Abstract
The purpose of this study was to test the hypothesis that blockade of α1-adrenergic receptors may suppress the excessive ethanol consumption associated with acute withdrawal in ethanol-dependent rats. Following the acquisition and stabilization of operant ethanol self-administration in male Wistar rats, dependence was induced in half the animals by subjecting them to a four-week intermittent vapor exposure period in which animals were exposed to ethanol vapor for 14 hours per day. Subsequent to dependence induction, the effect of prazosin (0.0, 0.25, 0.5, 1, 1.5 and 2.0 mg/kg IP) was tested on operant responding for ethanol in vapor-exposed and control rats during acute withdrawal. In ethanol-dependent animals, prazosin significantly suppressed responding at the 1.5 and 2.0 mg/kg doses, whereas only the 2.0 mg/kg dose was effective in nondependent animals, identifying an increase in the sensitivity to prazosin in dependent animals. Conversely, at the lowest dose tested (0.25 mg/kg), prazosin increased responding in nondependent animals, which is consistent with the effect of anxiolytics on ethanol self-administration in non-dependent animals. None of the doses tested reliably affected concurrent water self-administration. These results suggest the involvement of the noradrenergic system in the excessive alcohol drinking seen during acute withdrawal in ethanol-dependent rats.
Keywords: Ethanol, Reinforcement, Dependence, Withdrawal, Norepinephrine, Noradrenaline
Introduction
The acute effects of ethanol have been shown to be both rewarding and reinforcing in animal models such as the conditioned place preference (Bozarth, 1990; Walker and Ettenberg, 2007) and operant self-administration (Anderson and Thompson, 1974; Smith and Davis, 1974) paradigms. Ethanol’s effects on the central nervous system occur via a variety of pharmacological mechanisms, including alterations in the function of the cholinergic, dopaminergic, gamma-aminobutyric acid, glutamatergic, opioidergic, and serotonergic neurotransmitter systems (for review see (Eckardt et al., 1998). In the case of chronic ethanol exposure, during the transition to dependence, there is evidence implicating neuroadaptations in the substrates mediating the rewarding/reinforcing effects of ethanol (Koob, 2004; Koob and Weiss, 1992; McBride and Li, 1998; Siggins et al., 2003), as well as the recruitment of neurotransmitter systems distinct from those participating in the acute effects of ethanol (Valdez et al., 2002; Walker and Koob, 2007a) that could promote increased ethanol-taking behavior.
One neurotransmitter system that has been extensively evaluated for participating in dependence- and withdrawal-induced changes in brain and behavior that contribute to excessive drug intake is the noradrenergic system. It has been shown that the use of ligands targeting both pre- and postsynaptic noradrenergic receptor subtypes can attenuate both the physical and motivational components of enhanced drug ingestion that have been observed in opiate-dependent rats (Aston-Jones and Harris, 2004; Delfs et al., 2000; Maldonado, 1997). Although it has been shown previously that norepinephrine depletion produced by blockade of the synthetic pathway attenuates ethanol self-administration in rats (Amit et al., 1977; Brown et al., 1977; Davis et al., 1978), noradrenergic systems have received much less attention in ethanol-dependence. However, there is evidence suggesting that modulation of noradrenergic systems shows efficacy in alleviating certain aspects of ethanol withdrawal and dependence that could be related to enhanced ethanol consumption (Patkar et al., 2003; Rasmussen et al., 2006; Riihioja et al., 1997a; Riihioja et al., 1997b; Trzaskowska et al., 1986) from a negative reinforcement perspective (i.e., removal of negative stimuli translates into a positive event that is considered reinforcing).
Specifically, it has been shown that during withdrawal in ethanol-dependent humans, plasma norepinephrine levels are elevated (Patkar et al., 2003). When evaluating the role of increased norepinephrine levels during withdrawal in animals following multiple days of ethanol injections, it was shown that lesions produced by a selective noradrenergic neurotoxin suppressed the withdrawal symptoms that were normally exhibited by animals with an intact noradrenergic system (Trzaskowska et al., 1986). Pharmacologically, ligands targeting different receptor subtypes of the norepinephrine system have shown efficacy for reducing certain aspects of ethanol withdrawal symptomology. Postsynaptic β-adrenergic antagonists have been shown to reduce withdrawal-induced convulsions (Trzaskowska et al., 1986) and tremors (Riihioja et al., 1997b). Likewise, presynaptic α2-noradrenergic receptor agonists have been shown to reduce rigidity, tremor and irritability associated with ethanol withdrawal (Riihioja et al., 1997a; Riihioja et al., 1997b). Additionally, α1-adrenergic receptor antagonists have been shown to reduce the locomotor hyperactivity produced by ethanol withdrawal (Trzaskowska et al., 1986).
In contrast to many of the earlier studies which evaluated the role of noradrenergic systems in the physiological signs of ethanol withdrawal, the present experiment focused on the motivational impact of noradrenergic modulation in the context of withdrawal-induced increases in operant ethanol self-administration that have been previously observed during acute withdrawal in ethanol-dependent animals (Funk et al., 2006; Funk et al., 2007; O’Dell et al., 2004; Roberts et al., 1996; Roberts et al., 2000; Valdez et al., 2002; Walker and Koob, 2007a; Walker and Koob, 2007b). Specifically, the effect of prazosin, an α1-noradrenergic receptor antagonist, was evaluated for the ability to modulate operant ethanol self-administration in nondependent and ethanol-dependent animals during acute withdrawal. Because norepinephrine levels are elevated during withdrawal and ligands targeting norepinephrine receptors are effective in reducing withdrawal symptoms, it was hypothesized that prazosin would be more effective in reducing ethanol self-administration in ethanol-dependent as opposed to nondependent animals.
Material and Methods
Subjects
15 male Wistar rats (Charles River Laboratory, Kingston, NY) weighing approximately 200 g upon arrival (45 days old) were communally housed (2–3 per cage) with food and water available ad libitum. The animals were housed within a temperature-controlled (21.5 °C) vivarium that was maintained on a twelve hour light/dark cycle (lights on at 0800). Upon their arrival in the vivarium, animals were handled daily over a one-week period (until the onset of operant conditioning). The work described herein adheres to the guidelines stipulated in the NIH Guide for the Care and Use of Laboratory Animals and was reviewed and approved by The Scripps Research Institute’s Institutional Animal Care and Use Committee.
Operant Chambers
The operant chambers (Coulbourn Instruments, Allentown, PA) utilized in the present study had two retractable levers located 4 cm above a grid floor and 4.5 cm to each side of a two-well acrylic drinking cup that allowed for up to two solutions to be administered upon the pressing of the appropriate lever. Recording of operant responses and subsequent fluid delivery was controlled by custom software running on a PC computer. A lever-press resulted in the activation of a 15 rpm Razel syringe pump (Stanford, CT) which delivered 0.1ml of fluid to the appropriate well over 0.5 s. During the 0.5 s of pump activation, no responses were recorded. Operant chambers were individually housed in ventilated, sound-attenuated cubicles to minimize environmental disturbances.
Drugs
Prazosin was purchased from Sigma Chemical Co. (Saint Louis, MO). Prazosin was soluble in sterile water at 1 mg/2ml and was injected via an intraperitoneal (IP) route of administration 30 minutes prior to operant ethanol self-administration sessions.
Acquisition of Operant Ethanol Self-Administration
Acquisition of operant training was based on an adaptation of Samson’s sweetened fading procedure (Samson, 1986). Animals were allowed to initially respond for a sweetened solution (SS) consisting of 3% glucose and 0.125% saccharin. This solution serves as a potent reinforcer and makes it unnecessary to water restrict animals to induce the initial lever-pressing behavior. For one week, animals were trained to press on a continuous schedule of reinforcement (fixed-ratio 1, FR-1) for SS alone. The animals were then switched to a two-lever condition with SS + 10% EtOH (w/v; 10E) resulting from an operant response on one of the levers and water resulting from a response on the alternate lever, with the position of the SS + 10E lever and water lever being alternated from left to right each session. After one week of SS + 10E, animals were allowed to self-administer a 0.125% saccharin + 10E solution for four sessions at which point the animals were switched to a 10E solution. Animals were allowed to self-administer 10E and water to allow for stable self-administration rates prior to the onset of intermittent ethanol vapor exposure. Once stable ethanol self-administration had occurred, the animals were split into two groups that were matched for their ethanol self-administration. One of the groups (n=8) was designated as the ethanol vapor exposure group while the other (n=7) was to serve as the air-exposed control.
Ethanol Vapor Chamber Process
Ethanol vapor exposure has been shown to reliably allow for the titration of blood alcohol levels that are sufficient for inducing ethanol dependence (O’Dell et al., 2004; Roberts et al., 1996; Roberts et al., 2000; Walker and Koob, 2007a; Walker and Koob, 2007b). In this paradigm, blood alcohol levels can be easily titrated by the experimenter to fit established criterion and the animals show normal weight gain and are freely moving (Rogers et al., 1979). Standard rat cages were housed in separate clear plastic chambers that were sealed and ethanol vapor or air was pumped through the chambers. Ethanol vapor was created by dripping 95% ethanol into 2000ml Erlenmeyer flasks that remained at 50°C due to a warming tray. Air (11L/min) was passed over the bottom of the flask so that when the ethanol hits the warm glass and was vaporized, the air carried it into the vapor chamber. Alteration of the ethanol vapor concentration was accomplished by modulating the air flow carry the vapor into the chamber. Target Blood alcohol levels (BALs) were 150–200 mg% across the 4 week exposure period and were determined by sampling blood collected from the tail (0.5 ml) twice a week. Following centrifugation, plasma alcohol levels were determined using the Analox micro-stat GM7 (Analox Instr. Ltd.; Lunenberg, MA).
Animals were subjected to intermittent vapor exposure (14 hours on/10 hours off) over the course of four weeks. Intermittent vapor exposure has been shown to be more effective at inducing dependence (i.e., enhanced ethanol self-administration) when compared to continuous ethanol vapor exposure (O’Dell et al., 2004).
Post-Vapor Pharmacological Challenge of Ethanol Self-Administration Responding
Following the four week dependence induction period resulting from ethanol vapor exposure, all animals were tested in self-administration sessions on Tuesdays and Fridays at a time point corresponding to six hours into withdrawal (Walker and Koob, 2007a; Walker and Koob, 2007b) for the ethanol vapor-exposed animals (i.e., six hours after the ethanol vapor was terminated for that day) to confirm differences in baseline ethanol self-administration responding between the ethanol vapor-exposed and air-exposed groups and to allow for stable ethanol self-administration behavior. Following each test session, the animals were returned to the vapor chambers and allowed to re-experience ethanol vapor or air as described above. Once ethanol self-administration was stable for three days, two opioid antagonists (i.e., naltrexone and nalmefene) were tested over a one month period prior to the initiation of the present study (Walker and Koob, 2007a). To insure that the animals were still viable candidates for pharmacological manipulation in the present study, the animals continued with intermittent vapor exposure for two additional weeks, after which, a secondary baseline was conducted to confirm that differences between the air-exposed and vapor-exposed groups were maintained and comparable to self-administration levels from the initial post-vapor baseline. Once self-administration behavior was determined to be consistent with the initial baseline behavior, pharmacological challenges ensued. A prazosin dose-response curve (0.0 – 2 mg/kg, IP) was conducted according to a Latin square design with test days being carried out on Tuesdays and Fridays in order to minimize drug carryover effects. On all test days, blood was collected prior to the ethanol vapor termination for the test day to confirm that target BALs were met. Following the test trials, the animals were returned to the vapor chambers. In total, the animals were subjected to 16 weeks of intermittent vapor exposure from the beginning of dependence induction to the culmination of the experiment.
Statistical Analysis
To statistically confirm appropriate dependent-like acute withdrawal behavior, responding on the last three post-vapor ethanol self-administration sessions prior to pharmacological testing plus the re-baseline data were compared in ethanol vapor-exposed and air-exposed animals using a mixed-factor two-way analysis of variance (ANOVA). The between-subject factor was level of vapor exposure (i.e., vapor-exposed or air-exposed) and the within-subject factor was session. Following confirmation of dependent-like behavior, the ethanol-dependent and nondependent ethanol and water self-administration data following prazosin administration were analyzed using mixed-factor two-way ANOVAs with vapor-exposure as the between-subject and prazosin dose as the within-subject factor. The ethanol self-administration data for nondependent and ethanol-dependent animals were then individually analyzed using a repeated-measures one-way ANOVA. Post-hoc Least Significant Difference (LSD) tests were conducted if a main effect for dose was found. Separate two-way mixed factor ANOVAs were computed to compare the effects of vehicle treatments in non-dependent and ethanol-dependent animals to those effects produced by 0.25 mg/kg and 1.5 mg/kg prazosin; with level of dependence as the between-groups factor and drug as the within-subject factor. Post-hoc paired-sample t-tests were conducted on the appropriate pairings to evaluate changes from vehicle-treated responding if an interaction was found with the two-way ANOVA. To identify possible rate-dependent effects, a median split analysis was conducted on the dependent animal’s response rates which yielded high and low subgroups which were compared using an independent samples t-test. Subsequently, the percent change from baseline following 1.5 mg/kg prazosin administration was compared for the high and low responding group using an independent samples t-test.
Results
The data from the last three post-vapor period self-administration sessions prior to the onset of pharmacological challenges and the rebaseline session was analyzed using a mixed factor two-way ANOVA (see Figure 1). The analysis identified a main effect for level of vapor-exposure (F (1, 13) = 5.373, p < 0.05) which identified that the air- and vapor-exposed animals differed and that the induction of dependence was successful and continued to be expressed. There was no effect of session (F (3, 39) = 0.132, p > 0.05), nor was there an Exposure x Session interaction (F (3, 39) = 0.124, p > 0.05) indicating that self-administration was stable across days.
Figure 1.
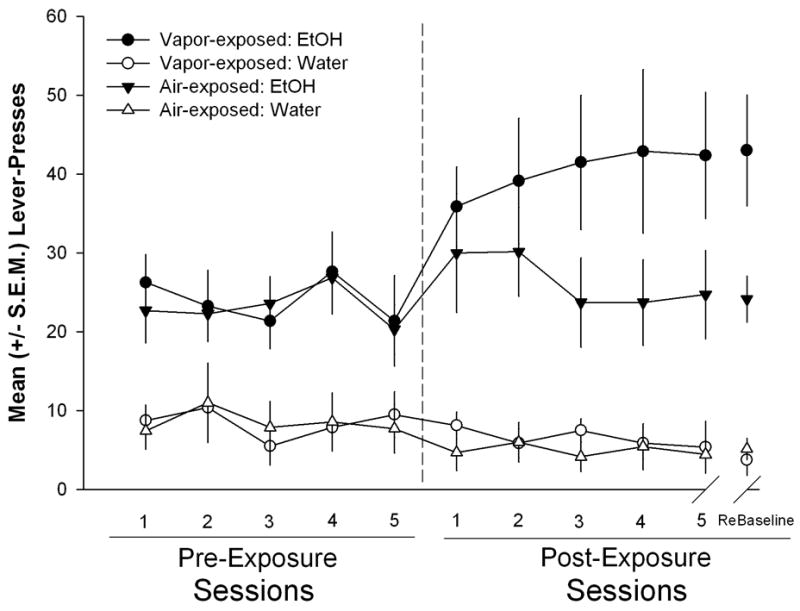
Left panel: Daily mean (±S.E.M) responses during 30 minutes sessions for ethanol and water prior to dependence induction for the ethanol vapor-exposed and air-exposed groups. Right panel: Mean (± S.E.M.) responses for ethanol and water during 30 minute sessions (occurring twice weekly) at a time-point corresponding to 6 h into withdrawal (i.e., acute withdrawal) following dependence induction. The vapor-exposed animals display escalated responding compared to the air-exposed controls.
The effects of prazosin on nondependent and dependent ethanol self-administration are presented in Figure 2. The data were analyzed using a mixed-factor two-way ANOVA. A main effect for Dose was found (F (5, 65) = 14.384, p<0.001) indicating that prazosin was effective in modulating ethanol self-administration. Moreover, a Dose × Exposure interaction (F (5, 65) = 2.378, p<0.05) was found which showed that prazosin affected nondependent and dependent animals differently. There was not a main effect of Exposure following prazosin administration (F (1, 13) = 3.483, p>0.05). To directly evaluate changes in self-administration induced by the different doses of prazosin, a one-way ANOVA was utilized for both nondependent and dependent data. In both groups of animals, a main effect of dose was identified (F (5, 41) = 5.636, p<0.01 and F (5, 47) = 8.847, p<0.001, respectively). Post-hoc LSD tests identified that both the 0.25 and 2.0 mg/kg doses of prazosin were significantly different (although in different directions - increased and decreased, respectively) from vehicle in nondependent animals while the 1.5 and 2.0 mg/kg doses significantly suppressed responding in ethanol-dependent animals when compared to vehicle. When a 2 × 2 ANOVA was computed on the vehicle- and 0.25 mg/kg-treated data from both nondependent and dependent groups of animals, only a main effect for Exposure was found (F(1, 13) = 11.572, p<0.05), whereas the 2 × 2 ANOVA comparing the vehicle-and 1.5 mg/kg-treated data (see Figure 4) identified a main effect of Dose and Exposure (F (1, 13) = 23.375, p<0.001 and (F (1,13) = 12.774, p<0.01), as well as a significant Dose × Exposure interaction (F (1, 13) = 9.036, p≤0.01). Post-hoc t-tests confirmed that the 1.5 mg/kg dose of prazosin significantly attenuated dependent responding compared to vehicle (t (7) = 6.252, p<0.001), whereas nondependent responding was unaffected (p>0.05).
Figure 2.

Mean (+S.E.M.) responses for ethanol during 30 minute sessions that occurred twice weekly following prazosin (0.0 – 2.0 mg/kg) administration in nondependent and ethanol-dependent animals during acute withdrawal. At higher doses, prazosin decreased ethanol self-administration in both nondependent and ethanol-dependent animals (*p<0.05 compared to air-exposed vehicle dose; # p<0.01 and ## p<0.001 compared to vapor-exposed vehicle dose). Water self-administration was unaffected by prazosin.
Figure 4.

Mean (±S.E.M.) responses for ethanol during 30 minutes sessions in nondependent and ethanol-dependent animals following 0.0 and 1.5 mg/kg prazosin. Prazosin (1.5 mg/kg) attenuated ethanol self-administration in ethanol-dependent animals (*** p<0.001), leaving nondependent self-administration intact.
The median split analysis of the dependent animals yielded two groups (high and low responders) with mean (±S.EM.) lever-presses (47.75 ± 2.98 and 32.75 ± 2.56, respectively) that displayed comparable decreases in responding following 1.5 mg/kg prazosin administration (66% ± 9% and 55% ± 3%, respectively). This further supports the hypothesis that prazosin acted independently of response rate in the dependent group.
Water self-administration was also evaluated using a mixed-factor two-way ANOVA. The analysis showed that prazosin did not affect water self-administration in the present study (see Figure 3) as identified by the lack of main effects for Dose (F (5, 65) = 1.322), p>0.05), Exposure (F (1, 13) = 0.797, p>0.05) or a Dose × Exposure interaction (F (5, 65) = 1.225, p>0.05).
Figure 3.
Mean (+S.E.M.) responses for water during 30 minute sessions that occurred twice weekly following prazosin (0.0 – 2.0 mg/kg) administration in nondependent and ethanol-dependent animals during acute withdrawal. There was no effect of prazosin on water self-administration in ethanol-dependent or nondependent rats.
Discussion
The purpose of the present experiment was to evaluate the involvement of noradrenergic systems in ethanol dependence. Consistent with previous reports, following a one-month intermittent ethanol vapor exposure regimen, dependent-like responding for ethanol was established as reflected by significant increases in operant ethanol self-administration for the vapor- as compared to air-exposed animals (O’Dell, et al., 2004; Funk, et al., 2006; Funk, et al., 2007; Walker and Koob, 2007b; Walker and Koob, 2007a). Once the induction of dependence had occurred and stable responding was established, pharmacological challenges using the α1-noradrenergic receptor antagonist prazosin were initiated.
Prazosin was administered prior to operant ethanol self-administration sessions that occurred at a time-point corresponding to six hours into withdrawal (i.e., six hours after the ethanol vapor was terminated for that day; acute withdrawal). Prazosin differentially affected nondependent and ethanol-dependent rats. Ethanol-dependent animals displayed attenuated responding for ethanol following the administration of 1.5 and 2.0 mg/kg prazosin that was suppressed to nondependent levels of self-administration. In contrast, nondependent responding for ethanol was increased following administration of the lowest (0.25 mg/kg) and decreased by the highest (2.0 mg/kg) dose of prazosin that was tested. None of the prazosin doses tested affected water self-administration.
The increased ethanol self-administration produced by the lowest dose of prazosin in nondependent animals could be reflective of anxiolysis induced by prazosin. Consistent with this hypothesis is the observation that classical anxiolytics (e.g., benzodiazepines) have the ability to increase non-dependent ethanol self-administration (Soderpalm and Hansen, 1998) and the same dose (0.25 mg/kg) of prazosin was able to attenuate the anxiogenic effects of nicotine administration in the elevated plus-maze (Zarrindast et al., 2000). An alternative explanation for the selective increases in self-administration observed in nondependent animals is that the low dose of prazosin is by some method increasing stress and thereby causing increased self-administration. However, because the dependent animals showed levels of responding that were comparable to baseline levels following administration of the lower prazosin doses, it would seem as that hypothesis is unlikely. The decreased ethanol self-administration produced by the blockade of norepinephrine transmission at the α1 receptor in both nondependent and ethanol-dependent animals during acute withdrawal is consistent with previous research showing attenuation of ethanol intake with blockade of noradrenergic neurotransmission (Amit et al., 1977; Brown et al., 1977; Davis et al., 1978).
When comparing the ability of prazosin to reduce ethanol consumption in nondependent and ethanol-dependent Wistar rats during acute withdrawal, differences in the dose required to suppress responding for ethanol were observed. Specifically, 1.5 mg/kg of prazosin was sufficient to reduce responding for ethanol in dependent animals to levels that were comparable to that of vehicle-treated nondependent animals. Conversely, the same dose of prazosin did not impact nondependent ethanol self-administration, however, when the dose was increased to 2.0 mg/kg suppression of responding was observed in non-dependent animals. Therefore, it appears as though there was a shift in sensitivity to α1-noradrenergic receptor antagonism for those animals that were ethanol-dependent, which is supported by the Dose x Exposure interaction that was observed when evaluating the vehicle- and 1.5 mg/kg prazosin-treated nondependent and ethanol-dependent during acute withdrawal.
The α1-adrenoceptors have been proposed to have a role in rate-dependent behavioral effects that occur from stimulation of noradrenergic systems (Stone and Quartermain, 2005). Consequently, an alternative hypothesis to explain the increased potency of prazosin in ethanol-dependent rats is that prazosin could have had rate-dependent effects. Because the dependent animals had a higher baseline response rate compared to the nondependent controls, it could be those higher response rates would be more susceptible to modulation by pharmacologically active compounds. To address this concern, a median split analysis was conducted on the dependent animals in which they were separated into ‘high’ and ‘low’ response groups. The median split yielded two groups (high and low) with mean (±S.EM.) lever-presses of 47.75 ± 2.98 and 32.75 ± 2.56, respectively. Prazosin induced a relatively equivalent decrease in percent change from baseline regardless of whether the animals had high or low response rates initially. Thus, prazosin suppressed responding comparably in animals regardless of whether they were high or low responders for ethanol which supports the finding that prazosin was acting independently of response rate and was more potent in ethanol-dependent animals.
The results of this experiment are consistent with those established when focusing on withdrawal symptoms in animals that were ethanol- or opiate- dependent. Both pre- and postsynaptic noradrenergic ligands that have the generalized effect of attenuating noradrenergic transmission (with antagonists at postsynaptic receptors or agonists at presynaptic inhibitory autoreceptors) are effective at reducing both the somatic and behavioral symptoms associated with acute withdrawal (Aston-Jones and Harris, 2004; Delfs et al., 2000; Ozdogan et al., 2003; Riihioja et al., 1997a; Riihioja et al., 1997b; Trzaskowska et al., 1986; Van der Laan, 1987). Although prazosin was administered peripherally in the present study, previous evidence has implicated central noradrenergic transmission as participating in withdrawal-induced behavioral changes (Aston-Jones et al., 1999; Aston-Jones and Harris, 2004; Maldonado, 1997). Specifically, the bed nucleus of the stria terminalis (BNST) has been heavily implicated as a primary substrate for enhanced norepinephrine release from neurons that comprise the ventral noradrenergic bundle and contribute to the anxiety and aversive states that are observed during withdrawal (Aston-Jones and Harris, 2004; Delfs et al., 2000), which is consistent with evidence showing that repeated ethanol exposure results in a sensitized noradrenergic system (Lanteri et al., 2007). Taking into consideration that brain norepinephrine systems are closely tied to the behavioral response to stress (Cecchi et al., 2002; Morilak et al., 2005), from a negative reinforcement perspective, it could be that the upregulation of norepinephrine systems (resulting in excessive norepinephrine release in the BNST) is contributing to the increased motivation to self-administer ethanol during withdrawal through the production of an aversive affective state that can be relieved by increased self-administration of ethanol. Therefore, by antagonizing the α1-adrenergic receptor, prazosin is able to alleviate the withdrawal-mediated aversion and consequently, the result is a decreased need to self-administer ethanol for animals that are dependent.
In summary, intermittent ethanol-vapor exposure was able to induce dependence in Wistar rats as reflected by increased self-administration of ethanol. The α1-adrenergic receptor antagonist prazosin was able to reduce self-administration of ethanol in both nondependent and ethanol-dependent rats during acute withdrawal. However, prazosin was more potent in ethanol-dependent animals which shows that alterations occurred in the norepinephrine system during the transition to dependence and that the α1-adrenergic receptor could be a pharmacotherapeutic target to treat chronic alcoholism.
Acknowledgments
Support for this research was provided by National Institute on Alcohol Abuse and Alcoholism grants awarded to GFK (AA012602) and a National Research Service Award awarded to BMW (AA014723) and The Pearson Center for Alcoholism and Addiction Research. We thank Dr. Zalman Amit for early discussions and encouragement in pursuing studies focusing on the interactions of norepinephrine and ethanol reinforcement. The authors would also like to thank Maury Cole for technical assistance and Mike Arends for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amit Z, Brown ZW, Levitan DE, Ogren SO. Noradrenergic mediation of the positive reinforcing properties of ethanol: I. Suppression of ethanol consumption in laboratory rats following dopamine-beta-hydroxylase inhibition. Arch Int Pharmacodyn Ther. 1977;230:65–75. [PubMed] [Google Scholar]
- Anderson WW, Thompson T. Ethanol self-administration in water satiated rats. Pharmacol Biochem Behav. 1974;2:447–454. doi: 10.1016/0091-3057(74)90002-1. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(Suppl 1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Bozarth MA. Evidence for the rewarding effects of ethanol using the conditioned place preference method. Pharmacol Biochem Behav. 1990;35:485–487. doi: 10.1016/0091-3057(90)90191-j. [DOI] [PubMed] [Google Scholar]
- Brown ZW, Amit Z, Levitan DE, Ogren SO, Sutherland EA. Noradrenergic mediation of the positive reinforcing properties of ethanol: II. Extinction of ethanol-drinking behavior in laboratory rats by inhibition of dopamine-beta-hydroxylase Implications for treatment procedures in human alcoholics. Arch Int Pharmacodyn Ther. 1977;230:76–82. [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Davis WM, Smith SG, Werner TE. Noradrenergic role in the self-administration of ethanol. Pharmacol Biochem Behav. 1978;9:369–374. doi: 10.1016/0091-3057(78)90298-8. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, ston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Koob GF, Weiss F. Neuropharmacology of cocaine and ethanol dependence. Recent Dev Alcohol. 1992;10:201–233. doi: 10.1007/978-1-4899-1648-8_11. [DOI] [PubMed] [Google Scholar]
- Lanteri C, Salomon L, Torrens Y, Glowinski J, Tassin JP. Drugs of Abuse Specifically Sensitize Noradrenergic and Serotonergic Neurons Via a Non-Dopaminergic Mechanism. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301548. [DOI] [PubMed] [Google Scholar]
- Maldonado R. Participation of noradrenergic pathways in the expression of opiate withdrawal: biochemical and pharmacological evidence. Neurosci Biobehav Rev. 1997;21:91–104. doi: 10.1016/0149-7634(95)00061-5. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Ozdogan UK, Lahdesmaki J, Scheinin M. Influence of prazosin and clonidine on morphine analgesia, tolerance and withdrawal in mice. Eur J Pharmacol. 2003;460:127–134. doi: 10.1016/s0014-2999(02)02961-8. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Gopalakrishnan R, Naik PC, Murray HW, Vergare MJ, Marsden CA. Changes in plasma noradrenaline and serotonin levels and craving during alcohol withdrawal. Alcohol Alcohol. 2003;38:224–231. doi: 10.1093/alcalc/agg055. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Wilkinson CW, Raskind MA. Chronic daily ethanol and withdrawal: 6 Effects on rat sympathoadrenal activity during “abstinence”. Alcohol. 2006;38:173–177. doi: 10.1016/j.alcohol.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riihioja P, Jaatinen P, Oksanen H, Haapalinna A, Heinonen E, Hervonen A. Dexmedetomidine alleviates ethanol withdrawal symptoms in the rat. Alcohol. 1997a;14:537–544. doi: 10.1016/s0741-8329(97)00044-x. [DOI] [PubMed] [Google Scholar]
- Riihioja P, Jaatinen P, Oksanen H, Haapalinna A, Heinonen E, Hervonen A. Dexmedetomidine, diazepam, and propranolol in the treatment of ethanol withdrawal symptoms in the rat. Alcohol Clin Exp Res. 1997b;21:804–808. [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Martin G, Roberto M, Nie Z, Madamba S, De LL. Glutamatergic transmission in opiate and alcohol dependence. Ann N Y Acad Sci. 2003;1003:196–211. doi: 10.1196/annals.1300.012. [DOI] [PubMed] [Google Scholar]
- Smith SG, Davis WM. Intravenous alcohol self-administration in the rat. Pharmacol Res Commun. 1974;6:379–402. doi: 10.1016/s0031-6989(74)80039-1. [DOI] [PubMed] [Google Scholar]
- Soderpalm AH, Hansen S. Benzodiazepines enhance the consumption and palatability of alcohol in the rat. Psychopharmacology (Berl) 1998;137:215–222. doi: 10.1007/s002130050613. [DOI] [PubMed] [Google Scholar]
- Stone EA, Quartermain D. Rate-dependent behavioral effects of stimulation of central motoric alpha(1)-adrenoceptors: hypothesized relation to depolarization blockade. Psychopharmacology (Berl) 2005;178:109–114. doi: 10.1007/s00213-004-2125-y. [DOI] [PubMed] [Google Scholar]
- Trzaskowska E, Pucilowski O, Dyr W, Kostowski W, Hauptmann M. Suppression of ethanol tolerance and dependence in rats treated with DSP-4, a noradrenergic neurotoxin. Drug Alcohol Depend. 1986;18:349–353. doi: 10.1016/0376-8716(86)90098-0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Van der Laan JW. Dopaminergic and alpha 1-adrenergic properties of B-HT920 revealed in morphine-dependent rats. Pharmacol Biochem Behav. 1987;26:265–269. doi: 10.1016/0091-3057(87)90116-x. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ettenberg A. Intracerebroventricular ethanol-induced conditioned place preferences are prevented by fluphenazine infusions into the nucleus accumbens of rats. Behav Neurosci. 2007;121:401–410. doi: 10.1037/0735-7044.121.2.401. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological Evidence for a Motivational Role of kappa-Opioid Systems in Ethanol Dependence. Neuropsychopharmacology. 2007a doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007b;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Homayoun H, Babaie A, Etminani A, Gharib B. Involvement of adrenergic and cholinergic systems in nicotine-induced anxiogenesis in mice. Eur J Pharmacol. 2000;407:145–158. doi: 10.1016/s0014-2999(00)00628-2. [DOI] [PubMed] [Google Scholar]


