Abstract
Objectives
To assess placental apoptosis at both mid-gestation and near term in an ovine model of placental insufficiency and IUGR (PI-IUGR).
Study Design
At 40 days gestational age (dGA), two groups of four ewes were exposed to hyperthermic (HT) conditions for either 55 days or 80 days to induce IUGR with necropsies at 95 (mid-gestation) and 130 dGA (term=140dGA), respectively. Blood gases were assessed and placental tissues obtained for apoptosis analyses.
Results
PI-IUGR pregnancies showed a: 1) decrease in fetal O2 saturation and pO2 (p<0.04), 2) increase in placental villi apoptosis (p≤3.0×10-7) at mid-gestation and near-term, 3) decrease of cotyledon XIAP protein at both gestational periods (p<0.04) with no differences in caruncle XIAP protein.
Conclusion
Placental villous apoptosis is increased at mid gestation and near-term in our ovine model of IUGR and that this increase is associated with a significant decrease in XIAP protein in the cotyledon of IUGR animals.
Introduction
Normal placentation and placental development are critical for a successful pregnancy and mediate important steps such as implantation, immune protection of the fetus, maternal blood flow to the placenta, and delivery of nutrients to the fetus. Abnormal placentation and maternal adaptation may result in pregnancy wastage and complications later in pregnancy such as preeclampsia (PE) and intrauterine growth restriction (IUGR) that are associated with long term adverse sequelae for the newborn and adult. 1-4 Programmed cell death or apoptosis is a component of normal development and differentiation in most tissues. 5;6 This is an active process of cellular destruction that serves an essential function in multi-cellular organisms. 7 Apoptosis is important during pregnancy particularly during implantation and placentation. 8 Placentae of growth-restricted pregnancies have demonstrated a number of pathologic findings such as reduced syncytiotrophoblast surface area, increased thickness of the exchange barrier formed by the trophoblast and fetal capillary endothelium and an increase in placental apoptosis at term. 5;6;9-14
The inhibitor of apoptosis proteins (IAPs) are a family of proteins that regulate cell death. 15-17 These proteins include the neuronal apoptosis inhibitor protein (NAIP), X-linked inhibitor of apoptosis protein (XIAP), c-Inhibitor of apoptosis 1 and 2 (c-IAP-1 and c-IAP-2), and survivin. 17;18 XIAP is the most potent member of the group IAPs that regulate cell death. 19 XIAP protects trophoblast cells from Fas-mediated apoptosis suggesting an important role for XIAP in the regulation of trophoblast apoptosis. 20 This protein is also present in trophoblast throughout placental development. Expression is significantly decreased near delivery when apoptosis is maximal, but little is known about apoptosis across gestation in pathologic pregnancies such as IUGR. 21
We chose to study apoptosis in an ovine model of IUGR induced by hyperthermic (HT) exposure. This established model has numerous features characteristic of IUGR in humans, including: asymmetric fetal growth and reduced placental mass, reduced uterine and umbilical blood flows, abnormal umbilical arterial and aortic Doppler velocimetry, and many others. 22-26 The process of placental apoptosis has not been evaluated in this model and because placental weight is reduced at both mid-gestation and near term in our ovine IUGR model, we hypothesize that hyperthermic exposure early in ovine pregnancy disrupts fetal and placental development and increases apoptosis in the placental villi at mid-gestation as well as near term in this model. We further hypothesize that with an increase in villous apoptosis there will be a concomitant decrease in the anti-apoptotic molecule XIAP, the expression of which also remains unknown. To test our hypotheses, apoptosis was examined in sheep whole placentomes by TUNEL assay while XIAP protein expression was determined in the placentomes after separation into cotyledon (fetal side) and caruncle (maternal side) component using western blot analysis. We also chose to study HT and control animals at two important points in ovine pregnancy, which were mid-gestation (95 dGA) when placental size is maximal and near-term (135 dGA) when fetal size is at its peak.
Materials and Methods
Animal care
This study was approved by the University of Colorado Health Sciences Center Animal Care and Use Committee. A total of 16 mixed-breed (Columbia-Rambouillet) ewes with time-dated singleton pregnancies were used in this study and equally divided into two groups based on gestational age at necropsy. In group one (Gp1), four ewes were housed in an environmental chamber for 55 days beginning at 40 days of gestation (dGA; term=147 days) and four ewes were housed at ambient temperature (20 ± 2 °C) to served as controls. Gp1 animals were euthanized at 95 dGA. In group 2 (Gp2), four ewes were exposed to HT conditions for 80 days and were removed to control conditions at approximately 120 days gestation. Four ewes were kept at ambient temperature for 130 dGA to use as controls. All animals from Gp2 were euthanized at 130 dGA (near-term). All ewes were pair-fed and offered water ad libitum. The environmental conditions to which the ewes were exposed are similar to that previously described 27 and consisted of the following: (1) temperature maintained at 40°C for 12 hours during the day and decreased to 35°C at night; (2) humidity was kept between 35% and 40%. Prior to necropsy, umbilical vein blood was sample for blood gas analysis using the ABL 520 Analyzer from Radiometer America, Inc. At the time of euthanization, fetal and placentome weights were recorded. The placentomes were divided into cotyledon (fetal) and caruncle (maternal) components, which were frozen in liquid nitrogen for western blot analysis. Placentomes were also sectioned and placed in 10% formalin and paraffin-embedded for histology and immunolocalization studies.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)
TUNEL was performed on paraffin-embedded whole placentomes sections. The TUNEL protocol was followed as suggested by the manufacturer (Chemicon, Inc.). Briefly, Slides were dewaxed with 100% xylene. Tissue slides were post-fixed using a solution of Ethanol:Acetic Acid (2:1) for 5 min. The equilibration buffer was added directly to the tissue slide for 10 seconds followed by incubation with the TdT enzyme for 1hr at 37°C. Following the enzyme treatment, the anti-digoxigenin conjugate was incubated on the slide for 30min. 4′,6-diamidino-2-phenylindole,dihydrochloride (DAPI) was used for nuclear staining in our slides followed by mounting with a glass coverslip. Slides were viewed using fluorescein excitation and emission filters. For apoptotic cells, the percent apoptosis was calculated in the placentomes as the number of TUNEL positive cells divided by the total number of cells in 20 to 30 fields.
Western blot analysis
Cotyledons and caruncles were homogenized in protein lysis buffer (10mM of PMSF, 10mM of Na3VO4, 1× triton TX-100, 150mM NaCl, 20mM Tris Base, 5μM of AEBSF, 5μ of EDTA, 10nM of E-64, 10nm of Leupeptin and 10ng/ml of Aprotinin). Protein tissue lysates (50μg) were separated on 10% SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were incubated with an antibody against mouse XIAP (at a dilution of 1:200) (Transduction Laboratories, Lexington, KY). A secondary anti-mouse Ig-HRP antibody (dilution 1:10,000) (Upstate Cell Signaling solutions, Lake Placid, NY) was incubated for 1 hour at room temperature. The membranes were incubated with chemiluminescent substrate (Pierce, Rockford, IL) for 5 minutes and the emission of light was digitally recorded by using a CCD camera. To determine loading consistencies, each membrane was stripped of antibodies and reprobed utilizing antibody against mouse beta-actin (dilution 1:4,000) (MP Biomedicals, Aurora Ohio) to determine the amount of total protein present in each lane. Presence of these proteins was confirmed by densitometry and quantified. Results were compared to the untreated controls.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on paraffin-embedded whole placentome sections. Slides were de-waxed with 100% xylene. Slide preparation and antigen retrieval were performed as previously described by Le Cras et al. 28 Slides were washed in PBS and sections were blocked for 1hr using 10% normal goat serum / PBS. Slides were incubated for one hour with a mouse monoclonal primary antibody against Pan-Cytokeratin (at a dilution of 1:500) (Sigma, Saint Louis, MO) for trophoblast localization, a mouse anti- XIAP (dilution of 1:500) (Transduction Laboratories, Lexington, KY) antibody or mouse IgG1 (dilution of 1:500) for negative control. Sections were washed in 1X PBS. Sections were then incubated for 45 minutes with a biotin-labeled anti-Mouse secondary antibody. Slides were washed in 1X PBS and incubated in streptavidin-biotin-horseradish peroxidase solution and developed with diaminobenzidine (DAB) or NovaRED using the Vectastain ABC, DAB and NovaRED kit (Vector Laboratories, Inc., Burlingame, CA). NovaRED was used to label the cytokeratin positive cells while DAB was used to stain for the XIAP positive cells in a serial placentome section. Hematoxylin was used as for nuclear couterstaining. Slides were mounted using Permount mounting media.
Statistical analysis
Data are shown as mean ± SE. Comparisons of the following end-points were made between control and IUGR pregnancies: fetal and placental weights, TUNEL positive cell ratio to all cells, blood gas values, and XIAP western blot analysis. Correlations were determined between O2 saturation and XIAP protein levels at both 95 and 130 dGA. The f-test was used to assess equality of variance. Differences between groups were determined using student's t-test with p<0.05 considered significant.
Results
HT exposed sheep showed a significant decrease in placental weight (2.4-fold; 440±50 vs. 186±18; p<0.004), but not fetal weight at mid-gestation (Gp1; see Figure 1). In contrast, the HT sheep in the near-term studies (Gp2) showed a significant decrease for both placental (2.0-fold; 348.7±21.02 vs. 168.7±43.2; p≤0.004) and fetal (1.8-fold; 2914±201g vs. 1718±433g; p≤0.008) weights. At both gestational time periods (95 and 130 dGA), there was a significant decrease in umbilical vein O2 saturation (p<0.04) and pO2 (p<0.03) associated with IUGR pregnancies (Table 1). There were no pregnancy losses in our studies.
Figure 1.
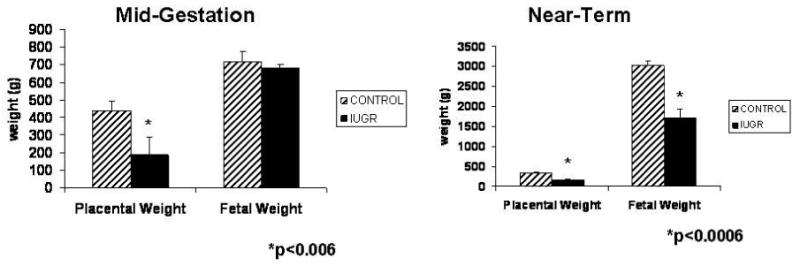
Fetal and placental weights at mid-gestation and near-term. A significant decrease in placental weight was observed in IUGR animals at both stages of pregnancy while fetal weight was significantly deceased only near-term.
Table 1. Fetal blood gas data of IUGR and control animals.
| Control | IUGR | P Value | |
|---|---|---|---|
| Blood gas data – Mid-gestation | |||
| pH | 7.26±0.02 | 7.17±0.05 | 0.117 |
| pCO2 (mmHg) | 61.93±2.06 | 68.04±7.13 | 0.302 |
| pO2 (mmHg) | 48.63±15.8 | 23.23±2.3 | 0.018 |
| O2 Saturation (%) | 55.3±0.061 | 38.1±0.047 | 0.035 |
| Blood gas data – Near-term | |||
| pH | 7.37±0.01 | 7.4±0.05 | 0.161 |
| pCO2 | 45.9±4.61 | 50.98±3.95 | 0.06 |
| pO2 (mmHg) | 18.9±1.47 | 13.9±1.9 | 0.001 |
| O2 Saturation (%) | 52.2±7.03 | 33.05±10.98 | 0.007 |
The TUNEL assay showed a significant increase in apoptosis (4-fold) during hyperthermia at mid-gestation in the villi of the sheep. A representative picture for TUNEL positive apoptotic cells is shown in figure 2 A for the Gp1 mid-gestation studies. Similar results were observed for apoptosis (2.4-fold increase) for Gp2 (near-term) HT sheep. A representative picture for the TUNEL assay at this point is shown in figure 2 B.
Figure 2.
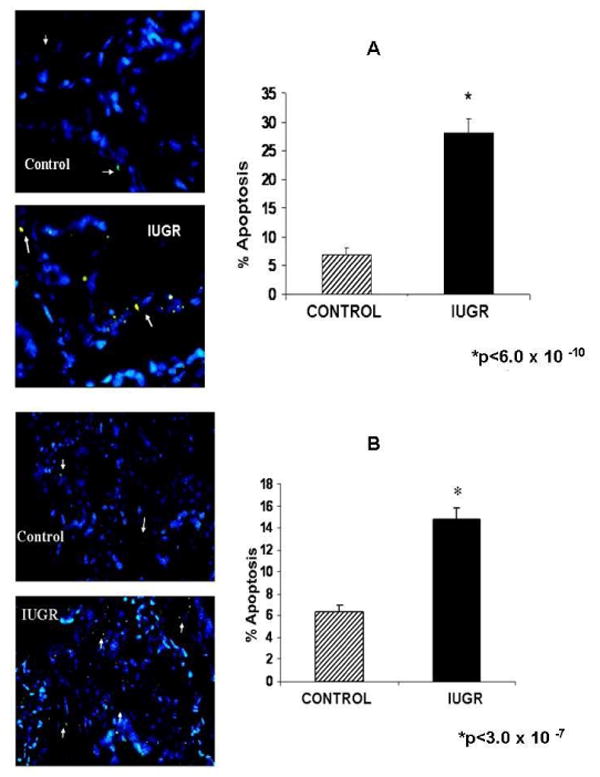
Apoptosis at mid-gestation and near-term placentae. A significant increase (4-fold) in apoptosis was observed at mid-gestation in the placental villi of IUGR sheep (A). Apoptosis was increased (2.4-fold) near-term in the IUGR sheep placental villi (B). White arrows indicate TUNEL positive cells.
XIAP protein was significantly decreased at both mid-gestation (1.7-fold) and near-term (2.4-fold) in the cotyledons of HT treated animals (Figure 3). In contrast, caruncle XIAP protein content was similar between treatments groups at both mid-gestation and near-term in the sheep (Figure 4).
Figure 3.
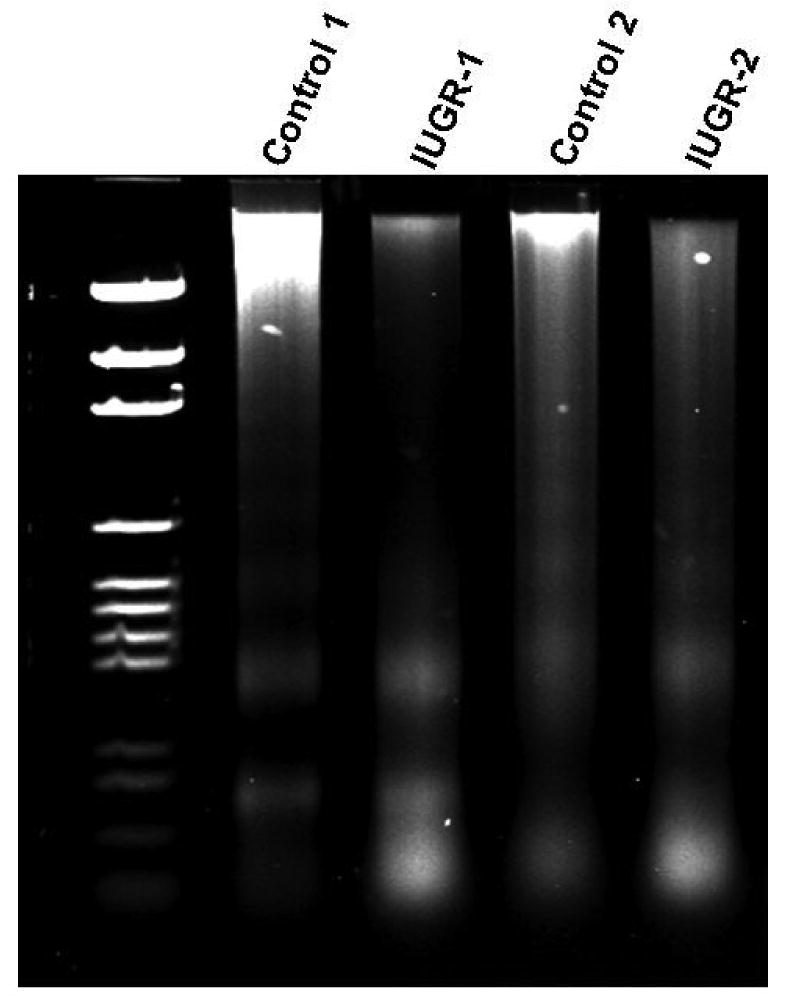
Mid-gestation cotyledon DNA degradation. A higher degree of DNA degradation was observed in the cotyledon of IUGR animals vs. controls.
Figure 4.
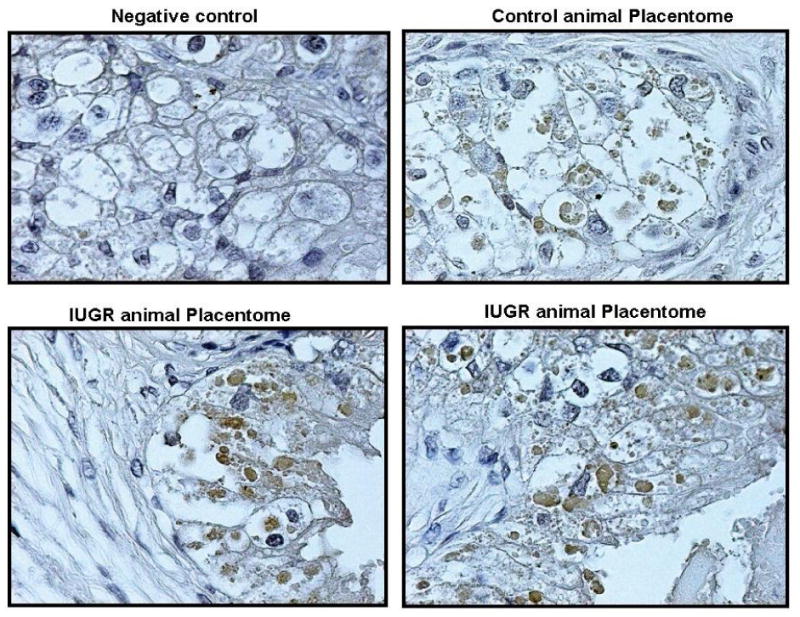
Cytokeratin 18 cleavage in the cotyledon of IUGR animals. Placentome sections were stained for M30 using DAB substrate. Control and IUGR placentomes were stained with this antibody. A higher DAB staining was observed in the placentomes of IUGR animals as compared to controls.
Figure 5 shows localization of XIAP protein in the placentome of treated and control animals (A and B). XIAP was co-localized to the cytokeratin positive cells in the villi of the ovine placentome (C and D). In these immunohistochemistry studies, NovaRED identifies cytokeratin positive cells indicating the trophoblast origin, and in a serial section using DAB, XIAP protein is co-localized to the cytokeratin positive cells. XIAP staining was found primarily in the trophoblast cells.
Figure 5.
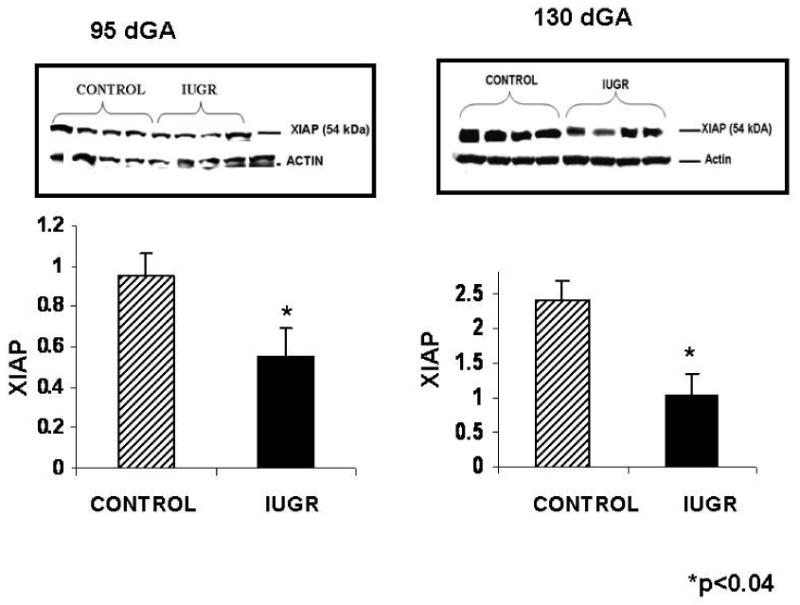
HT and control cotyledon XIAP protein at mid gestation and near-term. A 1.7-fold and 2.4-fold decrease was observed for XIAP protein in the cotyledon of HT IUGR animals during mid-gestation and near-term, respectively.
Correlations between cotyledon XIAP concentration and oxygen saturation between treatment groups at both 95 and 130 dGA is shown in Figure 6. At 95 dGA, a strong relationship between oxygen saturation and XIAP protein concentration was found for the control group, but the opposite was seen in the HT animals. At 130 dGA, the HT group also showed a strong inverse relationship between oxygen saturation and XIAP protein levels.
Figure 6.
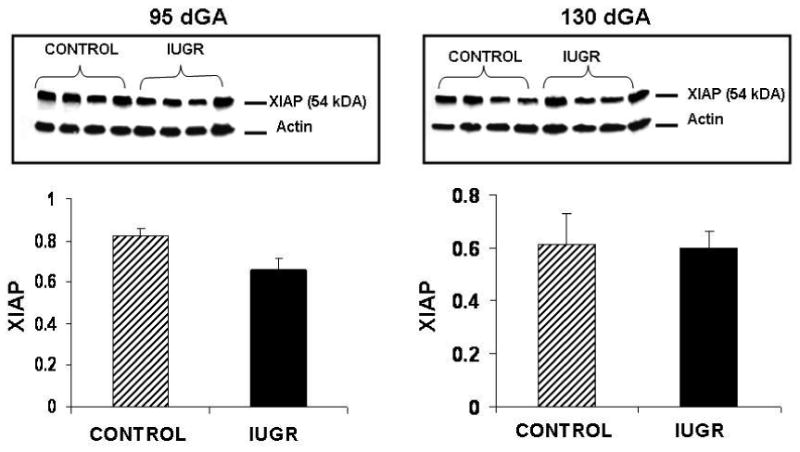
HT and control caruncle XIAP protein at mid gestation and near-term. XIAP protein did not change during mid-gestation or near-term in the caruncle of treated animals vs. controls.
Discussion
Compared to control pregnancies, we observed that placentome apoptosis was increased in the villous layer of hyperthermic exposed pregnancies at both mid-gestation and near-term. The near-term apoptotic result in our study is consistent with other studies showing an increase in placental apoptosis shown by TUNEL assay at term during human IUGR. 11 Interestingly, increased villous apoptosis was also observed at mid-gestation during IUGR in this model. To understand the apoptotic mechanism associated with this increase in apoptosis, XIAP protein levels were determined in the cotyledon and caruncle of treated and controls animals. We found that XIAP protein expression in the cotyledon was significantly decreased in HT treated animals as compared to controls for both gestational time periods. In contrast, there were no differences observed between treatment groups for caruncle XIAP protein at these time points. In addition, IHC experiments showed that XIAP was localized to the villous trophoblast of the placentome suggesting that the protective effect of this protein is preferentially expressed in the very metabolically active trophoblast cells. Umbilical vein cord gases demonstrated that the placental circulation is hypoxic at both mid and late gestation. We chose to discuss and asses blood tensions in the umbilical vein as this reflects blood coming directly from the placenta. This data suggests that the growth restricted placentae in this model of IUGR already transfer oxygen poorly. The apoptotic process which is active at mid-gestation may contribute to the poor trophoblast transfer function. However, the converse of this may also be true in that hypoxia is known to induce apoptosis in these cells. 5;6 When correlating oxygenation data with XIAP concentrations at 95 dGA, a strong positive correlation was noted in control pregnancies. We speculate that under normal conditions, higher oxygen levels allow for normal XIAP concentrations. In contrast, we observed XIAP concentrations to be inversely correlated with oxygen saturation levels in HT IUGR pregnancies at both 95 and 130 dGA. This association is opposite to that seen in controls and suggests that the hyperthermic process and associated hypoxia do not allow for the normal inhibitory activity on apoptosis by XIAP resulting in an early increase in apoptosis. While the number of animals for this correlation is small, the findings and implications are interesting, and in-vitro studies are in progress to confirm these effects of hypoxia on XIAP.
Insufficient or excessive apoptosis can contribute to pathological conditions such as cancer, AIDS and autoimmune disease. 29 Cell death or apoptosis had been shown to be present in the placenta during gestation suggesting a role for apoptosis during normal pregnancy. 14;30 Trophoblast are specialized epithelial cells that are critical for a successful pregnancy. These cells have specialized functions that facilitate the exchange of nutrients and wastes between maternal and fetal compartments. Aberrant trophoblast function and apoptosis are associated with clinical obstetric pathology such as that observed in pregnancies with isolated IUGR and in pregnancies associated with both IUGR and preeclampsia. 31;32 Increased trophoblast apoptosis is associated with IUGR in the humans at term. 5;6;6-14;33 In the current study, our TUNEL assay results suggest that apoptosis in the placenta occurs as a much earlier event than has been previously described in IUGR. We speculate that the increase in apoptosis could be a factor in the decreased placental weight observed in our model. Interestingly, at mid-gestation there were no differences in fetal weights, while a significant reduction in fetal weight was observed near-term. In contrast, placental weight showed to be reduced at both mid-gestation and near-term. This finding is also seen in several animal models of IUGR. For example, in the rat dietary restriction model by Ozaki et al, there was no significant decrease in fetal weight by day 20 of gestation, but growth restriction was present at birth. 34 Similar results have been described in the guinea pig IUGR model with uterine artery ligation. 35 The authors specified that placental weight was reduced prior to the fetal weight decrease observed at near-term. In an IGFII inactive IUGR model, placental weight was constantly decreased through mid and late gestation while fetal growth restriction was only seen towards the end of gestation. 36 Collectively, these results suggest that decreased placental weight at mid gestation precedes decreased fetal weight seen later in pregnancy. We found that placental apoptosis preceded the decreased fetal weight observed in this model of IUGR and this may partly be responsible for the decrease in placental weight at mid-gestation in this model and others described above. We speculate that the increase in mid-gestation cotyledon apoptosis may result in placental functional changes that fail to meet the fetal demands required for normal growth, particularly as the fetus just begins to enter the slope of maximal growth at this gestational age. The insufficient placental nutrient transfer, previously described in this model 22, subsequently leads to reduced fetal weight in late gestation.
In summary, the current study shows that apoptosis is increased in the cotyledon, which is seen in the villous layer of the placentome with no changes observed in the caruncle tissues. This suggests that hyperthermia preferentially impacts the fetal side of the placenta, and more specifically, the villous trophoblast. In addition, XIAP protein expression is decreased in the cotyledon at both mid-gestation and near-term in this model of IUGR and it is localized to the villous trophoblast in this tissue. Thus, we speculate that a possible mechanism for the increased apoptosis observed in the placenta of treated animals is secondary to a decrease in XIAP expression in the cotyledon of treated animals as compared to controls. To our knowledge this is the first report to show a decrease in XIAP protein associated with an increase in placental apoptosis during IUGR in animal or human studies. Further mechanistic studies are needed to determine the role of XIAP in the activation of caspases 3 and 9 in this model of IUGR in the sheep.
Figure 7.
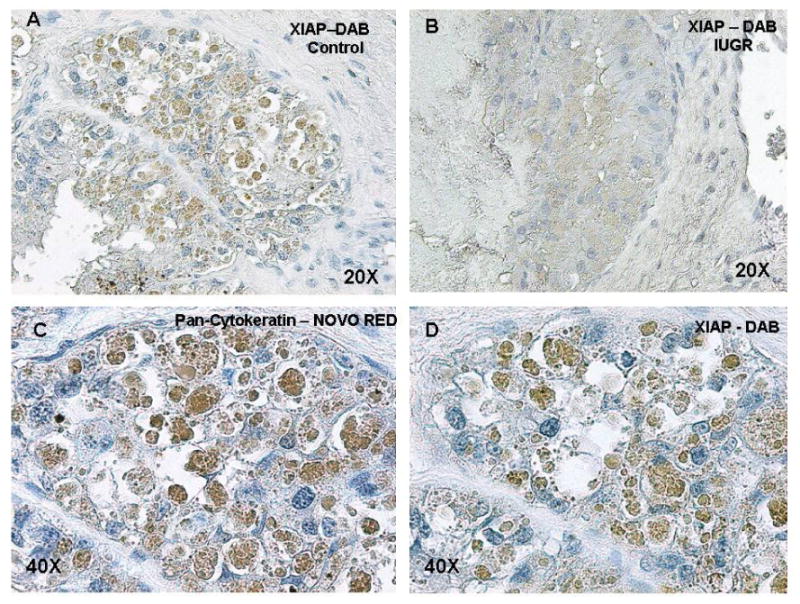
XIAP protein localization in the sheep placentome. Placentome sections stained with DAB demonstrated localization of XIAP protein to the villi of control and HT treated sheep placentomes (A and B). Serial placentome sections stained with NovaRED (trophoblast cells) and DAB (XIAP protein) demonstrated co-localization of XIAP protein to the trophoblast cells to the villi of the sheep placentome (C and D).
Figure 8.
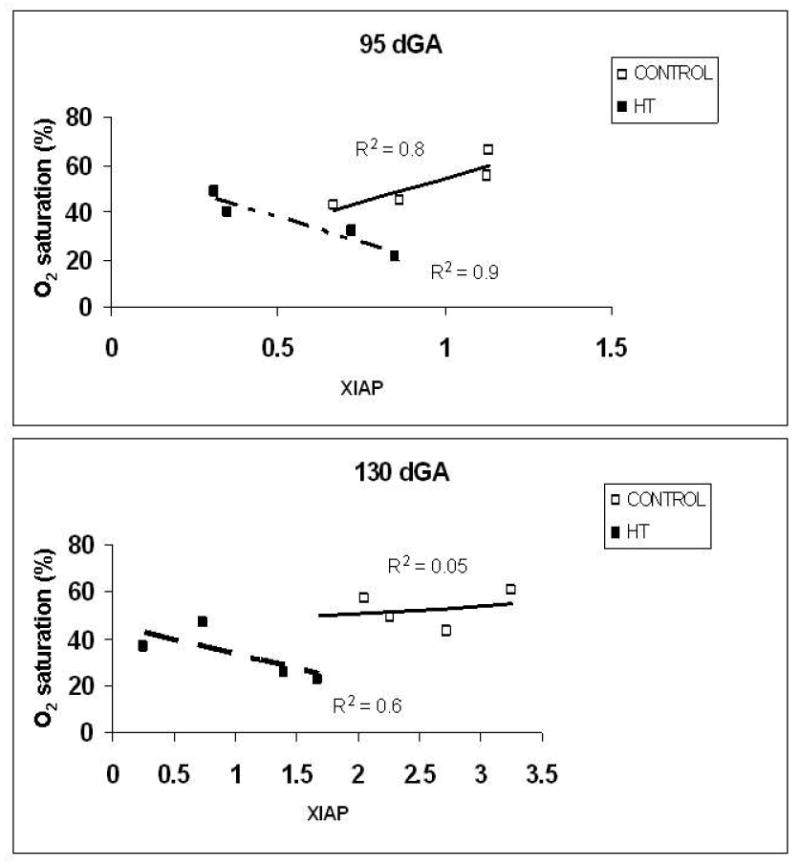
Correlation between XIAP and oxygen concentration between treatment groups. XIAP concentrations were increased in controls animals at both mid and near-term gestational points compared to HT groups. During HT treatment, XIAP concentration is inversely correlated with oxygen saturation at both time points.
Acknowledgments
This study was supported by the NIH grant HL071990.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–41. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Osmond C, Simmonds SJ, Wield GA. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ. 1993;306:422–26. doi: 10.1136/bmj.306.6875.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brar HS, Rutherford SE. Classification of intrauterine growth retardation. Semin Perinatol. 1988;12:2–10. [PubMed] [Google Scholar]
- 4.Pollack RN, Divon MY. Intrauterine growth retardation: definition, classification, and etiology. Clin Obstet Gynecol. 1992;35:99–107. doi: 10.1097/00003081-199203000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Levy R, Nelson DM. To be, or not to be, that is the question. Apoptosis in human trophoblast. Placenta. 2000;21:1–13. doi: 10.1053/plac.1999.0450. [DOI] [PubMed] [Google Scholar]
- 6.Levy R, Smith SD, Chandler K, Sadovsky Y, Nelson DM. Apoptosis in human cultured trophoblasts is enhanced by hypoxia and diminished by epidermal growth factor. Am J Physiol Cell Physiol. 2000;278:C982–C988. doi: 10.1152/ajpcell.2000.278.5.C982. [DOI] [PubMed] [Google Scholar]
- 7.Ratts VS, Tao XJ, Webster CB, Swanson PE, Smith SD, Brownbill P, et al. Expression of BCL-2, BAX and BAK in the trophoblast layer of the term human placenta: a unique model of apoptosis within a syncytium. Placenta. 2000;21:361–66. doi: 10.1053/plac.1999.0486. [DOI] [PubMed] [Google Scholar]
- 8.Akcali KC, Khan SA, Moulton BC. Effect of decidualization on the expression of bax and bcl-2 in the rat uterine endometrium. Endocrinology. 1996;137:3123–31. doi: 10.1210/endo.137.7.8770938. [DOI] [PubMed] [Google Scholar]
- 9.Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol. 2000;96:271–76. doi: 10.1016/s0029-7844(00)00895-4. [DOI] [PubMed] [Google Scholar]
- 10.DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155:293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–66. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 12.Leung DN, Smith SC, To KF, Sahota DS, Baker PN. Increased placental apoptosis in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 2001;184:1249–50. doi: 10.1067/mob.2001.112906. [DOI] [PubMed] [Google Scholar]
- 13.Mayhew TM, Ohadike C, Baker PN, Crocker IP, Mitchell C, Ong SS. Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta. 2003;24:219–26. doi: 10.1053/plac.2002.0900. [DOI] [PubMed] [Google Scholar]
- 14.Smith SC, Baker PN, Symonds EM. Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol. 1997;177:1395–401. doi: 10.1016/s0002-9378(97)70081-4. [DOI] [PubMed] [Google Scholar]
- 15.Holcik M, Korneluk RG. XIAP, the guardian angel. Nat Rev Mol Cell Biol. 2001;2:550–56. doi: 10.1038/35080103. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Sasaki H, Sheng YL, Schneiderman D, Xiao CW, Kotsuji F, et al. Apoptosis and chemoresistance in human ovarian cancer: is Xiap a determinant? Biol Signals Recept. 2000;9:122–30. doi: 10.1159/000014631. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki Y, Nakabayashi Y, Nakata K, Reed JC, Takahashi R. X-linked inhibitor of apoptosis protein (XIAP) inhibits caspase-3 and -7 in distinct modes. J Biol Chem. 2001;276:27058–63. doi: 10.1074/jbc.M102415200. [DOI] [PubMed] [Google Scholar]
- 18.Tran J, Rak J, Sheehan C, Saibil SD, LaCasse E, Korneluk RG, et al. Marked induction of the IAP family antiapoptotic proteins survivin and XIAP by VEGF in vascular endothelial cells. Biochem Biophys Res Commun. 1999;264:781–88. doi: 10.1006/bbrc.1999.1589. [DOI] [PubMed] [Google Scholar]
- 19.Hetts SW. To die or not to die: an overview of apoptosis and its role in disease. JAMA. 1998;279:300–07. doi: 10.1001/jama.279.4.300. [DOI] [PubMed] [Google Scholar]
- 20.Straszewski-Chavez SL, Abrahams VM, Funai EF, Mor G. X-linked inhibitor of apoptosis (XIAP) confers human trophoblast cell resistance to Fas-mediated apoptosis. Mol Hum Reprod. 2004;10:33–41. doi: 10.1093/molehr/gah001. [DOI] [PubMed] [Google Scholar]
- 21.Gruslin A, Qiu Q, Tsang BK. X-linked inhibitor of apoptosis protein expression and the regulation of apoptosis during human placental development. Biol Reprod. 2001;64:1264–72. doi: 10.1095/biolreprod64.4.1264. [DOI] [PubMed] [Google Scholar]
- 22.Anthony RV, Scheaffer AN, Wright CD, Regnault TR. Ruminant models of prenatal growth restriction. Reprod Suppl. 2003;61:183–94. [PubMed] [Google Scholar]
- 23.Bell AW, Wilkening RB, Meschia G. Some aspects of placental function in chronically heat-stressed ewes. J Dev Physiol. 1987;9:17–29. [PubMed] [Google Scholar]
- 24.Bell AW, McBride BW, Slepetis R, Early RJ, Currie WB. Chronic heat stress and prenatal development in sheep: I. Conceptus growth and maternal plasma hormones and metabolites. J Anim Sci. 1989;67:3289–99. doi: 10.2527/jas1989.67123289x. [DOI] [PubMed] [Google Scholar]
- 25.Galan HL, Hussey MJ, Barbera A, Ferrazzi E, Chung M, Hobbins JC, et al. Relationship of fetal growth to duration of heat stress in an ovine model of placental insufficiency. Am J Obstet Gynecol. 1999;180:1278–82. doi: 10.1016/s0002-9378(99)70629-0. [DOI] [PubMed] [Google Scholar]
- 26.Galan HL, Regnault TR, Le Cras TD, Tyson RW, Anthony RV, Wilkening RB, et al. Cotyledon and binucleate cell nitric oxide synthase expression in an ovine model of fetal growth restriction. J Appl Physiol. 2001;90:2420–26. doi: 10.1152/jappl.2001.90.6.2420. [DOI] [PubMed] [Google Scholar]
- 27.Regnault TR, Orbus RJ, Battaglia FC, Wilkening RB, Anthony RV. Altered arterial concentrations of placental hormones during maximal placental growth in a model of placental insufficiency. J Endocrinol. 1999;162:433–42. doi: 10.1677/joe.0.1620433. [DOI] [PubMed] [Google Scholar]
- 28.Le Cras TD, Tyler RC, Horan MP, Morris KG, Tuder RM, McMurtry IF, et al. Effects of chronic hypoxia and altered hemodynamics on endothelial nitric oxide synthase expression in the adult rat lung. J Clin Invest. 1998;101:795–801. doi: 10.1172/JCI786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789–95. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 30.Smith SC, Price E, Hewitt MJ, Symonds EM, Baker PN. Cellular proliferation in the placenta in normal human pregnancy and pregnancy complicated by intrauterine growth restriction. J Soc Gynecol Investig. 1998;5:317–23. doi: 10.1016/s1071-5576(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 31.Kilani RT, Mackova M, Davidge ST, Guilbert LJ. Effect of oxygen levels in villous trophoblast apoptosis. Placenta. 2003;24:826–34. doi: 10.1016/s0143-4004(03)00129-2. [DOI] [PubMed] [Google Scholar]
- 32.Krebs C, Longo LD, Leiser R. Term ovine placental vasculature: comparison of sea level and high altitude conditions by corrosion cast and histomorphometry. Placenta. 1997;18:43–51. doi: 10.1016/s0143-4004(97)90070-9. [DOI] [PubMed] [Google Scholar]
- 33.Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90:1274–81. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- 34.Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;530:141–52. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jansson T, Thordstein M, Kjellmer I. Placental blood flow and fetal weight following uterine artery ligation. Temporal aspects of intrauterine growth retardation in the guinea pig. Biol Neonate. 1986;49:172–80. doi: 10.1159/000242528. [DOI] [PubMed] [Google Scholar]


