Abstract
We have previously demonstrated that splenic B cells, transduced with peptide-IgG fusion proteins, are efficient tolerogenic APCs in vivo. Specific hyporesponsiveness to epitopes encoded in the peptide-IgG fusion protein has been achieved to over one dozen Ags, and clinical efficacy has been established in animal models for several autoimmune diseases and hemophilia. Previous studies also demonstrated that tolerance in this system requires MHC class II expression by the transduced B cells. Yet, the mechanisms of this B cell tolerogenic processing pathway remain unclear. In this study, we show that MHC class II molecules on tolerogenic B cells present epitopes derived from endogenous, but not exogenous (secreted), peptide-IgG fusion protein. These class II epitopes from the IgG fusion protein are processed in lysosomes/endosomes in an IFN-γ-inducible lysosomal thiol reductase-dependent manner. We suggest that the MHC class II presentation of endogenously produced fusion protein epitopes represents a novel mechanism for tolerance induced by peptide-IgG-transduced B cells. An understanding of this process might provide insights into central and peripheral tolerance induced by other professional and nonprofessional APCs.
The induction of both central and peripheral tolerance to control autoreactive T cells requires the expression of self Ag:MHC complexes on professional and/or nonprofessional APCs (1, 2). We have previously shown that splenic B cells, retrovirally transduced with a gene encoding a target Ag and IgG H chain fusion protein (peptide-IgG), are highly tolerogenic in vivo for the IgG-associated Ag (3–5). This in vivo tolerance-inducing system has shown efficacy in multiple animal models of autoimmune diseases, such as experimental autoimmune uveitis, diabetes, and experimental autoimmune encephalomyelitis, and in a mouse model for hemophilia (6–13). Further studies demonstrated that the expression of the MHC class II and B7 costimulatory molecules on the transduced (tolerogenic) B cells is required for tolerance induction (14, 15). Taken together, these results indicate that peptide-IgG B cells function as a tolerogenic APC to present target Ags directly on MHC class II.
Classically, endogenous and exogenous self or nonself Ags are degraded via proteasomes and lysosomes, and presented by APCs via MHC class I and class II molecules, respectively (16–18). Although they primarily present exogenous peptides, MHC class II molecules can also present endogenously derived peptides to CD4+ T cells. For example, measles and influenza A viral Ags are the earliest examples of cytosolic proteins being intracellularly processed and then presented by MHC class II molecules in a proteasome-independent manner (19, 20). Subsequent biochemical and genetic studies have indicated that endogenously derived peptides, such as cytoplasmic and nuclear Ags, account for up to 20% MHC class II epitopes (21–23).
Recent studies also have demonstrated that a broad variety of cytosolic viral, tumor, or self Ags is endogenously processed and then presented by MHC class II molecules in a number of cell lines, such as macrophages, B cells, dendritic cells, and epithelial cells (24–31). More importantly, MHC class II-restricted CD4+ T cell recognition of endogenously derived epitopes is altered by the manipulation of the formation of autophagosomes or the expression of lysosome-associated membrane protein (Lamp)3-2a and its ligand chaperone protein, the heat shock cognate protein (hsc) 70 (29, 30, 32). Based on these in vitro studies, two types of autophagy pathways, macroautophagy and chaperone-mediated autophagy, have been proposed as the mechanism for the delivery of intracellular proteins for MHC class II Ag presentation (33, 34). However, the molecular mechanisms of these endocytic pathways are largely unknown. In addition, due to the lack of an ideal in vivo model, the contribution of MHC class II presentation of endogenous Ags to immunity, particularly tolerance, remains unclear.
In this study, we have focused on the intracellular processing of peptide-IgG by tolerogenic B cells. Our results demonstrate that endogenously produced peptide-IgG is processed by tolerogenic B cells in the lysosomes/endosomes in an IFN-γ-inducible lysosomal thiol reductase (GILT)-dependent manner. This lysosomal processing of peptide-IgG then generates tolerogenic peptide:MHC class II complexes on B cells, most likely via chaperone-mediated autophagy. This intracellular processing pathway for endogenous peptide-IgG might represent a novel mechanism for the expression of self Ag:MHC class II complexes by APCs. In addition, our findings allow us potentially to improve this tolerance-inducing system for future clinical applications.
Materials and Methods
Mice
C57BL/6 and BALB/c mice were purchased from The Jackson Laboratory. GILT knockout (KO) mice were a gift from P. Cresswell (Yale University, New Haven, CT) (35) and have been backcrossed for at least 10 generations. All recipient animals were used at 3–6 wk of age and housed in pathogen-free microisolator cages in the University of Maryland animal facility.
Abs and peptides
The Y-Ae mAb, which recognizes Eα52–68:I-Ab peptide MHC complexes, is a gift from M. Jenkins (University of Minnesota Medical School; Minneapolis, MN). FITC anti-mouse Lamp-2 (CD107b) and PE anti-mouse CD19 mAbs were purchased from BD Pharmingen. The anti-EBV nuclear Ag 1 mAb 5F12 is a gift from M.-R. Chen (National Taiwan University; Taipei, Taiwan (ROC)) and C. Munz (Rockefeller University; New York, NY) (36). FITC anti-human Lamp-1 was purchased from eBioscience. Hen egg lysozyme (HEL) protein was purchased from Sigma-Aldrich. The Eα52–68 peptide, as well as peptides p12–26 and p73–88 of λ CI repressor protein (p1–102 domain) were synthesized at New England Peptide.
Virus-producing cell lines
The peptide-IgG1 H chain cDNA was subcloned into the murine Moloney leukemia retroviral vector (MBAE), as previously described (4, 5). Briefly, a construct Δss 1–102-IgG containing 1–102 fused with the IgG1 H chain, but without the signal sequence, was generated by deleting the signal sequence from the BSSK-IgG vector using a site-directed mutagenesis kit (Stratagene). The fusion cDNA constructs encoding Δss 1–102 or Eα red fluorescence protein (RFP) in frame with the IgG1 H chain were subsequently subcloned into the MBAE retroviral vector. Virus-producer cell lines were prepared by lipofection of GP-E86 packaging cells with the engineered constructs. Viral titers of these cell lines were ∼5 × 104 to 106 neomycin-resistant NIH3T3 CFU. These cell lines were cultured in DMEM (Invitrogen Life Technologies) supplemented with 10% FBS, 2 mM l-glutamine, and 2-ME. The cell lines were stably selected and then maintained in 0.6 mg/ml geneticin (Invitrogen Life Technologies).
B cell purification and retroviral transduction
Spleen B cells were purified to ∼95% homogeneity with anti-T cell Ab mixture (anti-Thy1, anti-CD4, and anti-CD8) plus complement (Low Tox M; Cedarlane Laboratories and Accurate Chemical & Scientific). Purified B cells were cultured with RPMI 1640 medium (Invitrogen Life Technologies) supplemented with 5% FBS, 2 mM l-glutamine, and 2-ME. B cells were prestimulated with 5 μg/ml bacterial LPS (Escherichia coli 055:B5; Sigma-Aldrich) overnight. Purified, LPS-stimulated B cells were infected in vitro via coculture with 1500 rad irradiated virus-producing packaging cells for 24 h in the presence of 6 μg/ml polybrene and 5 μg/ml LPS.
Tolerance induction and immune challenge
Normal recipient animals were injected via i.p. with 107 virally transduced B cells. Seven to 10 days following transduced B cell injection, animals were immunized in a hind footpad and the base of tail with 25 μg of indicated protein or peptide emulsified in CFA. Two weeks after immunization, serum samples were collected and assayed by ELISA for Ab titers or concentration. Ab titers were determined by the endpoint dilution method. Serial 3-fold dilutions were made in 2% BSA/PBS. Undiluted mouse serum was used as negative control. The endpoint titer represents the highest dilution of sample with an OD450 reading greater than the negative control. The total IgG concentrations were calculated using a standard curve generated with B3.11, a mAb specific for the 12–26 peptide. Cellular responses from draining popliteal and inguinal lymph nodes (LNs) were also assayed 2 wk after immunization. A total of 5 × 105 LN or splenic cells was seeded per well in 96-well plate in the presence of indicated concentration of Ag. After 48 h, cultures were pulsed with 1 μCi/well [3H]thymidine (Amersham Life Sciences) and incubated for an additional 16–20 h. Cells were then harvested on glass fiber filters, and incorporated [3H]thymidine was detected via gas scintillation counting in a Packard Matrix 9600 reader. Data are expressed as Δcpm (cpm by subtraction of the background (without Ag)). All tolerance induction experiments followed this protocol, unless stated otherwise.
Flow cytometry
Cells were incubated with 2.4G2 mAb (anti-CD16/32) for 15 min to block FcR, and then were stained with indicated Abs for 40 min at 4°C. To detect the Eα:I-Ab epitopes, B cells were stained with PE-CD19 and biotin-Y-Ae, followed with streptavidin-FITC. Propidium iodide buffer was used to gate live cells. To detect the Lamp-2, cells were fixed and permeabilized and then intracellularly stained with FITC anti-Lamp-2. Seminaphtharhodafluor (SNARF) (2 μM; Molecular Probes) was used to label cells for 10 min at 37°C, and then was washed with FBS-containing medium. Cells were analyzed on a FACScan or FACSCalibur flow cytometer (BD Biosciences). Data were analyzed by CellQuest software.
Fluorescence microscopy analysis
C57BL/6 B cells were stimulated with 5 μg/ml LPS overnight and then transduced with irradiated EαRFP-IgG-producing packaging cells or a mock control. After 24-h coculture, cells were collected and centrifuged over Lympholyte M (Cedarlane Laboratories) to remove dead cells. Cells were adhered to glass slides and then fixed with Cytofix/Cytoperm solution (BD Biosciences). Cells were blocked with rat serum and then stained with FITC anti-Lamp-2 (BD Pharmingen) for 40–60 min, followed by three washes in PBS. Finally, cells were mounted with FluorSave reagent (Calbiochem) and covered with cover slides. All steps were conducted at room temperature. Cells were analyzed with a Nikon Eclipse E800 microscope, and pictures were taken with the LaserSharp 2000 (Zeiss) software.
Statistics
Paired or unequal variances one-tailed Student's t test statistics were applied.
Results
Exogenous peptide-IgG is not required for tolerance induction
Use of the peptide-IgG B cell system for tolerance induction in vivo was based on the findings that B cells were highly efficient tolerogenic APCs (37–39) and that the IgG molecules were excellent tolerogenic carriers (3, 40–43). This model system has been shown to be effective with over one dozen Ags, and provided clinical efficacy in three autoimmune disease models and hemophilia (7–10, 12, 13, 44). The use of an IgG cassette in our model allowed us to insert different Ags to ask specific questions (45). We initially designed a secretory peptide-IgG fusion protein that includes a signal sequence for extracellular secretion, the target gene, and the murine IgG1 H chain backbone (Fig. 1A) (3–5). We originally hypothesized that the peptide-IgG protein expressed by transduced B cells assembles with the L chains in the endoplasmic reticulum (ER) of the host B cells and the fully assembled peptide-IgG molecules (H2:L2) are secreted. Secreted peptide-IgG would then be endocytosed and processed by B cells or neighboring APC as an exogenous Ag. However, transduced donor cells from MHC class II KO and B7 KO mice were not able to induce tolerance in vivo, suggesting peptide-IgG B cells tolerize T cells directly (14, 15). Moreover, B cells transduced with an Ig fusion lacking the signal sequence for secretion were still tolerogenic (Fig. 1, B and C). Thus, these results suggest that the secretion of peptide-IgG is not required for tolerance induction and that the peptide-IgG is processed by B cells as an endogenous protein, but not as an exogenous protein.
Figure 1.
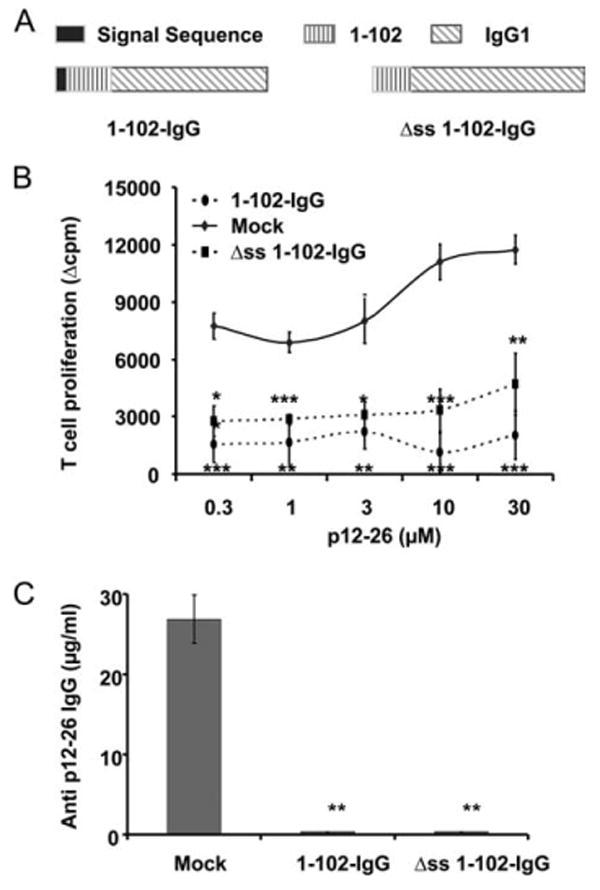
Secretion of peptide-IgG is not required for tolerance induction. A, The 1–102 cDNA was subcloned into the BSSK-IgG1 cassette, and then the 1–102-IgG1 fusion gene was subcloned into the MBAE retroviral vector. The signal sequence of IgG H chain was deleted to generate the Δss 1–102-IgG construct. B, Normal BALB/c recipients were injected i.p. with 107 Δss 1–102-IgG B cells, 1–102-IgG B cells, or an equal number of OVA-IgG B cells (mock control). On day 7 postinjection, animals were immunized in a hind footpad, and the base of tail with 25 μg of 12–26 peptide emulsified in CFA. Two weeks later, animals were sacrificed and T cells were isolated from draining LNs and assayed by [3H]thymidine incorporation. Data represent mean Δcpm ± SE for three or four animals. The background [3H]thymidine incorporation was in the range of 5,000–10,000 counts. This is representative of three independent experiments. ***, p < 0.001; **, p < 0.01; *, p ≤ 0.05. C, Animals were treated as described in B. Sera were collected 2 wk after immunization and analyzed by ELISA for total anti-12–26 IgG. B3.11 Ab was used as standard. This is representative of three independent experiments. **, p < 0.01.
Taken together, these observations indicate that the secretion of peptide-IgG is not required for tolerance induction. This finding led us to modify our original hypothesis to propose that peptide-IgG-expressing B cells function as APCs to directly present endogenously processed peptide-IgG to target T cells and induce tolerance.
Tolerogenic B cells directly present express endogenously derived peptide:MHC class II complexes
We previously demonstrated that MHC class II expression on the transduced B cells was absolutely required for tolerance induction in our system (33). To confirm that B cells directly present endogenously processed peptide and function as tolerogenic APC, we examined the expression of peptide:MHC class II complexes on transduced B cells. Eα is the α subunit of the I-Ed MHC class II molecules, and the Y-Ae mAb specifically recognizes the complexes of immunodominant peptide (residues 58–68) from α subunit of I-Ed presented in the context of I-Ab (that is, Eα p52–68: I-Ab) on APCs (46). The Y-Ae mAb has been used to track the pEα52–68:I-Ab expression by DCs in vivo (47). In this study, we used the Y-Ae mAb to examine the expression of pEα52–68:I-Ab complexes on tolerogenic B cells. First, to examine the expression of the pEα52–68:I-Ab on EαRFP-IgG B cells, we generated a construct (EαRFP-IgG) encoding the fusion protein of the pEα45–75 fragment, RFP, and the IgG H chain in the presence of the signal sequence. After 24-h incubation with irradiated EαRFP-IgG-producing packaging cells, B cells were collected and stained with the Y-Ae mAb. Compared with untransduced, LPS-activated B cells or a mock control (HEL-IgG) transduced B cells, a significant population (>20%) of EαRFP-IgG B cells is Y-Ae positive (Fig. 2A), suggesting that EαRFP-IgG fusion protein is processed and presented by B cells.
Figure 2.
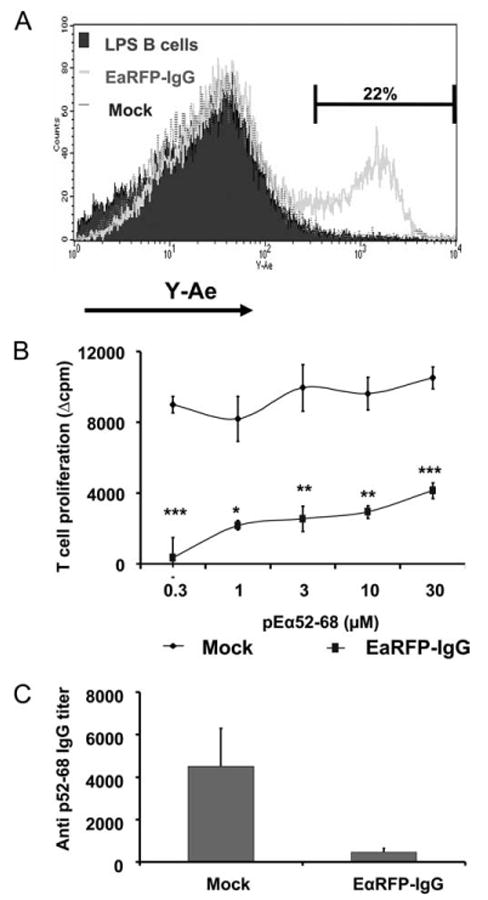
EαRFP-IgG-transduced B cells express Eα:I-Ab complexes. A, C57BL/6 B cells were stimulated with LPS for 24 h and then transduced with either EαRFP-IgG or HEL-IgG as a mock control. After 24-h coculture with irradiated packaging cells, B cells were collected and centrifuged over Lympholyte M to remove dead cells, and then stained with PE-CD19 and biotin-Y-Ae, followed by FITC-streptavidin. The expression of Y-Ae epitopes in CD19+ cells is shown. Nontransduced LPS B cells are depicted by the solid histogram; EαRFP-IgG-transduced B cells by the thick line; OVA-IgG-transduced B cells by the dotted line. This pattern is representative of four independent experiments. B, LPS-activated C57BL/6 B cells were transduced with EαRFP-IgG or OVA-IgG as a mock control. Normal C57BL/6 recipients were injected i.p. with 107 transduced B cells. On day 7 postinjection, animals were immunized in a hind footpad and the base of tail with 25 μg of pEα52–68 emulsified in CFA. Two weeks postimmunization, animals were sacrificed and draining LNs were removed. T cell proliferation was assayed by [3H]thymidine incorporation. Data represent mean Δcpm ± SE for three or five animals. The background [3H]thymidine incorporation was in the range of 3000–8000 counts. This is representative of three independent experiments. ***, p < 0.001; **, p < 0.01; *, p ≤ 0.05. C, Animals were treated as described in B. Sera were collected 2 wk after immunization and analyzed by ELISA for total p52–68 IgG. This is representative of three independent experiments.
Second, we tested tolerance to pEα52–68 induced by this construct expressed in B cells. As shown in Fig. 2B, mice that received EαRFP-IgG B cells exhibited significantly reduced T cell responsiveness to pEα52–68 compared with that of control animals. The Ab response to pEα52–68 was also reduced in animals receiving EαRFP-IgG B cells (Fig. 2C). These results indicate that the EαRFP-IgG-transduced B cells are tolerogenic for the peptide expressed endogenously by these cells.
To further examine the source of pEα52–68 (endogenous or exogenous EαRFP-IgG) that is presented by I-Ab molecules, we incubated untransduced B cells with EαRFP-IgG-transduced B cells and then examined the expression of Y-Ae epitopes on untransduced B cells. Untransduced B cells (as control) and EαRFP-IgG- or mock-transduced B cells were labeled with a red fluorescent intracellular dye SNARF after viral transduction. These three groups of SNARF+ B cells were then mixed in a 1:1 ratio with LPS-activated, untransduced B cells (SNARF−). After 24 h, cells were collected and stained with the Y-Ae mAb and analyzed by flow cytometry. SNARF+ (transduced) and SNARF− (untransduced) cells were gated, respectively. As shown in Fig. 3A, EαRFP-IgG B cells (SNARF+) expressed a high level of Y-Ae epitopes compared with the controls (untransduced or mock). However, SNARF− cells in all three groups only exhibited similar background levels of Y-Ae expression (Fig. 3A). The percentage of Y-Ae+ population in SNARF+ cells and the mean fluorescence intensity are listed in Fig. 3B. These results suggest that the pEα52–68:I-Ab complexes expressed on tolerogenic B cell surface are derived from an endogenous source, but not from an exogenous source (secretion and re-presentation by transduced B cells).
Figure 3.
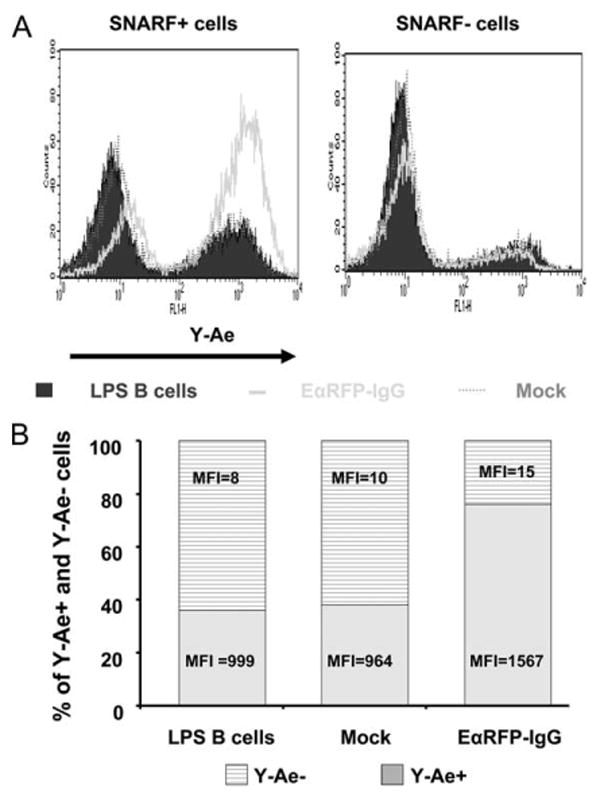
The Eα52–68:I-Ab complexes are derived from endogenous, but not exogenous, EαRFP-IgG. A, C57BL/6 B cells were stimulated with LPS for 24 h and then transduced with either EαRFP-IgG or HEL-IgG as a mock control. After 24-h coculture with irradiated packaging cells, B cells were collected and centrifuged over Lympholyte M to remove dead cells and then labeled with an intracellular dye SNARF (5 μM). SNARF-labeled untransduced LPS blasts, HEL-IgG, or EαRFP-IgG B cells were mixed with unlabeled, untransduced LPS B cells at 1:1 ratio. A total of 107 mixed cells was cultured in 5 ml of medium in 6-well plate. After 24 h, cells were collected and stained with biotin-Y-Ae mAb, followed by FITC-streptavidin. SNARF+ and SNARF− cells were gated and presented. Non-transduced LPS B cells are depicted by the solid histogram; HEL-IgG-transduced B cells by the dotted line; EαRFP-IgG-transduced B cells by the thick line. B, The percentages and mean fluorescence intensity (MFI) of Y-Ae+ and Y-Ae− cells in SNARF+ cells are indicated.
Lastly, to confirm that an exogenous source of peptide-IgG (secretion and re-presentation) does not contribute to generation of tolerogenic B cells, we transduced MHC class II KO B cells with a peptide (p1–102)-IgG construct that contains the signal sequence for extracellular secretion and is known to be secreted (45). We mixed these p1–102-IgG MHC class II KO B cells with activated, but nontransduced wild-type (WT) B cells for 24 h in vitro. The mixtures were then injected into WT recipients as donor B cells. If the transduced (MHC II KO) B cells secreted sufficient fusion Ig to be cross-presented by bystander (WT, MHC-sufficient) B cells, then tolerance would be observed. When the recipient animals were immunized with an immunodominant peptide from the 1–102 protein (p73–88), no tolerance was observed in terms of both cellular and humoral responsiveness (Fig. 4). Hence, even if transduced B cells produce and secrete peptide-IgG, this pathway does not contribute significantly to generation of pEα52–68:I-Ab complexes and to induction of tolerance. Rather, we conclude that tolerogenic B cells are generated by endocytic processing of endogenous peptide-IgG to directly present epitopes in a tolerogenic manner.
Figure 4.
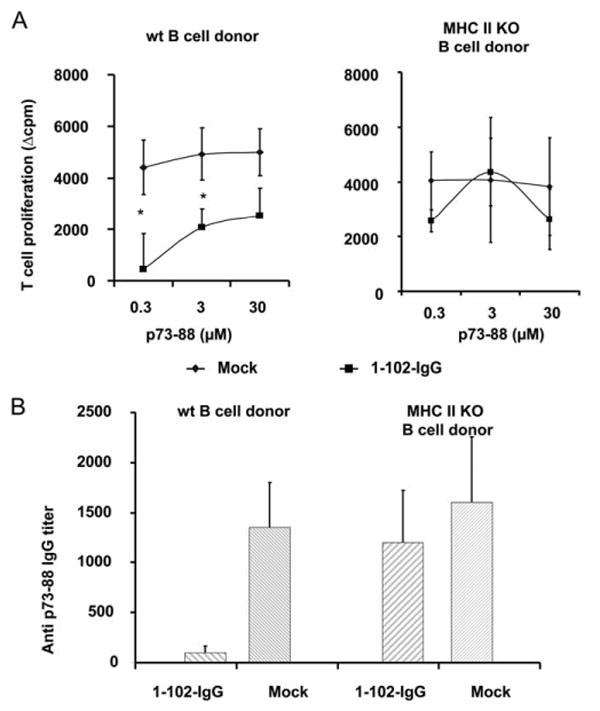
Tolerance is not induced by processing exogenous peptide-IgG. A, LPS-activated MHC class II KO or WT C57BL/6 B cells were transduced with 1–102-IgG or OVA-IgG as a mock control. After 24-h coculture with viral producing packaging cells, transduced B cells were collected and mixed at a 1:1 ratio with untransduced, LPS-activated B cells for another 24 h. A total of 107 mixed B cells was injected into normal C57BL/6 animals. On day 7 postinjection, animals were immunized in a hind footpad and the base of tail with 25 μg of p73–88 emulsified in CFA. Two weeks postimmunization, animals were sacrificed and draining LNs were removed. T cell proliferation to p73–88 was determined by [3H]thymidine incorporation. Left panel, Represents the mixture of transduced and untransduced WT C57BL/6 B cells as donors, whereas the right panel represents the mixture of transduced MHC class II KO and untransduced WT C57BL/6 B cells as donors. Values represent mean Δcpm ± SE for four animals. The background [3H]thymidine incorporation was in the range of 4,000–13,000 counts. *, p < 0.05. B, Animals were treated as described in A. Two weeks postimmunization, sera were assayed by the endpoint ELISA method. The Ab titer was determined by the highest dilution of samples, with an OD450 greater than the negative control (undiluted mouse serum). The result is shown as the mean ± SE of Ab titers.
Peptide-IgG localizes to late endosomes and lysosomes in tolerogenic B cells
Lamp-2a can mediate the delivery of cytosolic proteins to lysosomes through its ligand, chaperone protein hsc70; overexpression of human Lamp-2a in Chinese hamster ovary cells can upregulate this selective lysosomal proteolytic pathway (48, 49). This pathway is referred to as the chaperone-mediated autophagy. Zhou et al. (30) found that manipulation of Lamp-2a or hsc70 in a human B cell line could alter the MHC class II presentation of a cytoplasmic Ag glutamate decarboxylase, suggesting that chaperone-mediated autophagy could mediate the delivery of cytosolic proteins to endocytic compartments for subsequent MHC class II Ag presentation. In lieu of anti-Lamp-2a Ab, we used anti-Lamp-2 to examine the role of Lamp-2 proteins in sorting of peptide-IgG. First, we found that both resting and LPS-activated C57BL/6 (H2b haplotype) B cells express Lamp-2 (Fig. 5A), as well as in BALB/c (H2d haplotype) or B10.A (H2k/d haplotype) B cells (data not shown). To determine whether peptide-IgG is delivered to the late endosomes/lysosomes in tolerogenic B cells, a colocalization assay was performed. EαRFP-IgG B cells were stained with anti-Lamp-2, and the colocalization between Lamp-2 and RFP was examined under confocal fluorescence microscopy. As shown in Fig. 5B, EαRFP-IgG fusion protein detected by RFP fluorescence was colocalized with Lamp-2 in transduced B cells, demonstrating that the peptide-IgG fusion protein is delivered to the lysosomes/endosomes.
Figure 5.
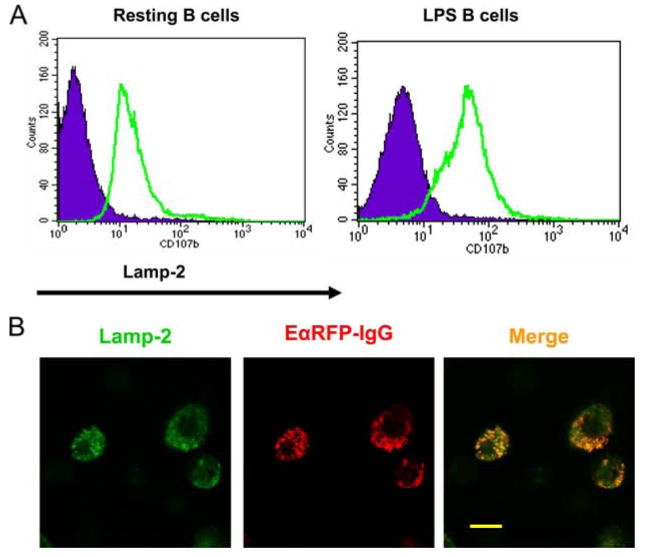
RFP is colocalized with Lamp-2 in EαRFP-IgG-transduced B cells. A, C57BL/6 splenic B cells were stimulated with LPS for 48 h. Cells were fixed, permeabilized, and then stained with FITC anti-Lamp-2 mAb (rat IgG2a). Isotype staining is depicted by the solid histogram, and Lamp-2 by the thick line. Data are representative of two independent experiments. B, LPS-activated C57BL/6 B cells were transduced with EαRFP-IgG or a mock control. After 24 h, cells were collected and centrifuged over Lympholyte M to remove dead cells. Cells were adhered to poly(l-lysine)-treated glass slides. Fixed and permeabilized cells were stained with FITC anti-Lamp-2 and analyzed by confocal fluorescence microscopy. Scale bars represent 10 μM. Representative fields from one experiment of two are shown.
The endogenous peptide-IgG is processed in the lysosomes/endosomes in a GILT-dependent manner
MHC class II epitopes are processed in the lysosomes. To further study how the endogenous peptide-IgG may be processed in the lysosomes, we used GILT KO B cells as donors to induce tolerance to two-model disulfide bond-containing or disulfide-free Ags, namely, HEL and p1–102, respectively. GILT is an enzyme that is constitutively expressed in the late endocytic compartments, such as lysosomes and mature endosomes of APCs in both human and mouse (35, 50). GILT functions in the reduction of inter- and intrachain disulfide bonds and facilitates the processing of disulfide bond-containing proteins. Thus, GILT KO mice have been shown to be defective in processing and presenting the HEL, which has four disulfide bonds (35). To determine whether GILT mediates the processing of disulfide bond-containing peptide-IgG in our gene therapy model, we transduced GILT KO or syngeneic WT C57BL/6 B cells with HEL-IgG (or a mock control; both have the signal sequence) and then transferred these B cells into normal C57BL/6 recipients. One week later, the recipients were immunized with HEL. As shown in Fig. 6A, mice that received HEL-IgG WT B cells exhibited a significantly reduced T cell responsiveness to HEL protein compared with that of control mice. In contrast, HEL-IgG GILT KO B cells failed to induce T cell tolerance to HEL protein (Fig. 6A). Furthermore, as shown in Fig. 6B, HEL-IgG WT B cells, but not GILT KO B cells, induced significantly lower primary Ab response against HEL protein. These data demonstrate that GILT expression is required for peptide-IgG processing and subsequent presentation on MHC class II molecules by tolerogenic B cells, suggesting that endogenously produced peptide-IgG is processed in the late endocytic compartments.
Figure 6.
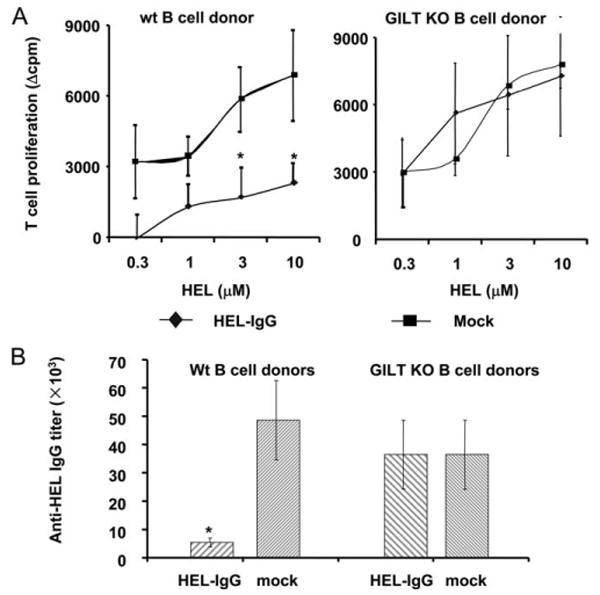
Transduced GILT KO B cells could not induce tolerance to disulfide bond-containing Ag HEL. A, LPS-activated GILT KO or WT C57BL/6 B cells were transduced with HEL-IgG or OVA-IgG as a mock control. Normal C57BL/6 recipients were injected i.p. with 107 transduced B cells. On day 7 postinjection, animals were immunized in a hind footpad and the base of tail with 25 μg of HEL protein emulsified in CFA. Two weeks postimmunization, animals were sacrificed and draining LNs were removed. T cell proliferation to HEL protein was determined by [3H]thymidine incorporation. Values represent mean Δcpm ± SE for four animals. The background [3H]thymidine incorporation was in the range of 13,000–20,000. Data are representative of four independent experiments. *, p < 0.05. B, Animals were treated as described in Fig. 6A. Two weeks postimmunization, sera were assayed by the endpoint ELISA method. The Ab titer was determined by the highest dilution of samples, with an OD450 greater than the negative control (undiluted mouse serum). The result is shown as the mean ± SE of Ab titers. Data are representative of four independent experiments.
In contrast, when this experiment was repeated with GILT KO or WT B cells transduced with disulfide bond-free Ag p1–102-IgG, tolerance was induced by both types of B cells. As shown in Fig. 7, mice that received p1–102-IgG WT C57BL/6 or GILT KO B cells significantly reduced T cell (Fig. 7A) and Ab (Fig. 7B) responses to the immunodominant H-2b epitope, p73–88, compared with those of control animals. These data demonstrate that GILT KO B cells could process and present Ag (disulfide bond free) and induce tolerance as efficiently as normal B cells. Therefore, disulfide bond processing by GILT appears to be a critical event for tolerance induction by transduced B cells and confirms that endogenous Ags undergo lysosomal/endosomal processing.
Figure 7.
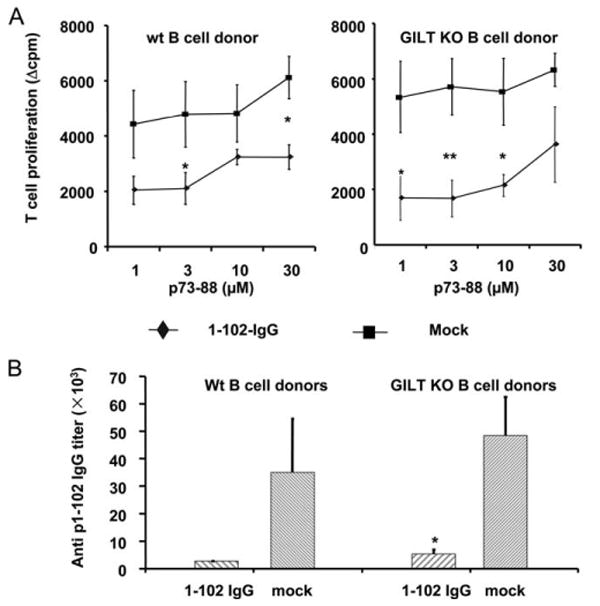
Transduced GILT KO B cells induce tolerance to disulfide bond-free Ag p1–102. A, LPS-activated GILT KO or WT C57BL/6 B cells were transduced with 1–102-IgG or OVA-IgG as a mock control. Normal C57BL/6 recipients were injected i.p. with 107 transduced B cells. On day 7 postinjection, animals were immunized in a hind footpad and the base of tail with 25 μg of p73–88 emulsified in CFA. Two weeks postimmunization, animals were sacrificed and draining LNs were removed. T cell proliferation to p73–88 was determined by [3H]thymidine incorporation. Values represent mean Δcpm ± SE for four animals. The background [3H]thymidine incorporation was in the range of 4,000–13,000. Data are representative of three independent experiments. **, p < 0.01; *, p < 0.05. B, Animals were treated as described in Fig. 7A. Two weeks postimmunization, sera were assayed by the endpoint ELISA method. The Ab titer was determined by the highest dilution of samples, with an OD450 greater than the negative control (undiluted mouse serum). The result is shown as the mean ± SE of Ab titers. Data are representative of three independent experiments.
Discussion
In this study, we show that endogenously produced peptide-IgG is processed by transduced splenic B cells in the lysosomes/endosomes in a GILT-dependent manner, and this endocytic processing pathway is essential for generating tolerogenic B cells. Our demonstration that peptide:MHC II complexes are exhibited on transduced B cells directly supports the hypothesis that transduced B cells are tolerogenic APCs.
The requirement of MHC class II and B7 molecules on tolerogenic bone marrow or B cells for tolerance induction suggests that peptide-IgG might be directly processed and presented as endogenous protein to target T cells (12, 14, 15). Nonetheless, with the current constructs, a secretory pathway cannot be ruled out as a mechanism of tolerance induction. If the expression level of peptide-IgG molecules is low, these secreted peptide-IgG molecules might not be captured by host APCs. Hence, even though the Fc/FcR interaction is not required for tolerance induction (51), transduced B cells might uptake freshly secreted peptide-IgG molecules by endocytosis or other unknown mechanisms and then re-present these molecules as exogenous Ag.
Our accumulated data, however, indicate that the secretion of peptide-IgG is not required for generating tolerogenic B cells. More importantly, the direct demonstration of pEα52–68:I-Ab epitopes on EαRFP-IgG-transduced B cells indicates that tolerogenic B cells process endogenous peptide-IgG and directly present peptide on MHC class II molecules. The pEα52–68 epitopes on tolerogenic B cell surface are not derived from an exogenous source. As a result, the secretion and/or re-presentation pathway does not contribute significantly to generation of the tolerogenic B cells. Together, these findings clearly suggest that tolerogenic B cells are most likely generated through intracellular processing of endogenously produced peptide-IgG. Moreover, the epitope:MHC class II complexes are essential for inducing tolerance. Indeed, in vitro treatment with the Y-Ae mAb completely abrogates the tolerogenicity of EαRFP-IgG B cells (data not shown).
GILT mediates the processing of endocytosed disulfide bond-containing Ags in lysosomes for MHC class II Ag presentation (35, 52–55). Thus, GILT KO APCs are deficient in presenting exogenous disulfide bonds containing protein HEL (35). In this study, we used GILT KO B cells to evaluate the role of lysosomal/endosomal processing of peptide-IgG in B cell-delivered tolerance induction. GILT KO B cells exhibited same efficacy as normal B cells in inducing tolerance to cysteine-free Ag, the λ cI protein p1–102, suggesting that the deficiency of GILT does not alter the normal Ag-presenting ability if the Ag does not contain any disulfide bonds. In addition, the reduction and unfolding of the IgG H chain carrier in lysosomes are independent of processing of the cargo Ag. However, GILT KO B cells failed to induce tolerance to disulfide bond-containing Ag, HEL, indicating that GILT plays a critical role in processing endogenous HEL-IgG. Because GILT is mainly expressed in lysosomes/endosomes, these results suggest that the intracellularly produced peptide-IgG is proteolytically processed in the lysosomal/endosomal compartments.
Even though the mechanisms of the sorting of cytosolic proteins to endocytic pathway for MHC class II presentation are not fully understood, two autophagy pathways (macroautophagy and chaperone-mediated autophagy) play a role in this intracellular delivery. Paludan et al. (32) reported that macroautophagy mediates the delivery of endogenous EBV nuclear Ag 1 for MHC class II presentation. In another study, Nimmerjahn et al. (27) found that autophagy mediates the delivery of a cytosolic protein neomycin phosphotransferase II for MHC class II presentation in an EBV-transformed B cell line, and that the inhibition of autophagy also altered specific T cell recognition. Unlike macroautophagy, which is a nonselective process to deliver cytosolic proteins to lysosomes or endosomes (56), chaperone-mediated autophagy is a selective process mediated through the interaction between the chaperone protein hsc70 and its receptor Lamp-2a (48). It was found that manipulation of the expression of Lamp-2a or hsc70 in a human B cell line could alter the MHC II-restricted CD4+ T cell recognition of a cytosolic protein glutamate decarboxylase, suggesting that chaperone-mediated autophagy is involved in delivery of cytosolic proteins to endocytic pathway of MHC class II presentation (30). However, other mechanisms might exist. In our system, colocalization between the peptide-IgG fusion protein and Lamp-2 in tolerogenic B cells indicates the sorting of peptide-IgG to the lysosomes/endosomes. More studies are required to clarify the mechanism(s) of intracellular delivery of peptide-IgG to late endocytic pathway.
The IgG H chain was initially used in this system because of its long t1/2 and its ability to interact with APCs via FcR and also to disseminate between the vasculature, although we now know that these functions of secreted IgG fusions are not necessary in our model. In the absence of IgG carrier, retrovirally transduced B cells could still induce tolerance to target Ag, but the degree and persistence of tolerance are lower than that induced by the peptide-IgG construct. Recently, we found that proper assembly of peptide-IgG with host L chains to form a whole Ig molecule in the ER is required for generating tolerogenic B cells (Y. Su and D. Scott, manuscript in preparation). Mutations in the hinge region of the IgG H chain carrier, which prevent its assembly with L chain, completely abrogate the tolerogenicity of peptide-IgG. Even though the molecular events that regulate the association of peptide-IgG and Lamp-2a in tolerogenic B cells are unclear, we propose that in tolerogenic B cells, endogenous Ag (without IgG H chain) and peptide-IgG are delivered to endocytic pathway via the same sorting machinery. The difference is that the peptide-IgG fusion protein has to undergo assembly in the ER. In addition, the peptide-IgG fusion protein might have a longer t1/2 in tolerogenic B cells than that of peptide alone. It was found that some long-lived cytosolic proteins could be degraded in lysosomes/endosomes, whereas short-lived proteins are degraded in the proteasomes (31). Hence, in our model, the IgG H chain carrier might promote tolerance induction by preventing associated cargo Ags from being rapidly degraded.
T cell activation requires the MHC recognition and B7 costimulation. In the absence of costimulatory molecules, APCs will induce T cell anergy. It is generally accepted that resting B cells act as tolerogenic APCs because they do not express high levels of costimulatory molecules, that is they lack signal 2 (38, 39). However, in our model, B cells have to be activated for the purpose of retroviral transduction. Our previous findings demonstrated that the expression of B7, particularly B7.2, by tolerogenic B cells is required for tolerance induction (15). In addition, in vivo anti-CTLA-4 treatment can interrupt the tolerogenicity of peptide-IgG B cells, suggesting B7 costimulatory molecules selectively interact with the inhibitory receptor CTLA-4 on helper or regulatory T cells (14). Taken together, we propose a model by which tolerogenic B cells induce peripheral T cell unresponsiveness: mitogen treatment stimulates tolerogenic B cells to express high-level B7 molecules that interact with CTLA-4 receptor on target T cells (perhaps regulatory T cells). At the same time, constitutively produced peptide-IgG undergoes endocytic processing pathway for generating peptide:MHC II complexes on the surface. With these two signals, target T cells are tolerized or regulatory T cells are activated or induced. Once induced, regulatory T cells can be maintained with basal B7 expression and constitutive expression of signal 1. The lysosomal processing of endogenous self-peptide-IgG is critical for generating the tolerogenic epitopes, and this may represent an important mechanism by which professional and nonprofessional APCs shape the T cell repertoire during central and peripheral tolerance.
Acknowledgments
We thank Damaris Lopez and Irina Mikhailenko for technical assistance, and Ai-Hong Zhang, Jonathan Skupsky, and Mark Saltis for critical reading of the manuscript.
Footnotes
This work was supported by a grant from National Institutes of Health (R01 AI035622-10).
Abbreviations used in this paper: Lamp, lysosome-associated membrane protein; ER, endoplasmic reticulum; GILT, IFN-γ-inducible lysosomal thiol reductase; HEL, hen egg lysozyme; hsc, heat shock cognate protein; KO, knockout; LN, lymph node; RFP, red fluorescence protein; WT, wild type; SNARF, seminaphtharhodafluor.
Disclosures: The authors have no financial conflict of interest.
References
- 1.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 2.Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 3.Zambidis ET, Scott DW. Epitope-specific tolerance induction with an engineered immunoglobulin. Proc Natl Acad Sci USA. 1996;93:5019–5024. doi: 10.1073/pnas.93.10.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zambidis ET, Kurup A, Scott DW. Genetically transferred central and peripheral immune tolerance via retroviral-mediated expression of immunogenic epitopes in hematopoietic progenitors or peripheral B lymphocytes. Mol Med. 1997;3:212–224. [PMC free article] [PubMed] [Google Scholar]
- 5.Zambidis ET, Barth RK, Scott DW. Both resting and activated B lymphocytes expressing engineered peptide-Ig molecules serve as highly efficient tolerogenic vehicles in immunocompetent adult recipients. J Immunol. 1997;158:2174–2182. [PubMed] [Google Scholar]
- 6.Melo ME, Qian J, El-Amine M, Agarwal RK, Soukhareva N, Kang Y, Scott DW. Gene transfer of Ig-fusion proteins into B cells prevents and treats autoimmune diseases. J Immunol. 2002;168:4788–4795. doi: 10.4049/jimmunol.168.9.4788. [DOI] [PubMed] [Google Scholar]
- 7.Xu B, Scott DW. A novel retroviral gene therapy approach to inhibit specific antibody production and suppress experimental autoimmune encephalomyelitis induced by MOG and MBP. Clin Immunol. 2004;111:47–52. doi: 10.1016/j.clim.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Xu B, Haviernik P, Wolfraim LA, Bunting KD, Scott DW. Bone marrow transplantation combined with gene therapy to induce antigen-specific tolerance and ameliorate EAE. Mol Ther. 2006;13:42–48. doi: 10.1016/j.ymthe.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Song L, Wang J, Wang R, Yu M, Sun Y, Han G, Li Y, Qian J, Scott DW, Kang Y, et al. Retroviral delivery of GAD-IgG fusion construct induces tolerance and modulates diabetes: a role for CD4+ regulatory T cells and TGF-β? Gene Ther. 2004;11:1487–1496. doi: 10.1038/sj.gt.3302327. [DOI] [PubMed] [Google Scholar]
- 10.Lei TC, Scott DW. Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood. 2005;105:4865–4870. doi: 10.1182/blood-2004-11-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang W, Karabekian Z, Xu Q, Viley AM, Scott DW. B-cell delivered gene transfer of human S-Ag-Ig fusion protein protects from experimental autoimmune uveitis. Clin Immunol. 2006;118:35–41. doi: 10.1016/j.clim.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Soukhareva N, Jiang Y, Scott DW. Treatment of diabetes in NOD mice by gene transfer of Ig-fusion proteins into B cells: role of T regulatory cells. Cell Immunol. 2006;240:41–46. doi: 10.1016/j.cellimm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal RK, Kang Y, Zambidis E, Scott DW, Chan CC, Caspi RR. Retroviral gene therapy with an immunoglobulin-antigen fusion construct protects from experimental autoimmune uveitis. J Clin Invest. 2000;106:245–252. doi: 10.1172/JCI9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Amine M, Melo M, Kang Y, Nguyen H, Qian J, Scott DW. Mechanisms of tolerance induction by a gene-transferred peptide-IgG fusion protein expressed in B lineage cells. J Immunol. 2000;165:5631–5636. doi: 10.4049/jimmunol.165.10.5631. [DOI] [PubMed] [Google Scholar]
- 15.Litzinger MT, Su Y, Lei TC, Soukhareva N, Scott DW. Mechanisms of gene therapy for tolerance: B7 signaling is required for peptide-IgG gene-transferred tolerance induction. J Immunol. 2005;175:780–787. doi: 10.4049/jimmunol.175.2.780. [DOI] [PubMed] [Google Scholar]
- 16.Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 17.Li P, Gregg JL, Wang N, Zhou D, O'Donnell P, Blum JS, Crotzer VL. Compartmentalization of class II antigen presentation: contribution of cytoplasmic and endosomal processing. Immunol Rev. 2005;207:206–217. doi: 10.1111/j.0105-2896.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 18.Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 19.Jaraquemada D, Marti M, Long EO. An endogenous processing pathway in vaccinia virus-infected cells for presentation of cytoplasmic antigens to class II-restricted T cells. J Exp Med. 1990;172:947–954. doi: 10.1084/jem.172.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson S, Sekaly RP, Jacobson CL, McFarland HF, Long EO. HLA class II-restricted presentation of cytoplasmic measles virus antigens to cytotoxic T cells. J Virol. 1989;63:1756–1762. doi: 10.1128/jvi.63.4.1756-1762.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dongre AR, Kovats S, deRoos P, McCormack AL, Nakagawa T, Paharkova-Vatchkova V, Eng J, Caldwell H, Yates JR, III, Rudensky AY. In vivo MHC class II presentation of cytosolic proteins revealed by rapid automated tandem mass spectrometry and functional analyses. Eur J Immunol. 2001;31:1485–1494. doi: 10.1002/1521-4141(200105)31:5<1485::AID-IMMU1485>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 22.Rudensky A, Preston-Hurlburt P, Hong SC, Barlow A, Janeway CA., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;353:622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- 23.Chicz RM, Urban RG, Gorga JC, Vignali DA, Lane WS, Strominger JL. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brazil MI, Weiss S, Stockinger B. Excessive degradation of intracellular protein in macrophages prevents presentation in the context of major histocompatibility complex class II molecules. Eur J Immunol. 1997;27:1506–1514. doi: 10.1002/eji.1830270629. [DOI] [PubMed] [Google Scholar]
- 25.Dorfel D, Appel S, Grunebach F, Weck MM, Muller MR, Heine A, Brossart P. Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro-transcribed MUC1 RNA. Blood. 2005;105:3199–3205. doi: 10.1182/blood-2004-09-3556. [DOI] [PubMed] [Google Scholar]
- 26.Lich JD, Elliott JF, Blum JS. Cytoplasmic processing is a prerequisite for presentation of an endogenous antigen by major histocompatibility complex class II proteins. J Exp Med. 2000;191:1513–1524. doi: 10.1084/jem.191.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nimmerjahn F, Milosevic S, Behrends U, Jaffee EM, Pardoll DM, Bornkamm GW, Mautner J. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur J Immunol. 2003;33:1250–1259. doi: 10.1002/eji.200323730. [DOI] [PubMed] [Google Scholar]
- 28.Qi L, Rojas JM, Ostrand-Rosenberg S. Tumor cells present MHC class II-restricted nuclear and mitochondrial antigens and are the predominant antigen presenting cells in vivo. J Immunol. 2000;165:5451–5461. doi: 10.4049/jimmunol.165.10.5451. [DOI] [PubMed] [Google Scholar]
- 29.Schmid D, Pypaert M, Munz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou D, Li P, Lin Y, Lott JM, Hislop AD, Canaday DH, Brutkiewicz RR, Blum JS. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22:571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Gueguen M, Long EO. Presentation of a cytosolic antigen by major histocompatibility complex class II molecules requires a long-lived form of the antigen. Proc Natl Acad Sci USA. 1996;93:14692–14697. doi: 10.1073/pnas.93.25.14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, Munz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 33.Strawbridge AB, Blum JS. Autophagy in MHC class II antigen processing. Curr Opin Immunol. 2007;19:87–92. doi: 10.1016/j.coi.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Munz C. Autophagy and antigen presentation. Cell Microbiol. 2006;8:891–898. doi: 10.1111/j.1462-5822.2006.00714.x. [DOI] [PubMed] [Google Scholar]
- 35.Maric M, Arunachalam B, Phan UT, Dong C, Garrett WS, Cannon KS, Alfonso C, Karlsson L, Flavell RA, Cresswell P. Defective antigen processing in GILT-free mice. Science. 2001;294:1361–1365. doi: 10.1126/science.1065500. [DOI] [PubMed] [Google Scholar]
- 36.Chen MR, Tsai CH, Wu FF, Kan SH, Yang CS, Chen JY. The major immunogenic epitopes of Epstein-Barr virus (EBV) nuclear antigen 1 are encoded by sequence domains which vary among nasopharyngeal carcinoma biopsies and EBV-associated cell lines. J Gen Virol. 1999;80:447–455. doi: 10.1099/0022-1317-80-2-447. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert KM, Weigle WO. Tolerogenicity of resting and activated B cells. J Exp Med. 1994;179:249–258. doi: 10.1084/jem.179.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 39.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aldo-Benson M, Borel Y. Loss of carrier-determined tolerance in vitro with loss of receptor blockade. J Immunol. 1976;116:223–226. [PubMed] [Google Scholar]
- 41.Borel Y, Kilham L, Hyslop N, Borel H. Isologous IgG-induced tolerance to benzyl penicilloyl. Nature. 1976;261:49–50. doi: 10.1038/261049a0. [DOI] [PubMed] [Google Scholar]
- 42.Paley RS, Leskowitz S, Borel Y. Effect on tolerance induction of the mode of attachment of the hapten carrier. J Immunol. 1975;115:1409–1413. [PubMed] [Google Scholar]
- 43.Venkataraman M, Aldo-Benson M, Borel Y, Scott DW. Persistence of antigen-binding cells with surface tolerogen: isologous versus heterologous immunoglobulin carriers. J Immunol. 1977;119:1006–1009. [PubMed] [Google Scholar]
- 44.Melo ME, El-Amine M, Tonnetti L, Fleischman L, Scott DW. Gene therapeutic approaches to induction and maintenance of tolerance. Int Rev Immunol. 2001;20:627–645. doi: 10.3109/08830180109045582. [DOI] [PubMed] [Google Scholar]
- 45.Kang Y, Melo M, Deng E, Tisch R, El-Amine M, Scott DW. Induction of hyporesponsiveness to intact foreign protein via retroviral-mediated gene expression: the IgG scaffold is important for induction and maintenance of immune hyporesponsiveness. Proc Natl Acad Sci USA. 1999;96:8609–8614. doi: 10.1073/pnas.96.15.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudensky A, Rath S, Preston-Hurlburt P, Murphy DB, Janeway CA., Jr On the complexity of self. Nature. 1991;353:660–662. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- 47.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 48.Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 49.Agarraberes FA, Terlecky SR, Dice JF. An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation. J Cell Biol. 1997;137:825–834. doi: 10.1083/jcb.137.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arunachalam B, Phan UT, Geuze HJ, Cresswell P. Enzymatic reduction of disulfide bonds in lysosomes: characterization of a γ-interferon-inducible lysosomal thiol reductase (GILT) Proc Natl Acad Sci USA. 2000;97:745–750. doi: 10.1073/pnas.97.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Amine M, Hinshaw JA, Scott DW. In vivo induction of tolerance by an Ig peptide is not affected by the deletion of FcR or a mutated IgG Fc fragment. Int Immunol. 2002;14:761–766. doi: 10.1093/intimm/dxf049. [DOI] [PubMed] [Google Scholar]
- 52.Phan UT, Maric M, Cresswell P. Disulfide reduction in major histocompatibility complex class II-restricted antigen processing by interferon-γ-inducible lysosomal thiol reductase. Methods Enzymol. 2002;348:43–48. doi: 10.1016/s0076-6879(02)48624-7. [DOI] [PubMed] [Google Scholar]
- 53.Lackman RL, Cresswell P. Exposure of the promonocytic cell line THP-1 to Escherichia coli induces IFN-γ-inducible lysosomal thiol reductase expression by inflammatory cytokines. J Immunol. 2006;177:4833–4840. doi: 10.4049/jimmunol.177.7.4833. [DOI] [PubMed] [Google Scholar]
- 54.Hastings KT, Lackman RL, Cresswell P. Functional requirements for the lysosomal thiol reductase GILT in MHC class II-restricted antigen processing. J Immunol. 2006;177:8569–8577. doi: 10.4049/jimmunol.177.12.8569. [DOI] [PubMed] [Google Scholar]
- 55.Haque MA, Li P, Jackson SK, Zarour HM, Hawes JW, Phan UT, Maric M, Cresswell P, Blum JS. Absence of γ-interferon-inducible lysosomal thiol reductase in melanomas disrupts T cell recognition of select immunodominant epitopes. J Exp Med. 2002;195:1267–1277. doi: 10.1084/jem.20011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agarraberes FA, Dice JF. Protein translocation across membranes. Biochim Biophys Acta. 2001;1513:1–24. doi: 10.1016/s0304-4157(01)00005-3. [DOI] [PubMed] [Google Scholar]


