SUMMARY
The mechanisms by which proneural basic helix-loop-helix (bHLH) factors control neurogenesis have been characterized, but it is not known how they specify neuronal cell-type identity. Here we provide evidence that two conserved serine residues on the bHLH factor neurogenin 2 (Ngn2), S231 and S234, are phosphorylated during motor neuron differentiation. In knock-in mice in which S231 and S234 of Ngn2 were mutated to alanines, neurogenesis occurs normally but motor neuron specification is impaired. The phosphorylation of Ngn2 at S231 and S234 facilitates the interaction of Ngn2 with LIM homeodomain transcription factors to specify motor neuron identity. The phosphorylation-dependent cooperativity between Ngn2 and homeodomain transcription factors represents a novel mode of transcriptional regulation, and may be a general mechanism by which the activities of bHLH and homeodomain proteins are temporally and spatially integrated to generate the wide diversity of cell types that are a hallmark of the nervous system.
INTRODUCTION
A key unresolved question in developmental neurobiology is how a relatively homogeneous population of neural stem cells/progenitors give rise to the diverse array of neuronal cell types that are present in the mature nervous system. The differentiation of neural progenitors to a specific type of neuron is known to involve two distinct but coordinated steps: the commitment to a neuronal fate and the establishment of cell-type identity (Bertrand et al., 2002). The identification of the molecular mechanisms that control these two steps is the subject of active investigation and in particular has been studied during spinal motor neuron development (Briscoe et al., 2000; Ericson et al., 1996; Jessell, 2000; Lee and Pfaff, 2003; Novitch et al., 2001; Vallstedt et al., 2001).
Spinal motor neurons are a group of cholinergic neurons located in the ventral horn of the spinal cord that control locomotion. The degeneration of motor neurons can lead to spinal muscular atrophy (SMA) in infants and amyotrophic lateral sclerosis (ALS) in adults (Cleveland, 1999; Monani, 2005). The treatment and eventual prevention of these devastating disorders will likely emerge from a better understanding of the molecular mechanisms that control motor neuron differentiation.
The basic helix-loop-helix (bHLH) transcription factor neurogenin 2 (Ngn2) regulates both the commitment of progenitor cells to a neuronal fate and the identity specification of spinal motor neurons (Mizuguchi et al., 2001; Novitch et al., 2001; Scardigli et al., 2001). While the mechanisms by which Ngn2 promotes neurogenesis have been characterized, little is known about how Ngn2 confers neuronal cell-type identity during spinal cord development. Ngn1 and Ngn2, two mammalian orthologs of the Drosophila proneural bHLH gene atonal, are expressed in overlapping patterns throughout the developing nervous system and act as important regulators of developmental neurogenesis (Fode et al., 2000; Ma et al., 1998). These factors have well-characterized roles in the process of neuronal differentiation—promoting cell cycle exit through up-regulation of the cyclin-dependent kinase inhibitor p27Kip1(Farah et al., 2000; Nguyen et al., 2006); inducing the expression of a cascade of late differentiation bHLH factors and pan-neuronal genes such as NeuroM, NeuroD and β-III-tubulin (Fode et al., 1998; Ma et al., 1998); suppressing gliogenesis by sequestering transcriptional coactivators essential for glial differentiation (Nieto et al., 2001; Sun et al., 2001); and promoting cortical neuron migration and dendritic growth via the modulation of RhoA GTPase activity (Ge et al., 2006; Hand et al., 2005). However, detailed analysis of mouse strains deficient for Ngn1 and Ngn2 has revealed that these factors have additional functions during development (Andersson et al., 2006; Hand et al., 2005; Kele et al., 2006; Scardigli et al., 2001). In Ngn2 null mice, motor neuron specification is compromised, whereas neurogenesis in the spinal cord is normal, likely due to the continued presence of Ngn1. By contrast, motor neuron differentiation in Ngn1 mutant mice occurs normally (Scardigli et al., 2001). These findings suggest that Ngn2 plays a unique and critical role in determining motor neuron cell-type identity. However, the molecular mechanisms used by Ngn2 to establish spinal motor neuron identity are not well understood and it is not clear how a single transcription factor can regulate both a general process such as neurogenesis and a more restricted process such as neuronal cell-type specification.
Recent advances have demonstrated that motor neuron differentiation is regulated by a complex interaction between extracellular and cell-intrinsic factors (Jessell, 2000). Secretion of the morphogen Sonic Hedgehog (Shh) from the notochord is essential for specifying multiple cell-types in the ventral neural tube, including motor neurons (Ericson et al., 1996; Jessell, 2000; Lu et al., 2002). Shh induces the region-specific expression of homeodomain transcription factors that play a key role in the establishment of distinct progenitor domains that give rise to the different types of neurons in the ventral neural tube (Briscoe et al., 2000; Shirasaki and Pfaff, 2002). Proliferating progenitors in different progenitor domains express unique combinations of LIM homeodomain (LIM-HD) transcription factors that function to establish neuronal cell-type identity as the progenitors exit the cell cycle (Ericson et al., 1992; Sharma et al., 1998; Tsuchida et al., 1994). The motor neuron progenitor (pMN) domain is marked by the expression of LIM-HD factors Lhx3 (Lim3) and Isl1 which form a transcription complex with LIM-HD-interacting transcription cofactor NLI (Ldb1/Chip/CLIM2) to establish motor neuron identity (Thaler et al. 2002).
The observation that certain LIM-HD factors are expressed in proliferating progenitors during neurogenesis raises the possibility that these factors might work together with Ngn2 to couple neurogenesis to the specification of neuronal cell-type identity (Bertrand et al., 2002; Shirasaki and Pfaff, 2002). In support of this idea, a recent study has shown that LIM-HD proteins can act cooperatively with the bHLH transcription factor NeuroM to promote motor neuron-specific gene HB9 expression (Lee and Pfaff, 2003). However, the mechanisms that control the interaction of LIM-HD proteins with proneural bHLH transcription factors to ensure that these interactions occur at the right time and place during spinal cord development remain to be characterized.
Here, we report that Ngn2 is phosphorylated on two key serine residues, S231 and S234, during motor neuron differentiation. We identify Shh as one of the extrinsic factors that regulate this process by inducing the expression of Ngn2 and initiating its subsequent phosphorylation by GSK3. The phosphorylation of Ngn2 at S231 and S234 is not required for Ngn2-dependent neurogenesis, but is critical for Ngn2-dependent motor neuron identity establishment. Phosphorylation of S231 and S234 promotes the interaction of Ngn2 with the LIM-HD transcription complex and thus facilitates the ability of these transcription factors to cooperatively promote motor neuron specification. These findings suggest a novel mode of transcriptional regulation that involves phosphorylation-dependent cooperativity between bHLH and homeodomain transcription factors to control neuronal cell-type specification during neural development.
RESULTS
Endogenous Ngn2 Is Phosphorylated During Motor Neuron Differentiation
To study the molecular mechanisms by which Ngn2 regulates neuronal cell-type specification, we hypothesized that extrinsic signal-controlled modifications at key sites on Ngn2 might regulate Ngn2’s ability to specify neuronal cell-type identity. In particular, we sought to identify amino acid sequences within Ngn2 that correspond to good consensus sites for protein phosphorylation. Sequence alignment of Ngn2 across species shows there are two highly conserved proline-directed serine residues, S231 and S234, at the C-terminus of Ngn2 that are similar in sequence to previously characterized sites of phosphorylation (Figure 1A).
Figure 1. Neurogenin 2 is Phosphorylated on S231 and S234 During Spinal Motor Neuron Differentiation.

(A) Sequence alignment showing S231 and S234 located near the C-terminus of Ngn2 are highly conserved across species. (B) Endogenous Ngn2 is phosphorylated on S231 and S234. Endogenous Ngn2 was immunoprecipitated from E11.5 mouse neural tube and telencephalon nuclear lysates with an anti-Ngn2 antibody crosslinked to protein A beads. The immunoprecipitaed proteins were left untreated or treated with alkaline phosphatase, separated on SDS-PAGE and blotted with the anti-Ngn2 or the anti-Ngn2 P-S231&S234 antibodies. (C-E) Endogenous Ngn2 is phosphorylated on S231 and S234 in spinal motor neuron progenitors. Coronal neural tube sections from E10 mouse embryos were stained with the anti-Ngn2 P-S231&S234 antibodies (red, C) and a monoclonal antibody recognizing motor neuron progenitor-specific transcription factor Olig2 (green, E). Merged image of anti-olig antibody and anti-Ngn2 P-S231&S234 antibody staining appears as yellow (D). (F-H) Ngn2 is phosphorylated on S231 and S234 in a subset of Ngn2-positive motor neuron progenitors. Coronal neural tube sections from E10 mouse embryos were stained with the anti-Ngn2 P-S231&S234 antibodies (red, F) and a rat anti-Ngn2 antibody (blue, H). Merged image of anti-Ngn2 P-S231&S234 antibody and anti- Ngn2 antibody staining appears as pink (G). Panels F through H correspond to the boxed area in panel C.
To test the possibility that Ngn2 is phosphorylated at these sites, or potentially other sites as well, we immunoprecipitated Ngn2 from E11.5 mouse embryonic neural tube and telencephalon nuclear lysates with a rabbit anti-Ngn2 antibody that specifically detects endogenous Ngn2 protein (Supplemental Figure 1). The immunoprecipitated proteins were separated by SDS-PAGE and visualized by using the anti-Ngn2 antibodies (Figure 1B). This analysis revealed that Ngn2 migrates on an SDS polyacrylamide gel as multiple bands, suggesting that Ngn2 is post-translationally modified. Alkaline phosphatase treatment of the immunoprecipitated Ngn2 resulted in the collapse of the more slowly migrating bands to a single band, suggesting that the slowly migrating bands are due to the phosphorylation of Ngn2 (Figure 1B).
To determine if Ngn2 is phosphorylated at S231 and S234, we generated phospho-specific antibodies that recognize Ngn2 only when it is phosphorylated at both S231 and S234 (anti-Ngn2 P-S231&S234 antibodies). Using non-phosphorylated or phosphorylated peptides that span S231 and S234 of Ngn2, and full length wild type or mutant versions of Ngn2 in which S231 and/or S234 were converted to alanines, we confirmed that the affinity-purified anti-Ngn2 P-S231&S234 antibodies specifically recognize Ngn2 that is phosphorylated on both S231 and S234, but not Ngn2 that is not phosphorylated or only phosphorylated on either S231 or S234 (Supplemental Figure 2). To test whether Ngn2 is phosphorylated on S231 and S234 in vivo, we immunoprecipitated endogenous Ngn2 from nuclear extracts of E11.5 mouse neural tube and telencephalon. Immunoprecipitated Ngn2 was left untreated or treated with alkaline phosphatase, fractionated on an SDS polyacrylamide gel and then processed for Western blotting using the anti-Ngn2 P-S231&S234 antibodies. We found that the anti-Ngn2 P-S231&S234 antibodies only detect endogenous Ngn2 prior to alkaline phosphatase treatment (Figure 1B). These findings suggest that endogenous Ngn2 is phosphorylated at S231 and S234 in the embryonic neural tube and/or telencephalon.
To investigate the temporal and spatial patterns of Ngn2 phosphorylation at S231 and S234, we stained coronal sections of E10 mouse neural tubes with the anti-Ngn2 P-S231&S234 antibodies. We focused our efforts on the developing neural tube because previous studies have shown that Ngn2 knock-out mice display a defect in spinal motor neuron differentiation. Intriguingly, immunostaining with the anti-Ngn2 P-S231&S234 antibodies revealed the presence of the S231 and S234 phosphorylated form of Ngn2 in the motor neuron progenitor domain of the ventral neural tube (Figure 1C). The staining pattern observed with the anti-Ngn2 P-S231&S234 antibodies partially overlapped with that detected with antibodies to the motor neuron progenitor marker Olig2 (Figure 1C-E). The anti-Ngn2 P-S231&S234 antibodies specifically stained a subset of differentiating motor neuron progenitors that express Ngn2 (stained with a rat anti-Ngn2 antibody) (Figure 1F-H). These findings indicate that endogenous Ngn2 is phosphorylated at S231 and S234 in a large proportion of spinal motor neuron progenitors in vivo, and suggest that the phosphorylation of Ngn2 at these sites could play a role in the process of motor neuron differentiation.
Spinal Motor Neuron Differentiation Is Defective in Ngn2S231A&S234A Knock-in Mice
To investigate the functional significance of Ngn2 phosphorylation at S231 and S234 during the course of normal development, we generated a knock-in mouse in which S231 and S234 in Ngn2 were converted to alanines (Ngn2S231&S234A) and thus refractory to phosphorylation. We constructed a targeting vector in which mutation of these two serines was introduced into the genomic Ngn2 sequence and linked to a positive neomycin selection marker (Neo) flanked by LoxP sites (Figure 2A). The targeting vector successfully recombined with the endogenous Ngn2 locus in mouse ES cells, as revealed by Southern blotting of genomic DNA obtained from targeted ES cells (Figure 2B). Two independent ES cell lines that were successfully targeted at the Ngn2 locus were used to generate the Ngn2S231&S234A knock-in mice following standard protocols.
Figure 2. Motor Neuron Differentiation Is Defective in the Ngn2S231A&S234A Knock-in Mice.
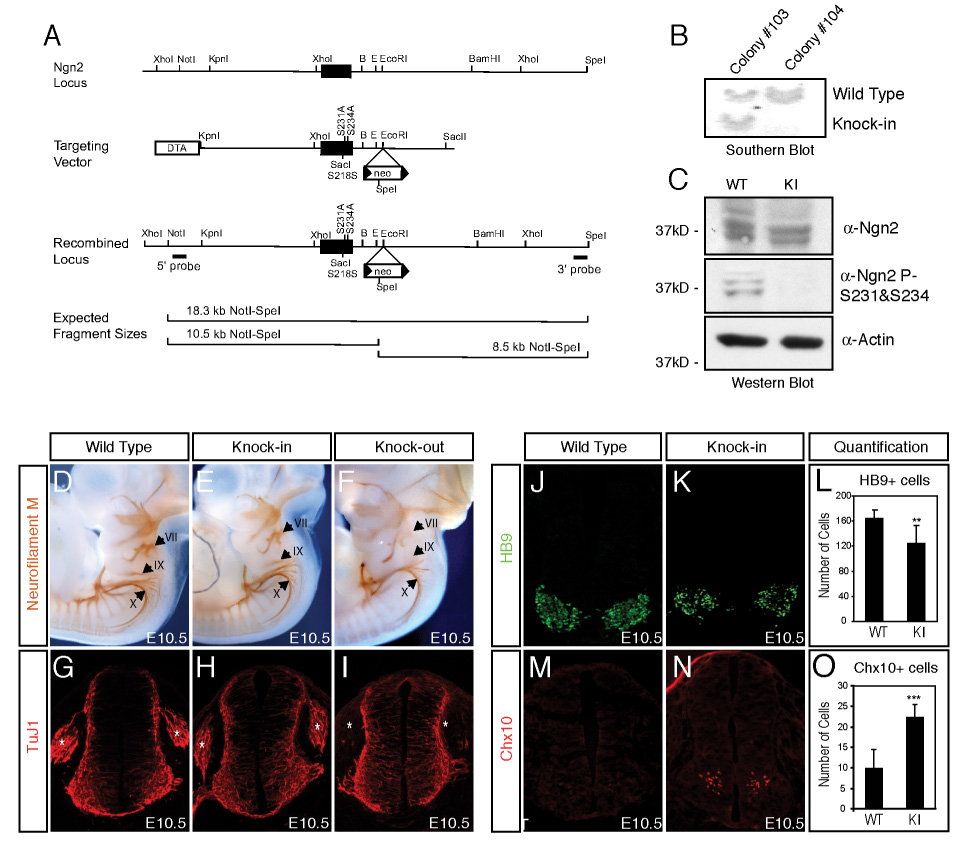
(A) Schematic representation of the mouse genomic region bearing the Ngn2 gene (top), the Ngn2S231A&S234A knock-in targeting vector (middle), and the Ngn2 locus after homologous recombination (bottom) with the targeting vector. The solid block represents the single exon encoding Ngn2, in which S231 and S234 have been mutated to alanines in the Ngn2S231A&S234A knock-in mice. 5’ and 3’ external probes are indicated and were used to detect the presence or absence of the recombined locus by Southern hybridization analysis of NotI plus SpeI digested genomic DNA. The expected NotI-SpeI restriction endonuclease fragments revealed by Southern blot analysis of wild type and targeted genomic DNA are shown. The positions of relevant restriction endonuclease sites are shown (B: BamHI; E: EcoRI). (B) Southern blot analysis showing successful targeting of the Ngn2S231A&S234A knock-in allele in mouse ES cells. Southern blot analysis of genomic DNA from targeted ES cell clones #103 and #104 digested with NotI and SpeI, and then probed with a 5’ external probe that recognizes an 18.3 kb wild type DNA fragment and a 10.5 kb DNA fragment that would only be present if the homologous recombination targeting event has occurred. (C) Absence of S231 and S234 phosphorylation in the Ngn2S231A&S234A knock-in mice. Lysates prepared from E11.5 neural tubes and telencephalons of wild type or Ngn2S231A&S234A knock-in mice were analyzed by immunoblotting using the anti-Ngn2 or the anti-Ngn2 P-S231&S234 antibodies. Note that the expression level of the Ngn2 protein is not altered in the Ngn2S231A&S234A knock-in mice compared to wild type mice. Bottom panel shows immunoblot using antibodies to actin as a loading control. (D-I) In the distal cranial ganglia (indicated by the arrows in D-F) and dorsal root ganglia (indicated by the stars in G-I), neurogenesis is defective in Ngn2 knock-out embryos, but not in wild type or Ngn2S231A&S234A knock-in embryos. E10.5 wild type, Ngn2S231A&S234A knock-in or Ngn2 knock-out embryos were subjected to whole-mount immunostaining with a monoclonal anti-neurofilament M antibody (D-F). Neurofilament M staining is detected in the anlagen and the nerve roots of the three distal cranial ganglia, including geniculate (VII) and petrosal (IX) and nodose (X) ganglia, in wild type (panel D) and Ngn2S231A&S234A knock-in embryos (panel E). In comparison neurofilament M staining is absent in the geniculate (VII) and petrosal (IX) ganglia in Ngn2 knock-out embryos (panel F). Neural tube sections at the hind limb bud level from E10.5 wild type (panel G), Ngn2S231A&S234A knock-in (panel H) and Ngn2 knock-out (panel I) embryos were stained with the anti-TuJ1 antibodies recognizing a pan-neuronal marker β-III tubulin. No TuJ1 staining was detected in the DRG in Ngn2 knock-out embryos, whereas in wild type and Ngn2S231A&S234A knock-in embryos TuJ1 staining was present in the DRG. The number of TuJ1 positive neurons present in the neural tubes of wild type and Ngn2S231A&S234A knock-in mice is not significantly different: wild type: 248.2±30.1; knock-in: 237.9±36.8. To assess the number of TuJ1-positive neurons in the neural tubes, Hoechst and TuJ1 staining of the same section was overlayed, and the number of TuJ1-positive nuclei was counted. (J-O) The number of spinal motor neurons is reduced and the number of V2 interneurons is significantly increased in the Ngn2S231A&S234A knock-in mice compared to wild type mice. Immunostaining of E10.5 neural tube sections from wild type (J, M) and Ngn2S231A&S234A knock-in (K,N) mice with antibodies that detect the motor neuron markers HB9 (green, J,K) or the V2 interneuron marker Chx10 (red, M,N). For the quantification five brachial level coronal neural tube sections from each pair of wild type or homozygous Ngn2S231A&S234A knock-in embryos were analyzed. Data are from at least four pairs of embryos with the similar number (30–32 pairs) of somites, and are means ± standard error of the mean (SEM). HB9 (panel L): wild type: 164.5±13.3; knock-in: 124.2±28.7, **: P<0.001, T-test. Chx10 (panel O): wild type: 9.8±4.7; knock-in: 22.3±3.2, ***: P<2.0E-5, T-test.
A key to the utility of the Ngn2S231&S234A knock-in mice for studying the importance of S231 and S234 phosphorylation for Ngn2 function is that the mutation of these amino acid residues to alanines does not affect the level of Ngn2 expression. This was tested by Western blotting of cell extracts of E11.5 embryonic neural tube and telencephalon obtained from wild type and Ngn2S231&S234A knock-in mice. We found that the levels of Ngn2 protein expressed in wild type and Ngn2S231&S234A knock-in mice are similar (Figure 2C). In contrast, Western blotting of the same lysates with the anti-Ngn2 P-S231&S234 antibodies revealed the presence of the S231 and S234 phosphorylated form of Ngn2 only in extracts prepared from wild type mice (Figure 2C), indicating that in Ngn2S231&S234A knock-in mice Ngn2 is not phosphorylated at these sites. In addition, the failure of the anti-Ngn2 P-S231&S234 antibodies to detect Ngn2 by Western blotting of extracts from Ngn2S231&S234A knock-in mice further validates the specificity of the anti-Ngn2 P-S231&S234 antibodies for the S231 and S234 phosphorylated form of Ngn2.
Homozygous Ngn2S231&S234A knock-in mice are viable and live to adulthood. This is in striking contrast to homozygous Ngn2 knock-out mice which die at birth, most likely due to defects in neurogenesis that lead to a loss of distal cranial sensory ganglia in Ngn2 knock-out mice (Figure 2D-F) (Fode et al., 1998; Ma et al., 1998). These findings suggest that the mutation of S231 and 234 to alanines does not disrupt all functions of Ngn2, and in particular may not affect the ability of Ngn2 to promote neurogenesis. Consistent with these conclusions we found that wild type Ngn2 and Ngn2S231&S234A bind to E-box-containing fragments of the NeuroD promoter, and induce mRNA synthesis from a NeuroD promoter-driven luciferase construct equally well (Supplemental Figure 3A-C). In addition, when transfected into cortical progenitors wild type Ngn2 and Ngn2S231&S234A were equally effective at inducing the differentiation of progenitors into β-III tubulin-expressing neurons (cells staining positive with anti-TuJ1 antibodies, Supplemental Figure 3D-G). To investigate whether Ngn2S231&S234A knock-in mice have a defect in neurogenesis, we examined the generation of distal cranial sensory ganglia by whole-mount neurofilament M staining and the formation of dorsal root ganglia by immunostaining with anti-TuJ1 antibodies. Neurogenesis in Ngn2S231&S234A knock-in mice appears normal in the distal geniculate (VII) and petrosal (IX) ganglia (Figure 2D-F) and the dorsal root ganglia (DRG) (Figure 2G-I), where neurogenesis is dependent on Ngn2 and is defective in Ngn2 knock-out mice (Figure 2F, I) (Fode et al., 1998; Ma et al., 1998). In addition, we also examined the number of neurons generated on brachial level neural tube sections from E10.5 wild type and Ngn2S231&S234A knock-in mice by staining these sections with both Hoechst dye and anti-TuJ1 antibodies and counting the number of TuJ1-positive nuclei. We found no significant difference in the number of neurons generated in the neural tube when wild type and Ngn2S231&S234A knock-in mice were compared (wild type:248.2±30.1; knock-in: 237.9±36.8). Together, these data suggest that conversion of Ngn2 S231 and S234 to alanines does not affect the transcription activation function of Ngn2 nor do these mutations impair the ability of Ngn2 to induce neurogenesis.
Although neurogenesis appears largely normal in homozygous Ngn2S231&S234A knock-in mice, 56% of these mice were observed to have a defect of forelimb clasping (Supplemental Figure 4A), suggesting that these mice might have impaired motor neuron function. To examine this possibility, we stained brachial level neural tube sections from E10.5 wild type and Ngn2S231&S234A knock-in mice with antibodies that recognize the motor neuron-specific transcription factor HB9 (Figure 2J, K). Quantification of the number of HB9-positive cells revealed significantly fewer motor neurons in E10.5 Ngn2S231A&S234A knock-in mice compared to wild type mice (wild type:164.5±13.3; knock-in: 124.2±28.7, **: P<0.001, T-test) (Figure 2L). To ensure that the decrease in the number of motor neurons detected in Ngn2S231&S234A knock-in mice is not due to a decrease in the expression of the HB9 gene in otherwise normal motor neurons, we stained the neural tube sections with antibodies that recognize a second motor neuron marker Isl1/2. We found that the number of neurons expressing Isl1/2 is also markedly reduced in E10.5 neural tubes of Ngn2S231A&S234A knock-in mice compared to wild type mice (wild type: 164.1±13.6; knock-in: 125.3±27.5, **: P<0.001, T-test). These findings suggest that the cell-type specification of motor neurons is impaired in Ngn2S231&S234A knock-in mice. We found that the extent of motor neuron loss in Ngn2S231&S234A knock-in mice is similar in magnitude to that seen in Ngn2 knock-out mice as measured by Isl-positive cells in E10.5 neural tubes (Supplemental Figure 4B), suggesting that the motor neuron identity specification function of Ngn2 is dependent upon the phosphorylation of Ngn2 on S231 and S234.
The reduction in the number of spinal motor neurons in Ngn2S231A&S234A knock-in mice could be due to a defect in neuronal cell-type specification, or an increase in the death of motor neurons. Using antibodies that detect cleaved caspase3, a marker of cells undergoing apoptosis, we failed to detect any increase in the death of motor neurons in Ngn2S231&S234A knock-in mice compared to wild type mice (data not shown). To test whether the defect in motor neuron differentiation in Ngn2S231A&S234A knock-in mice reflects a defect in cell-type specification, we examined the number of V2 interneurons generated in the knock-in mice. In the ventral neural tube, motor neurons and V2 interneurons are derived from adjacent progenitor domains. The expression of both LIM-HD factors Lhx3 and Isl1 is required to establish motor neuron identity, while only Lhx3 is needed to promote V2 interneuron differentiation (Lee and Pfaff, 2001; Thaler et al., 2002). Motor neuron progenitors express both Lhx3 and Isl1, which cooperate to induce the expression of HB9 to promote motor neuron differentiation and repress V2 interneuron gene expression in motor neuron progenitors (Arber et al., 1999; Thaler et al., 1999). Since HB9 expression is compromised in Ngn2S231A&S234A knock-in mice, we hypothesized that derepression of V2 interneuron gene expression might lead to ectopic V2 interneuron formation in these mice. To test this possibility, we stained E10.5 brachial level neural tube sections with the V2 interneuron-specific marker Chx10 (Figure 2M, N). When wild type and Ngn2S231A&S234A knock-in mice were compared, we found that the number of V2 interneurons in the Ngn2S231A&S234A knock-in mice was significantly increased (wild type: 9.8±4.7; knock-in: 22.3±3.2, ***: P<2.0E-5, T-test) (Figure 2O). We conclude that the decrease in the number of motor neurons in Ngn2S231&S234A knock-in mice is due to a defect in Ngn2-dependent motor neuron cell-type specification that results in a conversion of some motor neuron progenitors to the V2 interneuron fate. This conclusion is supported by additional experiments described below that have identified the mechanism by which Ngn2 specifies the formation of motor neurons.
Phosphorylation on S231 and S234 of Ngn2 Promotes the Cooperation between Ngn2 and LIM-HD Factors to Establish Motor Neuron Identity
We hypothesized that Ngn2 might regulate the formation of motor neurons by cooperating with LIM-HD factors Lhx3 and Isl1 that are known to promote motor neuron differentiation (Lee and Pfaff, 2003; Thaler et al., 2002). To test this possibility, we first examined the expression patterns of Ngn2, Lhx3 and Isl1/2 by immunostaining. We found that in mouse E10.5 ventral neural tube Ngn2, Lhx3 and Isl1/2 were coexpressed in the neural progenitors migrating away from the ventricular zone (Figure 3A-H). Then we assessed the ability of wild type Ngn2 or phosphorylation-deficient Ngn2 mutants to cooperate with LIM-HD factors in the promotion of motor neuron differentiation using the chick embryo in ovo electroporation assay. By monitoring the production of ectopic neuronal populations via immunostaining with neuronal cell-type specific markers, the chick neural tube assay has been used to assess the neurogenic potential of various transcription factors (Lee and Pfaff, 2003). We introduced into the chick neural tube either wild type Ngn2, or mutants of Ngn2 in which S231 and/or S234 were converted to alanines, together with LIM-HD factors Lhx3, Isl1 and adaptor NLI. NLI is a widely expressed adaptor protein that contains an N-terminal dimerization domain and C-terminal LIM interaction domain (Agulnick et al., 1996; Jurata et al., 1996). NLI binds directly to Isl1 which in turn interacts with Lhx3 to form a hexameric LIM-HD complex (2NLI:2Isl1:2Lhx3) to promote motor neuron identity specification (Thaler et al., 2002). We monitored the production of motor neurons by counting the number of neurons that express the motor neuron marker MNR2, a chick homeodomain protein that shares characteristics with mouse HB9 (Tanabe et al., 1998). To distinguish the effects of exogenously introduced Ngn2 from those of the endogenous Ngn2, we focused our analysis on dorsal spinal cord progenitors as these cells have the potential to develop into motor neurons, but do not express endogenous transcription factors that promote motor neuron differentiation (Ericson et al., 1992; Sharma et al., 1998). To increase the likelihood of co-expression of Ngn2 and the LIM-HD factors, a chimeric molecule DD-Isl1-Lhx3, which has been demonstrated to function as a self-dimerizing analog of the LIM-HD complex (Lee and Pfaff, 2003), was used in these experiments. As shown in Figure 3I the expression of wild type Ngn2, together with the LIM-HD transcription complex, effectively induces motor neuron differentiation in the dorsal spinal cord, as indicated by staining with the motor neuron specific marker MNR2 (Figure 3I). The mutation of S231 to an alanine in Ngn2 leads to a significant reduction in the Ngn2-dependent induction of motor neuron differentiation (Figure 3J). In contrast to the effect of converting S231 to an alanine, the mutation of S234 to an alanine has no significant effect on Ngn2’s ability to cooperate with LIM-HD transcription factors. However, the mutation of both S231 and S234 to alanines has a more dramatic effect on Ngn2-induced motor neuron differentiation, suggesting that the phosphorylation of Ngn2 at both S231 and 234 contributes to Ngn2’s ability to cooperate with LIM-HD transcription factors to promote motor neuron specification. We found that endogenous motor neuron formation in the ventral chick neural tube is not significantly affected by the expression of wild type or mutant Ngn2 (Figure 3I), suggesting that under these conditions the phosphorylation-deficient Ngn2 mutants do not interfere with endogenous motor neuron differentiation.
Figure 3. The Ngn2S231A&S234A Mutant does not cooperate with LIM-HD Factors to Specify Motor Neuron Identity.
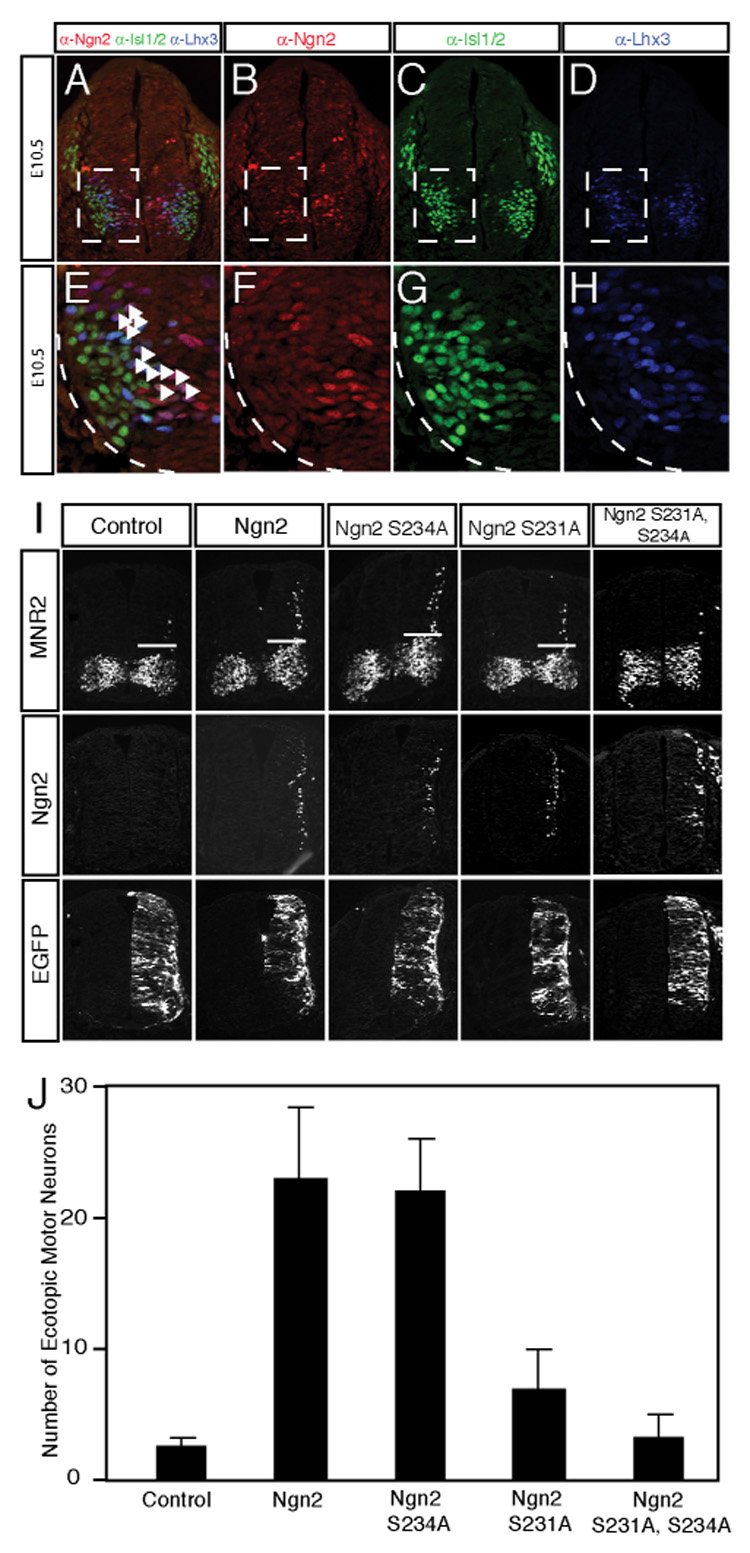
(A–H) Endogenous Ngn2, Isl1/2 and Lhx3 are coexpressed in differentiating motor neuron progenitors. Coronal sections from E10.5 mouse neural tube were stained with antibodies recognizing Ngn2 (red, B), Isl1/2 (green, C) and Lhx3 (blue, D). The overlap of staining by all three antibodies is shown in panel A. The boxed areas in panels A through D are enlarged in panels E through H, respectively. The white arrowheads in E correspond to the differentiating motor neuron progenitors. (I) The Ngn2S231A&S234A mutant is deficient in cooperating with LIM-HD factors to promote motor neuron differentiation in the chick neural tube. Embryonic chick neural tubes were electroporated with wild type or phosphorylation-deficient Ngn2 constructs together with LIM-HD complex NLI-Isl1-Lhx3 and pCS2-EGFP. Cross sections of electroporated chick neural tubes were stained with a monoclonal anti-MNR2 antibody or the rabbit anti-Ngn2 antibodies to detect ectopic motor neuron differentiation (above the white bars) or the expression of Ngn2, respectively. EGFP expression was measured as a control for the efficiency of electroporation in the assays. Images shown are representative from at least three experiments. (J) Quantitative analysis of MNR2-expressing motor neurons induced by wild type Ngn2 or phosphorylation-deficient Ngn2 mutants expressed together with LIM-HD factors. Data are from at least three experiments and are means ± SEM.
Phosphorylation on Ngn2 Affects its Interaction with the LIM-HD Transcription Complex
We next tested the possibility that in vertebrates Ngn2’s ability to cooperate with LIM-HD transcription factors to induce motor neuron differentiation reflects a physical interaction of Ngn2 with components of the LIM-HD complex, and investigated whether this interaction is facilitated by the phosphorylation of Ngn2 at S231 and S234. First we asked whether Ngn2 co-purifies with components of the LIM-HD complex. Nuclear extracts from E11.5 embryonic mouse neural tube and telencephalon were fractionated on a Superdex 200 gel filtration column and the column fractions were subjected to immunoblotting with the anti-Ngn2 antibodies. This analysis revealed that Ngn2 purifies as part of a high molecular weight protein complex that is approximately 600kD in size (Supplemental Figure 5). Western blot analysis showed that the adaptor protein NLI, LIM-HD factors Isl1/2 and Lhx3 co-purify with Ngn2 suggesting that these proteins might interact with one another (Supplemental Figure 5). The finding that Ngn2 and NLI interact was corroborated by co-immunoprecipitation experiments. When the anti-Ngn2 antibodies were incubated with 100μl E11.5 mouse telencephalon and neural tube nuclear extracts, we found by Western blotting with an anti-NLI antibody that NLI co-immunoprecipitates with Ngn2 (FIGURE 4A, B). This interaction is specific as no NLI was detected in the immunoprecipitates when the same nuclear extracts were immunoprecipitated using control antibodies. The co-immunoprecipitation of NLI with Ngn2 appears to be facilitated by the phosphorylation of Ngn2 at S231 and S234. We found that when Flag-tagged Ngn2 was transfected into HEK293T cells together with myc-tagged NLI, NLI was co-immunoprecipitated with wild type Ngn2 (Figure 4C). By contrast, Ngn2ΔC166, in which the C-terminus of Ngn2 beyond the bHLH domain was removed, or the phosphorylation-deficient Ngn2S231&S234A, both co-immunoprecipitated NLI to a much lesser extent than the wild type Ngn2 (Figure 4C-F). These findings suggest that the phosphorylation of Ngn2 at S231 and 234 facilitates the interaction of Ngn2 with NLI thereby promoting motor neuron differentiation.
Figure 4. Phosphorylation of Ngn2 on S231 and S234 Affects the Interaction between Ngn2 and the LIM-HD Transcription Complex.
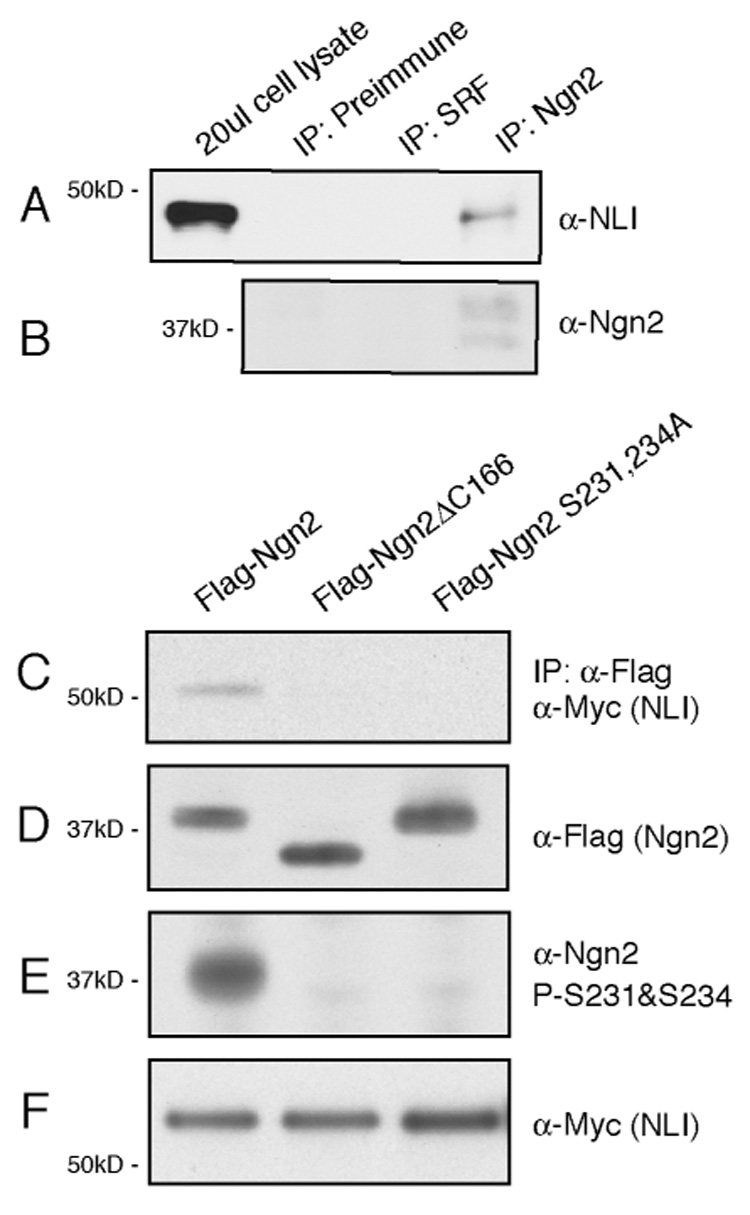
(A, B) Co-immunoprecipitation of endogenous Ngn2 and NLI from lysates prepared from E11.5 mouse neural tube and telencephalon using the anti-Ngn2 antibodies. NLI can be immunoprecipitated by the anti-Ngn2 antibodies but not by the preimmune serum or control antibodies that recognize serum responsive factor (SRF). (C-F) Phosphorylation of Ngn2 at S231 and S234 facilitates the interaction between Ngn2 and NLI. HEK293T cells were transfected with Myc-tagged NLI together with either Flag-tagged full-length Ngn2, Flag-tagged Ngn2ΔC166, in which the C-terminus of Ngn2 beyond the bHLH domain was removed, or Flag-tagged phosphorylation-deficient Ngn2S231&S234A. The transfected cells were lysed and the lysates were subject to immunoprecipitation with an anti-Flag antibody. The precipitated complexes were analyzed by immunoblotting using an anti-Myc antibody to detect the co-immunoprecipitated NLI protein (C). The expression levels of the various forms of Ngn2 were similar in 293T cells as shown by immunoblotting of the cell lysates using the anti-Flag (D). The phosphorylation of Ngn2 at S231 and S234 in 293T cells was confirmed by immunoblotting of the cell lysates with the anti-Ngn2 P-S231&S234 antibodies (E). The expression level of NLI in each sample was similarly indicated by western blotting with anti-Myc antibodies (F).
Ngn2 is Phosphorylated on S231 and S234 by GSK3 in vitro
Since the phosphorylation of S231 and S234 of Ngn2 plays a key role in promoting the development of motor neurons, we sought to identify the kinases that catalyze these events. We used Scansite 2.0, a computer program that identifies consensus sequences for phosphorylation by different protein kinases. This analysis suggested that the amino acids surrounding S231 and S234 of Ngn2 form a good consensus sequence for phosphorylation by the proline-directed kinase glycogen synthase kinase 3 (GSK3). GSK3 is a ubiquitously expressed serine/threonine kinase that has previously been implicated in sonic hedgehog (Shh) signaling in Drosophila (Jia et al., 2002; Price and Kalderon, 2002). Given the key role that Shh plays in motor neuron development in vertebrates, and the finding that the phosphorylation of Ngn2 at putative GSK3 phosphorylation sites promotes motor neuron differentiation, we asked if GSK3 phosphorylates Ngn2 to specify motor neuron cell-type identity.
To test whether GSK3 phosphorylates Ngn2 at S231 and/or S234 in vitro, we generated in bacteria recombinant wild type and mutant forms of Ngn2 in which S231 and/or S234 were replaced by alanine residues. Wild type Ngn2 was incubated with purified wild type GSK3, or heat-inactivated GSK3 kinase, and γ-32P-ATP. We found that wild type GSK3, but not the kinase-dead GSK3, phosphorylates Ngn2 (Figure 5A). In addition, when recombinant wild type and mutant forms of Ngn2 were phosphorylated by GSK3 in an in vitro kinase assay, Western blot analysis with the anti-Ngn2 P-S231&S234 antibodies revealed that GSK3 phosphorylates Ngn2 at S231 and S234 (Figure 5B, C). The Ngn2 that is phosphorylated on both S231 and S234 migrates as two distinct bands on SDS polyacrylamide gel (Figure 5B, C), suggesting that GSK3 phosphorylates Ngn2 on additional sites besides S231 and S234. The occurrence of the additional phosphorylation events may vary under different experimental conditions and consequently affect the migration pattern of Ngn2, which may in part account for the fact that endogenous Ngn2 from mouse neuronal lysates is observed to migrate differently in different experimental contexts.
Figure 5. GSK3 Phosphorylates Ngn2 on S231 and S234, and Sonic Hedgehog Induces Ngn2 Expression during Motor Neuron Differentiation.
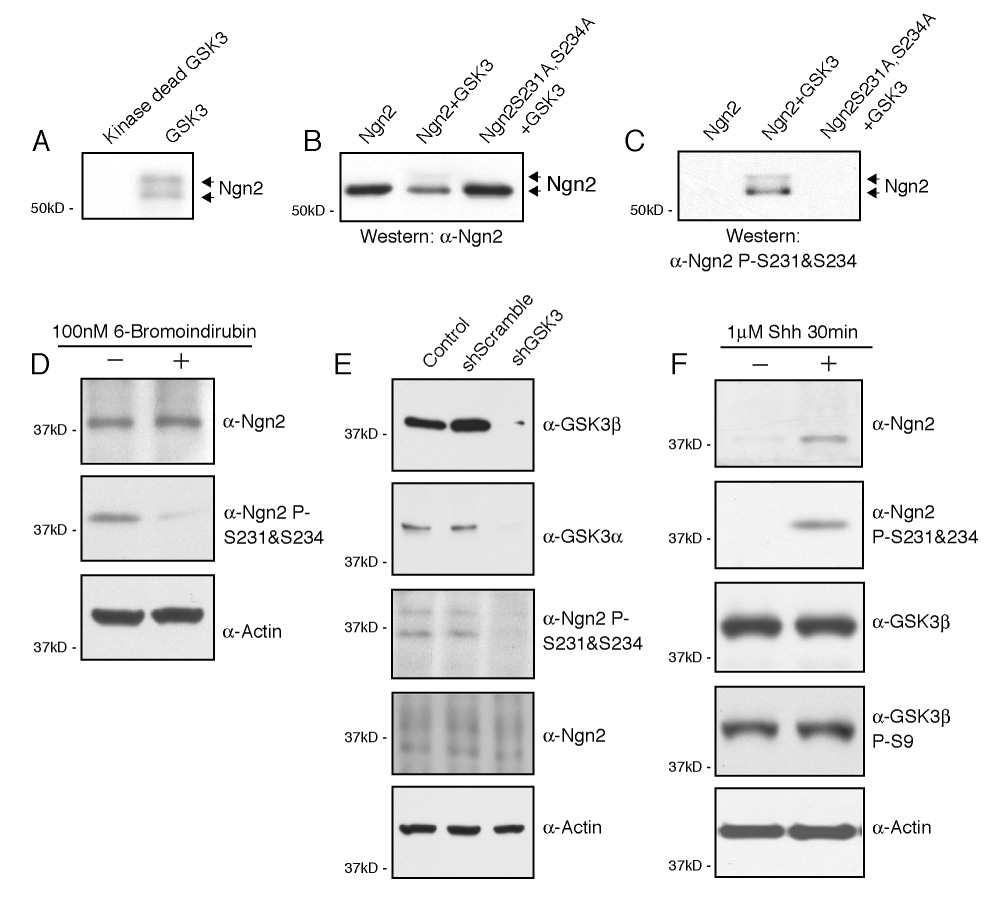
(A) GSK3 phosphorylates Ngn2 in vitro. Wild type recombinant Ngn2 protein generated in bacteria was incubated with purified active or heat-inactivated GSK3 kinase and γ-32P-ATP. Samples were separated on SDS-PAGE and radiolabeled Ngn2 was detected by autoradiography. (B–C) GSK3 phosphorylates Ngn2 on S231 and S234 in vitro. Recombinant wild type Ngn2 and the Ngn2S231&S234A mutant were in vitro phosphorylated by GSK3, and immunoblotted with the anti-Ngn2 (B) or anti-Ngn2 P-S231&S234 (C) antibodies. (D) The phosphorylation of endogenous Ngn2 on S231 and S234 is reduced by the GSK3 inhibitor 6-bromoindirubin in ES cell-derived motor neuron progenitors. ES cells were differentiated into motor neuron progenitors in the absence or presence of 100nM GSK3 inhibitor 6-Bromoindirubin. The expression level and phosphorylation status of Ngn2 in these ES cell-derived motor neuron progenitors were analyzed by Western blotting with the anti-Ngn2 or the anti-Ngn2 P-S231&S234 antibodies. (E) The phosphorylation of Ngn2 on S231 and S234 was significantly decreased in ES cell-derived motor neuron progenitors expressing GSK3 shRNA. ES cells were transfected with a shRNA construct that targets both GSK3α and GSK3β, or a construct encoding a scrambled hairpin, and then differentiated into motor neuron progenitors. The expression level of GSK3, Ngn2 and the phosphorylation status of Ngn2 were analyzed by immunoblotting with anti-GSK3α, anti-GSK3β, anti-Ngn2 or anti-Ngn2 P-S231&S234 antibodies. (F) Shh induces Ngn2 expression in ES cell-derived motor neuron progenitors and the induced Ngn2 is phosphorylated at S231 and S234. ES cell-derived motor neuron progenitors were treated with 1µM Shh for 30 minutes or left untreated. The expression level and phosphorylation status of Ngn2 and GSK3β were assessed by Western blotting with anti-Ngn2, anti-Ngn2 P-S231&S234, anti-GSK3β and anti-GSK3β P-S9 antibodies, respectively. Bottom panel shows Western blot with antibodies to actin as a loading control.
Endogenous Ngn2 is phosphorylated by GSK3 on S231 and S234 during motor neuron differentiation in vivo
To examine whether GSK3 mediates the phosphorylation of Ngn2 on S231 and S234 in cells, we generated motor neuron progenitors from mouse embryonic stem (ES) cells by exposing the ES cells to retinoic acid (RA) and Shh. The pathway of motor neuron generation from ES cells recapitulates the steps of motor neuron differentiation in vivo (Wichterle et al., 2002). Motor neurons progenitors derived from ES cells can integrate into existing neuronal circuits and form functional synapses that innervate limb muscles. Thus, ES cell-derived motor neuron progenitors are an excellent model for studying the signal transduction mechanisms that regulate motor neuron differentiation. Using an ES cell line that expresses EGFP under the control of motor neuron-specific promoter HB9, we successfully differentiated ES cells into EGFP-positive motor neuron progenitors. After differentiating for two days in the presence of RA and Shh, the ES cell-derived motor neuron progenitors express Ngn2 that is phosphorylated at S231 and S234, as detected by Western blotting with the anti-Ngn2 P-S231&S234 antibodies (Figure 5D). To test whether GSK3 phosphorylates Ngn2 in these developing motor neurons, we differentiated ES cells into motor neuron progenitors in the presence of the GSK3 inhibitor 6-Bromoindirubin-3′-acetoxime and assessed the phosphorylation status of Ngn2. We found that in the presence of 100nM 6-Bromoindirubin-3′-acetoxime, Ngn2 is expressed at normal levels but its phosphorylation at S231 and S234 is significantly reduced (Figure 5D). This suggests that GSK3 may phosphorylate Ngn2 on S231 and S234 during the differentiation of ES cells into motor neurons.
To further investigate the requirement of GSK3 for phosphorylating Ngn2 at S231 and S234, we knocked down the expression of endogenous GSK3 in mouse ES cells using vector-based RNAi constructs (Kim et al., 2006; Yu et al., 2003), and examined Ngn2 phosphorylation in these cells after exposure of the cells to RA and Shh for two days, a procedure that would normally induce Ngn2 expression. In vertebrates GSK3 has two isoforms, GSK3α and GSK3β, which have similar structures and functions (Doble and Woodgett, 2003; Jope and Johnson, 2004). By Western blotting with an anti-GSK3α antibody and an anti-GSK3β antibody we found that both GSK3α and GSK3β were effectively knocked down in ES cell-derived motor neuron progenitors by an shRNA that targets both isoforms of GSK3, but not by the scrambled hairpin. The knock-down effects of the RNAi construct persisted for more than 72 hours after transfection, allowing enough time for the ES cells to differentiate into Ngn2-expressing progenitors. We found that when the expression of GSK3 was effectively reduced, the phosphorylation of Ngn2 at S231 and S234, but not the level of Ngn2 expression was significantly decreased comparing to cells transfected with the scrambled shRNA (FIGURE 5E). These findings suggest that GSK3 mediates Ngn2 phosphorylation at S231 and S234 thereby promoting the association of Ngn2 with the LIM-HD factors during motor neuron differentiation. In addition to phosphorylating Ngn2, GSK3 has previously been suggested to phosphorylate Xenopus NeuroD to control the timing of neuronal differentiation in Xenopus retina (Moore et al., 2002). Given the diverse roles of GSK3 in development, it is possible that GSK3 regulates other aspects of motor neuron differentiation in addition to its effect on Ngn2 phosphorylation. Whether GSK3 phosphorylation of neurogenic bHLH factors is a general mechanism for controlling their functions awaits further study.
Given the critical role of GSK3/Shaggy in mediating Shh signaling in Drosophila (Jia et al., 2002;Price and Kalderon, 2002), and our observation that GSK3 phosphorylates Ngn2 on S231 and S234 during motor neuron differentiation, we considered the possibility that Shh might be one of the extrinsic factors that regulate the expression and/or phosphorylation status of Ngn2 at S231 and S234. To test this idea, mouse ES cells were differentiated into motor neuron progenitors and then stimulated with Shh. Nuclear lysates were prepared from these cells before, and after Shh treatment, and the Ngn2 expression level and phosphorylation status were assessed. We found that exposure to Shh led to a significant increase in Ngn2 protein in motor neuron progenitors, and the resulting Ngn2 was phosphorylated on both S231 and S234 (FIGURE 5F). Shh had no effect on the activity of GSK3 (Figure 5F), as indicated by Western blot analysis with a phospho-specific antibody recognizing phosphorylation on S9 of GSK3β. The phosphorylation of GSK3β at S9 has been shown to inversely correlate with GSK3β activity (Doble and Woodgett, 2003; Jope and Johnson, 2004). These findings suggest that Shh and GSK3 may function sequentially to coordinate motor neuron differentiation. Shh induces Ngn2 expression and newly synthesized Ngn2 is then phosphorylated by GSK3 on S231 and S234. The phosphorylation of Ngn2 facilitates the interaction of Ngn2 with LIM-HD factors that together activate genes that promote motor neuron specification.
DISCUSSION
Proneural bHLH transcription factors regulate many aspects of neural development, including neurogenesis, the timing of gliogenesis, and neuronal cell-type identity specification (Gowan et al., 2001; Guillemot et al., 1993; Hand et al., 2005; Johnson et al., 1990; Ma et al., 1996; Muroyama et al., 2005; Scardigli et al., 2001; Sun et al., 2001). While the mechanisms by which bHLH factors promote neurogenesis have been characterized, little is known about how these factors specify neuronal cell-type identity. In this study we have examined the mechanism by which Ngn2 promotes motor neuron cell-type specification. We find that Ngn2 is phosphorylated on two highly conserved residues, S231 and S234, by GSK3 during the formation of spinal motor neurons. These phosphorylation events promote the interaction of Ngn2 with LIM-HD transcription factors and thereby specify the formation of motor neurons. These findings have led us to propose a new mechanism of transcription factor action termed phosphorylation-dependent cooperativity that may allow a relatively small number of transcription factors to contribute to the generation of a diverse array of neuronal cell types. Phosphorylation-dependent cooperativity may be regulated by extrinsic factors such as growth factors, axon guidance factors, and neurotrophic factors so that transcription factor function is controlled temporally and spatially allowing a wide variety of neuronal cell types to be specified correctly and at the right time and place during brain development.
Phosphorylation on the C-Terminus of Ngn2 Is Critical for Its Function in Specifying Neuronal Cell-Type Identity but not in Promoting Neurogenesis
Proneural bHLH factors, such as Ngn2, promote neurogenesis by activating cascades of gene expression. The ability of Ngn2 to promote neurogenesis is dependent on the bHLH domain of Ngn2, which mediates both Ngn2’s interaction with the ubiquitously expressed E-protein, and the binding of the Ngn2/E-protein dimer to E-boxes within the promoters of Ngn2 target genes (Bertrand et al., 2002; Sun et al., 2001). Here we demonstrate that phosphorylation of S231 and S234 within the C-terminus of Ngn2, a region that is completely separate from the Ngn2 bHLH domain, is critical for neuronal cell-type specification. This is suggested by the findings that in Ngn2S231A&S234A knock-in mice motor neuron identity establishment is compromised and there is a significant increase in the number of V2 interneurons produced. Strikingly, the conversion of Ngn2 S231 and S234 to alanines has no effect on the ability of Ngn2 to promote neurogenesis indicating that the neurogenesis and neuronal cell-type specification functions of Ngn2 are separable. Thus it is possible that within a given motor neuron progenitor only a fraction of the total Ngn2 is phosphorylated at S231 and S234. The unphosphorylated Ngn2 in a motor neuron progenitor may regulate the expression of genes that control neurogenesis, while Ngn2 phosphorylated at S231 and S234 is targeted to the promoters of genes that specify motor neuron identity (Figure 6).
Figure 6. Model for Phosphorylation-Dependent Cooperativity between Ngn2 and LIM-HD Transcription Factors to Specify Motor Neuron Identity.
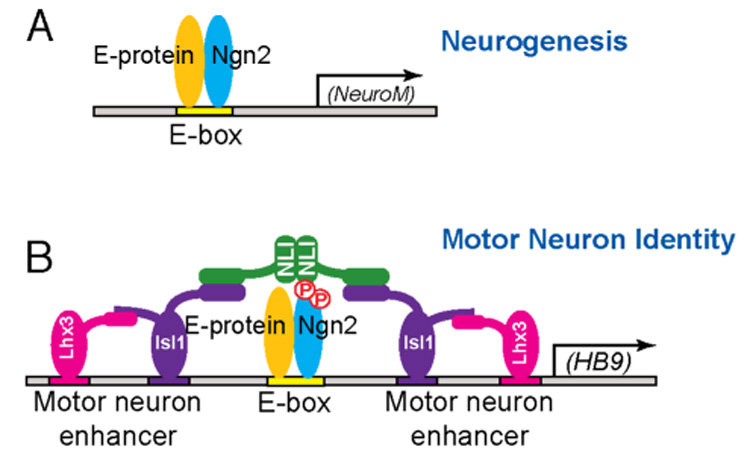
(A) To promote neurogenesis, Ngn2 dimerizes with E proteins to bind to consensus DNA motif E boxes in target promoters, and thereby activate the expression of genes such as NeuroM that induce neurogenesis. (B) During motor neuron identity specification, Ngn2 is phosphorylated on S231 and S234 by GSK3. These phosphorylation events facilitate the interaction between Ngn2 and LIM-HD transcription complexes to activate the expression of motor neuron-specific genes such as HB9. The Ngn2 S231&234 phosphorylation events are not required for Ngn2 induction of neurogenesis.
Additional post-translational modifications of Ngn2 within its C-terminal domain may contribute to the generation of the diverse cell types that are present in the nervous system. Consistent with this idea, there are other evolutionarily conserved serines, such as S215 and S205, within the C-terminus of Ngn2 that may be phosphorylated. It was recently reported that tyrosine residue Y241 on Ngn2, when mutated to a phenylalanine, results in defects in cortical neuron migration and dendritic arborization (Hand et al., 2005). These defects may result from a loss of phosphorylation of Ngn2 at Y241. It remains to be determined if the phosphorylation of Ngn2 at Y241, along with S231 and S234 phosphorylation, is a requisite step for the interaction of Ngn2 with LIM-HD proteins in motor neuron differentiation, or if the phosphorylation of Ngn2 at Y241 promotes Ngn2’s interaction with a distinct class of transcription factors to specify other neuronal subtypes. Nevertheless, our findings indicate that the differential phosphorylation of Ngn2 allows this transcription factor to regulate distinct aspects of neuronal differentiation.
The importance of the phosphorylation of Ngn2 at S231 and S234 for motor neuron specification was clearly demonstrated by the analysis of Ngn2S231A&S234A knock-in mice. We found a significant loss of motor neurons and an increase in the number of V2 interneurons in the knock-in embryos. This neuronal cell-type identity switch in Ngn2S231A&S234A knock-in mice is most likely due to the conversion of motor neurons to V2 interneurons resulting at least in part from a failure of Ngn2 to cooperate with LIM-HD proteins to activate the expression of HB9 which is required to suppress the V2 interneuron phenotype. The decreased expression of both HB9 and Isl1/2 in the spinal cord of Ngn2S231A&S234A knock-in mice and the increased expression of V2 interneuron markers suggest that the defect in motor neuron generation is more global than simply the loss of a few motor neuron markers.
It is notable that a subset rather than the entire population of motor neurons is lost in Ngn2S231A&S234A knock-in mice. One possible explanation for the partial loss of motor neurons in Ngn2S231A&S234A knock-in mice is that phosphorylation of Ngn2 on S231 and S234 is only required for the identity specification of a subgroup of motor neurons that eventually assemble into a specific motor column, for example the medial half of Median Motor Column (MMCm) that innervates axial muscles (Shirasaki and Pfaff, 2002). Interestingly, motor neurons from MMCm are known to express Lhx3 and Isl1, which synergize with Ngn2 to specify motor neuron identity. Further analysis of Ngn2S231A&S234A knock-in mice using markers that are selective to specific motor columns, as well as tracing of the trajectories of motor neurons in the knock-in mice, should allow us to determine the extent of the motor neuron defects that occur in the absence of Ngn2 phosphorylation at S231 and S234.
An alternative explanation for the partial loss of motor neurons in Ngn2S231A&S234A knock-in mice is that while Ngn2 plays a key role in motor neuron differentiation, in the absence of Ngn2 S231 and S234 phosphorylation, other bHLH factors such as NeuroM that typically act later than Ngn2 during development are able to compensate for Ngn2 and cooperate with LIM-HD factors. Consistent with this possibility, a previous study has shown that NeuroM, when over-expressed in the chick neural tube, can cooperate with LIM-HD proteins to promote motor neuron differentiation (Lee and Pfaff, 2003).
Phosphorylation-Dependent Cooperativity between Ngn2 and LIM-HD Factors
We have shown that Ngn2 phosphorylation at S231 and S234 regulates neuronal cell-type specification by promoting the interaction of Ngn2 with LIM-HD proteins and that this complex then activates the expression of motor neuron genes such as HB9. It will be of interest to characterize the nature of the interaction between Ngn2 and LIM-HD factors as it appears to represent a new mode of phosphorylation-dependent protein-protein interaction. It is through their ability to trigger the phosphorylation of transcription factors that cell extrinsic factors such as growth factors typically regulate cell intrinsic programs of gene expression that mediate the cell’s response to environmental signals. Growth factor-induced phosphorylation has previously been shown to modulate the activity of transcription factors by affecting their nuclear localization (Brunet et al., 1999), modulating their stability (Song et al., 1998), affecting their ability to interact with DNA, recruiting transcription cofactors (Chrivia et al., 1993), or switching a transcription repressor to an activator (Ju et al., 2004). Our finding that phosphorylation can also promote cooperative interactions between distinct classes of transcription factors suggests a mechanism by which a relatively small number of transcription factors and cell extrinsic factors might potentially specify a wide variety of neuronal cell types in the nervous system. The phosphorylation of Ngn2 at different sites within its C-terminus may allow Ngn2 to mix and match with distinct classes of homeodomain transcription factors thus greatly expanding the potential diversity of neuronal cell types generated.
How this might work is suggested by the nature of the interaction that S231 and S234- 24 phosphorylated form of Ngn2 makes with the LIM-HD complex. In motor neuron progenitors the LIM-HD complex is composed of six proteins (2NLI:2Isl1:2Lhx3). We have found that the LIM domain-binding adaptor protein NLI interacts with the S231 and S234-phosphorylated form of Ngn2. As NLI is known to associate with a number of transcription factors in addition to Isl1 and Lhx3 (Agulnick et al., 1996; Bach et al., 1997; Chen et al., 2002; Jurata et al., 1996; Ramain et al., 2000; Torigoi et al., 2000), this suggests that NLI may function to link C-terminal-phosphorylated Ngn2 with a variety of different transcription factors in different regions of the nervous system. This might then confer upon Ngn2 the ability to specify distinct neuronal cell types depending on which transcription factors are co-expressed with Ngn2 and NLI, and whether Ngn2 is phosphorylated at S231 and S234 or other sites within its C-terminus. In support of this idea, we have found that Ngn2 is phosphorylated at S231 and S234 in multiple regions of the brain in addition to the spinal cord. Future experiments will determine if in these various brain regions Ngn2 phosphorylation at S231 and S234, or at other sites, facilitates Ngn2’s cooperation with novel families of transcription factors to specify distinct types of neurons.
Regulation of GSK3-Mediated Phosphorylation of Ngn2
Given the importance of phosphorylation-dependent cooperativity between Ngn2 and LIM-HD factors for motor neuron development, we investigated the signal transduction pathways that control this process. We identify Shh as an extrinsic factor that promotes the cooperativity between Ngn2 and the LIM-HD complex by inducing Ngn2 expression. GSK3 then phosphorylates Ngn2 at S231 and S234. Additional mechanisms may control GSK3 activity so that Ngn2 is phosphorylated at the right time and place during motor neuron development and the level of Ngn2 phosphorylation is properly controlled. The activity of GSK3 is known to be negatively and positively regulated by extracellular stimuli (Doble and Woodgett, 2003; Jope and Johnson, 2004). It will be of interest to determine if factors such as Wnts, bone morphogenic proteins (BMPs) (Chizhikov and Millen, 2005; Logan and Nusse, 2004), or fibroblast growth factors (FGFs) (Jessell, 2000; Shirasaki et al., 2006), each of which are known to regulate both GSK3 function and spinal cord development, play a role in regulating GSK3 phosphorylation of Ngn2.
CONCLUSION
In this study we provide genetic, biochemical and cell biological evidence for a novel mode of transcriptional regulation that we have termed phosphorylation-dependent cooperativity. Through phosphorylation-dependent cooperativity Ngn2 and LIM-HD factors regulate the identity specification of motor neurons. However, given that there are well-conserved consensus sites for phosphorylation in the C-terminal regions of proneural bHLH factors in addition to Ngn2, it is likely that phosphorylation-dependent cooperativity is a general mechanism by which cell-extrinsic factors send signals to bHLH factors to promote the development of diverse cell types in the nervous system.
EXPERIMENTAL PROCEDURES
Generation of Ngn2S231A&S234A Knock-in Mice
The Ngn2S231A&S234A knock-in targeting vector was constructed by introducing mutations into the cloned genomic Ngn2 sequence using QuikChange (Stratagene) to convert S231 and S234 into alanines, and placing a loxP/PGKneo/loxP cassette into an EcoRI site at the end of the single exon that encodes Ngn2. A silent mutation (S218S) was also introduced to create a SacI site nearby to facilitate genotyping. The Ngn2S231A&S234A/loxP/PGKneo/loxP cassette was placed between a 5.6kb 5’ arm and a 2.2kb 3’ arm that were generated by PCR using wild type 129SVJ ES cell genomic DNA as template. The targeting construct was electroporated into 129SVJ ES cells and selected with G418. Eighteen ES cell clones carrying both the correctly targeted Ngn2S231A&S234A mutation and the loxP/PGKneo/loxP cassette were identified out of 208 clones (9.1%) by Southern blot analysis using external probes. Seven ES cell clones carrying wild type Ngn2 and the loxP/PGKneo/loxP cassette were identified out of 208 clones (3.4%). Two correctly recombined independent clones of ES cells were injected into C57BL/6J blastocysts to generate germline chimeric founders. One ES cell clone carrying a loxP/PGKneo/loxP cassette lacking the Ngn2S231A&S234A mutation was also injected to generate a control mouse line. Germline-transmitted chimeras were mated with transgenic mice expressing Cre recombinase under the control of ubiqitiously expressing E2A promoter to remove the PGKneo cassette. Mutant mice were genotyped by detecting the presence of the SacI site, the S231A&S234A point mutations and the LoxP site from PCR products covering the Ngn2S231A&S234A/loxP region.
In Ovo Electroporation
Chick eggs (Charles River) were incubated in a 38°C humidified chamber and embryos were staged according to Hamburger and Hamilton (HH). DNA constructs were injected into the lumens of HH stage 10 chick embryonic neural tubes. Electroporation was performed using a square wave electroporator (BTX). Electroporated embryos were incubated to allowed further development and incubated chicks were harvested and analyzed at HH stage 15 as previously described (Thaler et al., 2002).
Differentiation of Mouse ES Cells into Motor Neuron Progenitors
Details can be found in the Supplemental Data.
Immunohistochemistry
Mouse embryos were fixed by immersion in 4% paraformaldehyde from one to two hours at 4°C, depending on the age. The following primary antibodies were used: guinea pig anti-Chx10 (1:8000, Dr. Samuel L. Pfaff), rabbit anti-Hb9 (1:8000, Dr. Samuel L. Pfaff), rabbit anti-Isl1/2 (1:2500, Dr. Samuel L. Pfaff), rabbit anti-NLI (1:1000, Dr. Gordon N. Gill), mouse anti-Olig2 (1:1000, Dr. Charles D. Stiles), rabbit anti-GSK3α (1:500, Cell Signaling), rabbit anti-GSK3β (1:1000, Cell Signaling), mouse monoclonal (mAB) anti-TuJ1 (1:1000, Covance), mAB anti-Flag M2 (1:1000, Sigma), mAB anti-Myc 9E10 (1:1000, Santa Cruz Biotech) and rabbit anti-Myc (1:1000, Santa Cruz Biotech). mAbs against Lim3 (67.4E12), MNR2 (81.5C10), Isl1 (40.2D6) and neuronfilament M (2H3) were obtained from the Developmental Studies Hybridoma Bank. The rabbit anti-Ngn2 (1:500), rat anti-Ngn2 (1:200) and phospho-specific rabbit anti-Ngn2 P-S231&S234 antibodies (1:200) were generated and affinity-purified in the Greenberg laboratory as described (Brunet et al., 1999). Whole-mount neurofilament M immunostaining was performed as described (Gu et al., 2003).
Constructs
Details can be found in the Supplemental Data.
In Vitro Kinase Assay
Details can be found in the Supplemental Data.
Immunoblotting and Immunoprecipitation
Details can be found in the Supplemental Data.
Gel Filtration Column
Details can be found in the Supplemental Data.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Mental Retardation Developmental Disabilities Research Center Gene Manipulation Core Facility of Children’s Hospital Boston for their help with making the knock-in mice. We thank Dr. Gordon N. Gill for providing the anti-NLI antibody, Dr. Charles D. Stiles for the anti- Olig2 antibody, and Dr. William D. Snider and Dr. David L. Turner for GSK3 RNAi constructs. We thank members of the Greenberg laboratory for critically reading the manuscript. Y.C.M. was supported by fellowships from the American Cancer Society and the William Randolph Hearst Fund. This research was supported by National Institute of Neurological Disorders and Stroke (NINDS) grant R37NS037116 (S.L.P.), grant PO1NS047572 (M.E.G.), and a Mental Retardation Developmental Disabilities Research Center grant HD18655 (M.E.G.). M.E.G. acknowledges the generous support of the F.M. Kirby Foundation to the Children's Hospital F. M. Kirby Neurobiology Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agulnick AD, Taira M, Breen JJ, Tanaka T, Dawid IB, Westphal H. Interactions of the LIM-domain-binding factor Ldb1 with LIM homeodomain proteins. Nature. 1996;384:270–272. doi: 10.1038/384270a0. [DOI] [PubMed] [Google Scholar]
- Andersson E, Jensen JB, Parmar M, Guillemot F, Bjorklund A. Development of the mesencephalic dopaminergic neuron system is compromised in the absence of neurogenin 2. Development. 2006;133:507–516. doi: 10.1242/dev.02224. [DOI] [PubMed] [Google Scholar]
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Bach I, Carriere C, Ostendorff HP, Andersen B, Rosenfeld MG. A family of LIM domain-associated cofactors confer transcriptional synergism between LIM and Otx homeodomain proteins. Genes Dev. 1997;11:1370–1380. doi: 10.1101/gad.11.11.1370. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Chen L, Segal D, Hukriede NA, Podtelejnikov AV, Bayarsaihan D, Kennison JA, Ogryzko VV, Dawid IB, Westphal H. Ssdp proteins interact with the LIM-domain-binding protein Ldb1 to regulate development. Proc Natl Acad Sci U S A. 2002;99:14320–14325. doi: 10.1073/pnas.212532399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov VV, Millen KJ. Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev Biol. 2005;277:287–295. doi: 10.1016/j.ydbio.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Cleveland DW. From Charcot to SOD1: mechanisms of selective motor neuron death in ALS. Neuron. 1999;24:515–520. doi: 10.1016/s0896-6273(00)81108-3. [DOI] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- Ericson J, Thor S, Edlund T, Jessell TM, Yamada T. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science. 1992;256:1555–1560. doi: 10.1126/science.1350865. [DOI] [PubMed] [Google Scholar]
- Farah MH, Olson JM, Sucic HB, Hume RI, Tapscott SJ, Turner DL. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development. 2000;127:693–702. doi: 10.1242/dev.127.4.693. [DOI] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C, Guillemot F. The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron. 1998;20:483–494. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Fode C, Ma Q, Casarosa S, Ang SL, Anderson DJ, Guillemot F. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 2000;14:67–80. [PMC free article] [PubMed] [Google Scholar]
- Ge W, He F, Kim KJ, Blanchi B, Coskun V, Nguyen L, Wu X, Zhao J, Heng JI, Martinowich K, et al. Coupling of cell migration with neurogenesis by proneural bHLH factors. Proc Natl Acad Sci U S A. 2006;103:1319–1324. doi: 10.1073/pnas.0510419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, Odom R, Johnson JE. Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31:219–232. doi: 10.1016/s0896-6273(01)00367-1. [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Hand R, Bortone D, Mattar P, Nguyen L, Heng JI, Guerrier S, Boutt E, Peters E, Barnes AP, Parras C, et al. Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron. 2005;48:45–62. doi: 10.1016/j.neuron.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jia J, Amanai K, Wang G, Tang J, Wang B, Jiang J. Shaggy/GSK3 antagonizes Hedgehog signalling by regulating Cubitus interruptus. Nature. 2002;416:548–552. doi: 10.1038/nature733. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Birren SJ, Anderson DJ. Two rat homologues of Drosophila achaete-scute specifically expressed in neuronal precursors. Nature. 1990;346:858–861. doi: 10.1038/346858a0. [DOI] [PubMed] [Google Scholar]
- Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Ju BG, Solum D, Song EJ, Lee KJ, Rose DW, Glass CK, Rosenfeld MG. Activating the PARP-1 sensor component of the groucho/TLE1 corepressor complex mediates a CaMKinase IIdelta-dependent neurogenic gene activation pathway. Cell. 2004;119:815–829. doi: 10.1016/j.cell.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Jurata LW, Kenny DA, Gill GN. Nuclear LIM interactor, a rhombotin and LIM homeodomain interacting protein, is expressed early in neuronal development. Proc Natl Acad Sci U S A. 1996;93:11693–11698. doi: 10.1073/pnas.93.21.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kele J, Simplicio N, Ferri AL, Mira H, Guillemot F, Arenas E, Ang SL. Neurogenin 2 is required for the development of ventral midbrain dopaminergic neurons. Development. 2006;133:495–505. doi: 10.1242/dev.02223. [DOI] [PubMed] [Google Scholar]
- Kim WY, Zhou FQ, Zhou J, Yokota Y, Wang YM, Yoshimura T, Kaibuchi K, Woodgett JR, Anton ES, Snider WD. Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron. 2006;52:981–996. doi: 10.1016/j.neuron.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Pfaff SL. Transcriptional networks regulating neuronal identity in the developing spinal cord. Nat Neurosci. 2001;(4 Suppl):1183–1191. doi: 10.1038/nn750. [DOI] [PubMed] [Google Scholar]
- Lee SK, Pfaff SL. Synchronization of neurogenesis and motor neuron specification by direct coupling of bHLH and homeodomain transcription factors. Neuron. 2003;38:731–745. doi: 10.1016/s0896-6273(03)00296-4. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13:1717–1728. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- Ma YC, Huang J, Ali S, Lowry W, Huang XY. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Mizuguchi R, Sugimori M, Takebayashi H, Kosako H, Nagao M, Yoshida S, Nabeshima Y, Shimamura K, Nakafuku M. Combinatorial roles of olig2 and neurogenin2 in the coordinated induction of pan-neuronal and subtype-specific properties of motoneurons. Neuron. 2001;31:757–771. doi: 10.1016/s0896-6273(01)00413-5. [DOI] [PubMed] [Google Scholar]
- Monani UR. Spinal muscular atrophy: a deficiency in a ubiquitous protein; a motor neuron-specific disease. Neuron. 2005;48:885–896. doi: 10.1016/j.neuron.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Moore KB, Schneider ML, Vetter ML. Posttranslational mechanisms control the timing of bHLH function and regulate retinal cell fate. Neuron. 2002;34:183–195. doi: 10.1016/s0896-6273(02)00666-9. [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Fujiwara Y, Orkin SH, Rowitch DH. Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature. 2005;438:360–363. doi: 10.1038/nature04139. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Besson A, Heng JI, Schuurmans C, Teboul L, Parras C, Philpott A, Roberts JM, Guillemot F. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 2006;20:1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M, Schuurmans C, Britz O, Guillemot F. Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron. 2001;29:401–413. doi: 10.1016/s0896-6273(01)00214-8. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Chen AI, Jessell TM. Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron. 2001;31:773–789. doi: 10.1016/s0896-6273(01)00407-x. [DOI] [PubMed] [Google Scholar]
- Price MA, Kalderon D. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell. 2002;108:823–835. doi: 10.1016/s0092-8674(02)00664-5. [DOI] [PubMed] [Google Scholar]
- Ramain P, Khechumian R, Khechumian K, Arbogast N, Ackermann C, Heitzler P. Interactions between chip and the achaete/scute-daughterless heterodimers are required for pannier-driven proneural patterning. Mol Cell. 2000;6:781–790. doi: 10.1016/s1097-2765(05)00079-1. [DOI] [PubMed] [Google Scholar]
- Scardigli R, Schuurmans C, Gradwohl G, Guillemot F. Crossregulation between Neurogenin2 and pathways specifying neuronal identity in the spinal cord. Neuron. 2001;31:203–217. doi: 10.1016/s0896-6273(01)00358-0. [DOI] [PubMed] [Google Scholar]
- Sharma K, Sheng HZ, Lettieri K, Li H, Karavanov A, Potter S, Westphal H, Pfaff SL. LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons. Cell. 1998;95:817–828. doi: 10.1016/s0092-8674(00)81704-3. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Lewcock JW, Lettieri K, Pfaff SL. FGF as a target-derived chemoattractant for developing motor axons genetically programmed by the LIM code. Neuron. 2006;50:841–853. doi: 10.1016/j.neuron.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Song A, Wang Q, Goebl MG, Harrington MA. Phosphorylation of nuclear MyoD is required for its rapid degradation. Mol Cell Biol. 1998;18:4994–4999. doi: 10.1128/mcb.18.9.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, Fan G, Greenberg ME. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001;104:365–376. doi: 10.1016/s0092-8674(01)00224-0. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, William C, Jessell TM. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell. 1998;95:67–80. doi: 10.1016/s0092-8674(00)81783-3. [DOI] [PubMed] [Google Scholar]
- Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, Pfaff SL. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23:675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Lee SK, Jurata LW, Gill GN, Pfaff SL. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein-protein interactions. Cell. 2002;110:237–249. doi: 10.1016/s0092-8674(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Torigoi E, Bennani-Baiti IM, Rosen C, Gonzalez K, Morcillo P, Ptashne M, Dorsett D. Chip interacts with diverse homeodomain proteins and potentiates bicoid activity in vivo. Proc Natl Acad Sci U S A. 2000;97:2686–2691. doi: 10.1073/pnas.050586397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Muhr J, Pattyn A, Pierani A, Mendelsohn M, Sander M, Jessell TM, Ericson J. Different levels of repressor activity assign redundant and specific roles to Nkx6 genes in motor neuron and interneuron specification. Neuron. 2001;31:743–755. doi: 10.1016/s0896-6273(01)00412-3. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Yu JY, Taylor J, DeRuiter SL, Vojtek AB, Turner DL. Simultaneous inhibition of GSK3alpha and GSK3beta using hairpin siRNA expression vectors. Mol Ther. 2003;7:228–236. doi: 10.1016/s1525-0016(02)00037-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


