Abstract
Thymoquinone, a component derived from the medial plant Nigella sativa, has been used for medical purposes for more than two thousands of years. Recent studies reported that thymoquinone exhibited inhibitory effects on cell proliferation of many cancer cell lines and hormone-refractory prostate cancer by suppressing androgen receptor and E2F-1. Whether thymoquinone inhibits angiogenesis, the critical step of tumor growth and metastasis, is still unknown. In this study, we found that thymoquinone effectively inhibited human umbilical vein endothelial cell (HUVEC) migration, invasion, and tube formation. Thymoquinone inhibited cell proliferation and suppressed the activation of AKT and ERK. Thymoquinone blocked angiogenesis in vitro and in vivo, prevented tumor angiogenesis in a xenograft human prostate cancer (PC3) model in mouse and inhibited human prostate tumor growth at low dosage with almost no chemotoxicitical side effects. Furthermore, we observed that endothelial cells were more sensitive to thymoquinone-induced cell apoptosis, cell proliferation and migration inhibition compared to PC3 cancer cells. Thymoquinone inhibited VEGF-induced ERK activation, but showed no inhibitory effects on VEGF receptor 2 activation. Overall, our results indicate that thymoquinone inhibits tumor angiogenesis and tumor growth, and could be used as a potential drug candidate for cancer therapy.
Keywords: thymoquinone, angiogenesis inhibitor, tumor angiogenesis, HUVEC
Introduction
Thymoquinone (TQ, C10H12O2, molecular weight: 164.2), the main bioactive component of the volatile oil of the black seed (Nigella sativa, Ranunculaceae family), has been used as anti-oxidant, anti-inflammatory and antineoplastic medicines for more than two thousands of years (1, 2). Previous studies reported that thymoquinone exhibited inhibitory effects on cell proliferation of many types of cancer cell lines, including breast adenocarcinoma, ovarian adenocarcinoma (3), colorectal cancer (4), human pancreatic adenocarcinoma, uterine sarcoma (5), neoplastic keratinocytes (6), human osteosarcoma (7), fibrosarcoma, lung carcinoma (8), and so on. Recently, Ahmed et al reported that thymoquinone targeted androgen receptor and transcription factor E2F-1 and inhibited hormone-refractory prostate cancer (8). As angiogenesis is critically important for the growth of solid tumors not only by supplying with oxygen and nutrients for the survival of tumor cells but also by providing the route for metastatic spread. Therefore, angiogenesis has been an attractive target for tumor therapy (9, 10). The accumulated evidence has confirmed the importance of angiogenesis and validated the theory that inhibition of neovascularization is a promising anti-cancer strategy (11-13). Although Erdurmus M et al. reported that thymoquinone showed inhibitory effects on corneal neovascularization in the rat model (14), whether thymoquinone inhibits (tumor-)angiogenesis and suppresses prostate tumor growth through tumor-angiogenesis prevention are still not fully understood.
Endothelial cells play a major role in each step of tumor angiogenesis, including endothelial cell migration, proliferation, invasion, adhesion, and tube formation (15, 16). Among the endothelial cell signaling pathways that regulates endothelial cell migration, proliferation, growth and survival, the two major pathways are PI3K-AKT and Raf-MEK-ERK pathways. Activation of these two pathways in endothelial cells is necessary for angiogenesis (17, 18). AKT (protein kinase B), a serine/threonine-specific protein kinase, is a pivotal node involved in essential cellular functions of endothelial cells such as migration, growth, proliferation, apoptosis, and metabolism. AKT regulates endothelial nitric xoide (NO) synthase (eNOS) activation (19), stimulating vasodilation, vascular remodeling and angiogenesis (20). AKT signaling stimulates the production of hypoxia inducible factor α (HIFα) transcription factors, thereby mediates secretion of VEGF and other growth factors which are important proangiogenic factors (21, 22). Extracellular signal-related kinase (ERK) is a critical kinase in regulating endothelial cell cycle, proliferation, growth, migration, and apoptosis(23). Upon the extracellular growth factor stimulation, the activated ERK regulates its many substrates, such as NFκB and c-Jun, thereby regulates angiogenesis. ERK also is necessary for eNOS activation (17, 24).
Here, we have investigated whether thymoquinone inhibited angiogenesis through suppression of intracellular signaling pathways. We found that thymoquinone can inhibit HUVEC migration, invasion, proliferation, and tube formation by decreasing AKT/ERK activation. Thymoquinone also blocks angiogenic properties in both in vitro aortic ring assay and in vivo matrigel plug assays. Furthermore, thymoquinone inhibits tumor-angiogenesis and thereby prevents human prostate tumor growth at low dosage in xenograft mouse models.
Materials and Methods
Animals, Cell Lines, and Reagents
Severe combined immunodeficiency (SCID) male mice (5-6 week old) were purchased from National Cancer Institute. Thymoquinone was ordered from Sigma-Aldrich St. Louis, MO. Human umbilical vein endothelial cells (HUVEC) were kindly gifted from Dr. Xinli Wang (Cardiothoracic Surgery Division of the Michael E. DeBakey Department of Surgery at Baylor College of Medicine Hospital). The Human prostate cancer cell line (PC3) was purchased from the American Type Culture Collection (Manassas, A) and maintained in a mixture of RPMI-1460 medium and 10% fetal bovine serum. Matrigel was ordered from BD Biosciences, Bedford, MA. HTScan® VEGF receptor 2 kinase assay kit was ordered from Cell Signaling Technology. HRP labeled secondary antibody, TMB substrate and stop solution were kindly gifted by Cell Signaling Technology. Streptavidin coated yellow 96-well plates were kindly gifted by PerkinElmer Life Sciences.
Proliferation Assay
Cell proliferation assay with different concentration of thymoquinone was performed as following the manual (Promega, CellTiter 96 Aqueous One Solution Cell Proliferation Assay).
Flow Cytometry FACS Analysis
About 2×106 of either HUVEC or PC3 cells were treated with different concentrations of thymoquinone at 37°C, 5% CO2 incubator for 24 hours. The cells were collected, stained with propidium iodide, and subjected to the flow cytometry analysis. The percentage at SubG1 was defined as the apoptotic population.
Migration Assay
Migration assay was performed as previously described (25). HUVEC cells were allowed to grow to confluent on six-well plates precoated with 0.1% gelatin and inactivated by 0.1% mitomycin C as previously described. Monolayer cells were wounded by scratching with 1 ml pipette tips and washed twice with 1×PBS. Fresh endothelial cell growth medium (ECGM) was added with 4nM VEGF, which was received from NIH experimental branch, and different concentration of thymoquinone. Images were taken after 7-10 hours incubation at 37°C, 5% CO2 by Nikon digital camera. The migrated HUVEC cells were qualified by manual counting. Similar patterns of the inhibition effects were observed in three independent experiments.
Transwell Invasion Assay
The transwell (Corning Incorporated, USA) were coated with matrigel (BD Biosciences) and incubated at 37°C for 45 minutes. The bottom chambers (600μl) were filled with ECGM medium with 20% FBS supplemented with 4nM VEGF and the top chambers were seeded with 100μl ECGM medium and HUVEC cells (4×104 cell/well). The top and bottom chambers contained the same series of concentration of thymoquinone. HUVEC cells were allowed to migrate for 4 hours at 37°C, 5%CO2. After the incubation, cells on the top surface of the membrane (non-migrated) were scraped with a cotton swab. Cells on the bottom side of the membrane (migrated cells) were fixed with 4% paraformaldehyde for 20 minutes, washed three times with 1×PBS. The cells were stained by Hematoxylin and eosin (H&E) staining and then destained with 1×PBS (pH 7.4). The membranes were left to air dry at room temperature for 30 minutes. Images were taken using OLYMPUS inverted microscope. Three independent areas per well were counted and the mean number of migrated cells was calculated.
Tube Formation Assay
Matrigel was dissolved at 4°C for overnight, and each well of prechilled 24-well plates was coated with 100μl matrigel and incubated at 37°C for 45 minutes. HUVEC cells (4×104 cells) were added in 1 ml ECGM with various concentration of thymoquinone. After 12-16 hours of incubation at 37°C, 5% CO2, endothelial cell tube formation was assessed with an inverted photomicroscope. Tubular structures were quantified by manual counting of low power fields and percent inhibition was expressed using untreated wells as 100%.
Aortic Ring Assay
The aortic ring assay was performed as previously described(25).
Matrigel Plug Assay
Matrigel (0.5 ml/plug) containing 10 μM thymoquinone, 4nM VEGF, or a mixture of VEGF (4nM) and 1μM thymoquinone was injected subcutaneously in the midventral abdominal region of 5-6 week C57BL/6 mice (five mice for each group). After 7 days, the plugs were removed from the sacrificed mice. The matrigel plugs were fixed with formalin and embedded with paraffin. The 5μm sections were stained with H&E staining. The number of erythrocyte-filled blood vessels was counted (200 × fold magnification).
Western Blotting
A 200μg of total protein of the cells of each sample was performed for the immunoprecipitation with anti-c-Src antibody (Santa Cruz Biotech) and then followed by western blotting. The pTyr-antibody (Santa Cruz Biotech) was used for detecting c-Src phosphorylation and pFAK397 antibody (Cell Signaling) was blotted for FAK phosphorylation. AKT phosphorylation was examined using pSer473-AKT antibody (Cell Signaling). Anti-cleaved Caspase3 (Santa Cruz Biotech) was used for detecting apoptosis. Poly-ADP Ribose Polymerase (PARP) cleavage was detected by anti-PARP antibody (Zymed Laboratory).
Xenograft Mouse Model
The 5-6 week old SCID male mice weighing about 20g were divided into groups (five mice each group). PC3 cancer cells were subcutaneously injected (2×106 cell per mouse) into the mice. After the tumors had become established (about 50 mm3), the mice were subcutaneously injected with or without 6 mg/kg thymoquinone every day. The body weights and tumor sizes were recorded every day, and the tumor size was determined by a vernier caliper measurement. After 15 days, mice with subcutaneous tumors were sacrificed.
Histology and Immunohistochemistry
The tumors were removed, fixed with Histochoice® MB (Molecular Biology) tissue fixative (Amresco®), and embedded with parafilm. The 5μm sections were performed for the blood vessel staining (Chemicon International, blood vessel staining kit, peroxidase system). The number of blood vessel in 200× magnification was counted.
VEGF Receptor 2 Inhibition Assay
A 12.5 μl of the 4× reaction cocktail containing 100 ng VEGF Receptor 2 (supplied from the HTScan® VEGF receptor 2 kinase assay kit, Cell Signaling Technology, USA) was incubated with 12.5 μl/tube of series of concentration of thymoquinone for 5 minutes at room temperature. A 25 μl of 2× ATP/substrate peptide cocktail was added to the pre-incubated reaction cocktail/thymoquinone compound. After incubation at room temperature for 30 minutes, a 50 μl stop buffer (50 mM EDTA, pH 8) was added per tube to stop the reaction. Then 25 μl of each reaction was transferred with 75 μl H2O/well to a 96-well streptavidin-coated plate (PerkinElmer Life Sciences, USA) and incubated at room temperature for 60 minutes. After washed the wells three times with 200 μl/well PBS/T (0.05% Tween-20 in 1 × PBS), a 100 μl primary antibody (Phosphor-Tyrosine Monoclonal Antibody (P-Tyr-100), 1:1000 in PBS/T with 1% BSA) was added per well. After being incubated at room temperature for 60 minutes, the wells were washed three times with 200 μl PBS/T. A 100 μl diluted HRP labeled anti-mouse Ig G (1:500 in PBS/T with 1% BSA) was added per well. After incubation at room temperature for 30 minutes, the wells were washed five times with 200 μl PBS/T per well. Then a 100 μl/well TMB substrate was added per well and the plate was incubated at room temperature for 15 minutes. The stop solution (100μl/well) was added and mixed followed incubation for at room temperature 15 minutes. The plate was then detected at 405 nm with VERSAmax microplate reader (Molecular Devices) and the data were repeated 3 times.
Statistical Analysis
Three independent experiments were performed, and data were presented as mean ± standard error. Statistical significance of differences in control and sample groups was determined by using t- test (maximum p < 0.05).
Results
Thymoquinone Inhibits HUVEC Migration, Invasion, and Tube Formation
As endothelial cell migration is an important step of angiogenesis (26), we performed wound-healing migration assay to determine the effects of thymoquinone on HUVEC migration and found thymoquinone inhibited HUVEC migration in a concentration-dependent manner (Fig. 1A). Then, in the followed transwell assay showed in Fig.1B, thymoquinone significantly inhibited HUVEC invasion at 80-100 nM. In matrigel assay, we found that thymoquinone significantly blocked HEVEC tube formation at 100 nM (Fig. 1C).
Figure 1. Thymoquinone inhibits HUVEC migration, invasion, and tube formation.
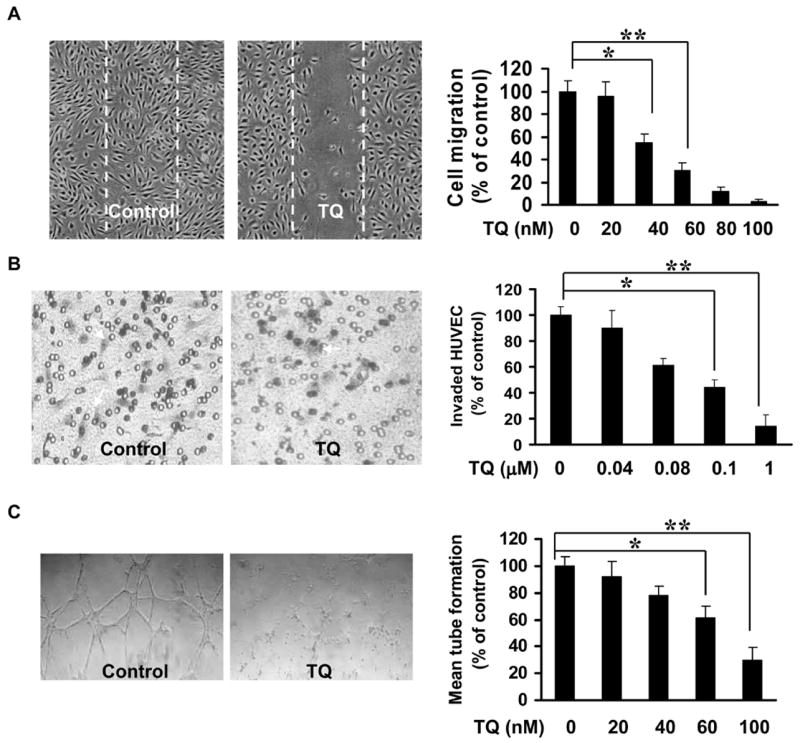
A, Inhibitory effect of thymoquinone on HUVEC migration. Inactivated HUVECs were performed wound-healing migration assays and the migrated cells were counted. B, Effect of thymoquinone on HUVEC invasion assay using transwell. The red-colored cells with irregular shape in the images were invaded cells attached on outside surface of the top chamber. C, Inhibitory effect of thymoquinone on HUVEC tubule-like-structure formation. Tubule like structure was quantified by manual counting of low power fields (25×). Percentage of inhibition percent was expressed using untreated wells as 100% (n = 3, * P<0.05, ** P<0.01).
Thymoquinone Inhibits Angiogenesis in Vitro and in Vivo
To ascertain the inhibitory effect of thymoquinone on angiogenesis, we performed aortic ring assay. As shown in Fig. 2A, thymoquinone inhibited micro-vessel growth in vitro from 50 nM to 100 nM (Fig. 2A) after 4 days incubation, suggesting thymoquinone inhibits angiogenesis in vitro. To confirm the anti-angiogenesis effects of thymoquinone in vivo, matrigel plug assays were performed with different concentrations. As shown in Fig. 2B, 1 μM thymoquinone significantly inhibited VEGF-induced angiogenesis while 10 μM thymoquinone almost completely abolished angiogenesis in the matrigel plug assays (Fig. 2B), indicating thymoquinone effectively inhibited angiogenesis in vivo.
Figure 2. Thymoquinone inhibits angiogenesis in vitro and in vivo.
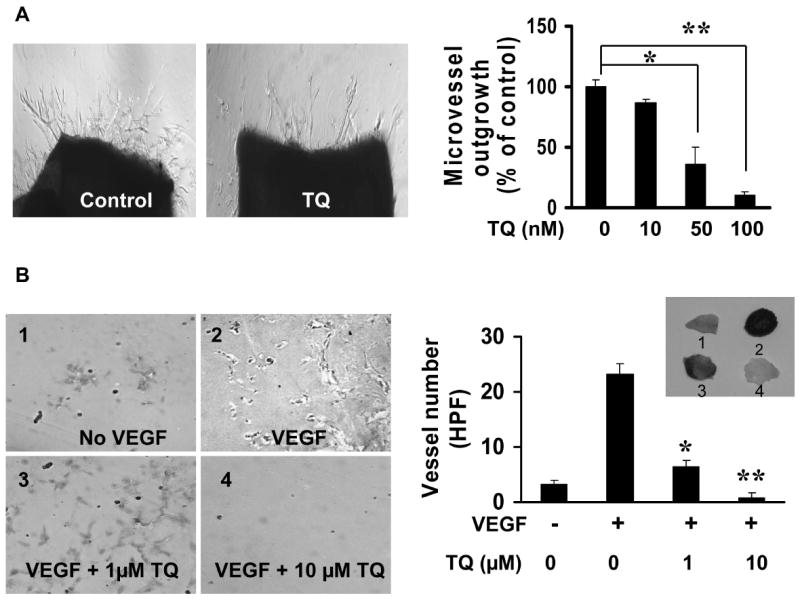
A, Effects of thymoquinone on angiogenesis in vitro. Images were taken with Olympus IX 70 invert microscope and micro-vessel outgrowth micro-vessels were counted as methods described (aortic ring number = 4, *, P <0.05). B, Inhibitory effects of thymoquinone on VEGF-induced angiogenesis in vivo (plug number = 5, *P<0.05, **P<0.01).
Thymoquinone Inhibits Tumor Angiogenesis and Arrests Prostate Tumor Growth
To investigate thymoquinone-induced inhibition of tumor-angiogenesis in vivo, we used the xenograft mouse model with human prostate cancer cells, PC3. PC3 cancer cells (2×106 PC3 cell per mouse) were injected subcutaneously into the mice (5 mice for each group). After the tumors had become established (about 50 mm3), the mice were subcutaneously injected with or without thymoquinone at a dosage of 6 mg/kg/day. After 15 days, mice were sacrificed and the tumors were removed. As shown in Fig. 3A and 3B, thymoquinone effectively prevented the tumor growth in both size (thymoquinone-treated group 184.2 ± 25.8 mm3 vs. control group 1,143.98 ± 169.14 mm3) and weight (thymoquinone-treated group 0.013 ± 0.002g vs. control group 0.3 ± 0.07g). In further analyses of the tumor sections treated and un-treated with thymoquinone, respectively, we found that thymoquinone significantly inhibited tumor angiogenesis as it decreased the number of blood vessels in the tumor [thymoquinone-treated group 2.8 ± 0.8/high field performance (HFP) vs. control group 17 ± 2.4 /HFP] (Fig. 3C). At the concentration used in the xenograft mouse model, thymoquinone shows no toxicity as measured by mouse body weights in control and thymoquinone-treated groups. The average mouse body weight of control group decreased from 22.28 ± 1.22 g to 21.24 ± 1.32g while that of thymoquinone treated group increased from 22 ± 1.5 g to 24.4 ± 1.2 g (Fig. 3D). The slight decrease of the control group is due to the growth of tumors in the xenograft mice.
Figure 3. Thymoquinone inhibits tumor-angiogenesis and prevents tumor growth in vivo.
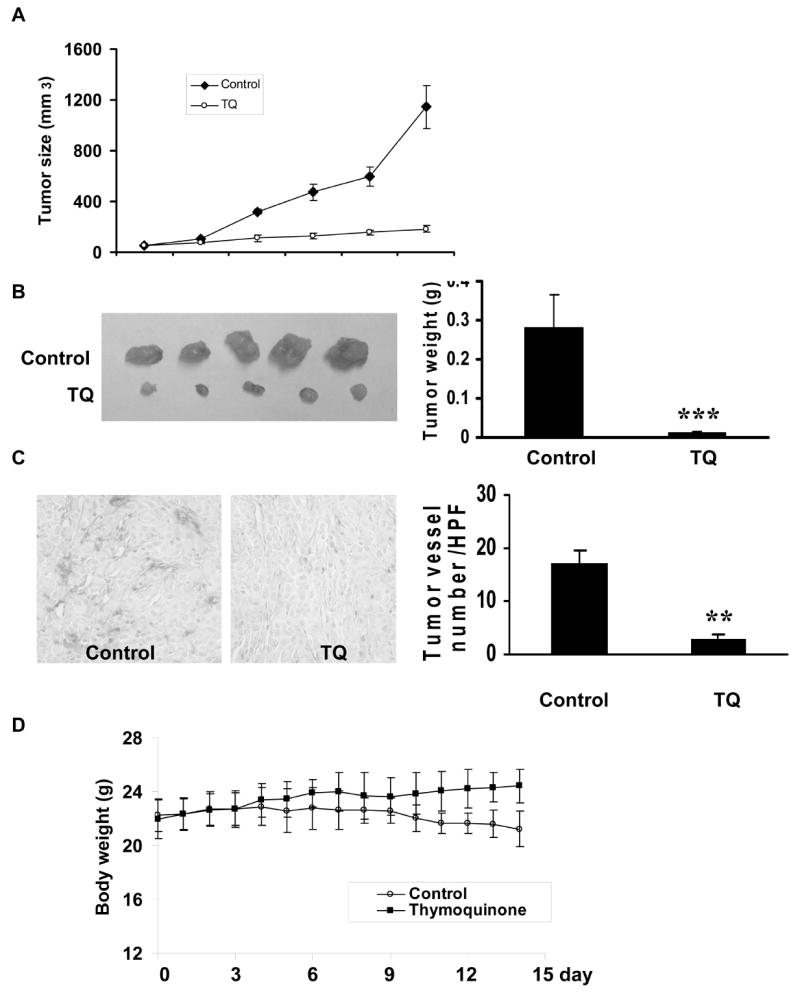
A, Effects of thymoquinone on tumor volume growth. B, Effects of thymoquinone on the increase of tumor weight. C, Inhibition of thymoquinone on tumor-angiogenesis. The number of micro blood vessels was significantly decreased. D, Effects of thymoquinone on mouse body growth (mouse number = 5, **P<0.01, ***P<0.001).
HUVECs are More Sensitive to Thymoquinone-induced Apoptosis and Inhibition in Cell Migration and Proliferation than PC3 Cancer Cells
To analyze whether the inhibition of tumor growth by thymoquinone primarily resulted from anti-angiogenesis, we evaluate whether thymoquinone has different effects on endothelial cells compared to cancer cells. We first compared the inhibition of thymoquinone on HUVEC and PC3 cell migration induced by VEGF. As shown in Fig. 4A, HUVECs were 10 fold more sensitive to thymoquinone inhibition on cell migration compared to PC3 cancer cells and the inhibitory effect of 0.1 μM in HUVECs is equivalent to 1 μM in PC3 tumor cells (Fig. 4A and 4B). Furthermore, we found that HUVECs were more sensitive than PC3 cancer cells to thymoquinone-induced inhibition in cell proliferation (Fig. 4C and 4D) and promotion in cell apoptosis assays (Table 1). These data indicate that HUVECs are more sensitive to thymoquinone-induced apoptosis and inhibition in cell migration and proliferation compared to PC3 cancer cells, suggesting thymoquinone may target tumor-angiogenesis at a lower dosage and then effectively inhibit tumor growth.
Figure 4. Sensitivity of HUVECs and tumor cells to thymoquinone.
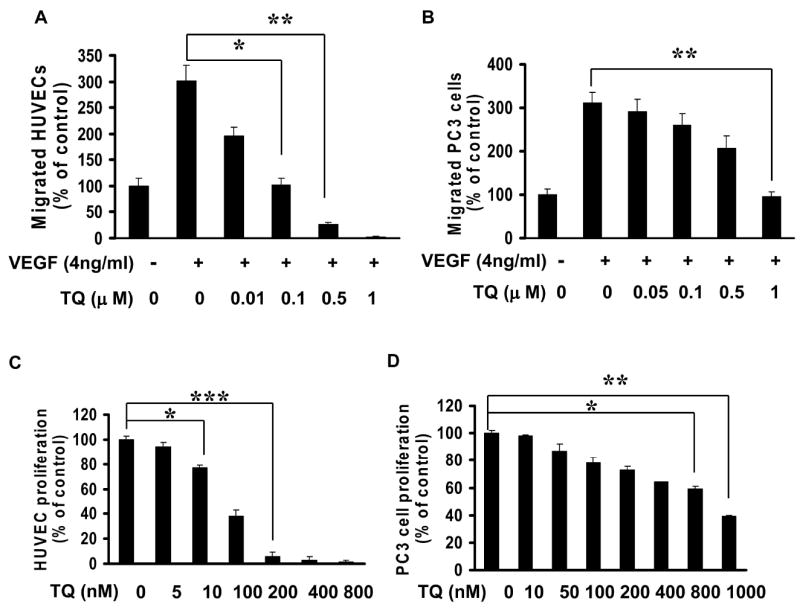
A-B, Effects of thymoquinone on cell migration of VEGF-induced HUVEC (A) and PC3 cancer cells (B). C-D, Inhibitory effects of thymoquinone on HUVEC (C) and PC3 (D) cancer cells proliferation (n= 3, *P<0.05, **P<0.01, ***P<0.001).
Table 1.
Thymoquinone activates apoptosis in HUVEC and PC3 cancer cells.
| Apoptotic population (% of total cells) | ||||
|---|---|---|---|---|
| Thymoquinone(nM) | 0 | 25 | 50 | 100 |
| PC3 | 2.3 ± 0.6 | 3.9 ± 0.5 | 3.5 ± 0.2 | 4.8 ± 1.8 |
| HUVEC | 2.7 ± 1.5 | 24.5 ± 1.2 | 28.4 ± 1.6 | 45 ± 2.6 |
Thymoquinone Suppresses VEGF Dependent ERK Activation, But is Not a VEGFR2 Inhibitor
VEGFR2 plays a major role in VEGF dependent angiogenesis. In order to investigate the molecular mechanism of thymoquinone-induced inhibition on VEGF dependent angiogenesis, we examined thymoquinone effects on VEGFR2 activation with a VEGFR2 specific activation assay. We found that thymoquinone showed very little inhibitory effects on VEGFR2 (Fig. 5A), suggesting that thymoquinone was not a direct VEGFR2 inhibitor. As shown in Fig. 5B, thymoquinone could effectively suppress VEGF dependent ERK activation at 10 nM. Taken together, thymoquinone inhibit angiogenesis by suppressing VEGF-induced ERK activation, but has no direct effect on VEGFR2 activation.
Figure 5. Effects of thymoquinone on VEGFR2 activation and signaling pathways.
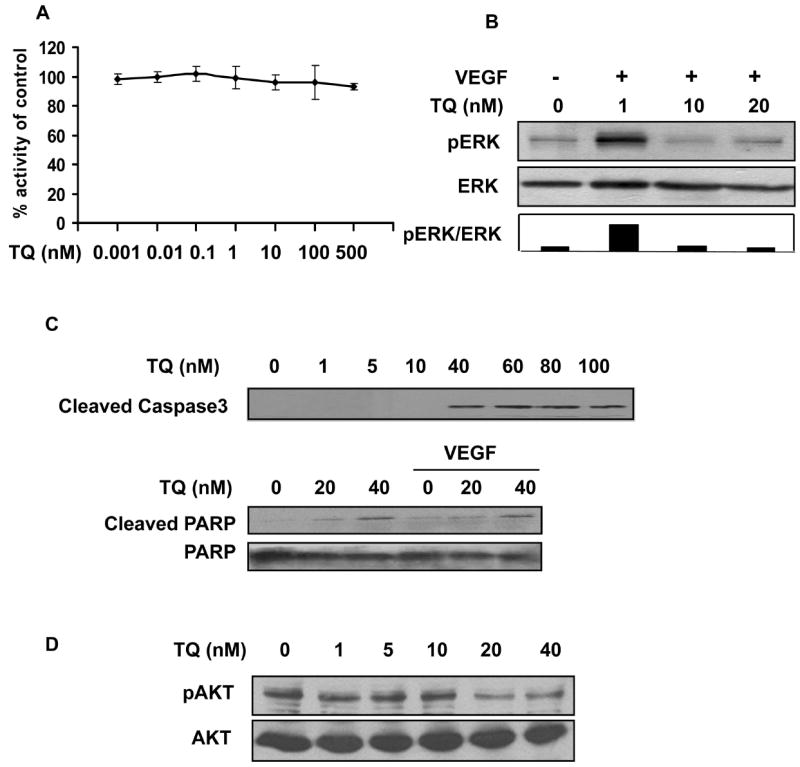
A, Thymoquinone has little effect of the activation of VEGFR2. B, Effects of thymoquinone on VEGF dependent ERK activation. C, Thymoquinone induces the levels of cleaved caspase 3 and the increased levels of cleaved PARP by thymoquinone are independent of VEGF in HUVECs. HUVECs were treated with thymoquinone at the indicated concentrations for 24 hours and whole cell proteins were performed western blotting. 4 ng/ml VEGF was used in the PARP cleavage assay. D, Thymoquinone inhibited that activation of AKT at 20-40 nM. HUVECs were treated with indicated amount of thymoquinone for 5 minutes.
Thymoquinone Induces Cell Apoptosis Pathways
We have shown that thymoquinone significantly induced HUVEC apoptosis in a dose dependent manner (Table 1). As shown in Fig. 5C, thymoquinone induced the activation of caspase3 cleavage at 40 nM and the data were confirmed by the increased cleavage of poly (ADP-ribose) polymerase (PAPR) in the absence or presence of VEGF. Furthermore, one of the key kinases involved in cell survival and apoptosis, Akt, was also significantly inhibited by thymoquinone at 20-40 nM (Fig. 5D).
Discussion
Based on our present results, we conclude that thymoquinone effectively inhibits endothelial cell migration, invasion, proliferation and tube formation, prevents angiogenesis in vitro and in vivo, and suppresses tumor-angiogenesis and tumor growth in vivo. Thymoquinone inhibits angiogenesis by suppressing the activation of VEGF-induced ERK and AKT, but is not a VEGFR2 inhibitor. This is the first report to comprehensively demonstrate that thymoquinone inhibits angiogenesis and tumor growth at low dosages by blocking tumor-angiogenesis.
The known angiogenesis inhibitors always inhibit one or several steps of angiogenesis by targeting endothelial cells (27). Here we not only demonstrated that thymoquinone effectively inhibited endothelial cell migration, invasion, proliferation and tube formation, but also identified that thymoquinone inhibited angiogenesis in vitro and in vivo, suggesting thymoquinone is an angiogenesis inhibitor. Furthermore, we found that thymoquinone inhibited tumor-angiogenesis and prevented prostate tumor growth in vivo at low dosage of 6 mg/kg/day in xenograft mouse model. We also elucidated that endothelial cells were more sensitive to thymoquinone-caused apoptosis (Table 1) and inhibition in cell migration and proliferation compared to PC3 cancer cells. As prostate cancer is predominantly a tumor of old men with limited treatment options for the coexisting illnesses, the lower dosage used, the less chemo-toxic side effects (11). Our data indicate that thymoquinone is a potential drug candidate for treating human prostate tumor.
AKT is a critical regulator generally involved in cell cycle, proliferation, and apoptosis through regulating gene expression, protein synthesis and transcription procedure (20). ERK is an important factor in mediating cell proliferation, survival and cell migration (17). Both AKT and ERK activation are necessary for essential cellular procedures of endothelial cells and angiogenesis (17). Here, we showed that thymoquinone inhibited the activation of both AKT and ERK (VEGF dependent or independent) in endothelial cells. However, thymoquinone had no inhibitory effects on VEGFR2 activation in a specific VEGFR2 assay. Other than VEGFs, there are many other pro-angiogenic growth factors, such as fibroblast growth factors (FGFs), placental growth factor (PlGF), and platelet-derived growth factor (PDGF) (28). Almost all of these pro-angiogenic growth factors regulate angiogenesis through AKT and ERK signaling pathways (28-30). Therefore, thymoquinone may inhibit angiogenesis/tumor angiogenesis through suppressing AKT/ERK signaling pathways, but not directly inhibit VEGFR2 activation.
The efficacy of angiogenesis inhibitors depend on the tumor stage: pre-malignant, small tumor and large tumor (31, 32). We concluded that thymoquinone can effectively inhibit prostate tumor growth at small tumor stage (50 mm3) at the dose of 6 mg/kg/day.
In summary, we systemically demonstrated that thymoquinone, the major biological-active component of the natural medicine of Nigella sativa, inhibited endothelial cell migration, invasion, proliferation and tube formation, effectively inhibited angiogenesis in vitro and in vivo, preventing tumor growth in a xenograft mouse model with a low dosage by blocking tumor angiogenesis. We identified that thymoquinone inhibited angiogenesis by suppressing AKT/ERK signaling pathway activation. Together, these data suggest that thymoquinone is a potential drug candidate for cancer chemotherapies with low chemotoxical side effects.
Acknowledgments
This work is supported partially by a grant (1R01CA106479 to M Liu) from National Cancer Institute, National Institutes of Health (NIH).
Abbreviation
- TQ
thymoquinone
- HUVEC
human umbilical vein endothelial cell
- PC3
human prostate cancer
- ERK
extracellular signal-related kinase
- ECGM
endothelial cell growth medium
- VEGFR2
vascular endothelial growth factor receptor 2
References
- 1.Trang NT, Wanner MJ, Phuong le VN, Koomen GJ, Dung NX. Thymoquinone from Eupatorium ayapana. Planta Med. 1993;59:99. doi: 10.1055/s-2006-959619. [DOI] [PubMed] [Google Scholar]
- 2.Hosseinzadeh H, Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine. 2004;11:56–64. doi: 10.1078/0944-7113-00376. [DOI] [PubMed] [Google Scholar]
- 3.Shoieb AM, Elgayyar M, Dudrick PS, Bell JL, Tithof PK. In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. Int J Oncol. 2003;22:107–13. [PubMed] [Google Scholar]
- 4.Gali-Muhtasib H, Diab-Assaf M, Boltze C, et al. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int J Oncol. 2004;25:857–66. [PubMed] [Google Scholar]
- 5.Worthen DR, Ghosheh OA, Crooks PA. The in vitro anti-tumor activity of some crude and purified components of blackseed, Nigella sativa L. Anticancer Res. 1998;18:1527–32. [PubMed] [Google Scholar]
- 6.Gali-Muhtasib HU, Abou Kheir WG, Kheir LA, Darwiche N, Crooks PA. Molecular pathway for thymoquinone-induced cell-cycle arrest and apoptosis in neoplastic keratinocytes. Anticancer Drugs. 2004;15:389–99. doi: 10.1097/00001813-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Roepke M, Diestel A, Bajbouj K, et al. Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol Ther. 2007;6:160–9. doi: 10.4161/cbt.6.2.3575. [DOI] [PubMed] [Google Scholar]
- 8.Kaseb AO, Chinnakannu K, Chen D, et al. Androgen receptor and E2F-1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer research. 2007;67:7782–8. doi: 10.1158/0008-5472.CAN-07-1483. [DOI] [PubMed] [Google Scholar]
- 9.Cooney MM, van Heeckeren W, Bhakta S, Ortiz J, Remick SC. Drug insight: vascular disrupting agents and angiogenesis--novel approaches for drug delivery. Nat Clin Pract Oncol. 2006;3:682–92. doi: 10.1038/ncponc0663. [DOI] [PubMed] [Google Scholar]
- 10.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 11.Lara PN, Jr, Twardowski P, Quinn DI. Angiogenesis-targeted therapies in prostate cancer. Clin Prostate Cancer. 2004;3:165–73. doi: 10.3816/cgc.2004.n.027. [DOI] [PubMed] [Google Scholar]
- 12.Gasparini G, Longo R, Toi M, Ferrara N. Angiogenic inhibitors: a new therapeutic strategy in oncology. Nat Clin Pract Oncol. 2005;2:562–77. doi: 10.1038/ncponc0342. [DOI] [PubMed] [Google Scholar]
- 13.Kerr DJ. Targeting angiogenesis in cancer: clinical development of bevacizumab. Nat Clin Pract Oncol. 2004;1:39–43. doi: 10.1038/ncponc0026. [DOI] [PubMed] [Google Scholar]
- 14.Erdurmus M, Yagci R, Yilmaz B, et al. Inhibitory effects of topical thymoquinone on corneal neovascularization. Cornea. 2007;26:715–9. doi: 10.1097/ICO.0b013e31804f5a45. [DOI] [PubMed] [Google Scholar]
- 15.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–45. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 16.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 17.Murphy DA, Makonnen S, Lassoued W, Feldman MD, Carter C, Lee WM. Inhibition of tumor endothelial ERK activation, angiogenesis, and tumor growth by sorafenib (BAY43-9006) Am J Pathol. 2006;169:1875–85. doi: 10.2353/ajpath.2006.050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somanath PR, Razorenova OV, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5:512–8. doi: 10.4161/cc.5.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–5. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 20.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 22.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–7. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang D, Ding Y, Luo WM, et al. Inhibition of MAPK kinase signaling pathways suppressed renal cell carcinoma growth and angiogenesis in vivo. Cancer research. 2008;68:81–8. doi: 10.1158/0008-5472.CAN-07-5311. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer research. 2006;66:11851–8. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 25.Yi T, Yi Z, Cho SG, et al. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer research. 2008;68:1843–50. doi: 10.1158/0008-5472.CAN-07-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park HJ, Zhang Y, Georgescu SP, Johnson KL, Kong D, Galper JB. Human umbilical vein endothelial cells and human dermal microvascular endothelial cells offer new insights into the relationship between lipid metabolism and angiogenesis. Stem Cell Rev. 2006;2:93–102. doi: 10.1007/s12015-006-0015-x. [DOI] [PubMed] [Google Scholar]
- 27.Kesisis G, Broxterman H, Giaccone G. Angiogenesis inhibitors. Drug selectivity and target specificity. Curr Pharm Des. 2007;13:2795–809. doi: 10.2174/138161207781757033. [DOI] [PubMed] [Google Scholar]
- 28.Perona R. Cell signalling: growth factors and tyrosine kinase receptors. Clin Transl Oncol. 2006;8:77–82. doi: 10.1007/s12094-006-0162-1. [DOI] [PubMed] [Google Scholar]
- 29.Lu B, Shinohara ET, Edwards E, Geng L, Tan J, Hallahan DE. The use of tyrosine kinase inhibitors in modifying the response of tumor microvasculature to radiotherapy. Technol Cancer Res Treat. 2005;4:691–8. doi: 10.1177/153303460500400614. [DOI] [PubMed] [Google Scholar]
- 30.Kim DW, Lu B, Hallahan DE. Receptor tyrosine kinase inhibitors as anti-angiogenic agents. Curr Opin Investig Drugs. 2004;5:597–604. [PubMed] [Google Scholar]
- 31.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 32.Folkman J. Is angiogenesis an organizing principle in biology and medicine? J Pediatr Surg. 2007;42:1–11. doi: 10.1016/j.jpedsurg.2006.09.048. [DOI] [PubMed] [Google Scholar]


