Abstract
Two exceptionally concise total syntheses of (–)- and ent-(+)-vindoline are detailed enlisting a diastereoselective tandem [4+2]/[3+2] cycloaddition of a 1,3,4-oxadiazole. The unique reaction cascade assembles the fully functionalized pentacyclic ring system of vindoline in a single step that forms four C–C bonds and three rings while introducing all the requisite functionality and setting all six stereocenters within the central ring including three contiguous and four total quaternary centers.
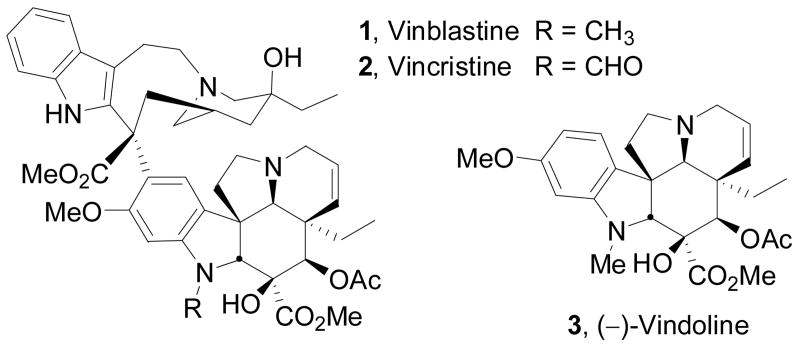
Vinblastine (1) and vincristine (2) constitute the most widely recognized members of the class of bisindole alkaloids as a result of their clinical use as antineoplastic drugs (Figure 1a).1 Originally isolated in trace quantities from Cantharanthus roseus (L.) G. Don,2 their biological properties were among the first to be shown to arise from inhibition of microtubule formation and mitosis that today is still regarded as one of the more successful drug targets for the treatment of cancer.3 In addition to being among the first natural products whose structures were determined by X-ray crystallography of a derivative, they were among the first for which X-ray was used to establish their absolute configuration.4 Vindoline (3)4,5 a major alkaloid of Cantharanthus roseus, constitutes the most complex half of vinblastine and serves as both a biosynthetic2 and synthetic6 precursor to the natural product.7
Figure 1.
Figure 1a. Natural product structures.
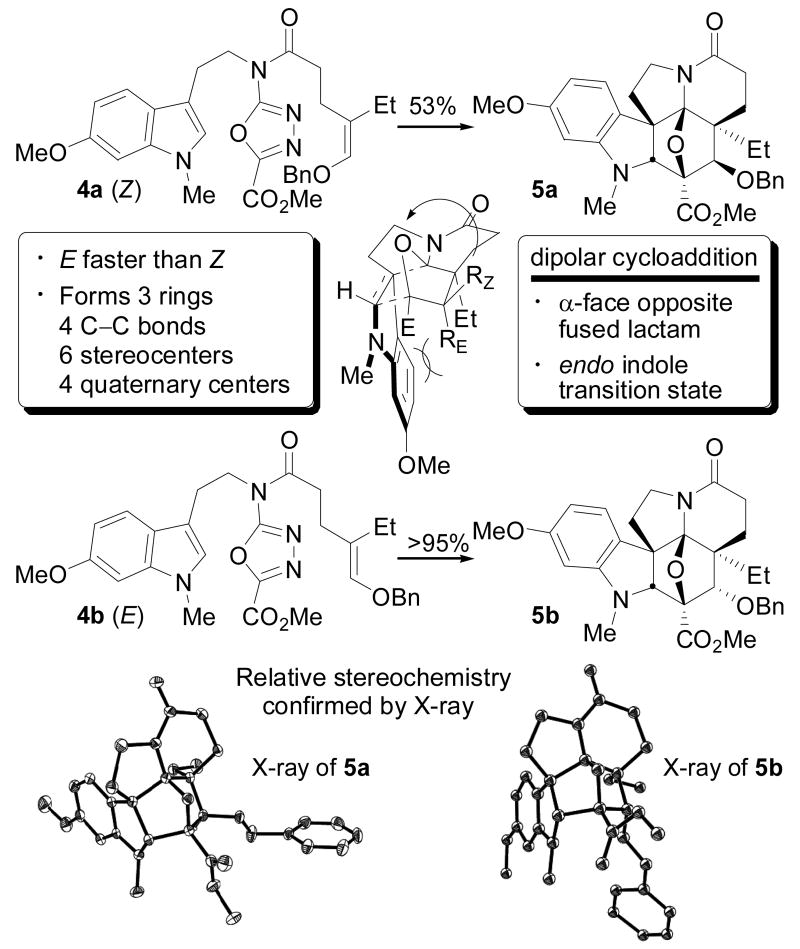
Figure 1b. Key cycloaddition cascade.
Herein, we detail two first generation total syntheses of vindoline that complement past efforts7,8,9 enlisting a unique tandem intramolecular [4+2]/[3+2] cycloaddition cascade of 1,3,4-oxadiazoles.10 In these studies, key issues regarding the scope and stereochemical features of the cycloaddition cascade were defined and its potential for use in the construction of the vindoline structure established. Thus, two concise routes to 3 are detailed enlisting the tandem cycloaddition reactions of either 1,3,4-oxadiazole 4a or 4b, each of which assembles the fully functionalized pentacyclic ring system of 3 with formation of four C–C bonds and three rings in a single step setting all six stereocenters within the central ring of vindoline including three contiguous and four total quaternary centers (Figure 1b).
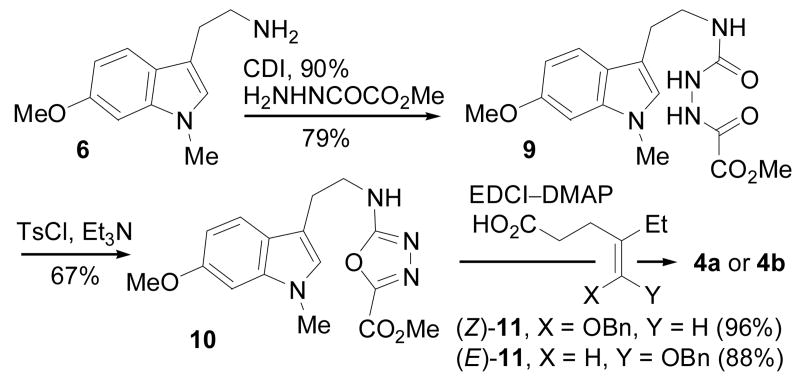
The preparation of the 1,3,4-oxadiazole precursors 4 is summarized in Scheme 1. Treatment of N-methyl-6-methoxytryptamine (6)11 with phenylcarbonate followed by hydrazine provided 7 (64%) which was coupled with methyl oxalate (EDCI, DMAP, CH2Cl2, 23 °C, 61%) to provide 9 (see supporting information). Alternatively, 6 was first treated with carbonyldiimidazole (CDI) to form 8 (90%) and subsequently treated with methyl oxalylhydrazide12 to furnish 9 (79%). Cyclization via dehydration of 9 (1.0 equiv of TsCl, 2.5 equiv of Et3N, CH2Cl2, 67%) provided 10 which was coupled with isomerically pure (Z)- or (E)-11 to provide 4a (96%) and 4b (88%).
Scheme 1.
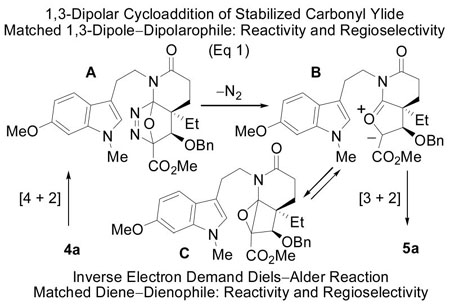
Both 4a and 4b undergo the tandem [4+2]/[3+2] cycloaddition cascade to give the pentacyclic products 5a and 5b, respectively, as single diastereomers (Figure 1b). The reaction leading to 5 is initiated by an intramolecular inverse electron demand Diels–Alder cycloaddition of the 1,3,4-oxadiazole with the tethered enol ether. Loss of N2 from the initial cycloadduct A provides the carbonyl ylide B (eq. 1), which undergoes a subsequent established13 1,3-dipolar cycloaddition with the tethered indole. Importantly, the diene and dienophile substituents complement and reinforce the [4+2] cycloaddition regioselectivity dictated by the linking tether, the intermediate 1,3-dipole is stabilized by the complementary substitution at the dipole termini, and the
tethered 1,3-dipolarophile (indole) complements the [3+2] cycloaddition regioselectivity that is dictated by the linker tether. The relative stereochemistry in each cycloadduct is controlled by a combination of (1) the dienophile geometry and (2) an exclusive endo indole [3+2] cycloaddition13 sterically directed to the α-face opposite the newly formed fused lactam. This endo diastereoselection for the 1,3-dipolar cycloaddition may be attributed to a conformational (strain) preference dictated by the dipolarophile tether since it mirrors the relative energy of the four possible products.10 The stereochemical assignments for 4a and 4b were based initially on their 1H NMR spectroscopic properties and unambiguously established by X-ray.14 Thus, the cycloaddition cascade of either 4a and 4b provides complete control of the intrinsic stereochemistry found in the pentacyclic skeleton of the Aspidosperma alkaloids, establishing all six stereocenters about the central six-membered ring and was designed to introduce essentially all the functionality found in 3. The distinction in the two cycloaddition cascades being that 4a permits the direct introduction of the naturally occurring C4 OAc β-stereochemistry, whereas 4b provides the C4 epimer requiring a subsequent inversion of configuration at this center. Although substrate 4a directly provides the preferred cycloadduct 5a for the use in the synthesis of 3, it proved to be more difficult to implement. The cyclization of 4a occurs in excellent yield when warmed in triisopropylbenzene (TIPB) for 60 h affording 5a as a single diastereomer (Figure 1b). Initial attempts to cyclize 4a provided 5a in low yield (<30% at >5 mM) which is in contrast to the cyclization of the 4b which provides the C4 diastereomer (84–99%). However, simple dilution of the reaction mixture led to improved yields (up to 53%). A study of this concentration effect is illustrated in Table 1 for both 4a and 4b, where a rather dramatic relationship between concentration and yield was found, especially for 4a, suggesting that a bimolecular 1,3-dipolar cycloaddition reaction of 4 may compete with the intramolecular cycloaddition cascade at the higher reaction concentrations. More significantly, we observed that the [4+2] cycloaddition is now the fast step in the reaction cascade and that the subsequent [3+2] cycloaddition is the slow step for 4a, a reversal of what is observed with 4b and other typical substrates.10 The net consequence is that longer reaction times and/or more vigorous reaction conditions, in conjunction with the use of a dilute reaction concentration, led to substantial improvements in the conversion of 4a. The origin of the distinctions in the slow step of the cycloaddition cascade 4a versus 4b is not yet clear. The 1,3-dipolar cycloaddition transition state for 4b embodies the more serious steric interactions disfavoring the indole endo approach on the α-face of the 1,3-dipole (Figure 1b), yet it progresses with a greater facility than that of 4a. It appears that the transition state for the 1,3-dipolar cycloaddition derived from 4a suffers a destabilizing electrostatic interaction of its central oxygen with the (Z)-OBn substituent (Figure 1b) that decelerates the reaction and/or that the (E)-OBn substituent of 4b stabilizes its transition state (transition state anomeric effect). However, it is also possible that the preferred stereochemistry of the corresponding cyclobutene epoxide C or their relative stability, a potential intermediate and reversible source of the 1,3-dipole, may dictate the relative ease of the 1,3-dipolar cycloaddition and studies to probe such questions are in progress.
Table 1.
Cycloaddition Concentration Dependence
| compound | conc. (mM) | % yield 5a or 5b |
|---|---|---|
| 4aa | 5 mM | 27-34% |
| 4aa | 1 mM | 43% |
| 4aa | 0.1 mM | 50% |
| 4aa | 0.05 mM | 53% |
| 4bb | 100 mM | 38% |
| 4bb | 50 mM | 58% |
| 4bb | 10 mM | 86% |
| 4bb | 5 mM | 90% |
| 4bb | 1 mM | 99% |
Condition: 230 °C, TIPB, 90 h.
Condition: 230 °C, TIPB, 20 h.
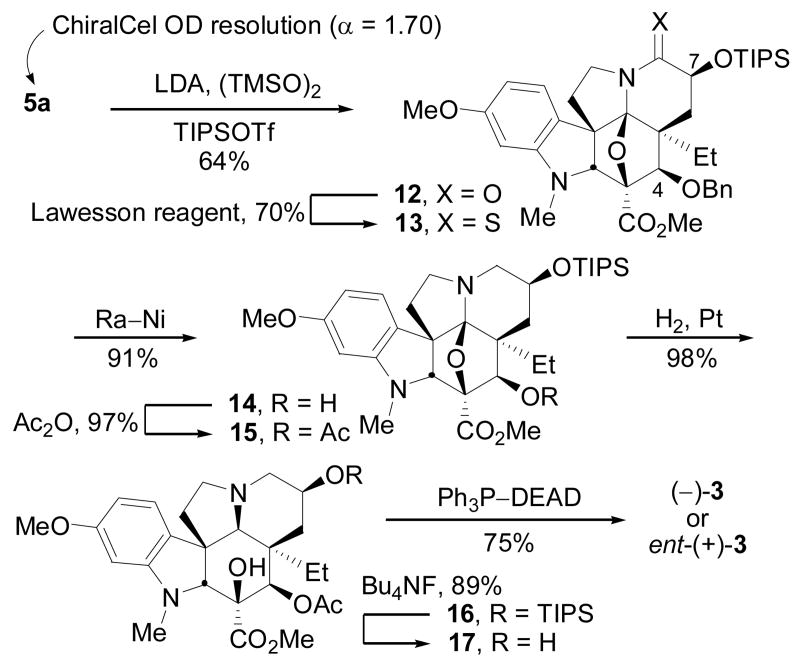
Although unanticipated, we found that the enantiomers of 5a could be easily separated on a semipreparative Chiralcel OD column (30% i-PrOH–hexanes, 2 × 25 cm) with a remarkable efficiency (α = 1.70, tR = 15.1 and 25.6 min, 10 mL/min) providing access to either of enantiomer on a preparatively useful scale (40–100 mg/injection). α-Hydroxylation of 5a was achieved best by treatment of the lactam enolate with TMSO–OTMS and was followed by a direct quench with TIPSOTf providing 12 (56–64%), Scheme 2 (natural enantiomer shown). The stereochemistry of the α-hydroxylation was clear from the 1H NMR of 12 (C7-H, J = 5.5, 11.7 Hz) and unambiguously determined by X-ray of the corresponding p-bromobenzoate.14 Conversion of 12 to thioamide 13 (70%) with Lawesson's reagent, reductive desulfurization with Ra–Ni conducted under conditions (15 h, 23 °C) that also served to cleave the benzyl ether provided 14 (91%), and subsequent acetylation of the resulting secondary alcohol afforded 15 (97%). Diastereoselective reductive cleavage of the oxido bridge upon catalytic hydrogenation (45 psi H2, PtO2, EtOAc) with reduction of the intermediate imminium ion from the α-face provided 16 (98%) analogous to observations first made by Padwa, 13 silyl ether cleavage (Bu4NF, 89%), and subsequent secondary alcohol activation and elimination of 17 (Ph3P, DEAD, THF, 23 °C) provided either (–)- or ent-(+)-vindoline (75%) identical in all respects with authentic material. The final conversion of 17 to 3 enlisting a Mitsunobu activation of the secondary alcohol for elimination may proceed via an intermediate aziridinium cation and relies on the reagent-derived base (EtO2CNHN−–CO2Et) to promote the regioselective elimination. This protocol proved much more effective and reproducible than alternative procedures that enlist reagents (e.g., Ph3P–CCl4)15 that generate reactive nucleophiles (e.g., Cl−) that can complete with the elimination reaction.
Scheme 2.
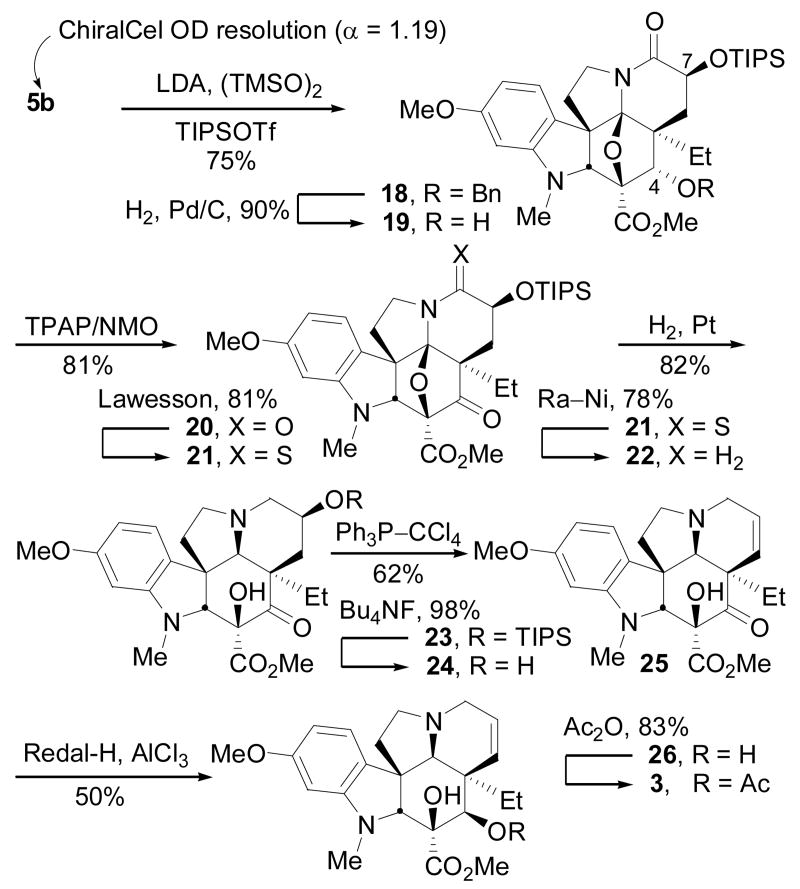
A second total synthesis of vindoline was accomplished utilizing cycloadduct 5b and was conducted while efforts to secure 5a were underway (Scheme 3). The advantage being that the key cycloaddition cascade proceeds with greater facility under milder conditions and in higher conversions (>95%), albeit in a route requiring inversion of the C4 stereochemistry. Thus, α-hydroxylation of 5b (LDA, TMSO–OTMS) followed by in situ treatment with TIPSOTf provided 18 in superb yield (75%). The stereochemistry of the intermediate alcohol, also obtained by a less effective reaction of the lactam enolate with Davis' reagent (40–50%), was established by 1H NMR (C7-H, J = 5.5, 12.1 Hz) and confirmed by X-ray.14 C4-Alcohol deprotection (H2, Pd/C, 90%) and oxidation of 19 with TPAP/NMO provided 20 (81%). Thiolactam formation (Lawesson's reagent, toluene, 81%) and Ra–Ni desulfurization of 21 (25 °C, 78%) preceded diastereoselective oxido bridge cleavage of 22 (H2, PtO2, MeOH, H+)13 to furnish 23 (82%). TIPS ether cleavage (Bu4NF, 98%) and treatment of 24 with Ph3P–CCl415 led to activation and elimination of the C7 alcohol to provide 25 (62%). Following the protocol of Büchi,8 diastereoselective C4 carbonyl reduction of 25 (Redal-H, AlCl3) and subsequent acetylation (Ac2O, 83%) of deacetylvindoline (26) furnished vindoline (3).
Scheme 3.
Supporting Information Available
Full experimental details are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We gratefully acknowledge the financial support of the National Institute of Health (CA42056) and the Skaggs Institute for Chemical Biology and wish to thank Y. Li for providing material at an intermediate stage of the studies.
References
- 1.Neuss N, Neuss MN. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. Academic; San Diego: 1990. p. 229. [Google Scholar]
- 2.a Noble RL, Beer CT, Cutts JH. Ann N Y Acad Sci. 1958;76:882. doi: 10.1111/j.1749-6632.1958.tb54906.x. [DOI] [PubMed] [Google Scholar]; b Noble RL. Lloydia. 1964;27:280. [Google Scholar]; c Svoboda GH, Nuess N, Gorman M. J Am Pharm Assoc Sci Ed. 1959;48:659. doi: 10.1002/jps.3030481115. [DOI] [PubMed] [Google Scholar]
- 3.a Owellen RI, Hartke CA, Dickerson RM, Haines FO. Cancer Res. 1976;36:1499. [PubMed] [Google Scholar]; b Pearce HL. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. Academic; San Diego: 1990. p. 145. [Google Scholar]
- 4.Moncrief JW, Lipscomb WN. J Am Chem Soc. 1965;87:4963. doi: 10.1021/ja00949a056. [DOI] [PubMed] [Google Scholar]
- 5.Gorman M, Neuss N, Biemann K. J Am Chem Soc. 1962;84:1058. [Google Scholar]
- 6.a Mangeney P, Andriamialisoa RZ, Langlois N, Langlois Y, Potier P. J Am Chem Soc. 1979;101:2243. doi: 10.1021/jo01336a006. [DOI] [PubMed] [Google Scholar]; b Kutney JP, Choi LSL, Nakano J, Tsukamoto H, McHugh M, Boulet CA. Heterocycles. 1988;27:1845. [Google Scholar]; c Kuehne ME, Matson PA, Bornmann WG. J Org Chem. 1991;56:513. [Google Scholar]; d Magnus P, Mendoza JS, Stamford A, Ladlow M, Willis P. J Am Chem Soc. 1992;114:10232. [Google Scholar]; e Yokoshima S, Ueda T, Kobayashi S, Sato A, Kuboyama T, Tokuyama H, Fukuyama T. J Am Chem Soc. 2002;124:2137. doi: 10.1021/ja0177049. [DOI] [PubMed] [Google Scholar]; Kuboyama T, Yokoshima S, Tokuyama H, Fukuyama T. Proc Natl Acad Sci USA. 2004;101:11966. doi: 10.1073/pnas.0401323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Review: Kuehne ME, Marko I. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. Academic; San Diego: 1990. p. 77.
- 8.Racemic total syntheses: Ando M, Büchi G, Ohnuma T. J Am Chem Soc. 1975;97:6880.Kutney JP, Bunzli-Trepp U, Chan KK, De Souza JP, Fujise Y, Honda T, Katsube J, Klein FK, Leutwiler A, Morehead S, Rohr M, Worth BR. J Am Chem Soc. 1978;100:4220.Andriamialisoa RZ, Langlois N, Langlois Y. J Org Chem. 1985;50:961. doi: 10.1021/jo01336a006. Formal racemic total syntheses: Ban Y, Sekine Y, Oishi T. Tetrahedron Lett. 1978;2:151.Takano S, Shishido K, Sato M, Yuta K, Ogasawara K. J Chem Soc Chem Commun. 1978:943.Takano S, Shishido K, Matsuzaka J, Sato M, Ogasawara K. Heterocycles. 1979;13:307.Danieli B, Lesma G, Palmisano G, Riva R. J Chem Soc Chem Commun. 1984:909.Danieli B, Lesma G, Palmisano G, Riva R. J Chem Soc Perkin Trans 1. 1987:155.Zhou S, Bommeziijn S, Murphy JA. Org Lett. 2002;4:443. doi: 10.1021/ol0171618.
- 9.Enantioselective total syntheses: Feldman PL, Rapoport H. J Am Chem Soc. 1987;109:1603.Kuehne ME, Podhorez DE, Mulamba T, Bornmann WG. J Org Chem. 1987;52:347.Kobayashi S, Ueda T, Fukuyama T. Synlett. 2000:883. Formal enantioselective total syntheses: Cardwell K, Hewitt B, Ladlow M, Magnus P. J Am Chem Soc. 1988;110:242.
- 10.a Wilkie GD, Elliott GI, Blagg BSJ, Wolkenberg S, Soenen DR, Miller MM, Pollack S, Boger DL. J Am Chem Soc. 2002;124:11292. doi: 10.1021/ja027533n. [DOI] [PubMed] [Google Scholar]; b Yuan ZQ, Ishikawa H, Boger DL. Org Lett. 2005;7:741. doi: 10.1021/ol050017s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takano S, Shishido K, Matsuzaka J, Sato M, Ogasawara K. Heterocycles. 1979;13:307. [Google Scholar]; An improved preparation is detailed in the supporting information.
- 12.Christl M, Lanzendoerfer U, Groetsch MM, Ditterich E, Hergmann J. Chem Ber. 1990;123:2031. [Google Scholar]
- 13.Padwa A, Price AT. J Org Chem. 1998;63:556. doi: 10.1021/jo971424n.1995;60:6258. Interestingly, intermolecular [3+2] variants proceed with indole exo cycloaddition directed to an analogous α-face, see: Muthusamy S, Gunanathan C, Babu SA. Tetrahedron Lett. 2001;42:523.
- 14.X-ray crystal structures of 5a (CCDC 253429), 5b (CCDC 253430), the C7 p-bromobenzoate of 12 (CCDC 255976), and the C7 free alcohol of 18 (CCDC 253428) have been deposited with the Cambridge Crystallographic Data Centre.
- 15.Kuehne ME, Okuniewicz FJ, Kirkemo CL, Bohnert JC. J Org Chem. 1982;47:1335. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full experimental details are provided. This material is available free of charge via the Internet at http://pubs.acs.org.