Essential hypertension is characterized by chronically elevated blood pressure with no specific underlying medical cause. Data on family history of affected individuals coupled with disease concordance rate in twins has established that both genetic and environmental factors determine susceptibility to hypertension. The heritability of hypertension is often cited in the range of ≈30% to 60%, with multiple contributory genes; additionally, ethnic and genetic heterogeneity participate in variable clinical presentation and drug response in hypertension, rendering genetic study of this disease a challenging task. Human population and animal studies have implicated several important etiologic pathways contributing to the clinical presentation of essential hypertension that enable functional candidate gene association studies, in addition to more comprehensive genome wide linkage or association studies. Recent results also suggest a complex genetic architecture for hypertension and its associated risk traits, including evidence for pleiotropy (one gene→multiple traits), epistasis (gene-by-gene interaction), and on occasion molecular heterosis (a more extreme phenotype for heterozygotes than either homozygote class). Here we highlight recent findings on the genetics of hypertension that may lead to new approaches for investigating the pathogenesis, diagnosis, treatment, and prognosis of the disease.
Manifestations of Heredity: Heritability, Twin Pairs, Genetic Pleiotropy, and “Intermediate Phenotypes” for Susceptibility to Hypertension
Although blood pressure displays substantial heritability, typically reported at ≈30% to 60%,1 hypertension is likely to be a heterogeneous phenotype (trait). Applying the concept of “intermediate phenotypes”2,3 might enhance risk assessment for the development of future hypertension and its consequences, thus enabling more timely diagnosis and management of even “prehypertensive” individuals. Such intermediate phenotypes may be influenced earlier and more proximately by the genome than are ultimate disease/clinical traits such as hypertension, and therefore may assist in discovery of hypertension-predisposition loci.2,3
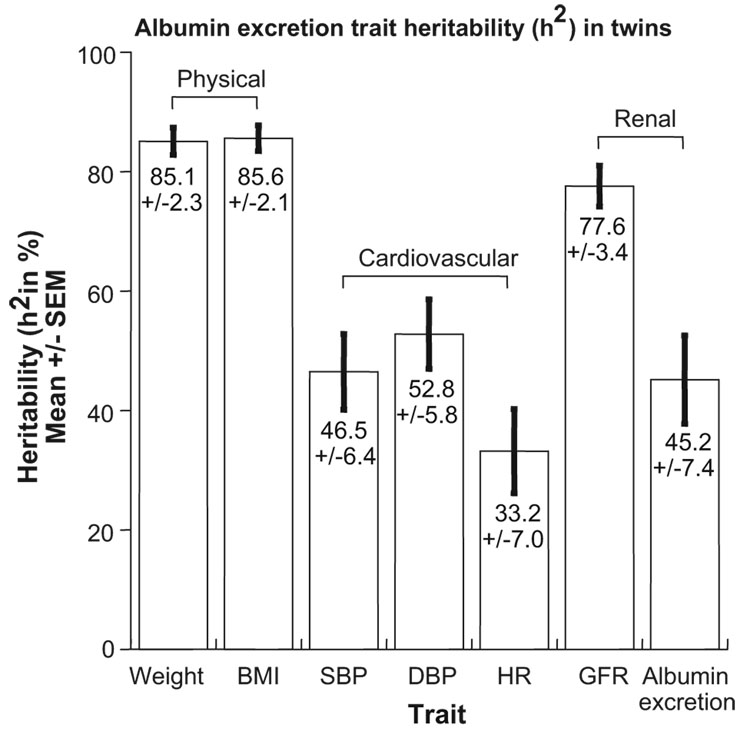
Intermediate traits in physical, physiological/hemodynamic,4 autonomic/sympathetic,5–8 metabolic,9 inflammatory,9 oxidative,5 endothelial,10,11 and renal12 pathways displayed significant heritability, typically exceeding that reported for blood pressure itself (eg, Figure 1). With the rapid advance of genome technologies, informed systematic phenotyping of sufficiently large numbers of subjects for genetic studies may now be the rate-limiting step in permitting substantive future advances in complex trait genetics.
Figure 1.
Heritability (h2) of physical, cardiovascular, and renal traits derived from studies in twin-pairs. Heritability is the % of trait variance accounted for by additive genetic variance. The cardiovascular and renal traits may be “intermediate phenotypes” for essential hypertension.12
Genome-Wide Linkage Studies: From Chromosomal Regions to Susceptibility Alleles
Identification of genes involved in complex genetic diseases, such as essential hypertension, might be difficult because of such factors as the number of loci involved and the possibility of small effects for each gene involved. Nonetheless, linkage offers the possibility of scanning the entire genome for contributory loci. A ≈400 to 800 microsatellite (≈5 to 10 cM) genome scan in 922 predominantly normotensive sibling pairs to position loci with suggestive linkage (LOD [loga-rithm of the odds for linkage] scores of 2 to 3) to DBP on chromosomes 5p13, 14q12, and 17p12.13 The NHLBI Family Blood Pressure Program (FBPP)14 used a 391 microsatellite (≈10 cM) genome scan in sibling pairs from >10 000 individuals in >3000 families to establish significant (LOD score >3) linkage for loci influencing pulse pressure on chromosomes 1, 7, 17, 19, 20, 17, and 21, with pulse pressure heritability (h2) estimates of 0.25 to 0.33.
Using a sibling-pair linkage study based on families ascertained on probands with established hypertension, excess allele sharing was found at the D18S474 marker on chromosome 18q21.1 in 56 pedigrees. The follow-up case-control analysis revealed association of hypertension with the Ring finger and KH-Domain-containing-2 gene (RKHD1). The mechanistic involvement of RKHD1 with hypertension is not understood.15
In a study of 2142 sibling pairs with evidence of linkage for hypertension on 5q13, the subsequent TDT (transmission disequilibrium test; association test controlling for family background) analysis revealed only borderline-significant evidence (P<0.07) for linkage and association at marker D5S2019 on 5q13, suggesting that this region may harbor a susceptibility gene with modest effects on hypertension.16 In the FBPP, linkage and subsequent positional candidate-based association studies demonstrated that chromosome 1q contains at least 3 genes associated with BP: ATP1B1, RGS5, and SELE. Individual variants in these 3 genes accounted for ≈2 to 5 mm Hg differences in mean systolic BP levels, with the cumulative effect reaching as high as ≈8 to 10 mmHg.17
Role of Systematic Polymorphism Discovery at Candidate Genetic Loci: Novel Susceptibility Alleles for Hypertension
The completion of human genome project and advances in public single nucleotide polymorphism (SNP) databases provide investigators with a screening set of variants for candidate gene studies. However, less than half of the ≈11.8 million variants in dbSNP have been validated as polymorphic, indicating that the true extent of variation in the human genome and across populations is still uncertain. Systematic SNP discovery can identify the actual extent of variation of any given candidate gene in the population of interest, especially for relatively uncommon alleles, which are likely to be underrepresented in the existing public databases.
Secretogranin II (SCG2), GTP cyclohydrolase 1 (GCH1), and tyrosine hydroxylase (TH) are examples of functional candidate genes at which systematic polymorphism discovery by resequencing was accomplished.8,10,18 Associations were found between TH promoter8 variant C-824T for urinary catecholamine excretion as well as for blood pressure response to environmental stress in twin pairs; and between SCG2 intronic SNP G735A and hypertension in blacks.18 The 3′-UTR C +243T variant of GCH1 had a significant overall effect on SBP, and a gene-by-sex interaction effect on DBP, with the predominant effect in women.10
Genome-Wide Association Studies: Initial Results in Hypertension
In the setting of substantial locus heterogeneity, allelic association studies may offer an increase in statistical power to detect disease predisposition genes, as compared with linkage approaches. Microsatellites (MS) DNA polymorphisms consist of variably repeating units of 1 to 5 base pairs. In a genome-wide study using 3 independent Japanese hypertension case-control samples, 54 MS markers were significant in all 3 stages of the pooling experiments, but only 19 (35%) of them were confirmed when individual typing was performed.19 In the NHLBI FBPP, family-based association analysis using 384 MS markers demonstrated 21 candidate loci for hypertension. Two loci, D3S2459 and D10S1412, were additionally confirmed in 2 linkage scans.20 However, MS markers may be too sparsely distributed to detect the great majority of genetic associations, given a typical extent of LD (linkage disequilibrium) at ≈30 to 50 kbp in subjects of European ancestry, and ≈3 to 10 kbp in subjects of African ancestry. Even 18 977 markers represents an average spacing of only ≈174 kbp across the ≈3.3 Gbp human genome, whereas the customary 400 to 800 MS markers used in genome-wide linkage scanning are spaced every ≈4000 to 8000 kbp (≈4 to 8 Mbp) across the genome.
A recent genome-wide association (GWA) study for susceptibility to several common diseases was conducted by the UK Wellcome Trust21; 500 568 SNPs on Affymetrix gene chips resulted in an average marker spacing of ≈6 kbp across the ≈3.3 Gbp human genome. ≈2000 individuals with hypertension and ≈3000 shared controls were studied to ensure adequate sample size for detection of even modest effects of susceptibility loci. In hypertension, the most significant SNPs, displaying a “moderate” level of association (probability values from 1×10−5 to 5×10−7), were not in genes from physiological pathways previously implicated, but instead included regions on chromosomes 1q43, 8q24, 10q11, 12p12, 12q23, 13q21, and 15q26. By contrast, if genome-wide significance was defined here as P<5×10−7, then no SNPs across the genome achieved “significant” effects on hypertension. What might account for this initially surprising lack of effects? One possibility is study design and selection of controls. Not all of the ≈3000 shared “controls” were suitable as controls for hypertension, because no blood pressure data were obtained in the “controls”, giving rise to the likelihood that up to ≈25% of such “controls” were in fact hypertensive, thus biasing this GWA result toward the null.
Of a note for studies of such a large number of SNPs (here: ≈0.5×106), the significance threshold to avoid false-positive conclusions in an unreplicated (initial) report must be adjusted appropriately; here “significance” was defined at P<5×10−7, a level not achieved for any locus in this blood pressure study. However, replication provides a potential way to circumvent such remarkably stringent significance thresholds, because the probabilities of independent replications are multiplicative. Indeed the Wellcome Trust study21 did successfully replicate hypertension associations previously found22 at the AGT (1q42-q43) and ADRB2 (5q32-q34) loci, albeit at only the P<0.05 level, thereby offering additional confirmation of the contribution of these loci to hypertension.
The Framingham Heart Study of the NHBLI23 completed a dense SNP scan on >1300 individuals with SBP and DBP followed for >25 years, as well as arterial stiffness phenotypes. At >70×105 SNPs, the average intermarker map density was ≈40 kbp. Although none of the associations achieved genome-wide significance (here stated as 4.4×10−8), at a more moderate level of stringency (P<10−5) there were 7 associations for SBP or DBP, and 5 for arterial stiffness traits. At prespecified candidate genes within the RAAS (ACE, AGT, AGTR1, CYP11B2, NR3C2, REN), there were nominal (P<0.05) associations with both SBP and DBP for AGT and NR3C2; the arterial stiffness traits also displayed nominal (P<0.05) associations with each of these loci. As a positive control, the Framingham study did replicate previous associations of chromosome 9p21 with coronary heart disease.24
Pathway-Wide Genomics in Population BP Extreme Samples: Gene-by-Sex Interactions on BP
An extreme-phenotype study design examined genotypes and haplotypes of 26 single nucleotide polymorphisms (SNPs) across the epithelial sodium channel γ-subunit gene (SCNN1G) using unrelated subjects within the upper and lower 10th %iles of the systolic pressure distribution of 2911 subjects from the Victoria Family Heart Study in Australia; the results indicated that common SNPs in the SCNN1G gene are associated with high systolic blood pressure in the general white population.25
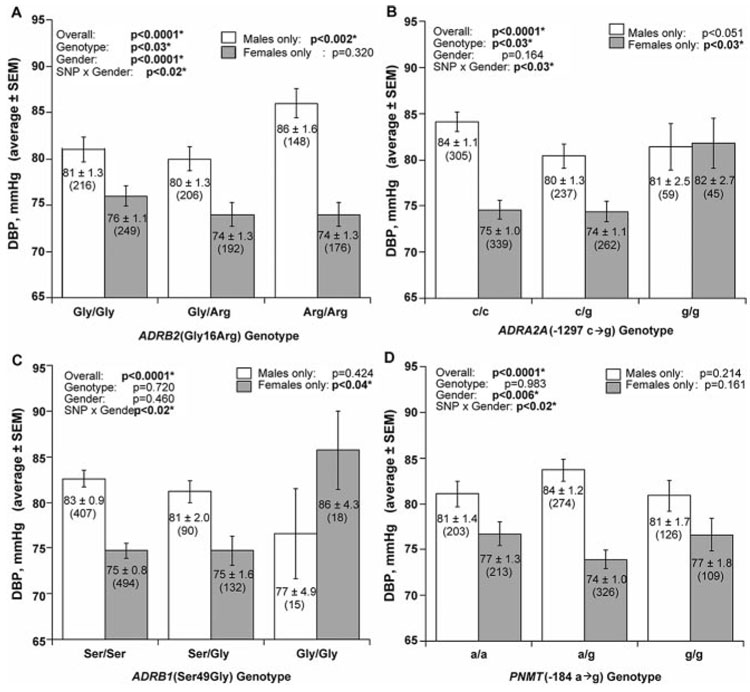
A large north American population-based study analyzed subjects falling within the upper and lower ≈5th %iles of DBP distribution among >50 000 individuals in a primary care population, to assess the role of genetic variation at 35 loci with known physiological roles in adrenergic or renal regulation of BP.22 In addition to confirming genotype-on-phenotype associations found previously, they discovered that sex-by-gene interactions (Figure 2) influence blood pressure. In females, variants at ADRB1 and ADRA2A were found to be hypertension susceptibility genes, whereas in men, the ADRB2 and AGT genes were associated with blood pressure. Consistent with the individual SNP analyses, an ADRA2A haplotype influenced BP only in women, whereas 2 AGT haplotypes affected BP only in men.22
Figure 2.
Gene-by-sex interaction on blood pressure. Examples of gene-by-sex interactions at 4 loci to influence diastolic blood pressure (DBP) in population extremes of blood pressure: ADRB2, ADRA2A, ADRB1, and PNMT.22
Sex-dependent genetic effects in hypertension were also noted for genetic variants at TH,8 chromogranin A (CHGA),26 GCH1,10 and the estrogen receptor genes (ERα and ERβ).27 Such findings raise the possibility that previous genetic studies failing to find associations with hypertension might have overlooked the importance of sex-by-gene interaction effects. Although variation in genes encoding for estrogen receptors showed differential effects on blood pressure in men versus women,27 suggesting a likely sex-dependent role for estrogen in hypertension, the precise mechanisms whereby sex and autosomal genes interact to influence susceptibility to hypertension is yet to be determined.
Sympathetic Nervous System: Adrenergic Pathway Polymorphism
The sympathetic nervous system is a primary regulator of acute changes in blood pressure. Adrenergic receptors have critical roles in regulating sympathetic neurotransmitter release and actions; hence genes encoding these receptors serve as functional candidate genes for hypertension. Among the many genes involved in this complex pathway, an intronic insertion/deletion variant in the Regulator of G protein Signaling (RGS2) gene was found to be an ethnicity-specific susceptibility gene for hypertension in blacks.28
On examining 25 SNPs identified in the α-2B adrenergic receptor gene (ADRA2B), no evidence was found to support ADRA2B as a major determinant of response to α-2-adrenergic blockade, or as a susceptibility gene to essential hypertension.29 In another study of α-2A and α-2C adrenergic receptor genes (ADRA2A, ADRA2C) with hypertension in 3398 men, no evidence was found for association with hypertension, untreated blood pressure, or cardiac functional alterations.30
Across 10 common variants in the β2-adrenoceptor (ADRB2) locus, no individual SNP showed association with hypertension, but a significant interaction between age and 1 common ADRB2 haplotype was present in white subjects. This haplotype was associated with protection against hypertension in younger (≤50 years) but not older (>50 years) subjects, suggesting that age is an important modifier for the effects of ADRB2 on the development of hypertension.31 Also within the adrenergic pathway, promoter SNP C-824T in TH,8 as well as the coding variant (catestatin Gly364Ser) of CHGA,26 had significant effects on basal blood pressure in the population.
In another disease population, ADRB2 was examined in patients with postural tachycardia syndrome (POTS); even though no association with the POTS trait was identified, higher catecholamine levels were observed in POTS patients compared with healthy controls. Further analysis showed that POTS patients who are homozygous in either codon 16 (Gly/Gly) or codon 27 (Glu/Glu) had lower catecholamine levels, with higher supine and upright blood pressures compared to other genotypes, suggesting that decreased β2-adrenoceptor-related vasodilation may contribute to the hemodynamic diversity of POTS.32
Renin-Angiotensin-Aldosterone-System (RAAS)
The renin-angiotensin-aldosterone-system (RAAS) is a BP-regulating pathway/cascade in series, beginning with the renin substrate angiotensinogen (AGT). In a study of AGT promoter variants coupled to transfected luciferase reporters, promoter positions −20 and −217 had the largest influence on AGT transcription in cell lines, whereas other promoter SNPs had substantially smaller impact. These data support the hypothesis that SNPs in the AGT promoter may act cell-specifically to differentially regulate the level of AGT transcription, thereby affecting hypertension risk.33,34
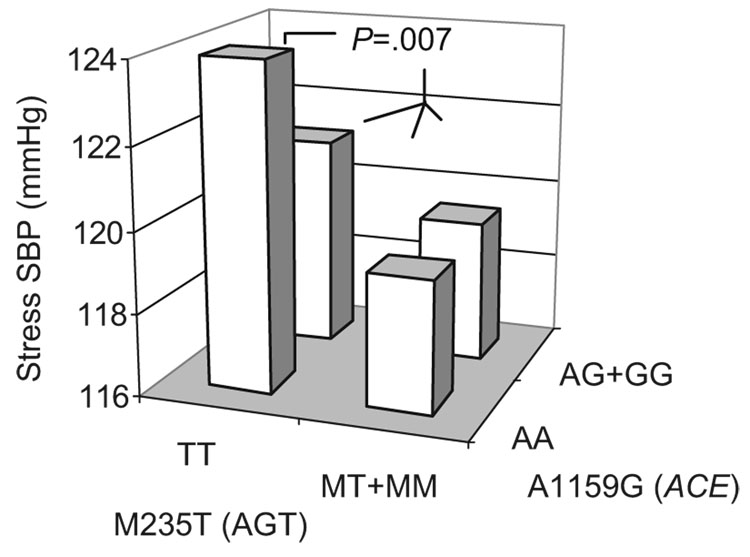
In a twin study of 4 major loci (AGT, ACE, AGTR1 and CYP11B2) in the RAAS pathway, AGT coding region variant Met235Thr and ACE promoter variant A-239T independently associated with blood pressure, but together they accounted for only ≈1% of SBP variation, whereas gene-by-gene (AGT-by-ACE, Figure 3), gene-by-gender, and gene-by-body mass index interactions accounted for up to ≈2.5% of resting BP and ≈7.3% of stress BP variations.35 In an association study between plasma aldosterone concentration and 13 SNPs in the steroid 11-βhydroxylase loci (CYP11B1, CYP11B2), aldosterone levels were associated with CYP11B2 T−344C, C595T, −4837C, and G4936A polymorphisms in multivariate analyses. The T−344C polymorphism remained associated after Bonferroni multiple-testing correction, and T−344C and renin activity interaction significantly predicted aldosterone, whereas T−344C and salt intake interaction determined BP. In vitro experiments showed that promoter activity of the −344C allele was influenced by angiotensin II stimulation, supporting the functional significance of T−344C in angiotensin II reactivity and hence salt sensitivity in this Japanese sample.36
Figure 3.
Epistasis in control of risk traits. Gene-by-gene inter-action between AGT and ACE to determine stress-induced BP increments in twin pairs.35
At the human type 1 angiotensin II receptor locus (AGTR1) on chromosome 3q, variant A1166C (rs5186) within the 3′-UTR of the is a target site for endogenous micro-RNA hsa-miR-155, encoded by a locus on chromosome 21. Hsa-miR-155 downregulates the expression of only the A1166, but not the 1166C allele. The reported 1166C allelic association with hypertension in many studies suggests that the 1166C allele may be functionally associated with hypertension through abrogating regulation by hsa-miR-155, thereby elevating AGTR1 expression.37 A study of 2 novel SNPs (G-1889C and A-1859G) upstream of the coding region in CYP11B1 showed that G-1889C was associated with decreased 11-βhydroxylase efficiency, as well as altered transcriptional response to stimulation by adrenocorticotropic hormone.38
On examining heritability of angiotensin I converting enzyme (ACE), angiotensin I converting enzyme 2 (ACE2), and beprilysin (NEP), genetic factors accounted for 24.5%, 67%, and 22.7% of the phenotypic variation in circulating ACE, ACE2, and NEP. Whereas ACE and NEP were associated with SBP and DBP in univariate analyses, only ACE was independently associated with BP after accounting for covariates and shared childhood household environment.39
Metabolism and Hypertension
The metabolic syndrome is a cluster of traits (obesity, atherogenic dyslipidemia, elevated blood pressure, insulin resistance, and a prothrombotic state) that may cooperate to confer increased risk of cardiovascular disease.9 Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α) is a transcription factor that regulates several metabolic processes, including mitochondrial biogenesis and respiration, hepatic gluconeogenesis, and muscle fiber-type switching. In Danish subjects, the Ser allele of Gly482Ser in PGC1-α associated with reduced risk of hypertension, as well as SBP and DBP.40
In the context of insulin sensitivity, 14 SNPs among hypertensive candidate genes (ACE, AGT, AGTRI, ADDI, NPPA, ADDRB2, SCNN1A, GNB3, and NOS3) were associated with the trait in 100 Mexican families.41 In a twin-pair study,9 C-reactive protein (CRP) concentration was substantially heritable (h2=56±7%), and shared joint genetic influences (pleiotropy, evidenced by genetic covariance) with several other features of the metabolic syndrome, including BMI, leptin, triglycerides, and blood pressure, as well as determination by several adrenergic pathway loci (TH, ADRB1, ADRB2).
Type 2 iodothyronine deiodinase (DIO2) activates thyroid hormone by converting the prohormone thyroxine to bioactive 3,3′,5-triiodothyronine; an excess of Ala92 carriers is found in hypertensive euthyroid adults.42 However, there was no evidence of association found between DIO2 and arterial hypertension in a sample of type 2 diabetes patients.43 Of 5 SNPs examined in the follicle-stimulating hormone (FSH) receptor gene (FSHR), the A allele of 5′ region variant rs1394205 showed association with decreased transcriptional activity in a FSHR promoter activity construct, lower serum estradiol levels, and increased susceptibility for hypertension in women.44
Cardio-Renal Target Organ Susceptibility Genes and Hypertension
Because hypertension is an established risk factor for cardio-vascular disease as well as end-stage renal disease, it is perhaps not surprising that essential hypertension shares common susceptibility genes with cardio-renal end-organ damage in the setting of hypertension. The frequency of the AGT Thr allele at Met235Thr was higher in blacks than whites, and independently associated with increased coronary events in postmyocardial infarction black patients.45 In patients with heart failure, AGT codon 235 Thr/Thr homozygotes were admitted to the hospital 3 years younger in age and 10 years earlier after initial hypertension diagnosis. Associations between the AGT 174 Met allele and history of heart failure as well as increased mortality at follow-up were also observed. High-risk combination genotypes were predictive of mortality.46 In another study, only one 3′ noncoding SNP (rs943580) in AGT was associated with transmitral early peak filling velocity.47
Among 80 cardiovascular candidate genes, APOE, SCN7A, and SLC20A1 associated with ventricular mass, whereas ADRB1 associated with relative wall thickness in blacks.48 Peter et al showed that the S/S genotype of rs20077647 in the promoter region of the estrogen receptor-alpha gene (ESR1) associated with LV mass and wall thickness.49 Corin activates pro-A-type and pro-B-type natriuretic peptides into biologically active molecules, and the natriuretic peptide system is known for its antihypertrophic effects; the Ile allele at Thr555Ile in Corin was an independent predictor of LV mass in subjects with high SBP. Interaction between 555Ile and SBP was also a significant predictor of LVM.50
Among renal disease candidate genes, the NEDD4L ubiquitin ligase influences sodium reabsorption in the distal nephron by controlling endocytosis of the epithelial Na+ channel. Indeed, several variants in NEDD4L ubiquitin ligase were associated with BP.51 An intronic variant, rs10177833 within the NaHCO3 cotransporter gene (SLC4A5), was associated with SBP and DBP both at baseline and at 10-year follow-up in 96 Utah pedigrees.52
The growth hormone (GH) secretagogue receptor (GHSR) is involved in release of GH, which influences left ventricular myocardial growth, structure, and function. Associations between 2 GHSR common haplotypes and LVM and geometry, independent of blood pressure and body mass in the general population, suggested involvement of GHSR variation in the pathogenesis of LVH.53
Complex Genetic Architecture: Pleiotropy, Epistasis, Heterosis, and Population Stratification
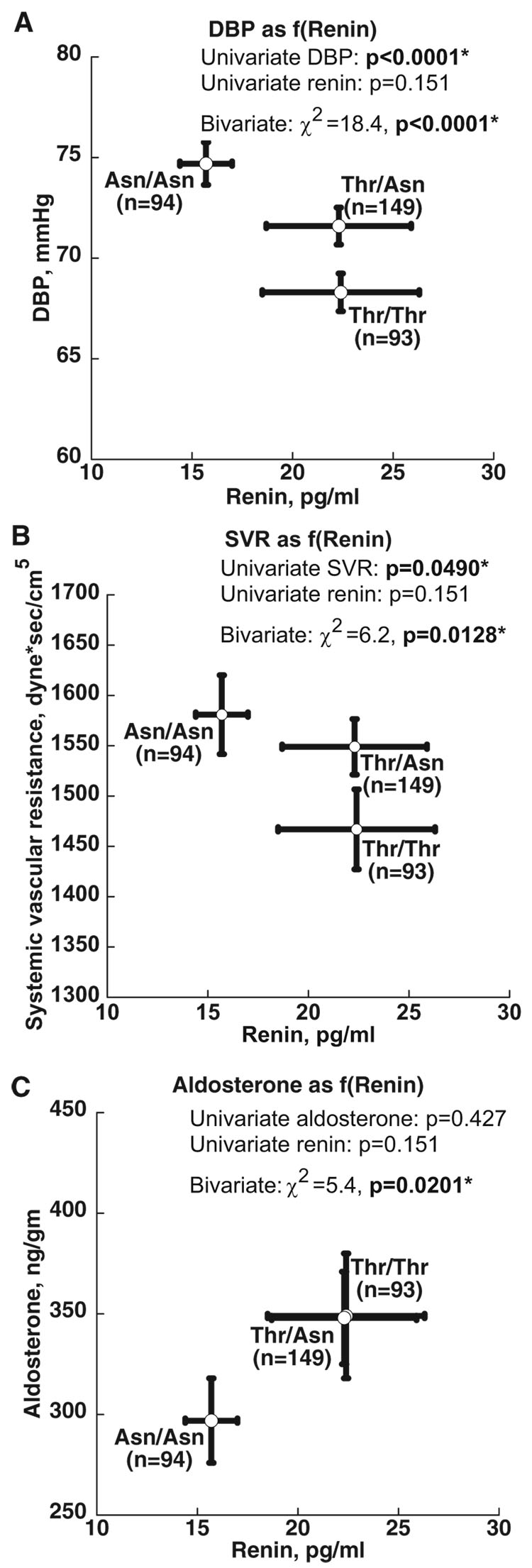
Single genes may affect multiple observable traits, a phenomenon known as pleiotropy A twin study demonstrated effects of pleiotropy for polymorphism Thr431Asn in the ROCK2 gene on renin, blood pressure, systemic vascular resistance (SVR), and aldosterone; in particular, Thr431Asn seemed to alter the coupling between renin and 3 of its downstream targets: aldosterone, SVR, and BP (Figure 4).4 In another example of genetic pleiotropy, On examining a bivariate genetic linkage demonstrated 3 novel trans-QTLs influencing sympathochromaffin exocytosis in twin and sibling pairs, on chromosomes 2, 7, and 13.
Figure 4.
Pleiotropy. ROCK2 (rho kinase) variant Thr431Asn: changing the coupling between renin and its targets: Blood pressure, aldosterone, and systemic vascular resistance. Bivariate (2 phenotype) genetic analyses in twin-pairs established pleiotropy (1 gene→more than 1 trait).4
Epistasis (gene-by-gene interaction), first defined by the English geneticist William Bateson in 1907, was demonstrated for circulating C-reactive protein (CRP) in a twin-pair study.9 With increasing numbers of the TH T−824 allele, CRP declines incrementally if the ADRB2 T−47C genotype is either T/C or C/C. However, on a background of ADRB2 T−47C T/T homozygosity, the effect of increasing TH T−824C minor (C) allele copy number was a sharper decline in CRP only for TH C/C homozygotes. Twin-pair studies also established epistasis of alleles at multiple loci encoding the renin-angiotensin-aldosterone system in determination of the blood pressure response to environmental stress.35
Molecular heterosis (more extreme phenotype for heterozygotes than either homozygote class, sometimes called “overdominance”) was noted in a study of ACE locus polymorphisms on response to ACE inhibition in hypertension. 54 Detection of heterosis explicitly requires analysis of diploid genotype (rather than simply allele) effects on a trait.55 To assess the genotype effect of the ACE locus on ACE inhibitor responsiveness, hypertensive patients in the African American Study of Kidney Disease and Hypertension trial (AASK) taking ACE inhibitor ramipril were genotyped at 3 intronic polymorphisms spanning the biologically active regions of the ACE locus. Either of 2 homozygous genotypes at G12269A responded significantly faster than G/A heterozygotes; similar associations were seen comparing homozygous to heterozygous ACE haplotypes, suggesting that the genetic phenomenon of heterosis may be an important determinant of responsiveness to an ACE inhibitor. Because the ACE enzyme may typically function as a homodimer,56 the possibility of ACE variant heterodimerization arises as a potential explanation for phenotypic heterosis in this setting.
Pharmacogenetics: Antihypertensive Drugs
Pharmacogenetic studies focus on determining how genetic variation affects drug response, with the goal of individualized (or “personalized”) antihypertensive therapy. In general, antihypertensive pharmacogenetics seems to be at a relatively early stage, without clear consensus yet on genetic predictors to be used routinely in clinical practice.57
A low-molecular weight heparin (LMWH) treatment trial for pregnant women with history of preeclampsia showed that LMWH reduced preeclampsia, poor outcomes, resistance of uteroplacental flow, and blood pressure in patients who were ACE Deletion/Deletion homozygotes.58 In a nonparametric linkage study of hypertensive-therapy-nonresponder groups, chromosome 2p was linked to RAAS agent (ACE inhibitor, angiotensin II type-1 receptor blocker, or adrenergic-blocker) nonresponsiveness, whereas 10q showed suggestive linkage to the calcium channel blocker/diuretic nonresponsiveness.59
In an association study between diuretic response and genes in renal sodium transport systems, polymorphisms in WNK1, ADRB2, and the epithelial sodium channel γ-subunit gene (SCNN1G) predicted interindividual differences in antihypertensive responses to hydrochlorothiazide.60 As noted above under genetic complexity, black hypertensive patients with ACE homozygous genotypes at G12269A (or haplotypes) responded to ramipril significantly faster than those with a heterozygous genotype or haplotype.54
Perspectives and Strategies
Human Genetic Study Designs
Recent genetic studies have identified or confirmed several chromosomal regions containing susceptibility alleles for essential hypertension. In the case of candidate genetic locus studies, the rationale for involvement of a specific gene product in hypertension may be apparent, whereas in hypothesis-free, genome-wide linkage or association studies, establishing the responsible variant in the associated chromosomal interval may be challenging.15 Particular problems in the linkage or association studies of the hereditary basis of complex traits (including hypertension) include likely phenotypic and genetic heterogeneity, complicated by problematic statistical power, sometimes resulting in inconsistent results or lack of replication.
In addition to large-scale, assumption-free, hypothesis-generating WGA study designs,21 smaller scale, investigator-initiated, hypothesis-driven research remains a mainstay of genetic approaches to the pathophysiology of essential hypertension.
Statistical Confidence
Achieving sufficient statistical power in newly emerging GWA studies is particularly challenging as a consequence of the very large number of genomic regions simultaneously examined for association; indeed, contemporary chip-based association strategies can now interrogate upwards of ≈5×105 SNP loci21 spanning ≈60×103 to ≈1×106 relatively independent LD blocks over the ≈3.3 Gbp human genome, and even denser SNP mapping is now in the works. Such density provokes severe adjustment of stringency thresholds for avoidance of Type I statistical errors; indeed, in a recent study21 the threshold for significance for ≈5×105 SNP comparisons was set at P<5×10−7. Although such stringent multiple comparisons standards are useful, ultimately more realistic standards may need to be used, such as the principles of the False Discovery Rate,61,62 to minimize false-negative conclusions.
A practical experimental alternative to extreme statistical stringency is replication,63 because the likelihood of finding significance in 2 independent studies represents the joint probability; hence, the probability values are essentially multiplicative. However, some studies may be difficult to replicate, because of size, cost, or unique patient populations.
Relative Pairs: Twins and Pedigrees (Families)
The role of relative pair studies in complex trait genetics is evolving, as association methods increasingly supplant linkage. However, even in the age of large scale genomic association studies, family members may provide useful information and enable valuable strategies, such as combined linkage with association analyses, or family-based case/control approaches to minimize the potentially artifactual effects of population stratification, such as the TDT or QTDT. In addition, twin and family studies offer the additional advantage of determining heritability (h2) for any trait, an important initial step in testing whether a trait is likely to be tractable to genetic investigation.2
The NHLBI has invested substantially since 1994 in its Family Blood Pressure Program (FBPP),14,20 an extensive series of 4 cooperating networks: GenNet (University of Michigan), GENOA (University of Texas at Houston), HyperGEN (University of Utah), and SAPPHIRe (Stanford). FBPP data are archived for collaborative studies at <http://www.biostat.wustl.edu/fbpp/FBPP.shtml>.20,64
Classical twin studies offer the many advantages of the twin pair approach, including estimation of heritability, pleiotropy, structural equation modeling, linkage, association, gene-by-environment interactions, and extension into pedigrees. Current twin studies actively pursuing the heredity of blood pressure include the Medical College of Georgia,1 the Free University of Amsterdam and Queensland Institute of Medical Research and University of Melbourne,13,65 and UCSD.8–10,12
Phenotypic Stratification of Hypertension
Essential hypertension is a common trait with late penetrance, multiple and heterogeneously correlated phenotypes, phenocopies as a result of secondary hypertension or environmental perturbation, and abrogation by other environmental influences. As compared to classical Mendelian syndromes, such “complex traits” may be only variable tractable to standard genetic investigation. An alternative approach to characterization of such ultimate disease traits is to use “intermediate phenotypes”,2,3,66–68 genetically simpler traits that may prove advantageous because of superior heritability, earlier and more consistent penetrance, and rational involvement in the pathogenesis of the disease state.
Genetic Complexity
Finally, genetic complexity in the form of potential effect modifiers such as interactions (sex-by-gene, gene-by-environment, gene-by-gene [epistasis]), pleiotropy (multiple traits consequent on one genetic variant), molecular heterosis (extreme phenotypic values in heterozygotes), and population stratification (admixture) will continue to necessitate consideration in experimental design, as well as appropriate stratification or covariate adjustment in genetic analyses of the hereditary basis of human blood pressure variation.
Acknowledgments
Sources of Funding This work was supported by National Institutes of Health, Department of Veterans Affairs, and the National Kidney Foundation.
Footnotes
Disclosures None.
References
- 1.Kupper N, Ge D, Treiber FA, Snieder H. Emergence of novel genetic effects on blood pressure and hemodynamics in adolescence: the Georgia Cardiovascular Twin Study. Hypertension. 2006;47:948–954. doi: 10.1161/01.HYP.0000217521.79447.9a. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor DT, Insel PA, Ziegler MG, Hook VY, Smith DW, Hamilton BA, Taylor PW, Parmer RJ. Heredity and the autonomic nervous system in human hypertension. Curr Hypertens Rep. 2000;2:16–22. doi: 10.1007/s11906-000-0053-8. [DOI] [PubMed] [Google Scholar]
- 3.Lillie EO, O’Connor DT. Early phenotypic changes in hypertension: a role for the autonomic nervous system and heredity. Hypertension. 2006;47:331–333. doi: 10.1161/01.HYP.0000203980.44717.aa. [DOI] [PubMed] [Google Scholar]
- 4.Seasholtz TM, Wessel J, Rao F, Rana BK, Khandrika S, Kennedy BP, Lillie EO, Ziegler MG, Smith DW, Schork NJ, Brown JH, O’Connor DT. Rho kinase polymorphism influences blood pressure and systemic vascular resistance in human twins: role of heredity. Hypertension. 2006;47:937–947. doi: 10.1161/01.HYP.0000217364.45622.f0. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy BP, Rao F, Botiglieri T, Sharma S, Lillie EO, Ziegler MG, O’Connor DT. Contributions of the sympathetic nervous system, glutathione, body mass and gender to blood pressure increase with normal aging: influence of heredity. J Hum Hypertens. 2005;19:951–969. doi: 10.1038/sj.jhh.1001912. [DOI] [PubMed] [Google Scholar]
- 6.Greenwood TA, Rao F, Stridsberg M, Mahapatra NR, Mahata M, Lillie EO, Mahata SK, Taupenot L, Schork NJ, O’Connor DT. Pleiotropic effects of novel trans-acting loci influencing human sympathochromaffin secretion. Physiol Genomics. 2006;25:470–479. doi: 10.1152/physiolgenomics.00295.2005. [DOI] [PubMed] [Google Scholar]
- 7.Valle A, O’Connor DT, Taylor P, Zhu G, Montgomery GW, Slagboom PE, Martin NG, Whitfield JB. Butyrylcholinesterase: association with the metabolic syndrome and identification of 2 gene loci affecting activity. Clin Chem. 2006;52:1014–1020. doi: 10.1373/clinchem.2005.065052. [DOI] [PubMed] [Google Scholar]
- 8.Rao F, Zhang L, Wessel J, Zhang K, Wen G, Kennedy BP, Rana BK, Das M, Rodriguez-Flores JL, Smith DW, Cadman PE, Salem RM, Mahata SK, Schork NJ, Taupenot L, Ziegler MG, O’Connor DT. Tyrosine hydroxylase, the rate-limiting enzyme in catecholamine biosynthesis: discovery of common human genetic variants governing transcription, autonomic activity, and blood pressure in vivo. Circulation. 2007;116:993–1006. doi: 10.1161/CIRCULATIONAHA.106.682302. [DOI] [PubMed] [Google Scholar]
- 9.Wessel J, Moratorio G, Rao F, Mahata M, Zhang L, Greene W, Rana BK, Kennedy BP, Khandrika S, Huang P, Lillie EO, Shih PA, Smith DW, Wen G, Hamilton BA, Ziegler MG, Witztum JL, Schork NJ, Schmid-Schonbein GW, O’Connor DT. C-reactive protein, an ‘intermediate phenotype’ for inflammation: human twin studies reveal heritability, association with blood pressure and the metabolic syndrome, and the influence of common polymorphism at catecholaminergic/betaadrenergic pathway loci. J Hypertens. 2007;25:329–343. doi: 10.1097/HJH.0b013e328011753e. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Rao F, Zhang K, Khandrika S, Das M, Vaingankar SM, Bao X, Rana BK, Smith DW, Wessel J, Salem RM, Rodriguez-Flores JL, Mahata SK, Schork NJ, Ziegler MG, O’Connor DT. Discovery of common human genetic variants of GTP cyclohydrolase 1 (GCH1) governing nitric oxide, autonomic activity, and cardiovascular risk. J Clin Invest. 2007;117:2658–2671. doi: 10.1172/JCI31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lillie EO, Mahata M, Khandrika S, Rao F, Bundey RA, Wen G, Chen Y, Taupenot L, Smith DW, Mahata SK, Ziegler MG, Cockburn M, Schork NJ, O’Connor DT. Heredity of endothelin secretion: human twin studies reveal the influence of polymorphism at the chromogranin A locus, a novel determinant of endothelial function. Circulation. 2007;115:2282–2291. doi: 10.1161/CIRCULATIONAHA.106.648345. [DOI] [PubMed] [Google Scholar]
- 12.Rao F, Wessel J, Wen G, Zhang L, Rana BK, Kennedy BP, Greenwood TA, Salem RM, Chen Y, Khandrika S, Hamilton BA, Smith DW, Holstein-Rathlou N-H, Ziegler MG, Schork NJ, O’Connor DT. Renal albumin excretion: twin studies identify influences of heredity, environment, and adrenergic pathway polymorphism. Hypertension. 2007;49:1015–1031. doi: 10.1161/HYPERTENSIONAHA.106.081679. [DOI] [PubMed] [Google Scholar]
- 13.Hottenga JJ, Whitfield JB, Posthuma D, Willemsen G, de Geus EJ, Martin NG, Boomsma DI. Genome-wide scan for blood pressure in Australian and Dutch subjects suggests linkage at 5P, 14Q, and 17P. Hypertension. 2007;49:832–838. doi: 10.1161/01.HYP.0000260092.93964.ed. [DOI] [PubMed] [Google Scholar]
- 14.FBPP investigators. Multi-center genetic study of hypertension: The Family Blood Pressure Program (FBPP) Hypertension. 2002;39:3–9. doi: 10.1161/hy1201.100415. [DOI] [PubMed] [Google Scholar]
- 15.Guzman B, Cormand B, Ribases M, Gonzalez-Nunez D, Botey A, Poch E. Implication of chromosome 18 in hypertension by sibling pair and association analyses: putative involvement of the RKHD1 gene. Hypertension. 2006;48:883–891. doi: 10.1161/01.HYP.0000244085.52918.a0. [DOI] [PubMed] [Google Scholar]
- 16.Munroe PB, Wallace C, Xue M-Z, Marcano ACB, Dobson RJ, Onipinla AK, Burke B, Gungadoo J, Newhouse SJ, Pembroke J, Brown M, Dominiczak AF, Samani NJ, Lathrop M, Connell J, Webster J, Clayton D, Farrall M, Mein CA, Caulfield M. Medical Research Council British Genetics of Hypertension S. Increased support for linkage of a novel locus on chromosome 5q13 for essential hypertension in the British Genetics of Hypertension Study. Hypertension. 2006;48:105–111. doi: 10.1161/01.HYP.0000228324.74255.f1. [DOI] [PubMed] [Google Scholar]
- 17.Chang YP, Liu X, Kim JD, Ikeda MA, Layton MR, Weder AB, Cooper RS, Kardia SL, Rao DC, Hunt SC, Luke A, Boerwinkle E, Chakravarti A. Multiple genes for essential-hypertension susceptibility on chromosome 1q. Am J Hum Genet. 2007;80:253–264. doi: 10.1086/510918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen G, Wessel J, Zhou W, Ehret GB, Rao F, Stridsberg M, Mahata SK, Gent PM, Das M, Cooper RS, Chakravarti A, Zhou H, Schork NJ, O’Connor DT, Hamilton BA. An ancestral variant of Secretogranin II confers regulation by PHOX2 transcription factors and association with hypertension. Hum Mol Genet. 2007;16:1752–1764. doi: 10.1093/hmg/ddm123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yatsu K, Mizuki N, Hirawa N, Oka A, Itoh N, Yamane T, Ogawa M, Shiwa T, Tabara Y, Ohno S, Soma M, Hata A, Nakao K, Ueshima H, Ogihara T, Tomoike H, Miki T, Kimura A, Mano S, Kulski JK, Umemura S, Inoko H. High-resolution mapping for essential hypertension using microsatellite markers. Hypertension. 2007;49:446–452. doi: 10.1161/01.HYP.0000257256.77680.02. [DOI] [PubMed] [Google Scholar]
- 20.Gu CC, Hunt SC, Kardia S, Turner ST, Chakravarti A, Schork N, Olshen R, Curb D, Jaquish C, Boerwinkle E, Rao DC. An investigation of genome-wide associations of hypertension with microsatellite markers in the family blood pressure program (FBPP) Hum Genet. 2007;121:577–590. doi: 10.1007/s00439-007-0349-8. [DOI] [PubMed] [Google Scholar]
- 21.Consortium WTCC. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rana BK, Insel PA, Payne SH, Abel K, Beutler E, Ziegler MG, Schork NJ, O’Connor DT. Population-based sample reveals gene-gender interactions in blood pressure in White Americans. Hypertension. 2007;49:96–106. doi: 10.1161/01.HYP.0000252029.35106.67. [DOI] [PubMed] [Google Scholar]
- 23.Levy D, Larson MG, Benjamin EJ, Newton-Cheh C, Wang TJ, Hwang SJ, Vasan RS, Mitchell GF. Framingham Heart Study 100K Project: genome-wide associations for blood pressure and arterial stiffness. BMC Med Genet. 2007;8:S3. doi: 10.1186/1471-2350-8-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larson MG, Atwood LD, Benjamin EJ, Cupples LA, D’Agostino RB, Sr, Fox CS, Govindaraju DR, Guo CY, Heard-Costa NL, Hwang SJ, Murabito JM, Newton-Cheh C, O’Donnell CJ, Seshadri S, Vasan RS, Wang TJ, Wolf PA, Levy D. Framingham Heart Study 100K project: genome-wide associations for cardiovascular disease outcomes. BMC Med Genet. 2007;8:S5. doi: 10.1186/1471-2350-8-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busst CJ, Scurrah KJ, Ellis JA, Harrap SB. Selective genotyping reveals association between the epithelial sodium channel gamma-subunit and systolic blood pressure. Hypertension. 2007;50:672–678. doi: 10.1161/HYPERTENSIONAHA.107.089128. [DOI] [PubMed] [Google Scholar]
- 26.Rao F, Wen G, Gayen JR, Das M, Vaingankar SM, Rana BK, Mahata M, Kennedy BP, Salem RM, Stridsberg M, Abel K, Smith DW, Eskin E, Schork NJ, Hamilton BA, Ziegler MG, Mahata SK, O’Connor DT. Catecholamine release-inhibitory peptide catestatin (chromogranin A(352–372)): naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation. 2007;115:2271–2281. doi: 10.1161/CIRCULATIONAHA.106.628859. [DOI] [PubMed] [Google Scholar]
- 27.Ellis JA, Infantino T, Harrap SB. Sex-dependent association of blood pressure with oestrogen receptor genes ERalpha and ERbeta. J Hypertens. 2004;22:1127–1131. doi: 10.1097/00004872-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Riddle EL, Rana BK, Murthy KK, Rao F, Eskin E, O’Connor DT, Insel PA. Polymorphisms and haplotypes of the regulator of G protein signaling-2 gene in normotensives and hypertensives. Hypertension. 2006;47:415–420. doi: 10.1161/01.HYP.0000200714.81990.61. [DOI] [PubMed] [Google Scholar]
- 29.Etzel JP, Rana BK, Wen G, Parmer RJ, Schork NJ, O’Connor DT, Insel PA. Genetic variation at the human alpha2B-adrenergic receptor locus: role in blood pressure variation and yohimbine response. Hypertension. 2005;45:1207–1213. doi: 10.1161/01.HYP.0000166721.42734.49. [DOI] [PubMed] [Google Scholar]
- 30.Li JL, Canham RM, Vongpatanasin W, Leonard D, Auchus RJ, Victor RG. Do allelic variants in alpha2A and alpha2C adrenergic receptors predispose to hypertension in blacks? Hypertension. 2006;47:1140–1146. doi: 10.1161/01.HYP.0000217972.80731.ef. [DOI] [PubMed] [Google Scholar]
- 31.Bao X, Mills PJ, Rana BK, Dimsdale JE, Schork NJ, Smith DW, Rao F, Milic M, O’Connor DT, Ziegler MG. Interactive effects of common beta2-adrenoceptor haplotypes and age on susceptibility to hypertension and receptor function. Hypertension. 2005;46:301–307. doi: 10.1161/01.HYP.0000175842.19266.95. [DOI] [PubMed] [Google Scholar]
- 32.Jacob G, Garland EM, Costa F, Stein CM, Xie HG, Robertson RM, Biaggioni I, Robertson D. Beta2-adrenoceptor genotype and function affect hemodynamic profile heterogeneity in postural tachycardia syndrome. Hypertension. 2006;47:421–427. doi: 10.1161/01.HYP.0000205120.46149.34. [DOI] [PubMed] [Google Scholar]
- 33.Dickson ME, Zimmerman MB, Rahmouni K, Sigmund CD. The −20 and −217 promoter variants dominate differential angiotensinogen haplotype regulation in angiotensinogen-expressing cells. Hypertension. 2007;49:631–639. doi: 10.1161/01.HYP.0000254350.62876.b1. [DOI] [PubMed] [Google Scholar]
- 34.Dickson ME, Sigmund CD. Genetic basis of hypertension: revisiting angiotensinogen. Hypertension. 2006;48:14–20. doi: 10.1161/01.HYP.0000227932.13687.60. [DOI] [PubMed] [Google Scholar]
- 35.Ge D, Zhu H, Huang Y, Treiber FA, Harshfield GA, Snieder H, Dong Y. Multilocus analyses of Renin-Angiotensin-aldosterone system gene variants on blood pressure at rest and during behavioral stress in young normotensive subjects. Hypertension. 2007;49:107–112. doi: 10.1161/01.HYP.0000251524.00326.e7. [DOI] [PubMed] [Google Scholar]
- 36.Iwai N, Kajimoto K, Tomoike H, Takashima N. Polymorphism of CYP11B2 determines salt sensitivity in Japanese. Hypertension. 2007;49:825–831. doi: 10.1161/01.HYP.0000258796.52134.26. [DOI] [PubMed] [Google Scholar]
- 37.Sethupathy P, Borel C, Gagnebin M, Grant GR, Deutsch S, Elton TS, Hatzigeorgiou AG, Antonarakis SE. Human microRNA-155 on chromosome 21 differentially interacts with its polymorphic target in the AGTR1 3′ untranslated region: a mechanism for functional single-nucleotide polymorphisms related to phenotypes. Am J Hum Genet. 2007;81:405–413. doi: 10.1086/519979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barr M, MacKenzie SM, Friel EC, Holloway CD, Wilkinson DM, Brain NJ, Ingram MC, Fraser R, Brown M, Samani NJ, Caulfield M, Munroe PB, Farrall M, Webster J, Clayton D, Dominiczak AF, Connell JM, Davies E. Polymorphic variation in the 11beta-hydroxylase gene associates with reduced 11-hydroxylase efficiency. Hypertension. 2007;49:113–119. doi: 10.1161/01.HYP.0000249904.93940.7a. [DOI] [PubMed] [Google Scholar]
- 39.Rice GI, Jones AL, Grant PJ, Carter AM, Turner AJ, Hooper NM. Circulating activities of angiotensin-converting enzyme, its homolog, angiotensin-converting enzyme 2, and neprilysin in a family study. Hypertension. 2006;48:914–920. doi: 10.1161/01.HYP.0000244543.91937.79. [DOI] [PubMed] [Google Scholar]
- 40.Andersen G, Wegner L, Jensen DP, Glumer C, Tarnow L, Drivsholm T, Poulsen P, Hansen SK, Nielsen EM, Ek J, Mouritzen P, Vaag A, Parving HH, Borch-Johnsen K, Jorgensen T, Hansen T, Pedersen O. PGC-1alpha Gly482Ser polymorphism associates with hypertension among Danish whites. Hypertension. 2005;45:565–570. doi: 10.1161/01.HYP.0000158946.53289.24. [DOI] [PubMed] [Google Scholar]
- 41.Guo X, Cheng S, Taylor KD, Cui J, Hughes R, Quinones MJ, Bulnes-Enriquez I, De la Rosa R, Aurea G, Yang H, Hsueh W, Rotter JI. Hypertension genes are genetic markers for insulin sensitivity and resistance. Hypertension. 2005;45:799–803. doi: 10.1161/01.HYP.0000154786.17416.ea. [DOI] [PubMed] [Google Scholar]
- 42.Gumieniak O, Perlstein TS, Williams JS, Hopkins PN, Brown NJ, Raby BA, Williams GH. Ala92 type 2 deiodinase allele increases risk for the development of hypertension. Hypertension. 2007;49:461–466. doi: 10.1161/01.HYP.0000256295.72185.fd. [DOI] [PubMed] [Google Scholar]
- 43.Canani LH, Leie MA, Machado WE, Capp C, Maia AL. Type 2 deiodinase Thr92Ala polymorphism is not associated with arterial hypertension in type 2 diabetes mellitus patients. Hypertension. 2007;49:e47–e48. doi: 10.1161/HYPERTENSIONAHA.107.088278. [DOI] [PubMed] [Google Scholar]
- 44.Nakayama T, Kuroi N, Sano M, Tabara Y, Katsuya T, Ogihara T, Makita Y, Hata A, Yamada M, Takahashi N, Hirawa N, Umemura S, Miki T, Soma M. Mutation of the follicle-stimulating hormone receptor gene 5′-untranslated region associated with female hypertension. Hypertension. 2006;48:512–518. doi: 10.1161/01.HYP.0000233877.84343.d7. [DOI] [PubMed] [Google Scholar]
- 45.Goldenberg I, Moss AJ, Ryan D, McNitt S, Eberly SW, Zareba W. Polymorphism in the angiotensinogen gene, hypertension, and ethnic differences in the risk of recurrent coronary events. Hypertension. 2006;48:693–699. doi: 10.1161/01.HYP.0000239204.41079.6b. [DOI] [PubMed] [Google Scholar]
- 46.Pilbrow AP, Palmer BR, Frampton CM, Yandle TG, Troughton RW, Campbell E, Skelton L, Lainchbury JG, Richards AM, Cameron VA. Angiotensinogen M235T and T174M gene polymorphisms in combination doubles the risk of mortality in heart failure. Hypertension. 2007;49:322–327. doi: 10.1161/01.HYP.0000253061.30170.68. [DOI] [PubMed] [Google Scholar]
- 47.Rasmussen-Torvik LJ, North KE, Gu CC, Lewis CE, Wilk JB, Chakravarti A, Chang YP, Miller MB, Li N, Devereux RB, Arnett DK. A population association study of angiotensinogen polymorphisms and haplotypes with left ventricular phenotypes. Hypertension. 2005;46:1294–1299. doi: 10.1161/01.HYP.0000192653.17209.84. [DOI] [PubMed] [Google Scholar]
- 48.Meyers KJ, Mosley TH, Fox E, Boerwinkle E, Arnett DK, Devereux RB, Kardia SL. Genetic variations associated with echocardiographic left ventricular traits in hypertensive blacks. Hypertension. 2007;49:992–999. doi: 10.1161/HYPERTENSIONAHA.106.081265. [DOI] [PubMed] [Google Scholar]
- 49.Peter I, Huggins GS, Shearman AM, Pollak A, Schmid CH, Cupples LA, Demissie S, Patten RD, Karas RH, Housman DE, Mendelsohn ME, Vasan S, Benjamin EJ. Age-related changes in echocardiographic measurements: association with variation in the estrogen receptor-alpha gene. Hypertension. 2007;49:1000–1006. doi: 10.1161/HYPERTENSIONAHA.106.083790. [DOI] [PubMed] [Google Scholar]
- 50.Rame JE, Drazner MH, Post W, Peshock R, Lima J, Cooper RS, Dries DL. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49:857–864. doi: 10.1161/01.HYP.0000258566.95867.9e. [DOI] [PubMed] [Google Scholar]
- 51.Russo CJ, Melista E, Cui J, DeStefano AL, Bakris GL, Manolis AJ, Gavras H, Baldwin CT. Association of NEDD4L ubiquitin ligase with essential hypertension. Hypertension. 2005;46:488–491. doi: 10.1161/01.HYP.0000178594.63193.c0. [DOI] [PubMed] [Google Scholar]
- 52.Hunt SC, Xin Y, Wu LL, Cawthon RM, Coon H, Hasstedt SJ, Hopkins PN. Sodium bicarbonate cotransporter polymorphisms are associated with baseline and 10-year follow-up blood pressures. Hypertension. 2006;47:532–536. doi: 10.1161/01.HYP.0000196949.26088.3c. [DOI] [PubMed] [Google Scholar]
- 53.Baessler A, Kwitek AE, Fischer M, Koehler M, Reinhard W, Erdmann J, Riegger G, Doering A, Schunkert H, Hengstenberg C. Association of the Ghrelin receptor gene region with left ventricular hypertrophy in the general population: results of the MONICA/KORA Augsburg Echocardiographic Substudy. Hypertension. 2006;47:920–927. doi: 10.1161/01.HYP.0000215180.32274.c8. [DOI] [PubMed] [Google Scholar]
- 54.Bhatnagar V, O’Connor DT, Schork NJ, Salem RM, Nievergelt CM, Rana BK, Smith DW, Bakris GL, Middleton JP, Norris KC, Wright JT, Cheek D, Hiremath L, Contreras G, Appel LJ, Lipkowitz MS. Angiotensin-converting enzyme gene polymorphism predicts the time-course of blood pressure response to angiotensin converting enzyme inhibition in the AASK trial. J Hypertens. 2007;25:2082–2092. doi: 10.1097/HJH.0b013e3282b9720e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Comings DE, MacMurray JP. Molecular heterosis: a review. Mol Genet Metab. 2000;71:19–31. doi: 10.1006/mgme.2000.3015. [DOI] [PubMed] [Google Scholar]
- 56.Kohlstedt K, Gershome C, Friedrich M, Muller-Esterl W, Alhenc-Gelas F, Busse R, Fleming I. Angiotensin-converting enzyme (ACE) dimerization is the initial step in the ACE inhibitor-induced ACE signaling cascade in endothelial cells. Mol Pharmacol. 2006;69:1725–1732. doi: 10.1124/mol.105.020636. [DOI] [PubMed] [Google Scholar]
- 57.Arnett DK, Claas SA, Glasser SP. Pharmacogenetics of antihypertensive treatment. Vascul Pharmacol. 2006;44:107–118. doi: 10.1016/j.vph.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 58.Mello G, Parretti E, Fatini C, Riviello C, Gensini F, Marchionni M, Scarselli GF, Gensini GF, Abbate R. Low-molecular-weight heparin lowers the recurrence rate of preeclampsia and restores the physiological vascular changes in angiotensin-converting enzyme DD women. Hypertension. 2005;45:86–91. doi: 10.1161/01.HYP.0000149950.05182.a3. [DOI] [PubMed] [Google Scholar]
- 59.Padmanabhan S, Wallace C, Munroe PB, Dobson R, Brown M, Samani N, Clayton D, Farrall M, Webster J, Lathrop M, Caulfield M, Dominiczak AF, Connell JM. Chromosome 2p shows significant linkage to antihypertensive response in the British Genetics of Hypertension Study. Hypertension. 2006;47:603–608. doi: 10.1161/01.HYP.0000197947.62601.9d. [DOI] [PubMed] [Google Scholar]
- 60.Turner ST, Schwartz GL, Chapman AB, Boerwinkle E. WNK1 kinase polymorphism and blood pressure response to a thiazide diuretic. Hypertension. 2005;46:758–765. doi: 10.1161/01.HYP.0000186240.81996.57. [DOI] [PubMed] [Google Scholar]
- 61.Benjamini Y, Yekutieli D. Quantitative trait Loci analysis using the false discovery rate. Genetics. 2005;171:783–790. doi: 10.1534/genetics.104.036699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabatti C, Service S, Freimer N. False discovery rate in linkage and association genome screens for complex disorders. Genetics. 2003;164:829–833. doi: 10.1093/genetics/164.2.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, Pericak-Vance MA, Gregory SG, Rioux JD, McCauley JL, Haines JL, Barcellos LF, Cree B, Oksenberg JR, Hauser SL. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 64.Bielinski SJ, Lynch AI, Miller MB, Weder A, Cooper R, Oberman A, Chen YD, Turner ST, Fornage M, Province M, Arnett DK. Genome-wide linkage analysis for loci affecting pulse pressure: the Family Blood Pressure Program. Hypertension. 2005;46:1286–1293. doi: 10.1161/01.HYP.0000191706.41980.29. [DOI] [PubMed] [Google Scholar]
- 65.Harrap SB, Cui JS, Wong ZY, Hopper JL. Familial and genomic analyses of postural changes in systolic and diastolic blood pressure. Hypertension. 2004;43:586–591. doi: 10.1161/01.HYP.0000118044.84189.44. [DOI] [PubMed] [Google Scholar]
- 66.Agarwal A, Williams GH, Fisher ND. Genetics of human hypertension. Trends Endocrinol Metab. 2005;16:127–133. doi: 10.1016/j.tem.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Cusi D, Taglietti MV. The time-honoured Galilean method and genetic association studies: the importance of hypothesis-driven selection of intermediate phenotypes in detecting genes associated to hypertension. J Hypertens. 2002;20:1703–1705. doi: 10.1097/00004872-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 68.Deschepper CF, Boutin-Ganache I, Zahabi A, Jiang Z. In search of cardiovascular candidate genes: interactions between phenotypes and genotypes. Hypertension. 2002;39:332–336. doi: 10.1161/hy0202.102787. [DOI] [PubMed] [Google Scholar]