | 0.6 | 0.6 | ||
| Ischemic heart disease | 0.94 [0.77, 1.15] | 0.5 | 2.06 [1.35, 3.15] | 0.001 | 0.002 | ||
| Congestive heart failure | 0.95 [0.77, 1.18] | 0.7 | 1.36 [0.84, 2.20] | 0.2 | 0.1 | ||
| Coronary Intervention | 1.31 [0.93, 1.85] | 0.1 | 1.24 [0.60, 2.57] | 0.6 | 0.7 | ||
| Cerebrovascular disease | 0.95 [0.71, 1.26] | 0.7 | 1.41 [0.73, 2.74] | 0.3 | 0.3 | ||
| COPD | 1.33 [1.06, 1.66] | 0.01 | 1.52 [0.87, 2.64] | 0.1 | 0.5 | ||
| Cancer | 1.04 [0.72, 1.50] | 0.8 | 0.85 [0.37, 1.94] | 0.7 | 0.7 | ||
CI=Confidence Interval; COPD=Chronic obstructive pulmonary disease.
Adjusted for age, sex, and race.
Discussion
In this national prospective cohort study of ESRD patients receiving dialysis, we found a high proportion of inaccurate self-report of several common chronic comorbid diseases, including cardiovascular disease. Importantly, we found a higher risk of death from any cause, in dialysis patients who inaccurately reported prevalent ischemic heart disease, coronary intervention, or chronic obstructive pulmonary disease compared with those who accurately reported their disease. This risk persisted for COPD and coronary intervention even after adjustment for demographic and clinical variables, although the risks were attenuated and no longer statistically significant. Peritoneal dialysis patients who inaccurately reported ischemic heart disease had a risk of death that was 2-fold higher than those who accurately reported their disease. To our knowledge, the association between inaccurate self-report of specific comorbid diseases and mortality has not been previously described in the ESRD population.
Characteristics of inaccurate self-reporters may identify patients who are at high risk for having disease but may not be aware of the implications of that disease or understand therapeutic recommendations. We found that, for many chronic diseases, inaccurate reporting was associated with older age. Previous studies have suggested that older age is associated with more accurate reporting of heart disease and hypertension(17, 18), with speculation that older patients are more aware of their health status and this results in improved accuracy of reporting. However, in the medically complex kidney disease population, this is not consistent with our findings. Other selected populations of the elderly have demonstrated that older age is associated with poor agreement between self-report and the medical record.(19, 20) We also have shown that for most diseases we evaluated inaccurate reporting was associated with either a greater number of comorbid diseases or a higher level of severity of disease. It may be that patients who are older, who also have been diagnosed with an average of five comorbid diseases, have a difficult time maintaining the broad knowledge base required to communicate these diverse conditions.
Fewer years of education was associated with inaccurate reporting of congestive heart failure. This condition may not always be associated with concrete diagnostic information. Therefore, less education may impair the understanding of the diagnosis and its varying presentations by the patient. Education has been found previously to be associated with inaccurate reporting for hypercholesterolemia(21), fractures(22), and comorbid diseases including heart failure, diabetes, ischemic heart disease, and cerebrovascular disease.(23) More specific testing of a patient’s general knowledge level, including health literacy, is needed to further clarify the relationship between adult learning capacity and inaccurate self-report of chronic diseases.
Peritoneal dialysis patients are a selected population who are often thought to be more likely to have a higher educational level and possibly more knowledge about their kidney disease.(24) In our study, for the diagnoses of congestive heart failure, cerebrovascular disease, and chronic obstructive pulmonary disease, inaccurate reporting was found to be associated with a lower proportion of peritoneal dialysis patients. Peritoneal dialysis patients were found to be more accurate self-reporters of some chronic diseases, which may represent more awareness and disease knowledge.
Our findings suggest that inaccurate self-report, or low awareness of chronic disease, may be associated with an increased risk of death by as much as 46% for coronary intervention, 34% for ischemic heart disease, and 40% for COPD. Lack of awareness and knowledge about these complex diagnoses may lead to ineffective application of health care provider recommendations, lower participation in daily self-management activities, and subsequently poor outcomes. There may also be other confounding variables that affect this relationship. Adjustment for demographic variables attenuates the relationship for ischemic heart disease and coronary intervention, but not for COPD. It may be that those patients who are older, have the most complex and severe combination of chronic comorbid diseases and are more likely to report inaccurately their disease status and also are at the highest risk of mortality. However, further adjustment for overall burden of chronic disease attenuated the hazard ratio, yet inaccurate reporting of these conditions remained positively, but not statistically significantly associated with a greater risk of death. These findings indicate that at least for selected comorbid diseases inaccurate self-report, or possible lack of disease awareness, may be associated with mortality. In turn, mortality may possibly be reduced by improving awareness of disease through directed patient education programs aimed to increase effective patient self-management or self-advocacy including interpretation and reporting of disease symptoms requiring medical attention.(25)
Awareness of ischemic heart disease may be of particular importance for patients who receive peritoneal dialysis. We found that even after adjusting for age and other demographic variables, that peritoneal dialysis patients who inaccurately report ischemic heart disease had a 2-fold higher risk of mortality compared to accurate self-reporters, while no difference was found among hemodialysis patients. As discussed previously, peritoneal dialysis patients are often thought to be more knowledgeable about their disease state. Inaccurate self-reporters may not have been adequately educated about their disease, or did not understand information they have received about their diagnosis. This may have been due to coexisting disease, such as cognitive impairment. Additionally, peritoneal dialysis may be selected as a dialysis modality when hemodynamic instability, often a result of cardiovascular disease, is present. There may be a subset of peritoneal dialysis patients who are recommended this modality because of clinical reasons and are not representative of the more common patient-directed choice of modality. Therefore, those patients who receive peritoneal dialysis, but are inaccurate self-reporters of heart disease, may be an easily identifiable sub-group that may benefit from additional patient education.
There are several limitations to our study. The definition of self-report in this study depended upon a baseline questionnaire that used disease terms, such as ‘chronic obstructive pulmonary disease’, that may have been less recognized by patients than common language, such as ‘lung disease’. The agreement between self-report and the medical record is similar to that found in other studies using a variety of terms to describe lung disease.(19, 20, 26–29) However, it is possible that if the term used were not recognized then a patient may be misclassified as inaccurate, when they actually were aware of their comorbid disease. Given the structure of the survey question it is less likely that an inaccurate patient would be misclassified as accurate. Although the misclassification is likely differential between inaccurate and accurate self-reporters, we hypothesized that accurate self-report would be associated with a lower risk of mortality and therefore, if patients were misclassified as inaccurate this would likely bias the result toward the null. The relationship between self-report accuracy and mortality may also be explained by residual confounding. There may be patient characteristics or disease severity that we are not able to capture in our analysis. However, models that included clinical markers (serum albumin, serum baseline C-reactive protein) did not substantially affect the described relationships. Previous studies have demonstrated that the medical record itself may have a substantial number of errors.(30–32) Because we use the medical record as the “gold standard” to which we compare self-report, it is possible that this assessment also has error. For some of the diseases the sample size of the inaccurate reporters was small which may have contributed to the lack of associations due to a lack of power to measure a difference between the inaccurate and accurate self-reporters. Finally, additional studies using this methodology of comparing self-report to the medical record to identify inaccurate and accurate self-reporters and then evaluating for associations with clinical outcomes, such as death, are needed for comparison and validation.
In summary, for ESRD patients, the accuracy of self-report is dependent upon the specific comorbid disease, and the characteristics of the patient, including their overall burden of illness. This nationally representative study of incident dialysis ESRD patients demonstrates that those who inaccurately report ischemic heart disease, coronary intervention, and chronic obstructive pulmonary disease may be at a higher risk of mortality compared to patients who accurately report their disease. There is a very high risk of death specifically in peritoneal dialysis patients inaccurately reporting ischemic heart disease. Identification of low awareness of a patient by inaccurate self-report is a rapid, inexpensive evaluation tool for clinicians. Patients with low awareness of disease are an identifiable target population for directed educational interventions that may reduce their overall risk of death. Patient education programs using diverse strategies to increase awareness and knowledge of chronic diseases have shown success at improving clinical outcomes.(33) For example, in patients with diabetes, participation in education programs have shown an associated reduction in glycosylated hemoglobin and improved systolic blood pressure.(33, 34) This suggests that interventions aimed to improve patient awareness and knowledge about chronic comorbid diseases may have important beneficial effects on clinical outcomes. It will be important to evaluate in future studies the effect of educational interventions on the mortality risk in inaccurate self-reporting kidney disease patients.
Acknowledgments
We thank the patients, staff, and medical directors of the participating clinics at Dialysis Clinic, Inc, New Haven CAPD, and St. Raphael’s hospital who contributed to the study.
Support: This research was supported by grant number RO1 DK 59616 from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Cavanaugh was supported by grant no. 5 T32 DK07732 and Dr. Powe is supported by grant K24DK02643 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Financial Disclosure: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 2.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. Jama. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 3.Joffres MR, Ghadirian P, Fodor JG, Petrasovits A, Chockalingam A, Hamet P. Awareness, treatment, and control of hypertension in Canada. Am J Hypertens. 1997;10:1097–1102. doi: 10.1016/s0895-7061(97)00224-0. [DOI] [PubMed] [Google Scholar]
- 4.Aranda JM, Jr, Vazquez R. Awareness of hypertension and diabetes in the Hispanic community. Clin Cornerstone. 2004;6:7–13. doi: 10.1016/s1098-3597(04)80060-7. discussion 14–15. [DOI] [PubMed] [Google Scholar]
- 5.Gnasso A, Calindro MC, Carallo C, et al. Awareness, treatment and control of hyperlipidaemia, hypertension and diabetes mellitus in a selected population of southern Italy. Eur J Epidemiol. 1997;13:421–428. doi: 10.1023/a:1007369203648. [DOI] [PubMed] [Google Scholar]
- 6.Maahs DM, Kinney GL, Wadwa P, et al. Hypertension prevalence, awareness, treatment, and control in an adult type 1 diabetes population and a comparable general population. Diabetes Care. 2005;28:301–306. doi: 10.2337/diacare.28.2.301. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Renal Data System. USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Am J Kidney Dis. 2007;49:S1–S296. [Google Scholar]
- 8.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 9.Levin A. Clinical epidemiology of cardiovascular disease in chronic kidney disease prior to dialysis. Semin Dial. 2003;16:101–105. doi: 10.1046/j.1525-139x.2003.16025.x. [DOI] [PubMed] [Google Scholar]
- 10.Merkin SS, Cavanaugh K, Longenecker JC, Fink NE, Levey AS, Powe NR. Agreement of self-reported comorbid conditions with medical and physician reports varied by disease among end-stage renal disease patients. J Clin Epidemiol. 2007;60:634–642. doi: 10.1016/j.jclinepi.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powe NR, Klag MJ, Sadler JH, et al. Choices for healthy outcomes in caring for end-stage renal disease. Seminars in Dialysis. 1996;9:9–11. [Google Scholar]
- 12.Imamura K, McKinnon M, Middleton R, Black N. Reliability of a comorbidity measure: the Index of Co-Existent Disease (ICED) J Clin Epidemiol. 1997;50:1011–1016. doi: 10.1016/s0895-4356(97)00128-5. [DOI] [PubMed] [Google Scholar]
- 13.Miskulin DC, Meyer KB, Athienites NV, et al. Comorbidity and other factors associated with modality selection in incident dialysis patients: the CHOICE Study. Choices for Healthy Outcomes in Caring for End-Stage Renal Disease. Am J Kidney Dis. 2002;39:324–336. doi: 10.1053/ajkd.2002.30552. [DOI] [PubMed] [Google Scholar]
- 14.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 15.Perkins AJ, Kroenke K, Unutzer J, et al. Common comorbidity scales were similar in their ability to predict health care costs and mortality. J Clin Epidemiol. 2004;57:1040–1048. doi: 10.1016/j.jclinepi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Localio AR, Berlin JA, Ten Have TR, Kimmel SE. Adjustments for center in multicenter studies: an overview. Annals of internal medicine. 2001;135:112–123. doi: 10.7326/0003-4819-135-2-200107170-00012. [DOI] [PubMed] [Google Scholar]
- 17.Bush TL, Miller SR, Golden AL, Hale WE. Self-report and medical record report agreement of selected medical conditions in the elderly. Am J Public Health. 1989;79:1554–1556. doi: 10.2105/ajph.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Sauver JL, Hagen PT, Cha SS, et al. Agreement between patient reports of cardiovascular disease and patient medical records. Mayo Clin Proc. 2005;80:203–210. doi: 10.4065/80.2.203. [DOI] [PubMed] [Google Scholar]
- 19.Kriegsman DM, Penninx BW, van Eijk JT, Boeke AJ, Deeg DJ. Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly. A study on the accuracy of patients’ self-reports and on determinants of inaccuracy. J Clin Epidemiol. 1996;49:1407–1417. doi: 10.1016/s0895-4356(96)00274-0. [DOI] [PubMed] [Google Scholar]
- 20.Simpson CF, Boyd CM, Carlson MC, Griswold ME, Guralnik JM, Fried LP. Agreement between self-report of disease diagnoses and medical record validation in disabled older women: factors that modify agreement. J Am Geriatr Soc. 2004;52:123–127. doi: 10.1111/j.1532-5415.2004.52021.x. [DOI] [PubMed] [Google Scholar]
- 21.Natarajan S, Lipsitz SR, Nietert PJ. Self-report of high cholesterol: determinants of validity in U.S. adults. Am J Prev Med. 2002;23:13–21. doi: 10.1016/s0749-3797(02)00446-4. [DOI] [PubMed] [Google Scholar]
- 22.Nevitt MC, Cummings SR, Browner WS, et al. The accuracy of self-report of fractures in elderly women: evidence from a prospective study. Am J Epidemiol. 1992;135:490–499. doi: 10.1093/oxfordjournals.aje.a116315. [DOI] [PubMed] [Google Scholar]
- 23.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Stack AG. Determinants of modality selection among incident US dialysis patients: results from a national study. J Am Soc Nephrol. 2002;13:1279–1287. doi: 10.1681/ASN.V1351279. [DOI] [PubMed] [Google Scholar]
- 25.Curtin RB, Mapes D, Schatell D, Burrows-Hudson S. Self-management in patients with end stage renal disease: exploring domains and dimensions. Nephrol Nurs J. 2005;32:389–395. [PubMed] [Google Scholar]
- 26.Chaudhry S, Jin L, Meltzer D. Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Med Care. 2005;43:607–615. doi: 10.1097/01.mlr.0000163658.65008.ec. [DOI] [PubMed] [Google Scholar]
- 27.Fowles JB, Fowler EJ, Craft C. Validation of claims diagnoses and self-reported conditions compared with medical records for selected chronic diseases. J Ambul Care Manage. 1998;21:24–34. doi: 10.1097/00004479-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Heliovaara M, Aromaa A, Klaukka T, Knekt P, Joukamaa M, Impivaara O. Reliability and validity of interview data on chronic diseases. The Mini-Finland Health Survey. J Clin Epidemiol. 1993;46:181–191. doi: 10.1016/0895-4356(93)90056-7. [DOI] [PubMed] [Google Scholar]
- 29.Skinner KM, Miller DR, Lincoln E, Lee A, Kazis LE. Concordance between respondent self-reports and medical records for chronic conditions: experience from the Veterans Health Study. J Ambul Care Manage. 2005;28:102–110. doi: 10.1097/00004479-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Stange KC, Zyzanski SJ, Smith TF, et al. How valid are medical records and patient questionnaires for physician profiling and health services research? A comparison with direct observation of patients visits. Med Care. 1998;36:851–867. doi: 10.1097/00005650-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Luck J, Peabody JW, Dresselhaus TR, Lee M, Glassman P. How well does chart abstraction measure quality? A prospective comparison of standardized patients with the medical record. Am J Med. 2000;108:642–649. doi: 10.1016/s0002-9343(00)00363-6. [DOI] [PubMed] [Google Scholar]
- 32.Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. Jama. 2000;283:1715–1722. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]
- 33.Warsi A, Wang PS, LaValley MP, Avorn J, Solomon DH. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med. 2004;164:1641–1649. doi: 10.1001/archinte.164.15.1641. [DOI] [PubMed] [Google Scholar]
- 34.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25:1159–1171. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]