Abstract
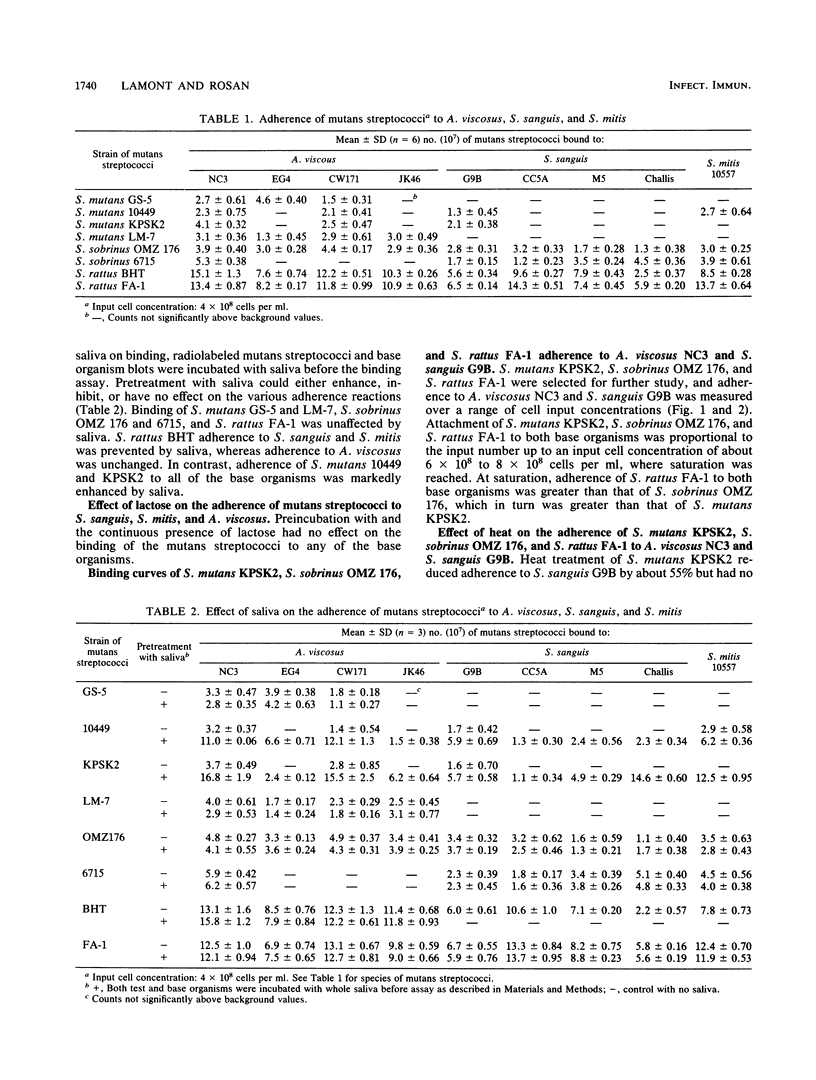
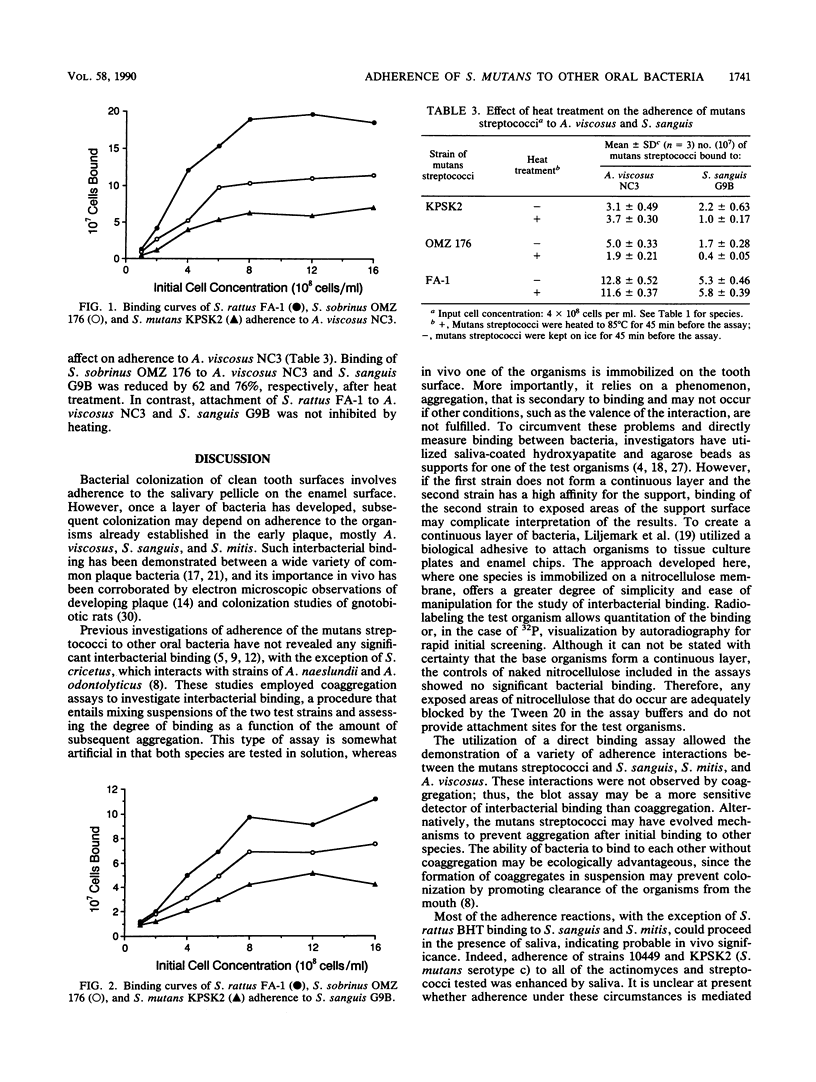
Adherence of mutans streptococci to strains of Actinomyces viscosus, Streptococcus sanguis, and Streptococcus mitis immobilized on a nitrocellulose membrane was measured. Strains of Streptococcus mutans, S. sobrinus, and S. rattus bound in a lactose-independent manner to a variety of the actinomyces and streptococci. Most of these reactions could proceed in the presence of whole saliva although adherence of S. rattus BHT to the streptococci was inhibited by salivary molecules. In contrast, adherence of S. mutans 10449 and KPSK2 to A. viscosus, S. sanguis, and S. mitis was enhanced by salivary molecules. S. mutans KPSK2, S. sobrinus OMZ 176, and S. rattus FA-1 binding to A. viscosus NC3 and S. sanguis G9B exhibited saturation kinetics. Adherence to A. viscosus NC3 was of a higher avidity than adherence to S. sanguis G9B. Attachment of S. mutans KPSK2 to S. sanguis G9B and of S. mutans OMZ 176 to A. viscosus NC3 and S. sanguis G9B was inhibited by heat treatment of the mutans streptococci. Attachment of S. mutans KPSK2 to A. viscosus NC3 and of S. rattus FA-1 to A. viscosus NC3 and S. sanguis G9B was unaffected by heat. These observations suggest that the mutans streptococci can adhere to a variety of early plaque bacteria by several distinct mechanisms. Such interactions may be important in the colonization of tooth surfaces by the mutans streptococci.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermans F., Klein J. P., Ogier J., Bazin H., Cormont F., Frank R. M. Purification and characterization of a saliva-interacting cell-wall protein from Streptococcus mutans serotype f by using monoclonal-antibody immunoaffinity chromatography. Biochem J. 1985 May 15;228(1):211–217. doi: 10.1042/bj2280211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H., Hageage G., Pollock F., Harr R. Plaque formation in vitro on wires by gram-negative oral microorganisms (Veillonella). Arch Oral Biol. 1970 Feb;15(2):127–133. doi: 10.1016/0003-9969(70)90048-8. [DOI] [PubMed] [Google Scholar]
- Ciardi J. E., McCray G. F., Kolenbrander P. E., Lau A. Cell-to-cell interaction of Streptococcus sanguis and Propionibacterium acnes on saliva-coated hydroxyapatite. Infect Immun. 1987 Jun;55(6):1441–1446. doi: 10.1128/iai.55.6.1441-1446.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E., Clark W. B., Curl S. H., Hurst-Calderone S., Sandberg A. L. Mutants of Actinomyces viscosus T14V lacking type 1, type 2, or both types of fimbriae. Infect Immun. 1988 Nov;56(11):2984–2989. doi: 10.1128/iai.56.11.2984-2989.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley P. J., Fischlschweiger W., Coleman S. E., Bleiweis A. S. Intergeneric bacterial coaggregations involving mutans streptococci and oral actinomyces. Infect Immun. 1987 Nov;55(11):2695–2700. doi: 10.1128/iai.55.11.2695-2700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Interbacterial aggregation of Actinomyces naeslundii and dental plaque streptococci. J Periodontal Res. 1977 Jan;12(1):11–20. doi: 10.1111/j.1600-0765.1977.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Forester H., Hunter N., Knox K. W. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J Gen Microbiol. 1983 Sep;129(9):2779–2788. doi: 10.1099/00221287-129-9-2779. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Adherent interactions which may affect microbial ecology in the mouth. J Dent Res. 1984 Mar;63(3):378–385. doi: 10.1177/00220345840630030401. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Holt R. G., Abiko Y., Saito S., Smorawinska M., Hansen J. B., Curtiss R., 3rd Streptococcus mutans genes that code for extracellular proteins in Escherichia coli K-12. Infect Immun. 1982 Oct;38(1):147–156. doi: 10.1128/iai.38.1.147-156.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. J. A special relationship between spherical and filamentous microorganisms in mature human dental plaque. Arch Oral Biol. 1972 Mar;17(3):613–616. doi: 10.1016/0003-9969(72)90081-7. [DOI] [PubMed] [Google Scholar]
- Jordan H. V. Cultural methods for the identification and quantitation of Streptococcus mutans and lactobacilli in oral samples. Oral Microbiol Immunol. 1986 Nov;1(1):23–30. doi: 10.1111/j.1399-302x.1986.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J., DiRienzo J. M. Isolation of a corncob (coaggregation) receptor polypeptide from Fusobacterium nucleatum. Infect Immun. 1989 Feb;57(2):331–337. doi: 10.1128/iai.57.2.331-337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Multigeneric aggregations among oral bacteria: a network of independent cell-to-cell interactions. J Bacteriol. 1986 Nov;168(2):851–859. doi: 10.1128/jb.168.2.851-859.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama K., Gibbons R. J. Inhibition of lactose-reversible adherence between Actinomyces viscosus and oral streptococci by salivary components. Caries Res. 1984;18(3):193–200. doi: 10.1159/000260765. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Coulter M. C., Fenner L. J., Skopek R. J., Schachtele C. F. Utilization of a continuous streptococcal surface to measure interbacterial adherence in vitro and in vivo. J Dent Res. 1988 Dec;67(12):1455–1460. doi: 10.1177/00220345880670120301. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier J. A., Klein J. P., Sommer P., Frank R. M. Identification and preliminary characterization of saliva-interacting surface antigens of Streptococcus mutans by immunoblotting, ligand blotting, and immunoprecipitation. Infect Immun. 1984 Jul;45(1):107–112. doi: 10.1128/iai.45.1.107-112.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H., Kato H., Okahashi N., Takahashi I., Hamada S., Koga T. Characterization of a cell-surface protein antigen of hydrophilic Streptococcus mutans strain GS-5. J Gen Microbiol. 1989 Apr;135(4):981–988. doi: 10.1099/00221287-135-4-981. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Lehner T. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch Oral Biol. 1978;23(1):7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Mansson-Rahemtulla B. Interaction between surface protein antigens of Streptococcus mutans and human salivary components. Oral Microbiol Immunol. 1989 Jun;4(2):106–111. doi: 10.1111/j.1399-302x.1989.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- Schwarz S., Ellen R. P., Grove D. A. Bacteroides gingivalis-Actinomyces viscosus cohesive interactions as measured by a quantitative binding assay. Infect Immun. 1987 Oct;55(10):2391–2397. doi: 10.1128/iai.55.10.2391-2397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staat R. H., Langley S. D., Doyle R. J. Streptococcus mutans adherence: presumptive evidence for protein-mediated attachment followed by glucan-dependent cellular accumulation. Infect Immun. 1980 Feb;27(2):675–681. doi: 10.1128/iai.27.2.675-681.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staat R. H., Peyton J. C. Adherence of oral streptococci: evidence for nonspecific adsorption to saliva-coated hydroxylapatite surfaces. Infect Immun. 1984 Jun;44(3):653–659. doi: 10.1128/iai.44.3.653-659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


