Abstract
The embryonic chick is able to regenerate the retina after it has been removed. We have previously shown that proliferating stem/progenitor cells present in the ciliary body/ciliary marginal zone (CB/CMZ) of the chick eye are responsible for regeneration, which can be induced by ectopic FGF2 or Shh. Here, we reveal the mechanisms showing how FGF2 and Shh signaling are interdependent during retina regeneration. If the FGF pathway is inhibited, regeneration stimulated by Shh is inhibited. Likewise, if the Hedgehog pathway is inhibited, regeneration stimulated by FGF2 is inhibited. We examined early signaling events in the CB/CMZ and found that FGF2 or Shh induced a robust Erk phosphorylation during the early stages of retina regeneration. Shh also upregulated the expression of several members of the FGF signaling pathway. We show that ectopic FGF2 or Shh overexpression increased the number of phosphohistone 3 (PH3) positive cells in the CB/CMZ and inhibition of either pathway decreased the number of PH3 positive cells. Additionally, both FGF and Hh signaling are required for cell survival in the CB/CMZ, while Hh and not FGF signaling plays a role in maintaining the identity of the retinal progenitor population in this region. Combined, our results support a model where the FGF and Hedgehog pathways work together to stimulate retina regeneration.
Keywords: Retina regeneration, CB/CMZ, Sonic hedgehog, FGF, Erk, Stem cell, Progenitor cell
Introduction
The embryonic chick retina is able to regenerate during a window of early development (Coulombre and Coulombre, 1965; 1970; Park and Hollenberg, 1989; 1991; Spence et al., 2004) from two distinct sources (reviewed in Del Rio-Tsonis and Tsonis, 2003; Tsonis and Del Rio-Tsonis, 2004; Haynes and Del Rio-Tsonis, 2004; Vergara et al., 2005). One source of regeneration is via the process of transdifferentiation of the retina pigmented epithelium (RPE). This type of regeneration can occur until approximately embryonic day 4.5 (E4.5) when an ectopic source of FGF is present (Park and Hollenberg, 1989; 1991, Spence et al., 2004). The other source of retina regeneration, which is the current focus of this work, consists of a pool of stem/progenitor cells located in the ciliary region of the chick eye. These cells proliferate and eventually differentiate as long as there is either ectopic FGF or ectopic Shh available (Spence et al., 2004). At E4 (time at which retinectomies are performed), the ciliary region is not well defined and contains the putative ciliary body and ciliary marginal zone (CB/CMZ), which is immunopositive for collagen IX, a CB/CMZ marker (Spence et al., 2004). In addition, at E4 most cells in the developing CB/CMZ co-express Pax6 and Chx10. This co-expression identifies the presence of retinal progenitor cells (Belecky-Adams et al., 1997; Fischer and Reh, 2000; Fischer et al., 2002; Spence et al., 2004). As the eye develops, the ciliary body (CB) and the ciliary marginal zone (CMZ) become well defined so that the CB is composed of a two layered epithelium, the pigmented epithelium (PE) and non-pigmented epithelium (NPE). The CMZ becomes a transitional zone between the peripheral retina and the CB (Perron et al., 1998; Fischer and Reh, 2000; 2003). Only amphibians, fish and birds house progenitor cells in the CMZ that are able to proliferate and add new neurons to the retina postnatally (reviewed in Haynes and Del Rio-Tsonis, 2004; and Hitchcock et al., 2004). In the chick, this region is only active for the first three weeks post-hatch (Fischer and Reh, 2000). Early post-hatch chicks also house stem cells in the CB that are able to proliferate in response to injection of exogenous growth factors such as insulin, EGF and FGF2 (Fischer and Reh, 2003).
Moshiri et al. (2005) have shown that Sonic Hedgehog (Shh) signaling is important in the CMZ as it is able to stimulate proliferation in the post-hatch chick eye. Interfering with the Hedgehog (Hh) pathway inhibits the ability of progenitor cells in this region to proliferate. Similarly, mice with overactive Hh signaling maintain a progenitor population in the CB. In fact, these progenitors are able to give rise to new neurons in an injured retina mouse model (Moshiri and Reh, 2004). In the embryonic chick, isolated cells from the anterior region of E9 eyes retain the ability to proliferate in vitro and when incubated in rotation culture assays, they form laminar structures containing all nuclear retinal cell types (Willbold and Layer, 1992). Consistent with these reports, we have observed that the chick can regenerate the retina from the CB/CMZ in vivo until at least E5 when stimulated with FGF2. However, the regeneration stimulated by FGF2 at E5 does not appear as robust as the regeneration stimulated by FGF2 at E4 (Spence and Del Rio-Tsonis, unpublished observations). Therefore, it appears that throughout development, the potential of the stem/progenitor cells in the CB/CMZ to regenerate lost or damaged retina is reduced, but not lost completely. We recently reported that both FGF2 and Shh are able to independently induce retina regeneration from the CB/CMZ in E4 chick eyes (Spence et al., 2004). Thus, both FGF and Shh are important in the regulation of stem/progenitor cells in the CB/CMZ of the embryonic and postnatal chick (Fischer et al., 2002; Fischer and Reh, 2003; Spence et al., 2004; Moshiri et al., 2004; 2005).
Despite the recent data on the role of Shh in regulating progenitor populations and proliferation in embryonic and post-hatch chicks and in mice, little is known about the mechanisms by which these cells are regulated. In this study, we used the embryonic chick eye as a model to study how stem/progenitor cells in the CB/CMZ are regulated by FGF2 and Shh, as well as the role of FGF and Hh in maintenance of stem/progenitor cells. We found that basal activity of both pathways is required for retina regeneration to take place, since inhibition of either pathway lead to a reduction of regeneration regardless of the treatment (FGF or Shh). In order to examine the relationship between the two signaling pathways, we developed an in vitro explant system to identify early signaling events stimulated by FGF2 or Shh. As expected, FGF2 activated a MAP Kinase signaling cascade that eventually leads to phosphorylation of Erk (pErk). Surprisingly, we also found that Shh leads to an increase in pErk and that this phosphorylation event is blocked by the translational inhibitor, cycloheximide. Using pharmacological inhibitors of both the FGF and Hh pathways, we show that Erk phosphorylation is reduced if either pathway is inhibited. In addition, we provide several lines of evidence suggesting that Shh induces pErk by upregulating FGF and FGFR expression, which leads to increased pErk, and ultimately, proliferation of the stem/progenitor cells in the CB/CMZ, leading to regeneration of the retina. Finally, we demonstrate that FGF and Shh are required for cell survival in the CB/CMZ after retina removal and this trait makes them both interdependent during chick retina regeneration.
Materials and Methods
Chick Embryos
Fertilized White Leghorn chicken eggs were purchased from the Ohio State University, Columbus, OH and incubated in a humidified rotating incubator at 38°C.
Preparation of FGF2, KAAD and other pharmacological agents for in vivo studies
Heparin coated polyacrylamide beads (Sigma) were washed 3 times in 1x PBS. FGF2 (R&D Systems) was resuspended in 1x PBS at a concentration of 1ug/ul. Heparin beads were then incubated with FGF2 for at least 2 hours before use. To inhibit the Hh pathway we used KAAD, a synthetic form of cyclopamine, which is more potent and not as toxic. A 1 mM KAAD (Toronto Research Chemicals) stock was prepared in 100% Ethanol. Affi-gel Blue beads (BioRad) were washed in 1x PBS and dehydrated through a series of ethanol washes of increasing concentration. KAAD stock solution was diluted to 200uM in DMSO and added to dried beads. Pharmacological inhibitors such as the FGFR inhibitor, PD173074 (Pfizer) and the MEK inhibitor, PD98059 (Calbiochem), were resuspended in DMSO at a concentration of 100mM and incubated in ethanol dehydrated Affi-gel Blue beads. Beads soaked in vehicle (DMSO) alone were used as controls where appropriate.
Retroviral Production, Titration and Infection
The production of RCAS viruses, as well as their titration and subsequent infection of chick embryos were performed as previously described in Spence et al. (2004). Replication competent Rcas (A) retrovirus engineered to express Shh was a generous gift from Cliff Tabin (Harvard University; Boston, MA). An Rcas construct expressing GFP, was a kind gift from Teri Belecky-Adams (IUPUI, Indianapolis, IN) and Ruben Adler (Johns Hopkins University, Baltimore, MD).
Surgical Procedures
A window was made in the egg shell using forceps, and microsurgical removal of the retina was carried out at E4 as previously described (Coulombre and Coulombre, 1965; Park and Hollenberg, 1989, Spence et al., 2004). The entire retina was removed and the CB/CMZ was left behind as this region is extremely sticky and difficult to get off. After retina removal eyes were incubated with either beads or viral vectors as described in Spence et al., 2004.
Tissue fixation and sectioning
Tissues processed for histology were fixed in Bouin’s fixative for at least 24 hours and embedded in paraffin wax. Transdifferentiated retina is easy to differentiate histologically from retina regenerated from the CB/CMZ as the former lacks RPE and forms a retina with a reverse orientation when compared to a normal developing retina. On the other hand, retina regenerated from the CB/CMZ has normal orientation and it is at least initially associated with the RPE. Tissues used for immunohistochemistry were fixed in 4% formaldehyde, cryoprotected in 30% sucrose and embedded in O.C.T. freezing medium (Sakura Finetek). All eyes used for histology and immunohistochemistry were sectioned at 10um.
Quantitation of Regeneration
To quantitatively measure the amount of retina that regenerated after the different treatments (figure 1), we analyzed the area of retina regenerated from 3 sections of 3 different eyes (n=9). Images were captured using a Magnafire image capture software. Captured images were opened in ImagePro, and the regenerating tissue was traced. The area of the trace was determined by ImagePro and the Student’s t-Test was used to assess significance. Error bars in figures represent Standard Error of the Mean (S.E.M.).
Figure 1. FGF and Hh signaling are required for retina regeneration in vivo.
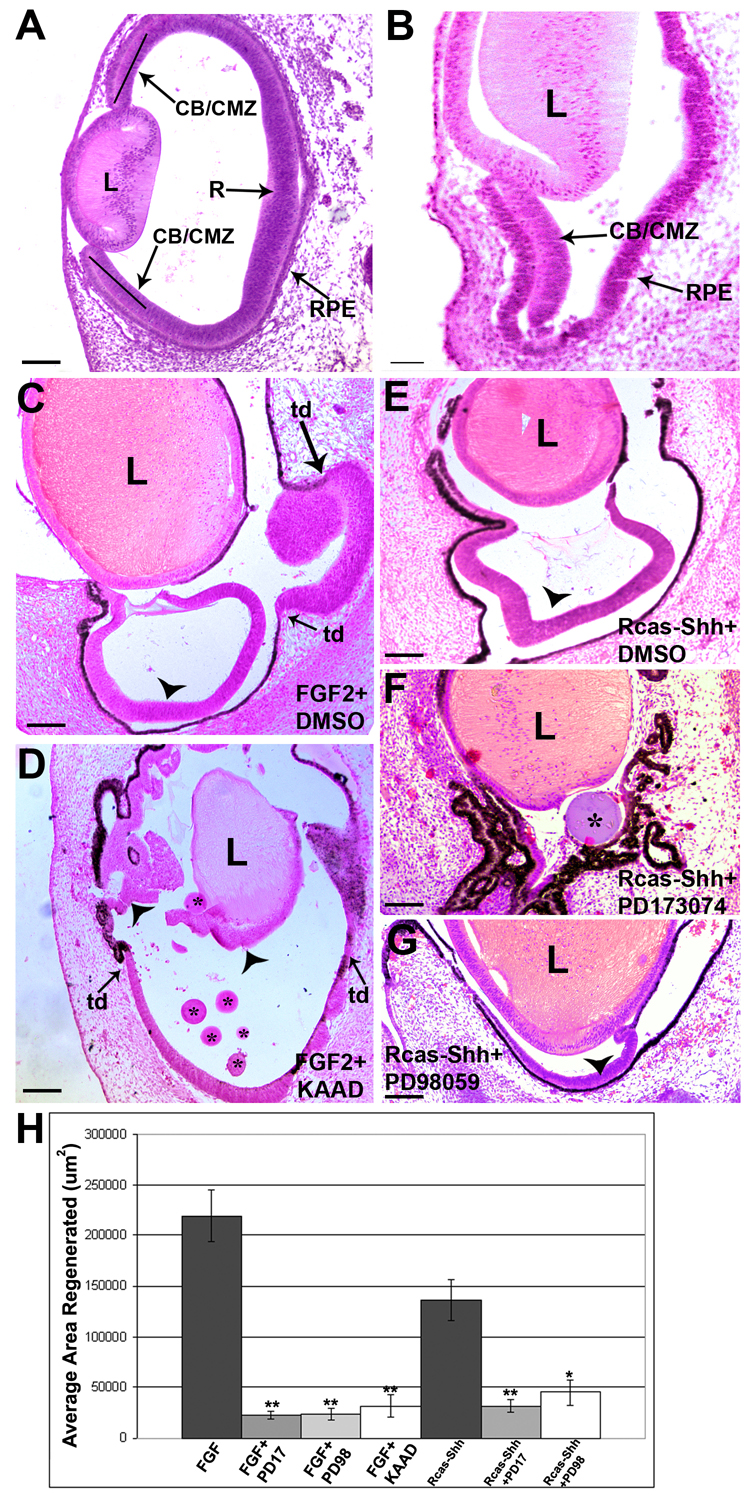
A. A histological section of an E4 chick eye at the stage at which retinectomies were performed.
B. E4 chick eye after the retina has been removed, leaving behind only the CB/CMZ, lens (L) and RPE. Note that the RPE is not heavily pigmented at this stage and has thickened.
C. E7 chick eye, 3 days after retinectomy and addition of FGF2 plus a control bead soaked in DMSO. In FGF2 treated eyes, regeneration from the CB/CMZ (arrowhead) and transdifferentiation (td) of the RPE can be observed.
D. Inhibiting the Hh pathway with beads soaked in 200uM KAAD decreases retina regeneration from the CB/CMZ (arrowheads) when stimulated with FGF2, 3 days after retina removal. Asterisks denote KAAD soaked beads. Note the arrowhead near the lens points to regeneration from the CB/CMZ that is closely associated with the lens. Transdifferentiating retina is denoted by (td) and arrows.
E. E7 chick eye, 3 days after retinectomy and addition of Rcas-Shh plus a control bead soaked in DMSO. Only regeneration from the CB/CMZ (arrowhead) can be observed.
F. Inhibition of the FGF receptors with beads soaked in 100mM PD173074 inhibits regeneration stimulated by Rcas-Shh, 3 days after retina removal. Asterisk denotes PD173074 soaked bead.
G. Inhibition of MEK with beads soaked in 100mM PD98059 decreases Rcas-Shh stimulated regeneration from the CB/CMZ (arrowhead) 3 days after retina removal. Scale bars in all panels represent 100um. R=retina; RPE=retina pigmented epithelium; L= lens; CB/CMZ= ciliary body/ciliary marginal zone; td= trans-differentiation.
H. The areas of regenerated retinal tissue from all treated eyes was traced and quantified using ImagePro as described under materials and methods. These quantitative results support the represenatative images shown in C–G. Error bars are S.E.M. * = p<0.05, ** = p<0.01.
In vitro CB/CMZ explant dissection and tissue culture
E4 chicks were placed in 1x Hanks Balanced Salt Solution (HBSS) and the embryonic membranes were removed. The heads were removed and placed in fresh 1x HBSS. For CB/CMZ explants, the cornea was carefully dissected away from the eye and the lens was then removed. Using microscissors, the anterior portion of the eye, corresponding to the presumptive CB/CMZ, was removed and placed in an eppendorf tube containing 1x HBSS and serum free “Reh’s” media (DMEM/F12 medium, 5mM Hepes, 0.11% NaHCO3, 0.6% glucose, penicillin 100 units/ml, streptomycin 100 ug/ml) (Fuhrmann et al., 2000) at a ratio of 4:1. The tissue was incubated at 37 °C.
RNA isolation of explants for Real-Time RTPCR
CB/CMZ explants were incubated for 4 hours as described above, at which time 10ug/ml FGF or 10ug/ml Shh-N was added to the culture and the tissue was incubated at 37°C for an additional 4 hours. RNA was then isolated using the Nucleospin II RNA isolation kit (BD Biosciences) following the manufacturers protocol.
Real-Time RTPCR (qPCR)
For RTPCR, RNA was reversed transcribed using ImpromII Reverse Transcriptase (Promega). Real-time PCR was carried out using iQ SYBR Green Master Mix (BioRad) on a RotorGene 3000 Real-Time PCR thermocycler. For PCR primers and annealing temperatures, see Supplemental Methods Table 1. For each sample, the target gene and an internal control were amplified. Quantitation of cDNA for each primer set was determined using the Pfaffl method (Pfaffl, 2001). All experiments described were repeated using at least two separate biological samples. Each biological sample was run in quadruplicate. Significant difference in gene expression between treated tissue and control tissue was determined using the Student’s t-Test with a sample number of at least 6 samples (n=6). Error bars in figures represent Standard Error of the Mean (S.E.M.).
Immunohistochemistry
Immunohistochemistry was carried out as previously described (Spence et al., 2004).
Western Blotting
Explants were cultured as described above. FGF2 or Shh-N peptide (R&D Systems) was added after the first 4 hours of incubation and cultured for an additional 4 hours. Inhibitors (see Table 2 in supplemental methods: Inhibitors and Antibodies) were added at various time points before FGF2 or Shh was added. The explants were spun down and the media removed. The explants were washed with 1x HBSS and resuspended in ice-cold RIPA buffer containing protease inhibitors and phosphatase inhibitors supplied in a RIPA lysis buffer kit (Santa Cruz Biotechnology). Explants were lysed using a sonicator, spun down at 4°C and the tissue lysate was transferred to a fresh tube on ice. Approximately 15ug of protein were mixed with 2x sample loading buffer, heated to 95°C for 5 min and separated by SDS-PAGE on a 10% acrylamide gel. Protein was transferred onto Immobillon-p membrane (Millipore) overnight, and proteins were detected using standard methods. Densitometry was performed using ImageQuant 5.2 software. To determine the relative amount of pErk, Western blot images of pErk and of actin (as a control) were scanned and densitometry comparisons were performed by dividing the density value of pErk by the density value of actin.
TUNEL assay and Phosphohistone 3
Cell death was assayed using the TUNEL in situ cell death kit (Roche) as previously described (Spence et al., 2004). Phosphorylated Histone 3 (PH3) labeling of mitotic cells was carried out using a standard immunohistochemical protocol using an anti-PH3 antibody (Upstate) diluted 1:200.
Cell Counting and Quantitation
Surgeries to remove the retina were performed on E4 embryos. Affigel Blue beads (BioRad) soaked in KAAD or PD173074 solution or DMSO (see Spence et al., 2004) were placed into the optic cups after retinectomy. Embryos were then incubated at 38°C for 4 or 24 hours. Subsequently, eyes were collected, fixed, embedded for frozen sectioning and sectioned at 12um thick. For each treatment, 3 separate eyes were collected and sectioned. For PH3 and TUNEL analysis at least 2 randomly selected sections from each eye were used (total of at least 6 sections from 3 eyes). TUNEL assay was performed on sections, and the total number of apoptotic cells in the NPE of the CB/CMZ per section was counted. In the case of the Rcas-GFP and Rcas-Shh experiments, virus was injected into the eye prior to the retinectomy (as described in the results) so the virus had time to infect the tissue and express ectopic GFP or Shh. Retinectomies were performed at E4 and embryos allowed to incubate for different times at 38°C, similar to the KAAD/ PD173074/DMSO experiments. Eyes were processed as described above. Phosphohistone 3 labeling of mitotic cells in Rcas infected eyes, as well as KAAD/PD173074/DMSO treated eyes was done using standard immunohistochemistry. Total number of PH3 positive cells in the NPE of the CB/CMZ per section was counted. Each eye has a dorsal and ventral CB/CMZ, both of which were counted for statistical analysis. For TUNEL and PH3 labeling statistical analysis was done using the Student’s t-Test. Sample number was at least 6, (n=6). Error bars in figures represent standard error of the mean (S.E.M).
Quantitation of Pax6/Chx10 positive cells in the CB/CMZ
Eyes that underwent retinectomy at E4 were treated with FGF, KAAD or DMSO (control). Three different eyes for each treatment were collected, sectioned and immunostained for Pax6/Chx10. Images of the eyes were then captured on a confocal microscope. Images were imported into Adobe Photoshop and at least 5 boxes of 500 um2 per section for the 3 different eyes were placed over the images. The number of cells expressing Pax6, Chx10 or co-expressing Pax6/Chx10 in each box were counted (at least 15 boxes were analyzed per treatment). The Student’s t-Test was used to determine statistical significance. Error bars in figures represent standard error of the mean (S.E.M).
Results
Shh and FGF are required for retina regeneration in vivo
We previously reported that ectopic FGF2 (also Figure 1C) or overexpression of Shh (also Figure 1E) are able to independently induce regeneration from the CB/CMZ of the embryonic chick (Spence et al., 2004). We have also reported that when regeneration is stimulated with Shh and the FGF pathway is inhibited with the FGFR antagonist PD173074, regeneration is significantly inhibited (also Figure 1F). To further explore if both the FGF and Shh pathways are required for regeneration from the CB/CMZ, we stimulated regeneration with FGF2 and simultaneously inhibited the Hh pathway with KAAD. Compared with FGF2 alone, regeneration from the CB/CMZ was not as robust 3 days after removal of the retina (compare Figure 1C to 1D). We quantified regeneration by measuring the total area of retina regenerated from the CB/CMZ using ImagePro (Figure 1H). Our statistical analysis showed that regeneration stimulated by FGF2 was significantly inhibited by KAAD (compare Figure 1C to 1D and 1H). In addition, as already reported, Shh stimulated regeneration was significantly inhibited by the FGFR antagonist PD173074 (compare Figure 1E to Figure 1F and 1H, and Spence et al., 2004). It is well documented that one of the pathways through which FGF2 works is the FGFR/MEK/Erk pathway in different cellular contexts (Galy et al., 2002; Rios-Muñoz et al., 2005). To expand on our findings that PD173074 is able to block Shh stimulated regeneration, we used a MEK inhibitor, PD98059, in combination with retroviral Shh overexpression (Rcas-Shh). Consistent with our FGFR antagonist results, inhibition of the downstream effector, MEK, was also able to reduce regeneration (compare Figure 1E to Figure 1G and 1H). From this set of experiments, it is clear that the Hh and FGF pathways are co-dependent. That is, when regeneration is stimulated via one pathway, the other pathway must be functional.
Shh induces Erk phosphorylation in the CB/CMZ
In order to determine if both the FGF and Hh pathways are working through a common mechanism to induce retina regeneration, we examined early signaling events in the CB/CMZ. To do this, we turned to an in vitro system, using isolated E4 CB/CMZ explants (see Materials and Methods). Since we initially showed that regeneration stimulated by Shh is inhibited using PD173074 (Figure 1, and Spence et al., 2004), we wanted to determine if Shh is able to activate the MAP kinase pathway. After placing our explants in culture media, we added increasing concentrations of FGF2 or Shh and assayed for pErk. We found that, compared to control, both FGF2 (10ug/ml) and Shh (5ug/ml and 10ug/ml) were able to robustly induce Erk phosphorylation after 4 hours of exposure (Figure 2A). The increase in pErk stimulated by Shh was somewhat surprising as Shh signaling does not usually act via the MAPK pathway. However, several recent reports support the activation of pErk after stimulating the Hh pathway. In one report transient overexpression of Gli1 in C3H10T1/2 cells increased the amount of pErk. Additionally, the pErk increase in C3H10T1/2 cells was inhibited with the MEK-inhibitor U0126 (Xie et al., 2001). In another report, Shh was able to stimulate proliferation of rat gastric cells via pErk activation which was also inhibited by a MEK-inhibitor PD98059 (Osawa et al., 2006).
Figure 2. Early signaling events in the CB/CMZ show an intimate connection between FGF and Shh signaling pathways.
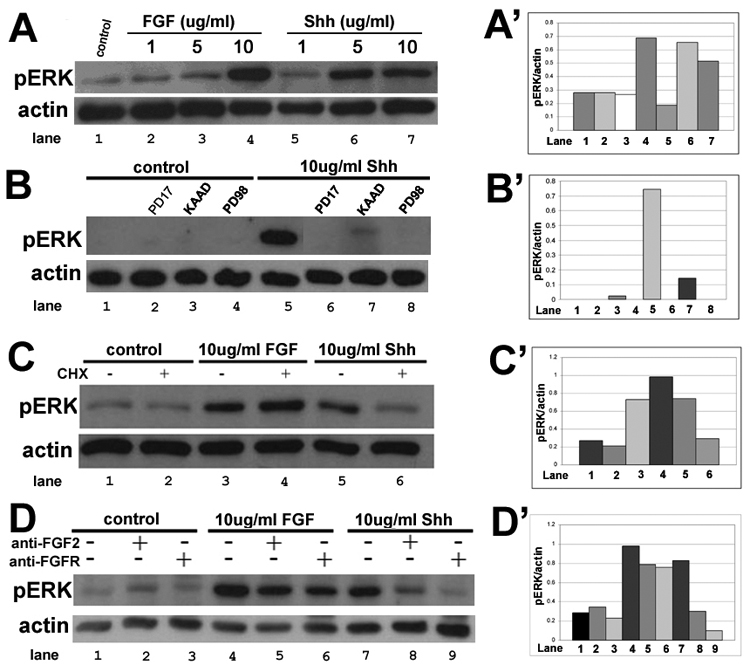
A. E4 CB/CMZ explants were used as either untreated tissue (control; lane 1), or tissue exposed to 1, 5 or 10 ug/ml of FGF2 (lane 2, 3, 4) or 1, 5 or 10ug/ml Shh peptide (lane 5, 6, 7). Explants were assayed for pErk using Western blot analysis. Actin was used as a loading control to show that similar amounts of protein were run in each lane. 10ug/ml of FGF2 and 5 and 10ug/ml of Shh were able to induce robust Erk phosphorylation.
A’. Densitometry showing the ratio of pErk/actin in A.
B. pErk levels were assayed in untreated E4 CB/CMZ explants (control; lane 1) or in E4 explants that were exposed to either FGFR inhibitor PD173074 (lane 2), Hh pathway inhibitor KAAD (lane 3) or MEK inhibitor PD98059 (lane 4). Explants were also exposed to 10ug/ml Shh alone (lane 5) or with PD173074 (lane 6), KAAD (lane 7) or PD98059 (lane 8). Shh was able to induce robust pErk levels, and this was abolished by inhibiting FGF/MAPK signaling or Hh signaling.
B’. Densitometry showing the ratio of pErk/actin in B.
C. E4 untreated CB/CMZ explants were used as control (lanes 1, 2). E4 explants were treated with 10ug/ml FGF2 (lanes 3, 4) or 10ug/ml Shh peptide (lanes 5, 6) for 4 hours. One hour prior to addition of growth factors, 100ug/ml of cycloheximide (CHX) was added. CHX did not decrease the amount of pErk in FGF2 treated explants (lane 3 vs. lane 4), but inhibited Erk phosphorylation in Shh treated explants (lane 5 vs. lane 6).
C’. Densitometry showing the ratio of pErk/actin in C.
D. E4 untreated CB/CMZ explants were used as control (lanes 1–3). E4 explants were treated with 10ug/ml FGF2 (lanes 3, 4) or 10ug/ml Shh (lanes 5, 6) for 4 hours. One hour prior to addition of growth factors, anti-FGFR or anti-FGF2 was added to the culture media at a dilution of 1:10. Both anti-FGF2 and anti-FGFR inhibited Erk phosphorylation stimulated by FGF2 (lanes 4–6) as well as Erk phosphorylation stimulated by Shh (lanes 7–9).
D’. Densitometry showing the ratio of pErk/actin in D.
We then carried out tissue explant experiments using 10ug/ml Shh in combination with PD173074 (FGFR inhibitor), PD98059 (MEK inhibitor) or KAAD (Hh inhibitor). We found that all three inhibitors are able to block pErk stimulated by Shh (Figure 2B). This suggests that Shh leads to activation of the MAPK pathway through FGF receptors, since inhibition of FGFR blocks pErk stimulated by Shh.
Shh stimulated pErk requires new protein synthesis
Shh works by binding its receptor, Patched (Ptc), which releases inhibition on a co-receptor, Smoothened, which then activates one of three Glis (Gli 1, 2 or 3). Gli then moves into the nucleus where it functions as a transcription factor to activate or repress transcription (reviewed in Ruiz i Altaba et al., 2003; Jacob and Briscoe, 2003). Since our data demonstrates that Shh induces pErk in E4 CB/CMZ explants (Figure 2A), and inhibiting either endogenous Shh or inhibiting the FGF/MAP Kinase pathway in Shh treated explants inhibits Erk phosporylation (Figure 2B), we hypothesized that Shh activates transcription and new protein synthesis which leads to increased MAPK signaling and increased pErk levels. To test this, we cultured CB/CMZ explants with FGF or Shh for 4 hours in the presence or absence of 100ug/ml cycloheximide (CHX), a protein synthesis inhibitor and examined Erk phosphorylation using Western blot analysis. As expected, pretreatment of tissue with cycloheximide did not reduce pErk levels stimulated by FGF2 since FGF2 binds to FGFRs and consequently elicits the signaling cascade that results in the phosporylation of Erk (Figure 2C lanes 3 and 4). In contrast, cycloheximide treatment was able to block the phosporylation of Erk in Shh treated CB/CMZ explants (Figure 2C lanes 5 and 6). This means that Shh needs to elicit new transcription and translation in order to have a protein product that will initiate a MAPK response. Indeed, pErk levels were similar to basal pErk levels in samples treated with cycloheximide prior to Shh exposure (Compare figure 2C lane 6 to lanes 1 and 2). The fact that Shh was able to provoke a protein synthesis dependent increase in pErk was unexpected as such an intimate relationship between the FGF and Hh pathway has not been well documented. This requirement for new protein synthesis seems to be very rapid. In the chick embryo it has been reported that new proteins are synthesized as early as 2 hours after exposure to developmental toxicants (Papaconstantinou et al., 2003) and that Heat Shock proteins reach maximum rates of accumulation after only 5 hours of exposure to Transforming Growth Factor-beta 1 (TGF beta1) in chicken embryo cells (Takenaka and Hightower, 1993).
Shh stimulated pErk is mediated by FGF signaling
Since protein synthesis inhibition stops Shh stimulated Erk phosphorylation (Figure 2C), and since blocking FGFRs using PD173074 inhibits Shh stimulated regeneration after 3 days (Figure 1) as well reducing levels of pErk stimulated by Shh after 4 hours in our in vitro explant system (Figure 2B), we hypothesized that Shh may be increasing mRNAs coding for growth factors, or their receptors in the CB/CMZ, which eventually leads to Erk activation. In order to test this hypothesis, we performed antibody blocking experiments using CB/CMZ explants treated with either FGF2 or Shh. In addition, FGF2 or FGFR blocking antibodies were added to the media and all treatments were assayed for pErk (Figure 2D). The FGFR antibody we used for blocking experiments has a high affinity for FGFR1 and a reduced affinity for FGFR2 as described by the manufacturer (Chemicon Antibody MAB125; Venkateswaran et al., 1992). This antibody has also been shown to inhibit FGF activity in chick Müller and retinal cells (Desire et al., 2000). In explants treated with FGF2, the anti-FGF2 and anti-FGFR treatments only slightly reduced pErk levels when compared to FGF2 treated CB/CMZ explants alone (Figure 2D, lane 4, 5, 6). This inhibition was not complete, and is likely explained by the ratio of FGF2: blocking antibody. Due to the high concentration of antibody used in this experiment (10 ug, a 1:10 dilution) we did not attempt to perform a competition experiment between the blocking antibodies and FGF2. In explants treated with Shh, both anti-FGF2 and anti-FGFR blocked pErk. In fact, both methods of blocking FGF signaling resulted in pErk levels that were similar to basal levels (Figure 2D, compare lane 8 and 9 to control lanes 1, 2 and 3). This set of experiments suggests that in the CB/CMZ, Shh indirectly signals via the FGF/FGFR pathway.
Shh upregulates FGF and FGFR1 mRNA
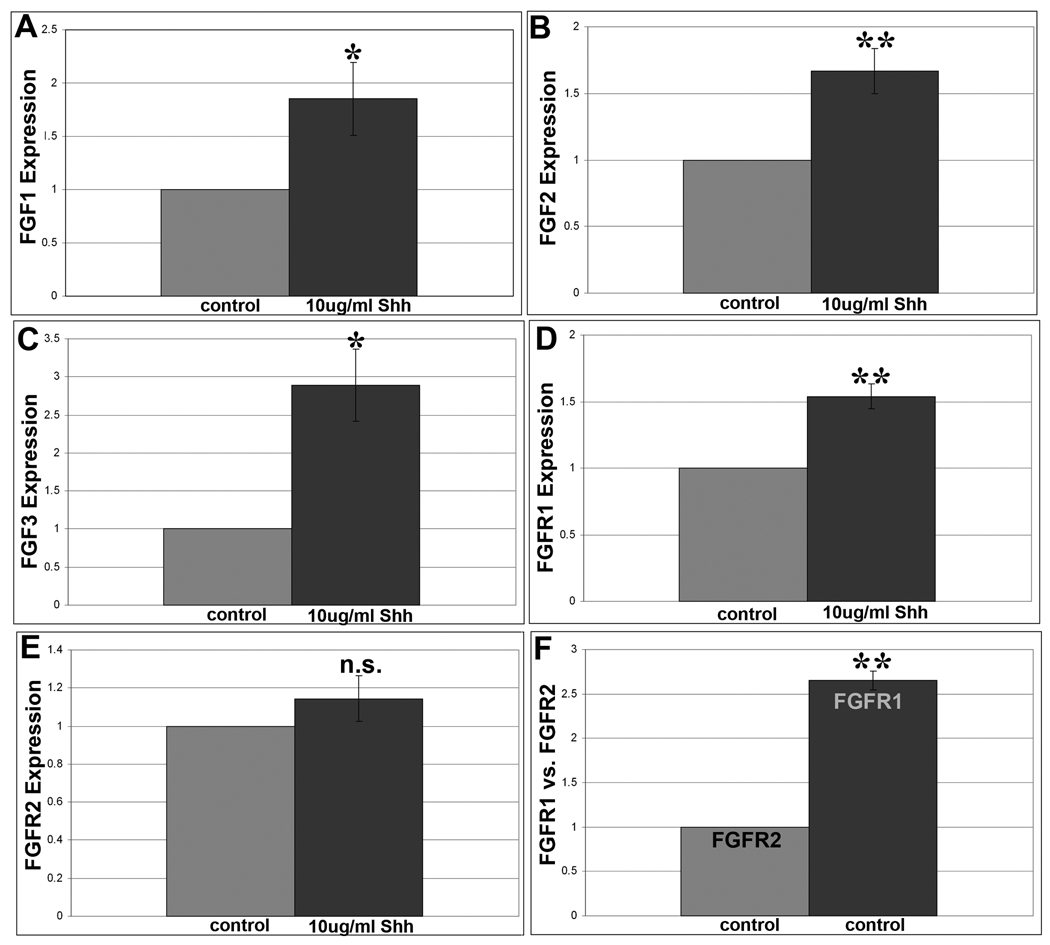
To more directly test how Shh may be activating the FGF pathway, we used quantitative real-time RTPCR to determine if exposure to Shh was able to cause an mRNA increase in any of the members of the FGF pathway. Specifically, we examined FGF receptors 1 and 2, since our antibody blocking experiments revealed that blocking FGFR 1 and 2 reduced Shh stimulated pErk (Figure 2D). We also examined all of the FGF ligands that are known in the chick. A list of results can be found in Supplemental Results Table 1. Here we focus only on results that showed a significant change in mRNA expression in response to Shh (Figure 3). We found that adding Shh to CB/CMZ explants significantly increased FGF1, FGF2, FGF3 and FGFR1 mRNA expression, but not FGFR2 or other FGF ligands (Figure 3A–E and Supplemental Results Table 1). Additionally, as expected, Shh was able to upregulate Ptc-1 mRNA in CB/CMZ explants (not shown).
Figure 3. Shh upregulates FGF 1, 2, 3 and FGFR1.
A. Quantitative real time RTPCR analysis of E4 CB/CMZ explants stimulated with Shh show that Shh treatment stimulated a significant increase in expression above control tissue for FGF1 (1.85 fold).
B. Shh triggered a significant increase in FGF2 expression above control tissue (1.65 fold).
C. Shh caused a significant increase in FGF3 expression above control tissue (2.89 fold).
D. Shh produced a significant increase in FGFR1 expression above control tissue (1.54 fold)
E. Shh did not trigger a significant increase in FGFR2 expression above control tissue.
F. In addition, in control E4 CB/CMZ explants, FGFR1 and FGFR2 levels were compared. FGFR1 was expressed more than 2.5 fold over FGFR2. Error bars are S.E.M. * = p<0.05, ** = p<0.01. n.s= not significant.
We also observed that in control CB/CMZ, FGFR1 was more robustly expressed than FGFR2 (Figure 3F). This was corroborated with immunohistochemical analysis of FGF receptor expression in the developing eye, where FGFR1 was abundant in the CB/CMZ while FGFR2 was barely detectable in the CB/CMZ (not shown). We were curious to know if the stem/progenitor cells in the CB/CMZ were being regulated specifically by FGFR1 since it is more robustly expressed. To do this, we removed the retina at E4 and added FGF7 (also called Keratinocyte Growth Factor, KGF) to the eye. FGF7 preferentially binds to FGFR2 isoform IIIb (FGFR2-IIIb) (Reviewed in Itoh and Ornitz, 2004; Mohammadi et al., 2005). After 3 days of exposure to FGF7, we observed some regeneration from the CB/CMZ (not shown), although this was not as robust as regeneration stimulated by FGF2. This indicates that signaling through multiple FGFRs may stimulate regeneration in the CB/CMZ. At this time, investigation of specific FGFR isoform activity is beyond the scope of this work. The fact that different FGFRs can be involved in inducing retina regeneration is likely correlated with a redundant role for FGFRs, a phenomenon seen in different developmental processes. For example, lung morphogenesis is disturbed only when both FGFR3 and FGFR4 are knocked out during development in mouse, indicating a compensatory developmental mechanism when only one gene is knocked out (Weinstein et al., 1998).
Since Shh was able to regulate components of the FGF signaling pathway, we carried out a similar experiment to determine if stimulating CB/CMZ explants with FGF2 was able to upregulate components of the Shh signaling pathway and activate Hh signaling. We chose to examine Ptc-1 expression, since it is known to be a downstream target of Shh signaling (Spence et al., 2004 and references therein). We found, by real time RT-PCR, that addition of FGF2 did not change the expression of Ptc-1 mRNA in CB/CMZ explants (not shown). From these results we determined that stimulating the CB/CMZ with FGF2 does not activate Hh signaling.
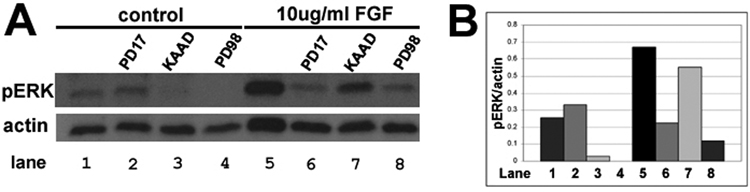
Hh signaling is required for basal pErk and Erk phosphorylation stimulated by FGF
To uncover the effect of the inhibitors used in Figure 1 and on FGF stimulated pErk levels, we added PD173074, PD98059 or KAAD along with 10ug/ml FGF2 to our explant culture system (Figure 4). As expected, FGF induced robust pErk (Figure 4A lane 5, and 4B), an effect that was inhibited by PD173074 and PD98059 (Figure 4A lanes 6 and 4B). In addition, the Hh pathway inhibitor KAAD was able to decrease pErk levels in the control (no FGF, KAAD alone Figure 4A lane 3, and 4B). KAAD only reduced pErk levels slightly in the FGF treated CB/CMZ explants (Figure 4A lane 7, and 4B). Therefore, inhibition of the Hh pathway reduced basal levels of pErk but did not considerably change FGF2 stimulated pErk.
Figure 4. Hh signaling is required for basal pErk as well as FGF induced pErk.
A. pErk levels were assayed in E4 untreated CB/CMZ explants (lane 1) or in explants that were exposed to the FGFR inhibitor PD173074 (lane 2), the Hh pathway inhibitor KAAD (lane 3) or the MEK inhibitor PD98059 (lane 4). Explants were also exposed to 10ug/ml FGF2 alone (lane 5) or with PD173074 (lane 6), KAAD (lane 7) or PD98059 (lane 8). Inhibiting the Hh pathway reduced the levels of both basal pErk and FGF induced pErk.
B. Densitometry showing the ratio of pErk/actin in A.
Shh and FGF2 stimulate proliferation in the CB/CMZ
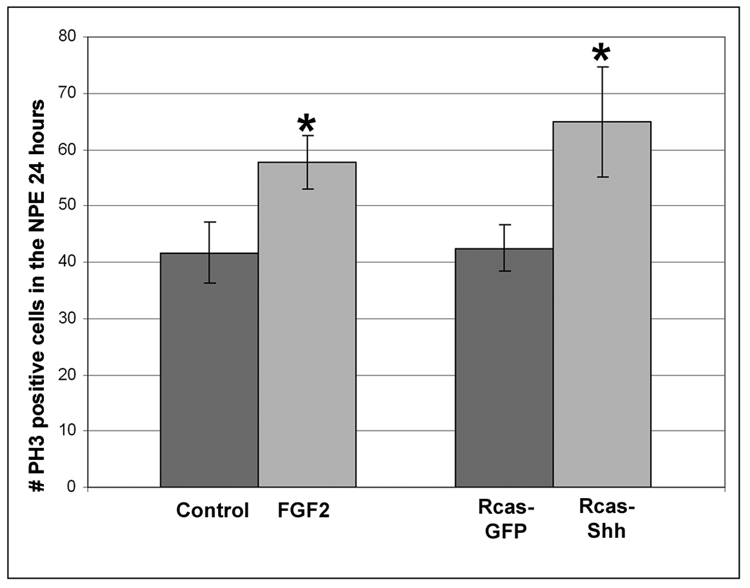
In order to further characterize early events stimulated by over-expression of Shh or ectopic FGF2 in the CB/CMZ, we examined cellular events induced by both factors in the CB/CMZ during retina regeneration. Specifically, we examined cell proliferation (Figure 5 and 6) and cell death (Figure 7). To investigate the outcome of exposing the CB/CMZ to ectopic FGF2, retinectomies were performed on E4 chick eyes and FGF2 was added to optic cups for 24 hours. Heparin beads were used in control experiments. Immunofluoresence labeling was performed on these eyes using an anti-PH3 antibody. The number of PH3 positive cells in the CB/CMZ was recorded (see materials and methods) during retina regeneration. A distinction was made between the positive cells present in the non-pigmented ciliary epithelium (NPE) and the pigmented ciliary epithelium (PE) of the CB/CMZ, since we have previously shown that it is the NPE that gives rise to the new retina (Spence et al., 2004). FGF2 treated eyes had significantly more PH3 positive cells in the NPE of the CB/CMZ than controls [(FGF2) 57.8 +/−4.8 vs. (control) 41.7 +/−5.4; p<0.05)] (Figure 5). To investigate the outcome of exposing the CB/CMZ to ectopic Shh, Rcas-Shh or control Rcas-GFP virus was injected at E3, the retina was removed at E4 and the eyes were collected 24 hours later. Over-expressing Shh significantly increased the number of PH3 positive cells in the CB/CMZ when compared to control RCAS-GFP infected eyes [(Rcas-Shh) 64.8 +/− 9.7 vs. (Rcas-GFP) 42.5 +/− 4.1; p<0.05] (Figure 5).
Figure 5. Increasing levels of FGF2 or Shh increases mitosis.
PH3 labeling of mitotic cells from E4 retinectomized eyes exposed to FGF2 or Heparin beads in vivo for 24 hours revealed that FGF2 significantly increased the number mitotic cells in the NPE of the CB/CMZ (left). Eyes were also injected with Rcas-Shh or Rcas-GFP at E3 and retinas removed at E4. Eyes were collected, sectioned and assayed for PH3 positive cells 24 hours after retinectomy. Ectopic Shh significantly increased the number of mitotic cells in the NPE of the CB/CMZ (right) *=p<0.05, n=6 sections from 3 eyes. Heparin beads represent the negative control for FGF treatments and Rcas-GFP the control for Shh treatments. Error bars are S.E.M.
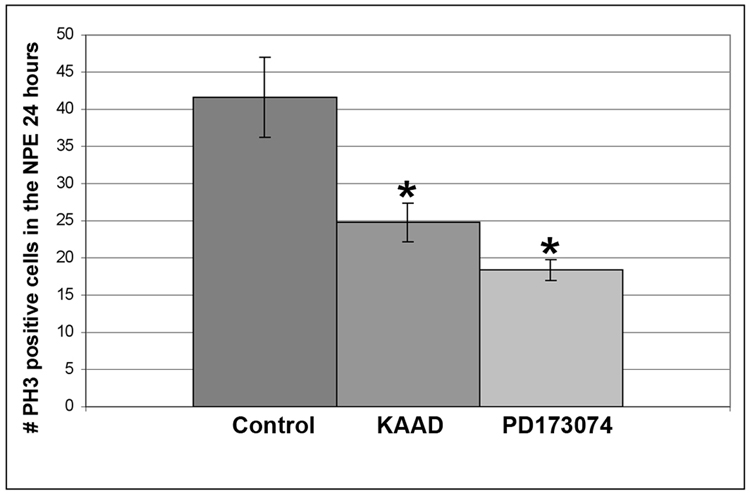
Figure 6. Hh and FGF signaling are required for basal proliferation in the NPE of the CB/CMZ.
Retinectomies were performed at E4 and chick eyes were exposed to 200uM KAAD, 50uM PD173074 or DMSO for 24 hours. PH3 labeling for mitotic cells showed that both KAAD (* = p<0.05) and PD173074 (*=p<0.01) reduced the number of mitotic cells in the NPE of the CB/CMZ. n=6 sections from 3 eyes. Error bars are S.E.M.
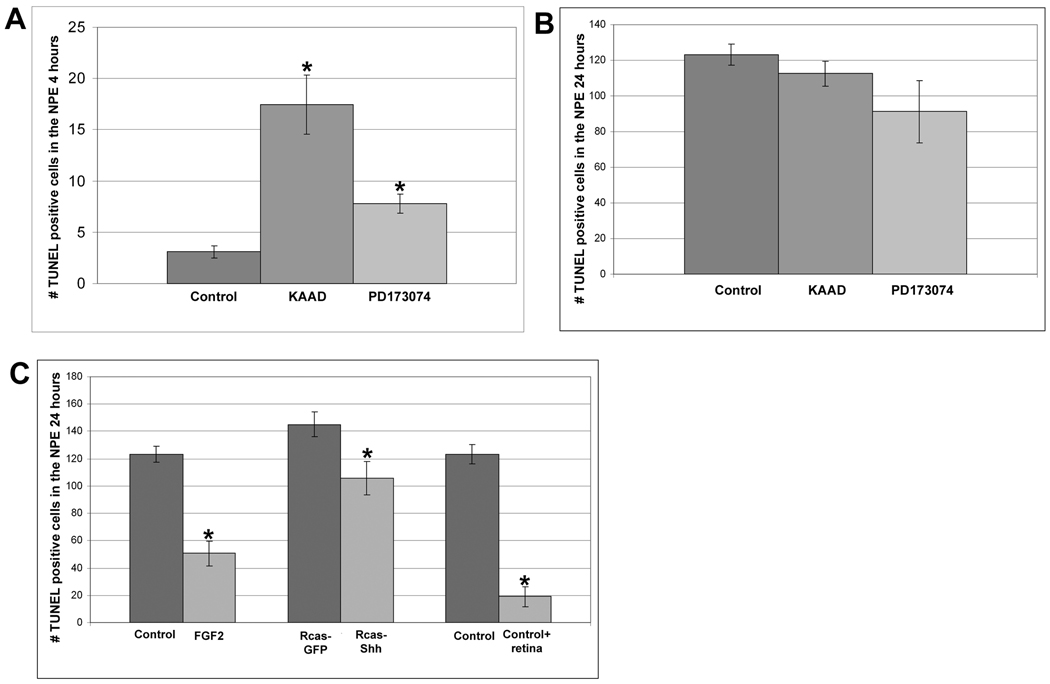
Figure 7. FGF and Hh signaling promote cell survival in the NPE of the CB/CMZ.
A. Retinectomies were performed at E4 and chick eyes were exposed to 200uM KAAD, 50uM PD173074 or DMSO control beads for 4 hours. Exposure to KAAD (* = p<0.01) and PD173074 (* = p<0.001) significantly increased the number of TUNEL positive cells in the NPE of the CB/CMZ, whereas exposure to PD173074 did not. n=6 sections from 3 eyes. Error bars are S.E.M.
B. Retinectomies were performed at E4 and chick eyes were exposed to 200uM KAAD, 50uM PD173074 or DMSO control beads for 24 hours. Exposure to KAAD and PD173074 did not increase the number of TUNEL positive cells in the NPE of the CB/CMZ, n=6 sections from 3 eyes. Error bars are S.E.M.
C. Retinectomy was performed on E4 chick eyes. Eyes were then exposed to FGF2 or DMSO control beads (left); Rcas-Shh or Rcas-GFP control (middle); DMSO control beads or DMSO control beads plus a piece of retina (right). Treatment with FGF (*=p<0.001), Rcas-Shh (*=p<0.05) or DMSO control plus retina (*=p<0.001) were all able to significantly reduce the number of TUNEL positive cells compared to their respective controls.
Endogenous Hh and FGF signaling are required for basal proliferation in the CB/CMZ
Since ectopic Shh or FGF2 is able to increase the number of PH3 positive cells in the CB/CMZ, we hypothesized that inhibiting the Hh pathway using KAAD or inhibiting FGF signaling using PD173074 may reduce the number of PH3 positive cells. To test this hypothesis, we removed the retina from E4 eyes and added DMSO control beads, 200 uM KAAD beads or 50uM PD173074 beads into the optic cup for 24 hours (Figure 6). Both KAAD and PD173074 treated eyes had a significant reduction in the number of PH3 positive cells compared to control (Figure 6) [(control) 41.7 +/− 5.4 vs. (KAAD) 24.8 +/− 2.6; p<0.05] [(control) 41.7 +/− 5.4 vs. (PD173074) 18.33 +/− 1.4; p<0.01].
Therefore, exposing the CB/CMZ to FGF2 or Shh during retina regeneration increases the number of PH3 positive cells, while removing endogenous FGF or Hh signaling using PD173074 and KAAD, respectively, reduces the number of PH3 positive cells.
Hh and FGF signaling are important for cell survival in the CB/CMZ
We wanted to examine the amount of cell death that would take place in the NPE of the CB/CMZ when the Hh or FGF pathways are inhibited during retina regeneration. We removed the retina from E4 chick eyes and added DMSO control beads, KAAD soaked beads (200uM) or PD173074 soaked beads (50uM) for 4 hours and assayed for cell death in the NPE of the CB/CMZ. We detected a significant increase in cell death when Hh was inhibited in the CB/CMZ compared to DMSO-control eyes [(control) 3.1 +/− 0.6 vs. (KAAD) 17.4 +/−2.9; p<0.001] (Figure 7A). We also detected a significant increase in cell death when the CB/CMZ was treated with PD173074, compared to control [(control) 3.1 +/− 0.6 vs. (PD173074) 7.8 +/− 0.9; p<0.001] (Figure 7A).
Because we observed an increase in cell death after inhibiting the FGF or Hh pathway for 4 hours, we hypothesized that the reduction in regeneration after exposure to the inhibitors for each pathway was in part due to the death of many of the cells in the CB/CMZ over time. To examine this possibility, we repeated the above experiment, except we collected the eyes after 24 hours of exposure to KAAD or PD173074. Compared to DMSO control, neither KAAD nor PD173074 induced a significant increase in TUNEL positive cells [(control) 123.3 +/− 5.9 vs. (KAAD) 112.5 +/− 7.1] [(control) 123.3 +/− 5.9 vs. (PD173074) 91.2 +/− 17.6] (Figure 7B).
There was a large increase in the number of TUNEL positive cells in the untreated eyes from 4 hours (3.1 +/− 0.6) (Figure 7A) to 24 hours (123.3 +/− 5.9) (Figure 7B). We thought that this large increase in cell death may be due to the absence of the retina and any growth and survival factors that it may provide the CB/CMZ. To determine if this was the case, we performed retinectomies at E4 and added either DMSO control beads or DMSO control beads plus a piece of the retina into the optic cup and collected the eyes after 24 hours. The number of apoptotic cells in the NPE of the CB/CMZ was recorded during retina regeneration (Figure 7C). We found that eyes with a piece of retina placed back into the optic cup had significantly fewer TUNEL positive cells than eyes that received DMSO control beads only [(control) 123.3 +/− 5.9 vs. (control + retina) 19 +/− 7.2; p<0.001]. This result indicates that the increase in cell death in the CB/CMZ between 4 and 24 hours is likely due to the removal of survival factors provided by the retina.
Since we observed an increase in the amount of cell death at 4 hours, but not 24 hours of treatment with either the Hh or FGF inhibitor, and since the removal of the retina is sufficient to increase the amount of cell death after 24 hours, we believe that the endogenous levels of FGF and Shh appear not to be sufficient to affect survival while they are sufficient to affect the number of proliferating cells. Another possibility could be that other critical retinal factors that contribute to survival are exhausted by 24 hours of retina removal and under these conditions changing the levels of endogenous Shh or FGF does not affect the number of apoptotic cells. We further hypothesized that both molecules and their signaling pathways may play a role in cell survival. To determine if this was the case, after removing the retina from E4 eyes we added DMSO control, or DMSO control plus FGF2 and collected after 24 hours of exposure. The NPE of the CB/CMZ was assayed for cell death. We found that FGF2 was able to act as a survival factor and significantly reduce the number of cells dying [(control) 123.3 +/− 5.9 vs. (FGF2) 50.7 +/−8.9; p<0.001] (Figure 7C). We repeated the above experiment, except we injected Rcas-GFP or Rcas-Shh at E3. Ectopic Shh was also able to act as a survival factor, significantly reducing the number of apoptotic cells in the NPE of the CB/CMZ during retina regeneration [(Rcas-GFP) 145 +/− 9.1 vs. (Rcas-Shh) 105.7 +/− 12.4; p<0.05] (Figure 7C).
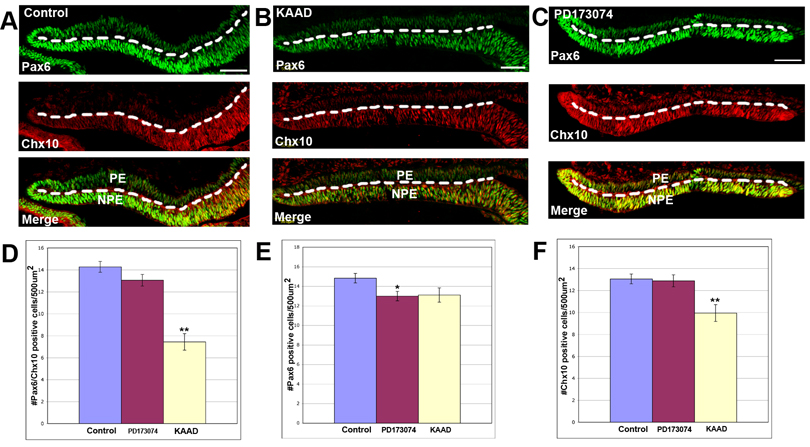
Maintenance of retinal progenitor cell markers in the CB/CMZ is disrupted by inhibiting Hh signaling, but not by inhibiting the FGF pathway
It was important to test if manipulating these pathways had any effect on the progenitor cell identity of the CB/CMZ as it has been previously described that Hh was required for stem/progenitor cell identity in different tissues (Ericson et al., 1996; 1997; Briscoe et al., 2000; Agius et al., 2004; Xu et al., 2005). To test whether Shh or FGF signaling is required for maintenance of retinal progenitor cell identity in the CB/CMZ, we performed retinectomies at E4 and we assayed DMSO control, KAAD or PD173074 treated eyes for Pax6/Chx10 co-expression. Cells that co-express Pax6/Chx10 are considered retinal progenitor cells (Belecky-Adams et al., 1997; Fischer and Reh, 2000; Spence et al., 2004). After 24 hours of exposure to DMSO control beads or 50uM PD173074, we did not find a clear difference in the pattern of Pax6/Chx10 labeling (Figure 8A and C and D), however PD173074 caused a significant decrease in the number Pax6 positive cells, but not in the number of Chx10 positive cells (Figure 8E and F). KAAD treated eyes on the other hand, showed a significant decrease in Pax6/Chx10 co-expressing cells (Figure 8B and D). Furthermore, the cells that no longer express a combination of genes have lost Chx10 expression (Figure 8F).
Figure 8. Maintenance of retinal progenitor cell markers is perturbed by Hh inhibition.
A. Retinectomies were performed on E4 chick eyes. Eyes were then exposed to DMSO for 24 hours. Many cells in the CB/CMZ are co-expressing Pax6 and Chx10 as determined by immunohistochemistry.
B. Operated eyes exposed to KAAD for 24 hours appear to have a difference in Pax6/Chx10 co-expression from control.
C. Operated eyes exposed to PD173074 for 24 hours shows no difference in Pax6/Chx10 co-expression from control.
D. Quantitation of the area in the NPE containing Pax6/Chx10 positive cells from eyes represented in A–C. Error bars are S.E.M. * = p<0.05.
E. Quantitation of the area in the NPE containing Pax6 positive cells from eyes represented in A–C. Error bars are S.E.M. * = p<0.05.
F. Quantitation of the area in the NPE containing Chx10 positive cells from eyes represented in A–C. Error bars are S.E.M. ** = p<0.001.
Dashed line marks the boundary between the NPE (below the line) and PE (above the line). PE = pigmented ciliary epithelium; NPE = non-pigmented ciliary epithelium; PE=pigmented epithelium; Scale bars = 50um and applies to all panels.
Discussion
Our experiments help define a complex mechanism by which Shh and FGF signaling interact to induce retina regeneration from the CB/CMZ. In this study, we present data that clearly points to cellular events that are “shared” by both pathways and are critical for retina regeneration.
A model for Shh and FGF-stimulated regeneration
Addition of FGF2 to the optic cup after the retina has been removed leads to increased proliferation (Figure 5) and induction of retina regeneration from the CB/CMZ (Figure 1 and Spence et al., 2004). Shh overexpression also leads to increased proliferation (Figure 5), increased FGF signaling (Figure 2), an increase in expression of FGF family members (Figure 3) and can induce retina regeneration from the CB/CMZ (Figure 1 and Spence et al., 2004).
There is an obvious model to explain how Shh overexpression is able to stimulate regeneration, where Shh acts as a regulator of FGF signaling in cells of the CB/CMZ. In this model, Shh overexpression causes an increase in FGF ligands and FGFR1 via new transcription as shown in Figure 3, and eventually new protein synthesis. This in turn increases the amount of FGF signaling, leading to an increase in pErk (Figure 2), and the increased levels of pErk leads to and an increase in the number of PH3 positive mitotic cells (Figure 5).
On the other hand, the co-dependence of FGF and Hh signaling during retina regeneration from the CB/CMZ could also be explained by the fact that both pathways play a role in survival of the CB/CMZ. Our results suggest that the acute effect of inhibiting either the FGF or Hh pathway in the CB/CMZ after retina removal is to decrease cell survival (Figure 7). The initial increase in cell death seen by inhibiting either pathway could explain the overall reduction of regeneration over time as well as the decreased pErk activity and reduced number of mitotic cells.
Finally, the CB/CMZ requires endogenous Hh signaling to maintain the identity of its progenitor cells. These progenitors can respond to exogenous growth factors like FGF to proliferate and give rise to new retina. Therefore, FGF and Hh signaling pathways are also interdependent in this case as one pathway is needed to maintain the identity of the progenitor population while the other is required to activate the proliferation of these cells to give rise to new retina.
FGF and Shh display proliferative effects during retina regeneration
FGF has been shown to control proliferation of many developing tissues and it is possible that FGF signaling acts upon Cyclin genes in this system, just as FGF does in for example the developing chick neural tube and during the regulation of cortical precursors (Lobjois et al., 2004; Li and Dicicco-Bloom, 2004). Shh could be controlling proliferation by inducing FGF signaling and subsequently activating cyclin genes indirectly. However, it is also possible that Shh may have an FGF independent role in regulating cell cycle progression since it has been demonstrated that Shh is able to regulate key cyclin genes or other important cell cycle regulators including phosphatases in different contexts (Dahmane and Ruiz-i- Altaba, 1999; Wallace, 1999; Wechsler-Reya and Scott, 1999; Kenney and Rowitch, 2000; Barnes et al., 2001; Kenney et al., 2003; Oliver et al., 2003; Cayuso et al., 2006; Yu et al., 2006).
Recent evidence from studies in the mouse retina have shown that activation of the Hh pathway acts in a cell autonomous manner to upregulate CyclinD1 and increase progenitor cell proliferation (Yu et al., 2006). Activation of Gli3 has also been shown to cause a cell autonomous increase in the number of PH3 positive cells in the developing chick neural tube (Cayuso et al., 2006). While our studies do not directly address whether Shh overexpression is acting cell autonomously to drive an increase in the number of 25 PH3 positive cells in the CB/CMZ, it is likely that Hh is able to signal in the PH3 positive mitotic cells, since these cells also show immunoreactivity for Gli1 and Gli3 showing that Hh signaling machinery is present in these cells (Supplemental Figure 1).
Shh is able to upregulate members of the FGF signaling cascade
As discussed previously, it is possible that one of the ways in which Shh stimulates the CB/CMZ to proliferate and give rise to a regenerated retina is by increasing various members of the FGF signaling pathway, which leads to an increase in FGF signaling activity (Figure 2 and 3). We do not suggest this is the only way in which progenitor cells in the CB/CMZ are regulated, as other molecules have been implicated in regulation of stem/progenitor cells in the CB/CMZ (Fischer and Reh, 2000; Zhao et al., 2002; Kubo et al., 2003 and 2005; Liu et al., 2003; Das et al., 2004; Haynes, Gutierrez and Del Rio-Tsonis, unpublished results), however, it is clear from our work that Shh is able to stimulate regeneration in an FGF-dependent manner. Although Shh mediated regulation of FGF1, 2, 3 and FGFR1 has not been previously documented, Shh is able to directly initiate FGF15 signaling in the diencephalon and midbrain in mice (Saitsu et al., 2005), and can also regulate FGF19 during forebrain development in Zebrafish (Miyake et al., 2005) supporting our observations that Shh induces expression of FGFs in the CB/CMZ of the embryonic chick.
Regeneration stimulated by FGF2 requires basal levels of Hh signaling
It is well documented that Hh signaling is required for cell proliferation in many different contexts. For example, Shh is required for cell proliferation of progenitors in the SVZ and neocortex in mice (Palma and Ruiz i Altaba, 2004; Palma et al., 2005). In other studies, removal of Shh signaling using conditional null mice for Shh and Smoothened reduced the number of neural progenitors in the SVZ and hippocampus postnatally. In these mice, reduced progenitor cell number was correlated with a marked increase in apoptosis (Machold et al., 2003). Other examples of Hh regulating proliferation and cell death are illustrated in studies on different types of cancer (Qualtrough et al., 2004; Romer et al., 2004; Sanchez et al., 2004; Sanchez and Ruiz i Altaba, 2005). Likewise, Hh signaling is also involved in progenitor cell proliferation and survival during eye development in several different organisms (Stenkamp et al., 2002 and reviewed in Amato et al., 2004 and Moshiri et al., 2004; Wang et al., 2005). Shh has also been implicated in controlling proliferation during regenerative processes. Inhibition of the Hh pathway during lens regeneration in the newt resulted in decreased proliferation and lens fiber differentiation in the lens vesicle (Tsonis et al., 2004). Similarly, treatment of regenerating axolotl tails with the Hh inhibitor cyclopamine significantly reduced blastema cell proliferation resulting in an incomplete regeneration of the tail (Schnapp et al., 2005). We have shown that Hh signaling has several different biological functions in the CB/CMZ during retina regeneration, consistent with its functions in different contexts and different organisms.
In addition to Shh being able to stimulate retina regeneration from the CB/CMZ, we have shown that FGF2 stimulated regeneration also requires basal Hh signaling (Figure 1, Figure 4 and Figure 8). We have shown that Shh is able to increase the number of mitotic cells as well as act as a survival factor in the CB/CMZ after retina removal (Figure 5 and 7C). Furthermore, removing basal levels of Hh signaling in the CB/CMZ during retina regeneration is sufficient to decrease basal levels of cell proliferation and to induce a wave of cell death soon after retina removal (Figure 6 and 7A- 4 hours). Endogenous Hh signaling is also required for the maintenance of the progenitor cell identity in the CB/CMZ (Figure 8). Therefore, this combination is likely responsible for the reduced regeneration observed in FGF- stimulated eyes that have also been treated with KAAD (Figure 1).
Maintenance of retinal progenitor cell markers in the CB/CMZ
Hh signaling has been shown to be important for progenitor cell maintenance in different cellular contexts. For example, a role for Hh signaling in progenitor cell maintenance has been shown in the developing retina where conditional ablation of Shh in mouse retinas led to a reduction of the retina progenitor (precursor) cell (RPC) pool (Wang et al., 2005). One of the best examples of Hh signaling specifying and maintaining the identity of neural progenitors takes place during neurogenesis where Shh regulates progenitor cell identity and neuronal fate in the ventral neural tube and developing spinal cord (Ericson et al. 1996; 1997; Briscoe et al., 2000; Agius et al., 2004). In our studies, we observed a significant decrease in Pax6/Chx10 positive cells in eyes that had been treated with KAAD for 24 hours compared to DMSO control treated eyes (Figure 8A, B and D). These eyes had a significantly reduced number of Chx10 positive cells (Figure 8F). On the other hand, inhibition of FGF signaling did not disrupt the retinal progenitor cell population compared to control tissue (Figure 8C and D). Our results suggest that only Shh regulates the identity of the retinal progenitor population in the CB/CMZ during the process of retina regeneration as defined by the co-expression of Pax6/Chx10.
Conclusion
We have demonstrated how both Shh and FGF signaling are interdependent during the process of retina regeneration in the embryonic chick. Although we do not rule out the possibility that other signaling pathways are involved in regulating the CB/CMZ, we propose a model for retina regeneration from the CB/CMZ where FGF and Shh are responsible for stimulating mitosis and proliferation, which leads to regeneration. Shh and FGF are able to induce regeneration from the CB/CMZ by increasing the number of mitotically active cells. The ability of either molecule to do this is dependent on the activity of each other.
In addition, both FGF and Shh signaling pathways are able to act as survival factors in the CB/CMZ. It is clear then, that disrupting FGF or Hh signaling can negatively affect the CB/CMZ by interfering with the ability of either pathway to stimulate proliferation and act as a survival factor.
Finally, endogenous Hh is required to maintain the identity of the retina progenitor population in the CB/CMZ and any disruption in this pathway will decrease the progenitors available to proliferate and participate in the process of regeneration.
Taken together, our results show a complex relationship between FGF and Shh that regulates stem/progenitor cells in the CB/CMZ of the embryonic chick during the process of retina regeneration.
Supplementary Material
Acknowledgments
We thank Pfizer for providing PD173074 and Dr. Cliff Tabin, Dr. Thomas Jessel, Dr. Ruben Adler and Dr. Teri Belecky–Adams for providing reagents used in this work. We also thank Dr. Tracy Haynes for assistance with the quantitation data, and Dr. Mike Robinson and M. Natalia Vergara for critical review of this manuscript. This work was supported by the Foundation Fighting Blindness Canada (FFB-C) student fellowship to JRS and NIH grant EY 014197 to KDRT.
Abbreviations
- CB
Ciliary Body
- CMZ
Ciliary Marginal Zone
- PE
Pigmented Epithelium
- NPE
Non-Pigmented Epithelium
- RPE
Retina Pigmented Epithelium
- FGF
Fibroblast Growth Factor
- FGFR
Fibroblast Growth Factor Receptor
- MAPK
Mitogen Actived Protein Kinase
- Erk
Extracellular Signal-Regulated Kinase
- pErk
phosphorylated ERK
- MEK
ERK Kinase
- Shh
Sonic Hedgehog
- Hh
Hedgehog
- Ptc
Patched
- PH3
Phosphohistone H3
References
- Agius E, Soukkarieh C, Danesin C, Kan P, Takebayashi H, Soula C, Cochard P. Converse control of oligodendrocyte and astrocyte lineage development by Sonic hedgehog in the chick spinal cord. Dev. Biol. 2004;270(2):308–321. doi: 10.1016/j.ydbio.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Amato MA, Boy S, Perron M. Hedgehog signaling in vertebrate eye development: a growing puzzle. Cell Mol. Life Sci. 2004;61(7–8):899–910. doi: 10.1007/s00018-003-3370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes EA, Kong M, Ollendorff V, Donoghue DJ. Patched1 interacts with cyclin B1 to regulate cell cycle progression. EMBO J. 2001;20(9):2214–2223. doi: 10.1093/emboj/20.9.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belecky-Adams T, Tomarev S, Li HS, Ploder L, McInnes RR, Sundin O, Adler R. Pax-6, Prox 1, and Chx10 homeobox gene expression correlates with phenotypic fate of retinal precursor cells. Inves.t Ophthalmol. Vis. Sci. 1997;38(7):1293–1303. [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101(4):435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Cayuso J, Ulloa F, Cox B, Briscoe J, Marti E. The Sonic hedgehog pathway independently controls the patterning, proliferation and survival of neuroepithelial cells by regulating Gli activity. Development. 2006;133(3):517–528. doi: 10.1242/dev.02228. [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Dev. Biol. 1965;12:79–92. doi: 10.1016/0012-1606(65)90022-9. [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Influence of mouse neural retina on regeneration of chick neural retina from chick embryonic pigmented epithelium. Nature. 1970;228(5271):559–560. doi: 10.1038/228559a0. [DOI] [PubMed] [Google Scholar]
- Das AV, James J, Zhao X, Rahnenfuhrer J, Ahmad I. Identification of c-Kit receptor as a regulator of adult neural stem cells in the mammalian eye: interactions with Notch signaling. Dev. Biol. 2004;273(1):87–105. doi: 10.1016/j.ydbio.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126(14):3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Tsonis PA. Eye regeneration at the molecular age. Dev. Dyn. 2003;226(2):211–224. doi: 10.1002/dvdy.10224. [DOI] [PubMed] [Google Scholar]
- Desire L, Courtois Y, Jeanny JC. Endogenous and exogenous Fibroblast Growth Factor 2 support survival of chick retinal neurons by control of neuronal bcl-xL and bcl-2 expression through a Fibroblast Growth Factor Receptor 1- and Erk-dependent pathway. J. Neurochem. 2000;75(1):151–163. doi: 10.1046/j.1471-4159.2000.0750151.x. [DOI] [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87(4):661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90(1):169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002;129(9):2283–2291. doi: 10.1242/dev.129.9.2283. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev. Biol. 2000;220:197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Growth factors induce neurogenesis in the ciliary body. Dev. Biol. 2003;259:225–240. doi: 10.1016/s0012-1606(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, Levine EM, Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development. 2000;127(21):4599–4609. doi: 10.1242/dev.127.21.4599. [DOI] [PubMed] [Google Scholar]
- Galy A, Neron B, Planque N, Saule S, Eychene A. Activated MAPK/ERK kinase (MEK-1) induces transdifferentiation of pigmented epithelium into neural retina. Dev. Biol. 2002;248(2):251–264. doi: 10.1006/dbio.2002.0736. [DOI] [PubMed] [Google Scholar]
- Haynes T, Del Rio-Tsonis K. Retina repair, stem cells and beyond. Curr Neurovasc. Re.s. 2004;1(3):231–239. doi: 10.2174/1567202043362216. [DOI] [PubMed] [Google Scholar]
- Hitchcock P, Ochocinska M, Sieh A, Otteson D. Persistent and injury-induced neurogenesis in the vertebrate retina. Prog. Retin. Eye Res. 2004;23(2):183–194. doi: 10.1016/j.preteyeres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20(11):563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Jacob J, Briscoe J. Gli proteins and the control of spinal-cord patterning. EMBO Rep. 2003;4(8):761–765. doi: 10.1038/sj.embor.embor896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney AM, Rowitch DH. Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mo.l Cell Biol. 2000;20(23):9055–9067. doi: 10.1128/mcb.20.23.9055-9067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130(1):15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130(3):587–598. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b inhibits differentiation of retinal progenitor cells in the absence of Notch activity by downregulating the expression of proneural genes. Development. 2005;132(12):2759–2770. doi: 10.1242/dev.01856. [DOI] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev. Dyn. 2003;227(3):323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- Li B, DiCicco-Bloom E. Basic fibroblast growth factor exhibits dual and rapid regulation of cyclin D1 and p27 to stimulate proliferation of rat cerebral cortical precursors. Dev. Neurosci. 2004;26(2–4):197–207. doi: 10.1159/000082137. [DOI] [PubMed] [Google Scholar]
- Lobjois V, Benazeraf B, Bertrand N, Medevielle F, Pituello F. Specific regulation of cyclins D1 and D2 by FGF and Shh signaling coordinates cell cycle progression, patterning, and differentiation during early steps of spinal cord development. Dev. Biol. 2004;273(2):195–209. doi: 10.1016/j.ydbio.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39(6):937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16(2):107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Miyake A, Nakayama Y, Konishi M, Itoh N. Fgf19 regulated by Hh signaling is required for zebrafish forebrain development. Dev. Bio. 2005;288(1):259–275. doi: 10.1016/j.ydbio.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Moshiri A, Close J, Reh TA. Retinal stem cells and regeneration. Int. J. Dev. Biol. 2004;48(8–9):1003–1014. doi: 10.1387/ijdb.041870am. [DOI] [PubMed] [Google Scholar]
- Moshiri A, McGuire CR, Reh TA. Sonic hedgehog regulates proliferation of the retinal ciliary marginal zone in posthatch chicks. Dev. Dyn. 2005;233(1):66–75. doi: 10.1002/dvdy.20299. [DOI] [PubMed] [Google Scholar]
- Moshiri A, Reh T. Persistent Progenitors at the Retinal Margin of ptc +/− Mice. J. Neurosci. 2004;24(1):229–237. doi: 10.1523/JNEUROSCI.2980-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver TG, Grasfeder LL, Carroll AL, Kaiser C, Gillingham CL, Lin SM, Wickramasinghe R, Scott MP, Wechsler-Reya RJ. Transcriptional profiling of the Sonic hedgehog response: a critical role for N-myc in proliferation of neuronal precursors. Proc. Natl. Acad. Sci. U S A. 2003;100(12):7331–7336. doi: 10.1073/pnas.0832317100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa H, Ohnishi H, Takano K, Noguti T, Mashima H, Hoshino H, Kita H, Sato K, Matsui H, Sugano K. Sonic hedgehog stimulates the proliferation of rat gastric mucosal cells through ERK activation by elevating intracellular calcium concentration. Biochem. Biophys. Res. Commun. 2006;344(2):680–687. doi: 10.1016/j.bbrc.2006.03.188. [DOI] [PubMed] [Google Scholar]
- Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132(2):335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma V, Ruiz i Altaba A. Hedgehog-GLI signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Development. 2004;131(2):337–345. doi: 10.1242/dev.00930. [DOI] [PubMed] [Google Scholar]
- Papaconstantinou AD, Brown KM, Noren BT, McAlister T, Fisher BR, Goering PL. Mercury, cadmium, and arsenite enhance heat shock protein synthesis in chick embryos prior to embryotoxicity. Birth Defects Res. B. De.v Reprod. Toxico. 2003;68(6):456–464. doi: 10.1002/bdrb.10044. [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ. Basic fibroblast growth factor induces retinal regeneration in vivo. Dev. Biol. 1989;134:201–205. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ. Induction of retinal regeneration in vivo by growth factors. Dev. Biol. 1991;148(1):322–333. doi: 10.1016/0012-1606(91)90341-y. [DOI] [PubMed] [Google Scholar]
- Perron M, Kanekar S, Vetter ML, Harris WA. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev. Biol. 1998;199(2):185–200. doi: 10.1006/dbio.1998.8939. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualtrough D, Buda A, Gaffield W, Williams AC, Paraskeva C. Hedgehog signalling in colorectal tumour cells: induction of apoptosis with cyclopamine treatment. In.t J. Cancer. 2004;110(6):831–837. doi: 10.1002/ijc.20227. [DOI] [PubMed] [Google Scholar]
- Rios-Muñoz W, Soto I, Duprey-Diaz MV, Blagburn J, Blanco RE. Fibroblast growth factor 2 applied to the optic nerve after axotomy increases Bcl-2 and decreases Bax in ganglion cells by activating the extracellular signal-regulated kinase signaling pathway. J. Neurochem. 2005;93(6):1422–1433. doi: 10.1111/j.1471-4159.2005.03129.x. [DOI] [PubMed] [Google Scholar]
- Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, Connelly M, Stewart CF, Gould S, Rubin LL, Curran T. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6(3):229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Nguyen V, Palma V. The emergent design of the neural tube: prepattern, SHH morphogen and GLI code. Curr. Opin. Genet. Dev. 2003;13(5):513–521. doi: 10.1016/j.gde.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Sanchez P, Hernandez AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, Ruiz i Altaba A. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Pro.c Natl. Acad. Sci. U SA. 2004;101(34):12561–12566. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez P, Ruiz i Altaba A. In vivo inhibition of endogenous brain tumors through systemic interference of Hedgehog signaling in mice. Mech Dev. 2005;122(2):223–230. doi: 10.1016/j.mod.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Saitsu H, Komada M, Suzuki M, Nakayama R, Motoyama J, Shiota K, Ishibashi M. Expression of the mouse Fgf15 gene is directly initiated by Sonic hedgehog signaling in the diencephalon and midbrain. Dev. Dyn. 2005;232(2):282–292. doi: 10.1002/dvdy.20236. [DOI] [PubMed] [Google Scholar]
- Schnapp E, Kragl M, Rubin L, Tanaka EM. Hedgehog signaling controls dorsoventral patterning, blastema cell proliferation and cartilage induction during axolotl tail regeneration. Development. 2005;132(14):3243–3253. doi: 10.1242/dev.01906. [DOI] [PubMed] [Google Scholar]
- Spence JR, Madhavan M, Ewing JD, Jones DK, Lehman BM, Del Rio-Tsonis K. The hedgehog pathway is a modulator of retina regeneration. Development. 2004;131(18):4607–4621. doi: 10.1242/dev.01298. [DOI] [PubMed] [Google Scholar]
- Stenkamp DL, Frey RA, Mallory DE, Shupe EE. Embryonic retinal gene expression in sonic-you mutant zebrafish. Dev. Dyn. 2002;225(3):344–350. doi: 10.1002/dvdy.10165. [DOI] [PubMed] [Google Scholar]
- Takenaka IM, Hightower LE. Regulation of chicken Hsp70 and Hsp90 family gene expression by transforming growth factor-beta 1. J. Cell Physiol. 1993;155(1):54–62. doi: 10.1002/jcp.1041550108. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Del Rio-Tsonis K. Lens and retina regeneration: transdifferentiation, stem cells and clinical applications. Exp. Eye Res. 2004;78(2):161–172. doi: 10.1016/j.exer.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Vergara MN, Spence JR, Madhavan M, Kramer EL, Call MK, Santiago WG, Vallance JE, Robbins DJ, Del Rio-Tsonis K. A novel role of the hedgehog pathway in lens regeneration. Dev. Biol. 2004;267(2):450–461. doi: 10.1016/j.ydbio.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Venkateswaran S, Blanckaert V, Schelling M. Production of anti-fibroblast growth factor receptor monoclonal antibodies by in vitro immunization. Hybridoma. 1992;11(6):729–739. doi: 10.1089/hyb.1992.11.729. [DOI] [PubMed] [Google Scholar]
- Vergara MN, Arsenijevic Y, Del Rio-Tsonis K. CNS regeneration: a morphogen's tale. J. Neurobiol. 2005;64(4):491–507. doi: 10.1002/neu.20158. [DOI] [PubMed] [Google Scholar]
- Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr. Biol. 1999;9(8):445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dakubo GD, Thurig S, Mazerolle CJ, Wallace VA. Retinal ganglion cell-derived sonic hedgehog locally controls proliferation and the timing of RGC development in the embryonic mouse retina. Development. 2005;132(22):5103–5113. doi: 10.1242/dev.02096. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development. 1998;125(18):3615–3623. doi: 10.1242/dev.125.18.3615. [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22(1):103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Willbold E, Layer PG. A hidden retinal regenerative capacity from the chick ciliary margin is reactivated in vitro, that is accompanied by down-regulation of butyrylcholinesterase. European J. Neur. 1992;4:210–220. doi: 10.1111/j.1460-9568.1992.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Xie J, Aszterbaum M, Zhang X, Bonifas JM, Zachary C, Epstein E, McCormick F. A role of PDGFRalpha in basal cell carcinoma proliferation. Proc. Natl. Acad. Sc. USA. 2001;98(16):9255–9259. doi: 10.1073/pnas.151173398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wonders CP, Anderson SA. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development. 2005;132(22):4987–4998. doi: 10.1242/dev.02090. [DOI] [PubMed] [Google Scholar]
- Yu C, Mazerolle CJ, Thurig S, Wang Y, Pacal M, Bremner R, Wallace VA. Direct and indirect effects of hedgehog pathway activation in the mammalian retina. Mol Cell Neurosci. 2006;32(3):274–282. doi: 10.1016/j.mcn.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Zhao S, Chen Q, Hung FC, Overbeek PA. BMP signaling is required for development of the ciliary body. Development. 2002;129(19):4435–4442. doi: 10.1242/dev.129.19.4435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.