Abstract
Conventional substance abuse treatments have only had limited success for drugs such as cocaine, nicotine, methamphetamine, and phencyclidine. New approaches, including vaccination to block the effects of these drugs on the brain, are in advanced stages of development. Although several potential mechanisms for the effects of anti-drug vaccines have been suggested, the most straightforward and intuitive mechanism involves binding of the drug by antibodies in the bloodstream, thereby blocking entry and/or reducing the rate of entry of the drug into the central nervous system. The benefits of such antibodies on drug pharmacodynamics will be influenced by both the quantitative and the qualitative properties of the antibodies. The sum of these effects will determine the success of the clinical applications of anti-drug vaccines in addiction medicine. This review will discuss these issues and present the current status of vaccine development for nicotine, cocaine, methamphetamine, phencyclidine, and morphine.
Keywords: Substance Abuse, Vaccination, Theory, Cocaine, Methamphetamine, Nicotine, Phencyclidine, Morphine
INTRODUCTION
Substance abuse is a driving factor for many social ills that continue to plague almost every country in the world. Addiction provides the basis for the demand that underpins enormous flows of money and materiel across national borders into large cities, but increasingly into many suburban and rural areas as well. As an illegal activity, substance abuse results in crime, accidents, and social and economic disruptions which affect individuals, families, and entire communities. The prevalence of illicit drug experimentation and subsequent addictive use in the United States and around the world has progressed apace. Despite huge expenditures of time and money to combat drug addiction and drug trafficking (the War on Drugs) 1, billions of dollars continue to flow into the pockets of the people who grow, process and distribute these substances. Indeed, the love of money may be the root of all evil 2, but sadly, drug addicts water that root because of their drive to obtain the drugs they need. Simply proscribing drug use is no match for this drive, which leads to illicit behaviors by many addicts when the money to buy drugs almost inevitably runs out 3. At least a third of all federal and state prisoners for property crimes report that their offenses were done to obtain money for drugs 4. In 2004, more than 3% of people over the age of 12 in the United States used cocaine and/or methamphetamine, with the attendant negative consequences on education, employment, health, and behavior 1. Similar levels of abuse occur elsewhere in the developed world 5, underscoring the need for new approaches to address this problem. If an individual user could be made less susceptible to the psychological and physiological reinforcement from drug ingestion, the bonds of addiction might be more easily broken. Medications like naltrexone have benefited individual alcoholics, probably by reducing the reward that comes from ingesting alcohol and thus reducing dependence 6. However, the effects of this drug are transient, and require ongoing compliance to ensure benefits during periods when a subject ingests alcohol 7. Recently, a slow release form of naltrexone has been developed to enhance adherence, making it more effective in a substantial number of patients in Western countries8. Drug substitution can also assist in maintenance and/or withdrawal from smoking (nicotine patches or gum) 9 and opiate abuse (methodone) 10, but benefits in these addictions also require high levels of patient compliance. In addition, such pharmacological approaches are too expensive in developing countries, including China, which now faces a rapidly expanding epidemic of heroin abuse (see review by Liu and colleagues, this volume) 11. At present, there are no similarly effective pharmacological agents for cocaine or methamphetamine. A persistent reduction in the reward sensation from these drugs might be achieved, however, by an entirely different method: blocking the passage of the drugs into the brain with antibodies elicited by therapeutic vaccines. Such vaccines could also be valuable treatment tools for nicotine and morphine class drugs, especially in the developing world. This review will first discuss the immunological parameters governing the effects of the antibodies elicited by anti-drug vaccines on the pharmacodynamics of abused drugs. We will then review the current status of clinical vaccines, and will discuss pertinent animal studies. Vaccination resulting in long-term inhibition of the pharmacologic actions of abused drugs has great potential for assisting the motivated addict to begin and sustain abstinence from his specific substance of abuse.
ANTIBODY BINDING THEORY
If drug-conjugated vaccines can produce a high level of specific IgG antibodies, these molecules will bind and hold drug molecules within the circulation, preventing its access to the brain (see review by Rose, this volume). This blockade will have the effect of obviating the pharmacologic effects of the rapid rise of receptor occupation in the brain which is required to elicit maximal pleasurable and reinforcing effects of many abused drugs 12. Cocaine and methamphetamine, for example, bind dopamine transporters, while morphine and heroin metabolites bind mu opiate receptors. For each of these agents, both the rate and magnitude of receptor occupation determines the psychological experience from a dose of the drug. To understand how vaccination can effect a blockade of drug function, both the affinity of the antibody (e.g., how well the antibody can bind to a particular drug of interest) and the total amount of the antibody in circulation must be known in order to determine the percentage of a given amount of drug that can be bound. There will be a range of drug concentrations in different addicts, depending on the specific drug of abuse, the prior frequency and length of use, and the concurrent use of other drugs. Using cocaine as an example, published studies have clearly demonstrated that cocaine peak concentrations of 0.5 μM are pharmacologically active in addicts who have withdrawn from the drug 13. Under similar conditions, methamphetamine 14 and phencyclidine 15 have been shown to be pharmacologically active in the 0.5 to 1 μM range. Nicotine 16 and morphine levels can be active in similar concentrations, but in abusers, morphine is often found at higher concentrations17.
Fundamental features of IgG and hapten interactions have been described in detail elsewhere 18. A summary of the affinity calculation equations pertinent to this discussion follows: The single site interaction of two molecules (A + B ↔ AB) at equilibrium is governed by the law of mass action. The equilibrium constant (Ka) expressing the relationship of the bound (AB) and free species under specific concentration conditions (e.g., with the concentration of free species A represented by [A]) is defined by the equation:
The drugs of interest to this discussion are small molecules, and as a result, the binding of the drug (or hapten, a small single specificity antigen) and an antibody at one combining site can be described as a simple, bimolecular interaction. The association equilibrium binding constant Ka describes the combining site affinity, or strength of binding, and the larger the Ka, the stronger the binding. For a single combining site interaction, Ka is called the intrinsic affinity. IgG antibodies have 2 combining sites per molecule, while IgM antibodies have 10. The expressions that govern their behavior in solution are thus more complex, even though binding to and release from each individual combining site is largely independent of actions at other site. For IgG, the major antibody species of interest for this discussion, an antibody molecule and the target drug can combine to produce antibody-drug complexes with either one or two drug molecules bound. These complexes are generated by separate binding events, e.g., for A representing a divalent antibody molecule and D representing the drug molecule: A + D ↔ AD1, and AD1 + D ↔ AD1D2. Each interaction displays its own equilibrium binding equation as shown below, where [A] represents the concentration of unbound antibody, [D] represents that of free drug, [AD1] represents the concentration of antibody with one combining site occupied, and [AD1D2] represents that of antibody with both combining sites occupied:
As derived elsewhere 19, these values differ in a predictable way from the intrinsic affinity constant Ka. K1, which dominates at low drug concentrations, is related to the intrinsic combining site affinity by this equation: K1 = 2 × Ka . However, K2 reflects less avid binding (K2 = Ka /2) because only the second antibody binding site is available for binding the drug molecule when the first combining site is occupied, but once both sites are occupied, either drug molecule is equally likely to leave the antibody. When the concentration of a drug is relatively high in comparison to that of the antibody, a large fraction of the combining sites are occupied and K2 dominates the binding. Since K1 dominates at lower drug concentrations, the effective binding affinity of drug to antibody changes with the relative concentrations of the antibody and the drug. For the purpose of this discussion, the drug binding needed in the context of abused drugs will largely be governed by the K2 affinity of IgG antibodies. IgM, which usually has a combining site intrinsic affinity in the primary antibody response range (see below), can bind only relatively small amounts of drug using less than half of its sites in the low concentrations that can be achieved for IgM. For simplicity, we will not include IgM in the following discussions and will instead focus on IgG.
With the properties of antibody and hapten binding behavior defined, the percentage of a particular starting concentration of drug that is left unbound in solution as a function of the quality (affinity) and quantity (concentration) of the antibody can be directly calculated. For various concentrations of a drug in solution, Figure 1A shows the percent of unbound drug (Y axis) at equilibrium in the presence of increasing IgG antibody concentrations (X axis) with an average K2 affinity of 10 μM−1. For example, at an initial drug concentration of 1 μM, it can be calculated that 45 μg/mL will bind approximately 80% of the target drug in the bloodstream. At higher affinities (Figure 1B, 100 μM−1 K2 affinity), 80% of a 1.5 μM concentration can be bound by 45 μg/mL of antibody. At high drug concentrations, the curves become essentially linear so that there is little effect of affinity since all available antibody binding sites are occupied (e.g., at 2 and 2.5 μM initial drug concentrations, Figure 1A and 1B).
Figure 1. Drug Dose Effect on Binding at 10 and 100 μM−1 K2 Affinities.
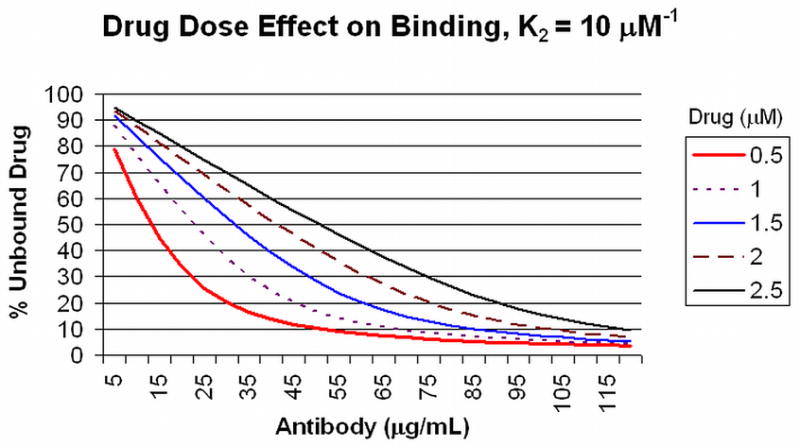
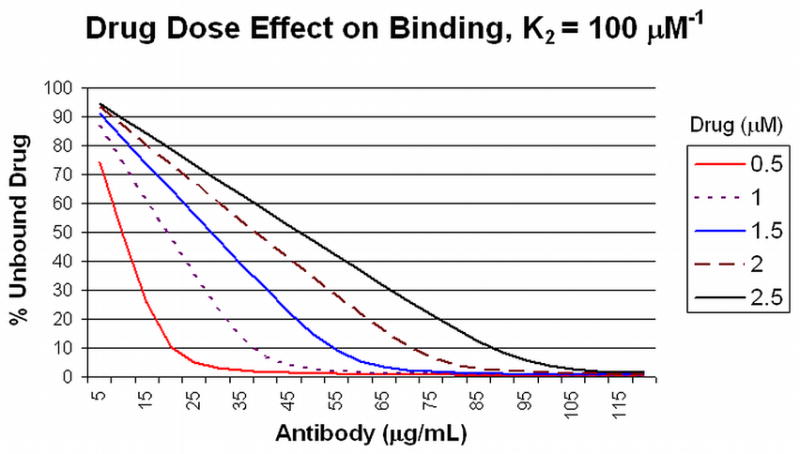
Panel A: The amount of unbound drug (Y axis) at equilibrium for different initial concentrations of drug (0.5 to 2.5 μM) is plotted against the amount of antibody (X axis) with a K2 binding affinity of 10 μM−1 (see text for discussion). Panel B: The same conditions are plotted for antibody with a K2 binding affinity of 100 μM−1.
Antibodies with average binding affinities of this magnitude and higher usually are expected in secondary responses to booster vaccine doses 20, when antibody concentrations of >40 μg/mL can be achieved from a good drug conjugate vaccine response in humans 21. Low affinity antibodies, such as those produced in response to the first dose of a vaccine that typically display affinities of about 1 μM−1) or antibodies present at low concentrations will not contribute significantly to drug binding in circulation at the relevant drug concentrations, as shown in Figure 2. In this figure, the different curves represent unbound drug at a total concentration of 0.5 μM, with increasing amounts of antibody and increasing K2 affinities. Even 45 μg/mL of antibody with a K2 affinity of 1.0 μM−1 will bind only 50% of the drug. As a result of these calculations, one can readily predict the amount of antibody that will need to be achieved in order to bind a defined proportion of drug at different initial drug concentrations. Antibodies with K2 affinities of 100 μM−1 and concentrations of 90 μg/mL will bind 90% of an initial 2.5 μM concentration of drug, for example (Figure 3). This antibody concentration can be achieved after booster injections in individuals who have received appropriately constructed conjugated vaccine with effective carriers and effective adjuvants. Achieving such high level responses in vaccine recipients should greatly reduce both the rate and the total drug accumulation in the brain from single doses.
Figure 2. Affinity Dependence of Binding at 0.5 μM.
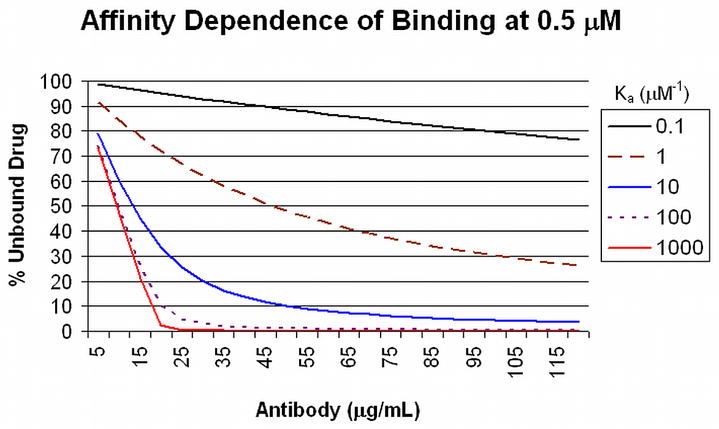
Drug Unbound drug at equilibrium from a starting concentration of 0.5 μM (Y axis) is plotted against the amount of antibody (X axis) for a range of K2 binding affinities (0.1 to 1000 μM−1).
Figure 3. Drug Dose and Binding Goal, K2 = 100 μM−1.
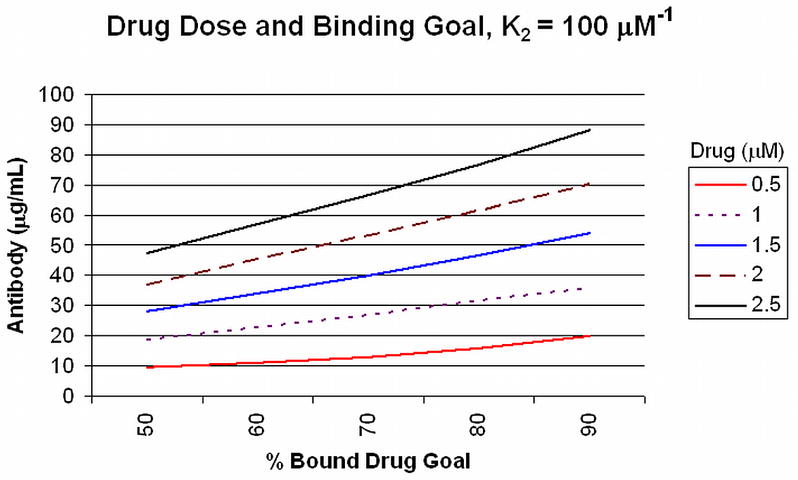
The amount of specific antibody (Y axis) required to achieve a specified proportion of drug binding (50–90%, X axis) is plotted for a range of initial drug concentrations (0.5 to 2.5 μM).
Another theoretical consideration for drug binding is the speed with which the antibodies can bind to a drug and thereby prevent its entry into the CNS. Most drugs of abuse are very rapidly taken up from the bloodstream; for example, some physiological and subjective effects from smoked cocaine or methamphetamine can be detected within 2 minutes after exposure 13. Antibody binding to hapten has been carefully studied over the years, and the “on” rate (the initial attachment of an antibody combining site to its specific target molecule) has been determined empirically in a number of model systems 22, 23. These initial antibody binding rates for these small molecules have been shown to be quite rapid for the purposes of this discussion. Under physiological conditions, essentially all binding in a well mixed sample occurs in less than one second 18. For ordinary administration routes and doses, the on rate of antibody binding is expected to be fast enough that drug binding will take place well before the transfer of large amounts of drug from the bloodstream to the brain begins to occur.
ANTIBODY EFFECTS ON DRUG PHARMACODYNAMICS AND PHARMACOKINETICS
Most current ideas about reward from addictive substances combine aspects of “rate” and equilibrium binding pharmacological theories to explain observations relating to drug action. The influence of both drug concentrations and pharmacodynamic features for cocaine, methamphetamine and opiods are important for their physiological and subjective effects. Thus, a marked reduction in free drug concentration in the blood by binding to specific antibodies would significantly inhibit drug action in the brain (e.g., if 75–90% of the drug is bound by high affinity antibody), since IgG-bound drug cannot readily cross the normal, uninflamed blood-brain barrier. In addition, it is also well known that the rate of increase in receptor occupation in the CNS has a profound influence on the subjective effects of each of these drugs 24. Thus, less dramatic binding of the drug even by lower affinity antibody (e.g., resulting in 50% binding) could still have a substantial influence on the rate of entry of free drug into the CNS. Even if the eventual total accumulation in the brain is similar to what would be achieved in the absence of antibody, the subjective CNS effects of the drug may be substantially or completely blunted, since the rate of receptor occupancy would be significantly reduced.
The pharmacokinetics of antibody-bound drug are thus related to the effects of antibody binding on a drug’s metabolism, tissue distribution, and elimination pathways, as well as the antibody’s intrinsic half life and the effect of drug binding on antibody half life through changes in the antibody structure, if any. Antibody binding to morphine, for example, prolongs morphine’s terminal half life in the blood stream of experimental animals by 2–3 fold 25, with essentially no effect on drug metabolism. In a vaccine study in rats, on the other hand, antibody binding of cocaine was shown to have little effect on cocaine half life or metabolism, as well as no effect on clearance of the antibody 26.
Some antibodies can display catalytic properties, however. Selected monoclonal antibodies can enhance cocaine hydrolysis and thus speed the metabolic degradation of this drug in vivo 27. However, a vaccine that would elicit such catalytic antibodies from active immunization would be very difficult to design, given the broad variation in the structural features of the polyclonal antibodies elicited through immunization. Similar to morphine and in contrast to cocaine, anti-methamphetamine antibodies have been shown to decrease methamphetamine clearance, prolong methamphetamine concentrations in serum, reduce conversion to amphetamine, and increase uptake in reticuloendothelial tissues like the liver 28. These features may well be related to the longer biological half life displayed by methamphetamine in comparison to cocaine, especially to the fact that a substantial fraction of methamphetamine is not metabolized, but rather excreted unchanged in the urine. Nicotine also has a longer half life in the body than cocaine 29, and the effects of antibodies on nicotine pharmacokinetics have been shown to resemble those on methamphetamine, with higher plasma concentrations after nicotine doses 30–32, and a half life that is prolonged 3–6 fold.
A theoretical concern is that the potential high affinity of the anti-drug antibodies for drug metabolites, which, if present in high concentrations, may reduce the amount of antibody available for native drug binding. This is particularly a concern with cocaine. Benzoylecgonine is produced by hydrolysis of cocaine’s methyl ester moiety, is structurally very similar to cocaine, and is essentially inactive pharmacologically. Heavy use of cocaine will result in substantial concentrations of benzoylecgonine in plasma, up to 10 fold higher than the peak cocaine concentrations 33. Other metabolites, such as ecgonine methyl ester, and norcocaine are present in concentrations lower than cocaine itself. The half life of benzoylecgonine is longer than that of cocaine, contributing to the high concentrations observed 34. Nicotine and its major metabolites, e.g., cotinine, also present concerns regarding antibody binding competition. On the other hand, significant cross reactivity of anti-methamphetamine antibodies with amphetamine, a major methamphetamine metabolite, would be very desirable since amphetamine itself is pharmacologically active. Similarly, heroin is rapidly metabolized to 6-acetylmorphine and morphine in both the periphery and in the CNS 35. Both of these heroin metabolites are pharmacologically active. Fortunately, morphine conjugate vaccines can elicit antibodies capable of recognizing all three compounds 36.
STATUS OF SPECIFIC DRUG VACCINES
Clinical trials with nicotine and cocaine vaccines have shown considerable promise, but still need improvement in the magnitude and consistency of the antibody responses. Antibody strategies for methamphetamine, morphine, and phencyclidine are still in preclinical development. A table of vaccines in clinical studies is show below (Table I).
TABLE I.
Current Status of Drug Vaccines in Clinical Trials
| Vaccine | Drug-Carrier | Human Studies |
|---|---|---|
| TA-NIC | Nicotine - cholera toxin b | Phase I, Phase II |
| TA-CD | Cocaine - cholera toxin b | Phase I, Phase IIa, Phase IIb |
| NicVax | Nicotine - Pseudomonas exoprotein A. | Phase I, Phase II |
| NicQb | Nicotine – virus like particle | Phase I, Phase II, Phase IIb |
NICOTINE
Nicotine is a very important therapeutic vaccine target, given the well known adverse health consequences of smoking 9, which kills over 400,000 people in the U. S. annually, and at least 10 times that many around the world. Although a large proportion of smokers would like to quit the habit, most have a very difficult time doing so due to the addictive properties of nicotine. Numerous medications, counseling programs, and other treatments are available to help addicted individuals stop smoking, but the success rate for breaking addiction to nicotine in long term smokers is disappointingly low 37.
The nicotine vaccines now advancing through clinical trials offer some new hope for motivated individuals to finally kick the habit (see also Rose, this volume). As discussed above, specific antibodies in sufficient quantity to reduce free nicotine concentrations in the blood inhibit the magnitude and rate of accumulation of nicotine in the brain. These beneficial effects have been shown clearly in animal models regarding administered nicotine 38–40. Levels of nicotine in the plasma of smokers are in the appropriate range for the effects discussed in the theory section41; however, unlike cocaine and methamphetamine, where abuse usually results in sporadically administered doses, nicotine is most often abused with very frequent small doses, as in the “pack a day” smoker. As a result, the total accumulated dose can be quite large and produce persistently high plasma concentrations over long time periods. On the other hand, for a motivated smoker who has quit temporarily, relapse to smoking often occurs from taking a few puffs or smoking a few cigarettes after a period of abstinence. As Mark Twain famously said, “It’s easy to quit smoking. I’ve done it hundreds of times.” In that context, the presence of substantial quantities of antinicotine antibodies could be expected to inhibit the reinforcing response to a modest nicotine exposure, reducing the risk of relapse.
All three of the current nicotine conjugate vaccines in clinical trials (NicVax, NicQb and TANIC) were well tolerated in phase 1 trials with no evidence of untoward cross reactivity with endogenous neurotransmitters or other signaling molecules. In an animal model, NicVax stimulated high level antibody responses up to 130 μg/mL with a reasonable average binding affinity (26 μM−1), and a low cross reactivity with major nicotine metabolites (cotinine and nicotine-N-oxide)42. In humans, these antibody concentrations should be very effective at binding the expected quantities of nicotine absorbed from smoking a few cigarettes21. In fact data reported from a clinical study that was designed to test safety in an escalating dose design demonstrated that more subjects with high antibody responses quit smoking during the trial than those with lower antibody responses. These observations were striking since subjects were not specifically asked to quit smoking.
The NicQb vaccine also elicited significant quantities of anti-nicotine antibodies 43. Subjects who were among the upper third in their ability to mount antibody responses displayed quit rates that were almost double those of placebo-treated subjects (57% vs 31%).
Subjects in the TA-NIC vaccine trial were immunized with 4 doses over the first 8 weeks and then given a booster dose at 32 weeks. All subjects were encouraged to quit smoking after 12 weeks of the trial. At 12 months, the quit rate in the highest dose group considerably exceeded the control group (38% vs 8%) 44.
These studies thus suggest that high antibody titers correlate with smoking cessation. Evaluations of the nicotine conjugate vaccines are moving forward with phase IIb/III trials that aim to document efficacy as well as safety planned for NicQb 45.
COCAINE
Government surveys indicate that 2.4 million or more Americans age 12 or older are current users of cocaine 1. Its use has penetrated all levels of society, and the ills it has created are evident in both crime (about 30% of federal and state prisoners were regular cocaine users before incarceration) and health statistics (from overcrowded emergency rooms to individual acute psychotic reactions, heart attacks, or strokes). As with tobacco abuse, a substantial number of addicted users eventually gain the desire but lack the ability to stop using the drug.
Behavioral interventions can be helpful in treating cocaine addiction in a limited number of abusers, but currently there are no approved medications to treat this disorder. Conjugate vaccines for cocaine abuse are in development. Both phase I and II trials have been completed using a cholera toxin B conjugated COC preparation (TA-CD) 46, 47 (Martel, et al, 2008 submitted). Animal studies have shown that adequate amounts of antibody reduce cocaine uptake in the brain of rats and demonstrate good inhibition of both locomotor activity and reinstatement of cocaine self administration 26, 48–50. For the purposes of human applications, the latter is probably the most important finding, since, as with nicotine, the most important likely benefit of vaccination would be to inhibit reinforcing effects of cocaine reexposure after a period of abstinence. If we could substantially block the pleasurable effects of such reexposures and reduce the subsequent craving response, it would help a great deal in preventing a resumption of regular drug use for those motivated users who succumb temporarily to the temptation of use brought on by social pressures or an individual stressful event.
In human studies, phase I and early phase II trials of the TA-CD vaccine immunogenicity, safety, and efficacy, the vaccine showed reductions in cocaine effects during human laboratory cocaine administration and in cocaine use in outpatient studies 47, 50, 51. The vaccine was tolerated with no serious adverse effects during 12 months of follow-up 51. In a Phase IIa, 14 week trial of eighteen cocaine dependent subjects in early recovery, the vaccine was well tolerated at two dose levels (100 μg × 4 injections, or 400 μg × 5 injections). Cocaine-specific antibodies persisted in sera of immunized individuals for at least six months 47. Furthermore, subjects who received the higher dose of vaccine displayed significantly higher mean antibody titers and were more likely to maintain cocaine-free urines than individuals who received the lower dose group 47. In a Phase IIb study with the TA-CD vaccine in 114 methadone clinic patients, substantial quantities of antibody were elicited in about one third of patients (17/53 vaccinated subjects who could be evaluated), with IgG concentrations above 40 μg/mL (Martell, 2008 submitted). Higher levels of antibody were correlated with periods of reduced cocaine use in a substantial number of these subjects, as determined by urine monitoring.
Of particular interest in these trials were observations that about 25% of vaccinated patients had very low IgG responses to vaccination yet displayed substantial levels of anti-cocaine IgM antibodies at baseline. Deng, et al. 52 reported detection of antibodies against cocaine in unvaccinated addicts, and demonstrated that covalent conjugates of cocaine with native serum proteins were likely to have formed in such individuals. Exactly how such spontaneous anticocaine immune responses might lead to relative unresponsiveness to vaccination is an area of current investigation in our laboratories. Taken together, these results demonstrate that a cocaine specific vaccine can elicit an immunologic response sufficient to reduce cocaine usage and attenuate the self-reported psychological effects of cocaine. It may be possible to override the effects of the vaccine by increasing the amount of cocaine used; a few subjects increased cocaine use during the period of high antibody titers, as indicated by quantitative testing of their urine for the major excreted cocaine matabolite benzoylecgonine. A cocaine vaccine might thus be of primary utility in abusers who are motivated to quit. Other conjugate anti-cocaine vaccines are in preclinical development.
PHENCYCLIDINE
Phencyclidine (PCP) is an N-methyl-D-aspartate (NMDA) receptor antagonist that became a popular drug of abuse in some areas of the US, and spawned production of many related chemical derivatives (“designer drugs”). While PCP dependence per se has been relatively uncommon, acute toxicity from phencyclidine remains an important problem. Vaccine development for PCP thus has had a goal of eliciting antibodies having a broad specificity for binding to a variety of PCP-related compounds. Animal studies with PCP conjugate vaccines have shown that substantial quantities of antibodies can reduce the accumulation of PCP in the brain 53, 54. Such antibodies can block the behavioral effects of PCP on locomotor activity and posturing 55, 56. Passively administered polyclonal and monoclonal anti-PCP antibodies can also reverse the toxic effects of high PCP doses 55, 56, suggesting that they may prove useful in treating patients who overdose on this drug. Interestingly, protection against PCP CNS effects from passively infused antibodies lasts longer than would be predicted based on drug pharmacokinetics and antibody half life. Although the mechanism of this effect remains obscure, protection can persist for up to a month after administration, despite continuous infusion of PCP at a rate that far exceeds the binding capacity of the antibody 53. Further development of a clinically effective vaccine may have to await development of vaccines for other, more common drugs of abuse, however, given the modest size of the market for potential active or passive anti-PCP therapeutics.
METHAMPHETAMINE
Methamphetamine is a widely abused stimulant, with 1.3 million current year users in 2005 1. Methamphetamine use engenders substantial negative consequences for both the individual user’s health and for society in general. It is a stimulant that blocks plasma membrane monoamine transporters as well as causing the release of monoamines from synaptic vesicles, leading to activation of dopamine and other monamine receptors in the CNS in ways that overlap with cocaine. Methamphetamine is also highly addictive; individuals with the motivation to discontinue methamphetamine often find it exceptionally difficult to stop. In contrast to cocaine, which is rapidly metabolized to inactive major metabolites, methamphetamine has a longer half life and is metabolized in part to amphetamine, which is also a powerful stimulant. With no medications currently approved for treatment of addiction to methamphetamine, it is also an important target for a therapeutic vaccine, particularly if high level cross-reactivity for amphetamine can be elicited. It is a small molecule with a molecular weight of 149, but like nicotine (with a molecular weight of 162) and the other drugs discussed, appropriate conjugation to a carrier protein permits antibody responses to it as an antigenic hapten 21.
Animal studies of methamphetamine vaccines are in the early stages, although passive administration of monoclonal antibodies have been shown to reduce methamphetamine self-administration in rats 57, and to reduce locomotor activity in rats given high dose methamphetamine 58, 59.
Recent studies in our laboratory have shown that high titer antibodies can be elicited by administration of methamphetamine conjugates in mice, depending on the conjugate construction and the adjuvants used. The antibodies produced inhibit methamphetamine-stimulated locomotor activity to a degree that is roughly proportional to the amount of antibody, using methamphetamine doses selected to model those expected in drug abusers (Orson, unpublished). Thus, successful development of methamphetamine-targeted vaccines would be expected to provide an effective clinical tool to assist in the management of those addicted to this highly addictive stimulant.
HEROIN AND MORPHINE
Since the introduction of methadone and other pharmacological agents for opiate addictions, many substance abuse programs for opiates have been successfully implemented in developed countries 10. These pharmacological successes reduced efforts to create vaccines against heroin and morphine 60, 61.
However, as drug abuse has increased in the developing world in ways that act as a vector for the spread of HIV/AIDS, there is now a renewed interest in such vaccines against opiates. The economics of pharmacological and behavioral treatments make current substance abuse programs less feasible than vaccination in some settings. For example, in the early 1980s, an unprecedented era of drug abuse began to unfold in China, particularly involving heroin (see review by Liu and colleagues, this volume). The number of registered drug users there increased more than 10-fold from 70,000 in 1990 to more than one million by the end of 2004 62. Heroin accounted for 75–85% of all drug use 11, and more than half (51.2%) of the Chinese people who are HIV positive them are intravenous drugs users 63. Similar observations have been made in India 64 and Russia 65. If a vaccine can be developed to slow or even reverse opiate abuse, the consequences for criminal behavior, social stability, and transmission of HIV/AIDS and other blood born or sexually transmitted diseases would be enormous 3.
Early studies had shown that conjugation of morphine to proteins through a derivative of its 6-hydroxyl group produced a vaccine that could elicit a polyclonal antibody response that bound heroin and 6-acetylmorphine, as well as morphine itself 66. This cross reactivity is critically important, since heroin is a prodrug that is rapidly converted to the pharmacologically active opiates 6-acetyl morphine and morphine by esterases present in both the periphery and the central nervous system 35. Anton and Lef 67 among others 68 recently demonstrated high level antibody responses in rodents to a 6-succinylmorphine conjugate vaccine. Earlier experiments by others in rodents showed antibody blockade with efficacy that was directly related to the plasma levels of morphine and the antibody 60. Most importantly, self-administration of heroin could be reduced in rhesus monkeys after similar active immunizations 61. Berkowitz and Spector 36 demonstrated that this effect was at least in part due to sequestration of the drug in the blood by antibody binding with correspondingly-prolonged drug half lives. Labeled morphine could be displaced from this binding by later addition of unlabeled morphine, indicating that bound drug is eventually depleted by gradual release with subsequent metabolism and/or elimination. In these studies, the authors also demonstrated that the antibodies were saturable, so that higher doses of drug could overcome the binding capacity of circulating antibodies. Since the range of opiate doses used by human opiate abusers can be very broad 17, it will be critical for vaccines to be capable of eliciting large quantities of high quality antibody for treatment of heroin or morphine addicts.
CONCLUSIONS
A number of addictive drugs have pharmacokinetic and pharmacodynamic characteristics that make them viable targets for vaccine development. On a theoretical basis, from the known properties of antibodies and the drug concentrations in blood expected for the abused drugs discussed, the quantity and quality of antibody elicited by an efficacious vaccine should be sufficient to reduce or block drug effects, often by both reducing and slowing of the accumulation of drug in the brain. Animal studies with several conjugate drug vaccines and human studies with nicotine and cocaine vaccines have all shown promising results. Blocking immediate behavioral and toxic drug effects is valuable, but even more promising from the addiction perspective is the inhibition of drug reinforcement, or craving, which will be necessary to help prevent relapse to drug use by individuals motivated to quit. According to some animal experiments, the effects on reinforcement may not require levels of antibody blocking as high as would be expected for inhibition of the acute drug effects, a property that could dramatically extend the benefits of this approach to therapy. Drug vaccines should also very effectively complement current counseling programs and potential future small molecule medications that may be developed to treat the growing worldwide problems posed by the addictions. Advances in vaccine conjugate design, carrier protein use, and especially adjuvant optimization will significantly enhance the quantity and quality of the antibodies produced, allowing drug vaccines to become useful clinical tools for the treatment of substance abuse.
Acknowledgments
Supported by the Department of Veterans Affairs (VA) Merit Review Program and VISN 16 Mental Illness Research, Education and Clinical Center (MIRECC), the VA National Substance Use Disorders Quality Enhancement Research Initiative (QUERI), and the National Institute on Drug Abuse grants K05 DA 0454 (TRK), P50-DA18197.
ABBREVIATIONS
- COC
Cocaine
- CNS
Central Nervous System
- CTB
Cholera Toxin B
- ELISA
Enzyme Linked Immunosorbent Assay
- MA
Methamphetamine
- NIC
Nicotine
- PCP
Phencyclidine
References
- 1.National Survey on Drug Use and Health: National Findings. [Accessed June 2007];Office of Applied Studies, NSDUH Series H-30, DHHA Publication No. SMA 06-4194, Rockville, MD. 2006 Available at http://www.drugabusestatistics.samhsa.gov/
- 2.Paul S. 1 Timothy 6:10, Bible (King James Version) [Accessed January 2008]; Available at http://www.holybible.com/resources/KJV_DFND/index.php?Book=68&mode=4&BookTitle=1%20Timothy&Chapter=6.
- 3.DeBeck K, et al. Income generating activities of people who inject drugs. Drug Alcohol Depend. 2007;91:50–6. doi: 10.1016/j.drugalcdep.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drug Related Crime. [Accessed December 2007];2006 Available at http://www.whitehousedrugpolicy.gov/publications/factsht/crime/index.html.
- 5.EMCDDA Annual report 2006. [Accessed June 2007];2006 Available at http://www.emcdda.europa.eu/
- 6.Mitchell JM, et al. Endogenous opioid blockade and impulsive responding in alcoholics and healthy controls. Neuropsychopharmacology. 2007;32:439–49. doi: 10.1038/sj.npp.1301226. [DOI] [PubMed] [Google Scholar]
- 7.Sinclair JD, Alho H, Shinderman M. Naltrexone for alcohol dependence. N Engl J Med. 2002;346:1329–31. author reply 1329–31. [PubMed] [Google Scholar]
- 8.Johnson BA. A synopsis of the pharmacological rationale, properties and therapeutic effects of depot preparations of naltrexone for treating alcohol dependence. Expert Opin Pharmacother. 2006;7:1065–73. doi: 10.1517/14656566.7.8.1065. [DOI] [PubMed] [Google Scholar]
- 9.Tobacco Use in America. [Accessed June 2007];2001 Available at http://www.oas.samhsa.gov/NHSDA/tobacco/chapter1.htm.
- 10.Millson P, et al. Reduction in injection-related HIV risk after 6 months in a low-threshold methadone treatment program. AIDS Educ Prev. 2007;19:124–36. doi: 10.1521/aeap.2007.19.2.124. [DOI] [PubMed] [Google Scholar]
- 11.Liu ZM, et al. Epidemiological features of drug abusers in China. Journal of Drug Abuse Prevention and Treatment. 2002;8:27–30. [Google Scholar]
- 12.Kosten T, Owens SM. Immunotherapy for the treatment of drug abuse. Pharmacol Ther. 2005;108:76–85. doi: 10.1016/j.pharmthera.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins AJ, et al. Correlation between pharmacological effects and plasma cocaine concentrations after smoked administration. J Anal Toxicol. 2002;26:382–92. doi: 10.1093/jat/26.7.382. [DOI] [PubMed] [Google Scholar]
- 14.Cook CE, et al. Pharmacokinetics of methamphetamine self-administered to human subjects by smoking S-(+)-methamphetamine hydrochloride. Drug Metab Dispos. 1993;21:717–23. [PubMed] [Google Scholar]
- 15.Kunsman GW, et al. Phencyclidine blood concentrations in DRE cases. J Anal Toxicol. 1997;21:498–502. doi: 10.1093/jat/21.6.498. [DOI] [PubMed] [Google Scholar]
- 16.Russell MA, Feyerabend C, Cole PV. Plasma nicotine levels after cigarette smoking and chewing nicotine gum. Br Med J. 1976;1:1043–6. doi: 10.1136/bmj.1.6017.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rook EJ, et al. Pharmacokinetics and pharmacodynamics of high doses of pharmaceutically prepared heroin, by intravenous or by inhalation route in opioid-dependent patients. Basic Clin Pharmacol Toxicol. 2006;98:86–96. doi: 10.1111/j.1742-7843.2006.pto_233.x. [DOI] [PubMed] [Google Scholar]
- 18.Day ED. Advanced Immunochemistry. Wiley-Liss; New York: 1990. [Google Scholar]
- 19.Eisen HN. Immunology. Harper & Row; New York: 1980. [Google Scholar]
- 20.Eisen HN, Siskind GW. Variations in Affinities of Antibodies During the Immune Response. Biochemistry. 1964;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- 21.Hatsukami DK, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther. 2005;78:456–67. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Barbet J, et al. Structural requirements for recognition of vasopressin by antibody; thermodynamic and kinetic characteristics of the interaction. Mol Immunol. 1981;18:439–46. doi: 10.1016/0161-5890(81)90106-1. [DOI] [PubMed] [Google Scholar]
- 23.Smith TW, Skubitz KM. Kinetics in interactions between antibodies and haptens. Biochemistry. 1975;14:1496–1502. doi: 10.1021/bi00678a023. [DOI] [PubMed] [Google Scholar]
- 24.Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J Exp Anal Behav. 1973;20:119–29. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill JH, et al. Delayed clearance of morphine from the circulation of rabbits immunized with morphine-6-hemisuccinate bovine serum albumin. J Immunol. 1975;114:1363–8. [PubMed] [Google Scholar]
- 26.Fox BS, et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med. 1996;2:1129–32. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- 27.Matsushita M, et al. Cocaine catalytic antibodies: the primary importance of linker effects. Bioorg Med Chem Lett. 2001;11:87–90. doi: 10.1016/s0960-894x(00)00659-4. [DOI] [PubMed] [Google Scholar]
- 28.Laurenzana EM, et al. Use of anti-(+)-methamphetamine monoclonal antibody to significantly alter (+)-methamphetamine and (+)-amphetamine disposition in rats. Drug Metab Dispos. 2003;31:1320–6. doi: 10.1124/dmd.31.11.1320. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Stable EJ, et al. Nicotine metabolism and intake in black and white smokers. Jama. 1998;280:152–6. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 30.Keyler DE, et al. Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine Tob Res. 1999;1:241–9. doi: 10.1080/14622299050011361. [DOI] [PubMed] [Google Scholar]
- 31.Keyler DE, et al. Monoclonal nicotine-specific antibodies reduce nicotine distribution to brain in rats: dose- and affinity-response relationships. Drug Metab Dispos. 2005;33:1056–61. doi: 10.1124/dmd.105.004234. [DOI] [PubMed] [Google Scholar]
- 32.Pentel PR, et al. Differential effects of passive immunization with nicotine-specific antibodies on the acute and chronic distribution of nicotine to brain in rats. J Pharmacol Exp Ther. 2006;317:660–6. doi: 10.1124/jpet.105.097873. [DOI] [PubMed] [Google Scholar]
- 33.Williams RH, et al. Cocaine and its major metabolites in plasma and urine samples from patients in an urban emergency medicine setting. J Anal Toxicol. 2000;24:478–81. doi: 10.1093/jat/24.7.478. [DOI] [PubMed] [Google Scholar]
- 34.Jufer RA, et al. Elimination of cocaine and metabolites in plasma, saliva, and urine following repeated oral administration to human volunteers. J Anal Toxicol. 2000;24:467–77. doi: 10.1093/jat/24.7.467. [DOI] [PubMed] [Google Scholar]
- 35.Inturrisi CE, et al. Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci. 1983;33 Suppl 1:773–6. doi: 10.1016/0024-3205(83)90616-1. [DOI] [PubMed] [Google Scholar]
- 36.Berkowitz BA, Ceretta KV, Spector S. Influence of active and passive immunity on the disposition of dihydromorphine-H3. Life Sci. 1974;15:1017–28. doi: 10.1016/0024-3205(74)90016-2. [DOI] [PubMed] [Google Scholar]
- 37.Fiore MC, et al. Treating Tobacco Use and Dependence. 2000. [Accessed June 2007]. Available at. [Google Scholar]
- 38.Hieda Y, et al. Vaccination against nicotine during continued nicotine administration in rats: immunogenicity of the vaccine and effects on nicotine distribution to brain. Int J Immunopharmacol. 2000;22:809–19. doi: 10.1016/s0192-0561(00)00042-4. [DOI] [PubMed] [Google Scholar]
- 39.Hieda Y, et al. Immunization of rats reduces nicotine distribution to brain. Psychopharmacology (Berl) 1999;143:150–7. doi: 10.1007/s002130050930. [DOI] [PubMed] [Google Scholar]
- 40.Pentel PR, et al. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol Biochem Behav. 2000;65:191–8. doi: 10.1016/s0091-3057(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 41.Sobue S, et al. Comparison of nicotine pharmacokinetics in healthy Japanese male smokers following application of the transdermal nicotine patch and cigarette smoking. Biol Pharm Bull. 2006;29:1068–73. doi: 10.1248/bpb.29.1068. [DOI] [PubMed] [Google Scholar]
- 42.Hieda Y, et al. Active immunization alters the plasma nicotine concentration in rats. J Pharmacol Exp Ther. 1997;283:1076–81. [PubMed] [Google Scholar]
- 43.Maurer P, et al. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. Eur J Immunol. 2005;35:2031–40. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- 44.LeSage MG, Keyler DE, Pentel PR. Current status of immunologic approaches to treating tobacco dependence: vaccines and nicotine-specific antibodies. Aaps J. 2006;8:E65–75. doi: 10.1208/aapsj080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heading CE. Drug evaluation: CYT-002-NicQb, a therapeutic vaccine for the treatment of nicotine addiction. Curr Opin Investig Drugs. 2007;8:71–7. [PubMed] [Google Scholar]
- 46.Haney M, Kosten TR. Therapeutic vaccines for substance dependence. Expert Rev Vaccines. 2004;3:11–8. doi: 10.1586/14760584.3.1.11. [DOI] [PubMed] [Google Scholar]
- 47.Martell BA, et al. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58:158–64. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 48.Carrera MR, et al. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378:727–30. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- 49.Carrera MR, et al. Cocaine vaccines: antibody protection against relapse in a rat model. Proc Natl Acad Sci U S A. 2000;97:6202–6. doi: 10.1073/pnas.97.11.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kantak KM, et al. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berl) 2000;148:251–62. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- 51.Kosten TR, et al. Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine. 2002;20:1196–204. doi: 10.1016/s0264-410x(01)00425-x. [DOI] [PubMed] [Google Scholar]
- 52.Deng SX, et al. Covalent modification of proteins by cocaine. Proc Natl Acad Sci U S A. 2002;99:3412–6. doi: 10.1073/pnas.042700599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Proksch JW, Gentry WB, Owens SM. Anti-phencyclidine monoclonal antibodies provide long-term reductions in brain phencyclidine concentrations during chronic phencyclidine administration in rats. J Pharmacol Exp Ther. 2000;292:831–7. [PubMed] [Google Scholar]
- 54.Valentine JL, Owens SM. Antiphencyclidine monoclonal antibody therapy significantly changes phencyclidine concentrations in brain and other tissues in rats. J Pharmacol Exp Ther. 1996;278:717–24. [PubMed] [Google Scholar]
- 55.Hardin JS, et al. Pharmacodynamics of a monoclonal antiphencyclidine Fab with broad selectivity for phencyclidine-like drugs. J Pharmacol Exp Ther. 1998;285:1113–22. [PubMed] [Google Scholar]
- 56.Valentine JL, et al. Antiphencyclidine monoclonal Fab fragments reverse phencyclidine-induced behavioral effects and ataxia in rats. J Pharmacol Exp Ther. 1996;278:709–16. [PubMed] [Google Scholar]
- 57.McMillan DE, et al. Effects of murine-derived anti-methamphetamine monoclonal antibodies on (+)-methamphetamine self-administration in the rat. J Pharmacol Exp Ther. 2004;309:1248–55. doi: 10.1124/jpet.103.061762. [DOI] [PubMed] [Google Scholar]
- 58.Byrnes-Blake KA, et al. Monoclonal IgG affinity and treatment time alters antagonism of (+)-methamphetamine effects in rats. Eur J Pharmacol. 2005;521:86–94. doi: 10.1016/j.ejphar.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 59.Gentry WB, et al. Safety and efficiency of an anti-(+)-methamphetamine monoclonal antibody in the protection against cardiovascular and central nervous system effects of (+)-methamphetamine in rats. Int Immunopharmacol. 2006;6:968–77. doi: 10.1016/j.intimp.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Berkowitz B, Spector S. Evidence for active immunity to morphine in mice. Science. 1972;178:1290–2. doi: 10.1126/science.178.4067.1290. [DOI] [PubMed] [Google Scholar]
- 61.Bonese KF, et al. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature. 1974;252:708–10. doi: 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- 62.Fang YX, et al. Recent trends in drug abuse in China. Acta Pharmacol Sin. 2006;27:140–4. doi: 10.1111/j.1745-7254.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 63.Tang YL, et al. Opiate addiction in China: current situation and treatments. Addiction. 2006;101:657–65. doi: 10.1111/j.1360-0443.2006.01367.x. [DOI] [PubMed] [Google Scholar]
- 64.Kermode M, et al. My first time: initiation into injecting drug use in Manipur and Nagaland, north-east India. Harm Reduct J. 2007;4:19. doi: 10.1186/1477-7517-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heimer R, et al. Spatial distribution of HIV prevalence and incidence among injection drugs users in St Petersburg: implications for HIV transmission. Aids. 2008;22:123–30. doi: 10.1097/QAD.0b013e3282f244ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wainer BH, et al. A measurement of the specificities of antibodies to morphine-6-succinyl-BSA by competitive inhibition of 14 C-morphine binding. J Immunol. 1973;110:667–73. [PubMed] [Google Scholar]
- 67.Anton B, Leff P. A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine. 2006;24:3232–40. doi: 10.1016/j.vaccine.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 68.Akbarzadeh A, et al. Design and synthesis of a morphine-6-succinyl-bovine serum albumin hapten for vaccine development. Biotechnol Appl Biochem. 1999;30(Pt 2):139–46. [PubMed] [Google Scholar]


