Abstract
The random-zero sphygmomanometer has been widely used in observational studies and clinical trials for blood pressure measurement. We examined the agreement of blood pressure measurements between random-zero and standard mercury sphygmomanometers among 2,007 Chinese study participants aged 15–60 years. Three blood pressure readings were obtained by trained observers using random-zero and standard mercury sphygmomanometers, respectively, in a random order. Overall, blood pressure readings obtained using the random-zero device were significantly lower than those obtained with the standard mercury sphygmomanometer, with a mean difference ranging from −3.0 to −2.7 mm Hg for systolic and −1.4 to −0.9 mm Hg for diastolic blood pressure (all p <0.01). Correlation coefficients between mean blood pressure measurements obtained using the random-zero and standard mercury sphygmomanometers were high (0.90 for systolic and 0.85 for diastolic blood pressure, both p< 0.0001). In conclusion, our study indicated that there was strong agreement between blood pressure measurements obtained using the random-zero and standard mercury sphygmomanometers although blood pressure values were on average lower with the random-zero sphygmomanometer.
Keywords: blood pressure measurements, random-zero sphygmomanometer, standard mercury sphygmomanometer, agreement
INTRODUCTION
Accurate measurement of blood pressure (BP) is crucially important to classify individuals, to ascertain BP-related risk, and to guide management. The classic approach to BP measurement is based on use of the standard mercury sphygmomanometer, which has long been regarded as the gold standard for BP measurements in clinical practice since its first description in 1896 (1–3). The two most common sources of inter-observer variation in the measurement of BP using the standard mercury sphygmomanometer are measurement bias and digit preference (1, 4–6). The random-zero sphygmomanometer was introduced as a modified instrument to improve the quality of BP measurement by reducing observer bias (7,8). It has been widely used in BP clinical trials and observational epidemiologic studies.
Some initial studies assured the accuracy of the random-zero sphygmomanometer and documented that it reduced observer bias and digit preference (9,10). Lately, clinical studies have reported significant underestimation of both systolic and diastolic BP by the random-zero sphygmomanometer (4,11–14). Compared with the standard mercury sphygmomanometer, the random-zero sphygmomanometer has been reported to underestimate systolic BP by 0.9 to 3.8 mm Hg, and diastolic BP by 0.7 to 7.5 mm Hg (4,11,15–19). Previous studies, however, have had small sample sizes. In addition, no study has examined the agreement between BPs measured by the random-zero and standard mercury sphygmomanometer in an Asian-population. We examined the agreement of BP measurements by the random-zero sphygmomanometer and the standard mercury sphygmomanometer among 2,007 Chinese adults who were participants in the Genetic Epidemiology Network of Salt Sensitivity (GenSalt) study.
METHODS
Study Population
The GenSalt study was conducted in north China from October 2003 to July 2005. Study participants (n=3,153) were recruited from rural areas in Hebei, Henan, Shandong, Shaanxi, and Jiangsu provinces. A community-based BP screening was conducted among persons aged 18 years or older in the study villages to identify potential probands and their families for the study. Detailed eligibility criteria for study participation have been published (20). In general, individuals were excluded from the study if they had stage-2 hypertension (systolic BP ≥160 and/or diastolic BP ≥100 mm Hg), a history of clinical cardiovascular disease or diabetes, used antihypertensive medications, consumed alcohol frequently, were pregnant, or were currently following a low-sodium diet. A total of 2,007 study participants aged 16–60 years were selected to have 3 random-zero and 3 standard BP measurements on the same day and were used for the current analyses.
Institutional Review Boards at the Tulane University Health Sciences Center, Washington University School of Medicine, University of Texas Health Sciences Center at Houston, Chinese Academy of Medical Sciences, and Chinese National Human Genomic Center at Beijing approved the GenSalt study. Written informed consents were obtained from each participant.
Data Collection
The GenSalt study included a 3-day baseline observation. A standard questionnaire was administered by a trained staff member to collect information on demographic characteristics, personal and family medical history, and lifestyle risk factors (including cigarette smoking, alcohol consumption, and physical activity) on the first day of baseline observation. Three random-zero BP measurements were obtained using a Hawksley random-zero sphygmomanometer (Hawksley & Sons Ltd, Lancing, UK; zero range 0–20 mmHg) on each day of baseline observation and 3 standard BP measurements were obtained using a PyMah mercury sphygmomanometer (Pymah Corporation, Flemington, NJ) on day 2 or 3 of baseline observation. The Hawksley random-zero sphygmomanometers were calibrated against a standard mercury sphygmomanometer weekly. The random-zero and standard BP measurements were obtained within one hour of each other. The order of the random-zero or standard mercury sphygmomanometer BP measurements was random. Body weight, height, and waist girth were also obtained according to a standard protocol.
BP was measured by trained and certified observers according to a common protocol adapted from procedures recommended by the American Heart Association (21). BP was measured with the participant in the sitting position after 5 minutes of rest. In addition, participants were advised to avoid alcohol, cigarette smoking, coffee/tea, and exercise for at least 30 minutes prior to their BP measurement. One of four cuff sizes (pediatric, regular adult, large, or thigh) was chosen on the basis of the circumference of the participant’s arm (21). All BP observers participated in a special training session on the use of a standardized protocol for measurement of BP. Satisfactory performance during a written test on knowledge of preparing study participants for BP measurement, selecting the correct cuff size, and using standard techniques for BP measurement, during a standardized videotape examination and during concordant measurements of BP with an instructor, were required for certification as a GenSalt BP observer.
Statistical Analysis
Mean BP values were calculated based on the 3 readings obtained using the random-zero or standard mercury sphygmomanometer. Differences are presented as random-zero BP values minus standard mercury BP values. A paired t test was used to determine whether differences in BP obtained with the random-zero and standard mercury methods were significantly different from zero. The Chi-square test was used to determine whether there were significant differences in digit preference for the BP readings obtained the two sphygmomanometers. The mean BP values obtained using the standard mercury sphygmomanometer were plotted against the mean BP values obtained using the random-zero sphygmomanometer to assess their level of agreement. In addition, the difference of mean systolic or diastolic BP measurements recorded by the two sphygmomanometers was plotted against an average of the BP measurements obtained with both sphygmomanometers (22). A multivariate linear regression analysis was conducted to explore the determinants of BP difference between the two sphygmomanometers. Two tailed p values were calculated and p <0.05 was considered to be statistically significant. All analyses were performed using the SAS statistical software version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Characteristics of the 2,007 study participants are presented in Table 1. Mean age of the participants was 40.1 years (ranging from 16 to 60 years) and 52.4% of the participants were men. Mean random-zero BP was 117.8 mm Hg for systolic and 73.9 mm Hg for diastolic BP. Mean standard BP was 120.1 mm Hg for systolic and 74.7 mm Hg for diastolic BP.
Table 1.
Characteristics of 2,007 Study Participants with Blood Pressure Measurements by Random-zero and Standard Mercury Sphygmomanometers
| Characteristics | Mean* or Percentage |
|---|---|
| Age, year | 40.1 ± 10.3 |
| Men, % | 52.4 |
| Body mass index, kg/m2 | 23.4 ± 3.2 |
| Physical activity, met/day | 63.4 ± 21.1 |
| Arm circumference, cm | 28.4 ± 2.9 |
| Waist circumference, cm | 80.5 ± 10.0 |
| Blood pressure by random-zero sphygmomanometer, mm Hg | |
| Systolic | 117.8 ± 15.1 |
| Diastolic | 73.9 ± 10.5 |
| Blood pressure by standard mercury sphygmomanometer, mm Hg | |
| Systolic | 120.1 ± 15.6 |
| Diastolic | 74.7 ± 10.9 |
mean ± standard deviation
The distribution of digit preference was not statistically significantly different between the two sphygmomanometers for systolic (χ2= 26.9, p=0.3) and diastolic (χ2=13.1, p=0.7) BP. The percentage of readings with 0, 2, 4, 6, and 8 as the terminal digit was 20.4, 18.0, 20.8, 20.4, and 20.5 for the random-zero systolic BP measurements and 23.2, 18.5, 20.0, 21.7, and 16.7 for the standard mercury systolic BP measurements. The corresponding percentages were 24.4, 16.7, 19.6, 19.8, and 19.5 for the random-zero diastolic BP measurements and 24.3, 16.9, 19.5, 20.1, and 19.3 for the standard diastolic BP measurements, respectively.
Mean differences in BP measurements obtained with the random-zero and standard mercury sphygmomanometers are shown in Table 2. Overall, BP readings obtained with the random-zero sphygmomanometer were significantly lower than the corresponding readings using the standard mercury sphygmomanometer, with a mean difference ranging from −3.1 to −2.7 mm Hg for systolic BP and −1.3 to −0.9 mm Hg for diastolic BP (all p <0.01). The lower BP values obtained using the random-zero sphygmomanometer were consistently identified in gender, age, body weight, and physical activity subgroups. For example, the mean differences (95% CI) in systolic and diastolic BP were −2.9 (−3.3, −2.5) and −1.0 (−1.4, −0.5) among those <40 year old, and −2.8 (−3.3, −2.2) and −1.1 (−1.5, −0.6) among those ≥40 year old; −2.5 (−3.0, −1.9) and −0.9 (−1.3, −0.4) among those with body mass index <23.0 kg/m2 and −3.2 (−3.7, −2.7) and −1.3 (−1.7, −0.9) among those with body mass index ≥23.0 kg/m2; and −2.6 (−3.0, −2.1) and −1.0 (−1.4, −0.5) among those with physical activity <56.4 MET/day and −3.1 (−3.6, −2.6) and −1.1 (−1.6, −0.7) among those with physical activity ≥56.4 MET/day.
Table 2.
Mean Difference in Blood Pressure Measurements by Random-zero and Standard Mercury Sphygmomanometers
| Systolic, mm Hg | Diastolic, mm Hg | |||
|---|---|---|---|---|
| Reading No. | ||||
| Mean ± SD | Mean Difference (95% CI) | Mean ± SD | Mean Difference (95% CI) | |
| Overall | ||||
| 1st | ||||
| Random-zero | 117.7 ± 15.5 | −3.1 (−3.4, −2.7) ** | 73.6 ± 11.2 | −1.3 (−1.6, −1.0) ** |
| Standard | 120.7 ± 15.8 | 75.0 ± 11.1 | ||
| 2nd | ||||
| Random-zero | 117.2 ± 15.5 | −2.8 (−3.1, −2.4) ** | 73.7 ± 11.1 | −1.0 (−1.3, −0.6) ** |
| Standard | 120.0 ± 15.7 | 74.7 ± 11.1 | ||
| 3rd | ||||
| Random-zero | 116.9 ± 15.3 | −2.7 (−3.0, −2.3) ** | 73.6 ± 10.9 | −0.9 (−1.2, −0.6) ** |
| Standard | 119.6 ± 15.6 | 74.5 ± 11.0 | ||
|
| ||||
| Men | ||||
| 1st | ||||
| Random-zero | 119.2 ± 14.1 | −2.8 (−3.2, −2.3) ** | 75.3 ± 10.7 | −1.3 (−1.8, −0.9) ** |
| Standard | 122.0 ± 14.5 | 76.6 ± 10.8 | ||
| 2nd | ||||
| Random-zero | 118.8 ± 14.0 | −2.5 (−3.0, −2.1) ** | 75.5 ± 10.5 | −1.0 (−1.5, −0.6) ** |
| Standard | 121.3 ± 14.5 | 76.5 ± 10.8 | ||
| 3rd | ||||
| Random-zero | 118.3 ± 13.9 | −2.7 (−3.1, −2.2) ** | 75.4 ± 10.6 | −1.0 (−1.5, −0.6) ** |
| Standard | 121.0 ± 14.4 | 76.4 ± 10.7 | ||
|
| ||||
| Women | ||||
| 1st | ||||
| Random-zero | 116.0 ± 16.9 | −3.4 (−4.0, −2.9) ** | 71.8 ± 11.4 | −1.4 (−1.8, −0.8) ** |
| Standard | 119.4 ± 17.1 | 73.2 ± 11.2 | ||
| 2nd | ||||
| Random-zero | 115.5 ± 16.8 | −3.0 (−3.6, −2.5) ** | 71.8 ± 11.3 | −0.9 (−1.3, −0.4) * |
| Standard | 118.5 ± 16.9 | 72.7 ± 11.1 | ||
| 3rd | ||||
| Random-zero | 115.3 ± 16.6 | −2.8 (−3.3, −2.2) ** | 71.8 ± 10.9 | −0.7 (−1.1, −0.3) * |
| Standard | 118.1 ± 16.8 | 72.5 ± 10.9 | ||
SD = standard deviation; CI = confidence interval.
p<0.01,
p<0.0001 for comparisons between blood pressure values using the random zero sphygmomanometer vs. standard mercury sphygmomanometer.
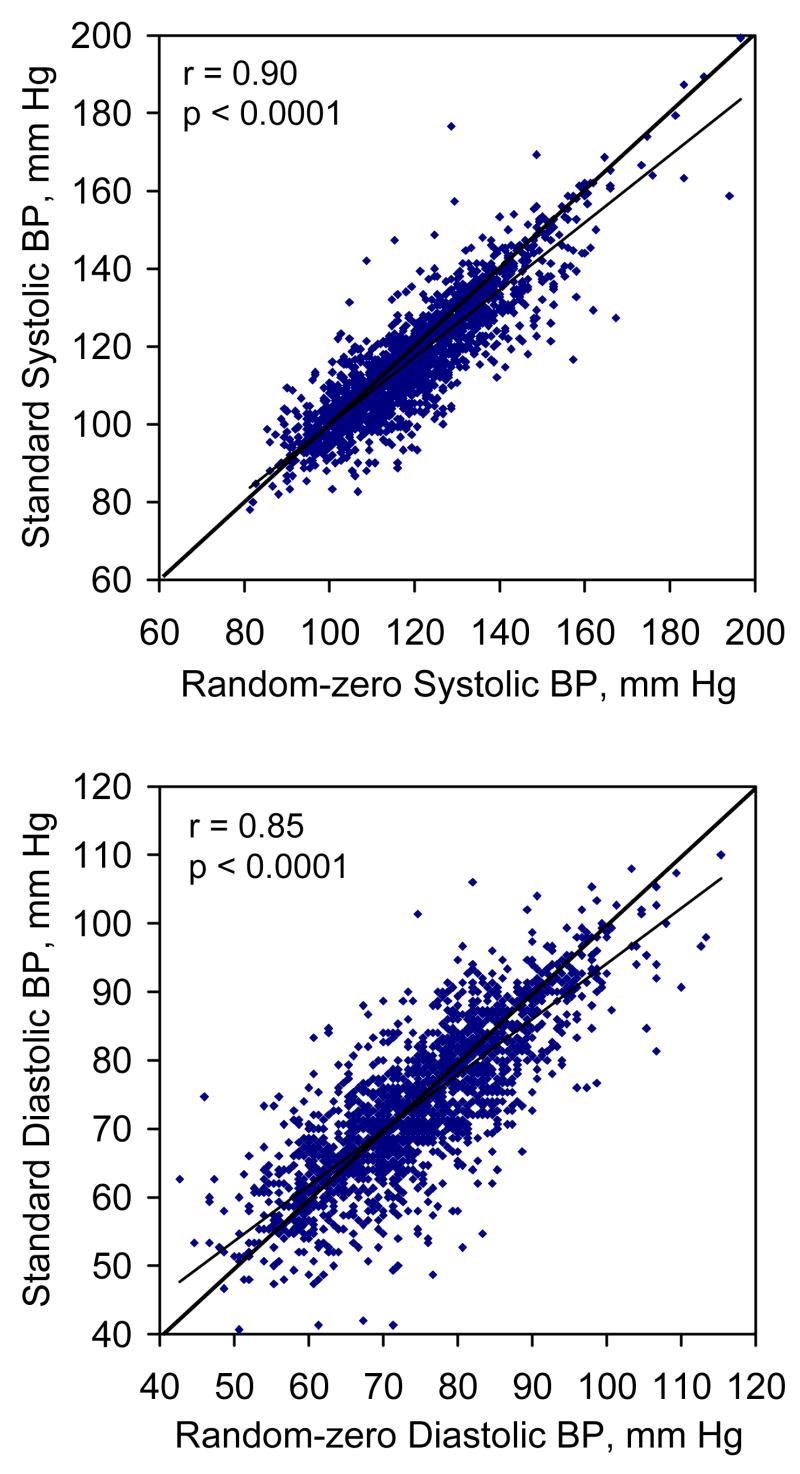
Scatter plots of BP measurements obtained using the random-zero sphygmomanometer against BP measurements obtained using the standard mercury sphygmomanometer are shown in Figure 1 (upper panel for systolic and lower panel for diastolic pressure). There was a strong linear relationship between BP measurements obtained using both measurement methods, with slightly more points being scattered below the line of identity. Estimated correlation coefficients between the random-zero and standard BP measurements were 0.90 for systolic and 0.85 for diastolic (both p< 0.0001), respectively.
Figure 1.
Scatter plots of standard blood pressure by random-zero blood pressure (upper panel for systolic pressure and lower panel for diastolic pressure). Standard systolic blood pressure = 10.56 + 0.93 random-zero systolic blood pressure; standard diastolic blood pressure = 8.92 + 0.89 random-zero diastolic blood pressure.
Figure 2 shows plots of the difference in BP between the two sphygmomanometers against an average of the BP obtained with the two sphygmomanometers (upper panel for systolic and lower panel for diastolic). On average, systolic BP was 2.83 (standard deviation 7.49) mm Hg lower for random-zero sphygmomanometer measurements compared to readings obtained using the standard mercury sphygmomanometer and the corresponding difference was 1.05 (standard deviation 6.66) mm Hg for diastolic BP. There were no special patterns between differences in BP and average of BP by the two sphygmomanometers.
Figure 2.
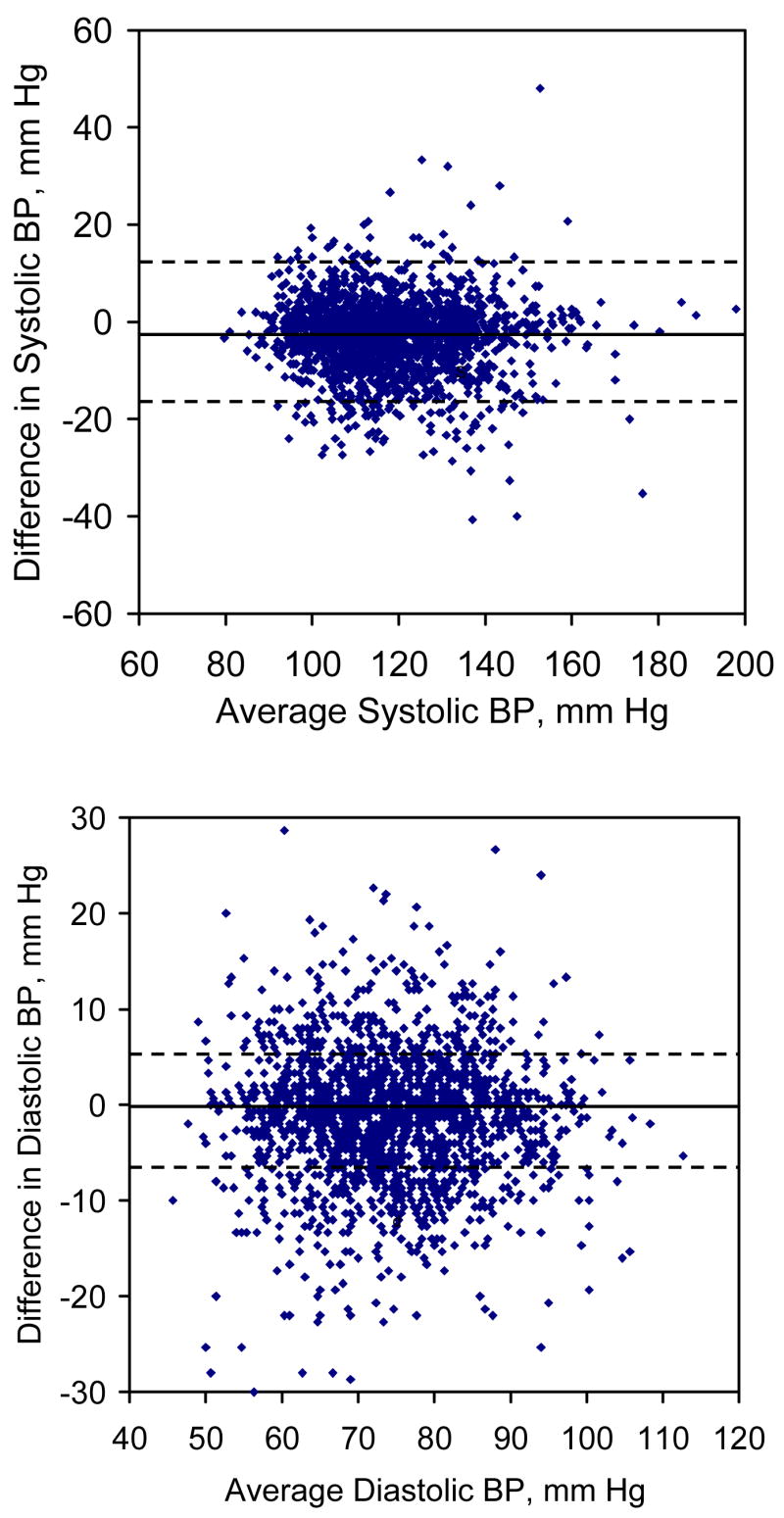
Scatter plots of difference in blood pressure measurements (random-zero – standard mercury) by average blood pressure levels from the two sphygmomanometers (upper panel for systolic and lower panel for diastolic blood pressure). The solid line indicates the mean blood pressure difference between random-zero and standard mercury sphygmomanometers. The dotted line indicates 1.96 times the standard deviation of this difference.
Multivariate linear regression analyses of the differences in systolic and diastolic BP obtained with the two sphygmomanometers are presented in Table 3. Age was inversely associated with systolic BP differences while body-mass index and physical activity were positively related to systolic BP difference. Only body-mass index was related to diastolic BP difference. Gender and average BP values were not associated with either systolic or diastolic BP difference.
Table 3.
Multivariate linear regression analyses of systolic and diastolic blood pressure difference by random-zero and standard mercury sphygmomanometers
| Systolic | Diastolic | |||
|---|---|---|---|---|
| Study variable | ||||
| Difference* (95% CI) | p-value | Difference* (95% CI) | p-value | |
| Age, year | −0.05 (−0.08, −0.01) | 0.007 | −0.02 (−0.05, −0.01) | 0.21 |
| Men | 0.58 (−0.09, 1.27) | 0.09 | −0.21 (−0.80, 0.44) | 0.58 |
| Body mass index, kg/m2 | 0.13 (0.02, 0.24) | 0.017 | 0.10 (0.00, 0.20) | 0.05 |
| Physical activity, MET/day | 0.02 (0.01, 0.04) | 0.01 | 0.01 (−0.01, 0.02) | 0.38 |
| Average blood pressure, mm Hg | 0.02 (−0.00, 0.05) | 0.06 | 0.00 (−0.03, 0.03) | 0.86 |
Differences are presented as mean random-zero blood pressure values minus mean standard mercury blood pressure values.
DISCUSSION
High BP is a major public health challenge, which affects approximately 1 billion individuals worldwide and is the leading preventable risk factor for premature death (23,24). Accurate measurements of BP are important for appropriate diagnosis and management of hypertension. The random-zero and standard mercury sphygmomanometers are used frequently in clinical practice and research. In the current study, our findings showed a small, but significantly lower value of systolic and diastolic BP for readings obtained with the random-zero sphygmomanometer compared to those obtained using the standard mercury sphygmomanometer. This difference has important implications for the clinical evaluation of patients with hypertension. Underestimation of true BP values, even small, can misclassify millions of individuals. For example, one study indicated that systematically underestimating BP values by 5 mm Hg would result in 21 million persons, who would benefit from drug treatment for hypertension, being misclassified as normotensive and, therefore, not receiving treatment (25). However, readings obtained using the standard mercury sphygmomanometer might not be a gold standard of true BP either, although the current guidelines are mostly based on mercury measurements.
Consistent underestimation of BP by the random-zero sphygmomanometer has been suggested in previous reports (4,17,18,26). Two previous studies have suggested greater underestimation for diastolic than systolic BP (17,18). However, our study and another by Parker et al (4) suggest greater underestimation for systolic than for diastolic BP. In addition, our study suggested that underestimation of BP by the random-zero sphygmomanometer was slightly greater among those who were younger, heavier, and more physically active for systolic and heavier for diastolic. However, the absolute differences among sub-groups were small and clinical implications were unclear.
Underestimation of BP by the random-zero sphygmomanometer may result from observer, technique, and instrument factors (10). Accurate recording of final random-zero values in order to obtain the true corrected BP readings is extremely important when the random-zero sphygmomanometer is used (27). Overestimation of the random-zero values would lead to underestimation of the final corrected BP values. This could occur when sufficient time is not allowed for the reserve mercury chamber to fill before deflation is started or sufficient care is not taken to ensure that complete deflation has occurred while recording the random-zero number. In addition, the design of the random-zero sphygmomanometer might also be responsible for the underestimation of BP due to the increased height of mercury in the random-zero manometer tube (15,27).
It is evident that the random-zero sphygmomanometer minimizes but does not eliminate digit preference (27). Like Parker et al (4), we did not find a significant difference in the percent distribution of digit preference with the two sphygmomanometers for either systolic or diastolic BP values. This suggests that digit preference can be decreased using the standard mercury sphygmomanometer with appropriate training and correct usage.
There are several advantages of the random-zero sphygmomanometer. It minimizes digit preference and reduces or eliminates bias resulting from knowledge of earlier readings, making blind duplicate readings more feasible (27). However, the random-zero sphygmomanometer is approximately 10 times more expensive than the standard mercury device. In addition, it is more difficult to maintain and transport and those who use it need more intensive training because of its complexity. The high agreement between the two measurements found in our study suggested that observer bias associated with the standard mercury sphygmomanometer could be avoided by appropriate training. Therefore, the standard mercury sphygmomanometer should be the preferable choice in the general clinical setting in the US and elsewhere.
Currently, aneroid sphygmomanometers and automated BP measuring devices are widely used in clinical settings (28). A few studies reported that aneroid sphygmomanometers provided inaccurate BP measurements (28,29) while others indicated that aneroid sphygmomanometers could provide accurate measurements if a proper maintenance protocol was followed (30). Likewise, the agreement of BP measurements between the automated devices and the mercury sphygmomanometers was inconsistent in the previous studies (28,31–34). The mercury sphygmomanometers still should be the “golden standard” for BP measurements in the clinical practice.
In conclusion, our study documented strong agreement between BP readings obtained using the random-zero and standard mercury sphygmomanometer. In addition, our study demonstrated no significant difference for digit preference between the two sphygmomanometers. However, systolic and diastolic BP measurements obtained using the random-zero sphygmomanometer appears to underestimate the corresponding results obtained with the standard mercury sphygmomanometer. Choice of sphygmomanometer may depend on the purpose and setting of the BP measurement. In clinical practice, the random-zero sphygmomanometer seems to offer no significant advantage over the standard sphygmomanometer for the diagnosis and management of hypertension. However, in clinical trials where the net change in BP is the primary outcome and avoidance of observer bias is the main concern, the random-zero sphygmomanometer provides a valid tool for BP measurement.
Acknowledgments
The Genetic Epidemiology Network of Salt Sensitivity (GenSalt) is supported by a cooperative agreement project grant (U01HL072507) from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland. The authors wish to express their sincere appreciation to the GenSalt study participants for their participation and cooperation in this project.
References
- 1.Parati G, Faini A, Castiglioni P. Accuracy of blood pressure measurement: sphygmomanometer calibration and beyond. J Hypertens. 2006;24:1915–8. doi: 10.1097/01.hjh.0000244935.19299.f5. [DOI] [PubMed] [Google Scholar]
- 2.Riva-Rocci S. Un nuovo sfigmomanometro. Gaz Med di Torino. 1896;50:981–96. [Google Scholar]
- 3.Parati G, Mancia G. History of blood pressure measurement from the pre- Riva-Rocci era to the 20-first century. In: Birkenhager WH, Reid JL, editors. Handbook of hypertension. Vol. 22. Amsterdam: Elsevier BV; 2004. pp. 3–32. [Google Scholar]
- 4.Parker D, Liu K, Dyer AR, et al. A comparison of the random-zero and standard mercury sphygmomanometers. Hypertension. 1988;11:269–72. doi: 10.1161/01.hyp.11.3.269. [DOI] [PubMed] [Google Scholar]
- 5.Jones DW, Appel LJ, Sheps SG, et al. Measuring blood pressure accurately. JAMA. 2003;289:1027–30. doi: 10.1001/jama.289.8.1027. [DOI] [PubMed] [Google Scholar]
- 6.Turner MJ, Irwig L, Bune AJ, et al. Lack of sphygmomanometer calibration causes over-and under-detection of hypertension-a computer simulation study. J Hypertens. 2006;24:1931–8. doi: 10.1097/01.hjh.0000244940.11675.82. [DOI] [PubMed] [Google Scholar]
- 7.Wright BM, Dore CF. A random zero sphygmomanometer. Lancet. 1970;i:337–8. doi: 10.1016/s0140-6736(70)90709-9. [DOI] [PubMed] [Google Scholar]
- 8.Lawson M, Fredericks S, Johnston A. The Hawksley random zero sphygmomanometer: unbiased assessment of blood pressure. J Hum Hypertens. 1993;7:97. [Google Scholar]
- 9.Birkett NJ. Potential problems with the random-zero sphygmomanometer. Hypertension. 1994;23:254–7. doi: 10.1161/01.hyp.23.2.254. [DOI] [PubMed] [Google Scholar]
- 10.Churchill D, Beevers DG. Has the random zero sphygmomanometer been exonerated. J Hum Hypertens. 1997;11:73–4. doi: 10.1038/sj.jhh.1000379. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien E, Mee F, Atkins N, et al. Inaccuracy of the Hawksley random zero sphygmomanometer. Lancet. 1990;336:1465–8. doi: 10.1016/0140-6736(90)93177-q. [DOI] [PubMed] [Google Scholar]
- 12.Gillman MW, Cook NR. Blood pressure measurement in childhood epidemiological studies. Circulation. 1995;92:1049–57. doi: 10.1161/01.cir.92.4.1049. [DOI] [PubMed] [Google Scholar]
- 13.Kim JW, Bosworth HB, Voils CI, et al. How well do clinic-based blood pressure measurements agree with the mercury standard. J Gen Intern Med. 2005;20:647–9. doi: 10.1111/j.1525-1497.2005.0105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGurk C, Nugent A, McAuley D, et al. Sources of inaccuracy in the use of the Hawksley random-zero sphygmomanometer. J Hypertens. 1997;15:1379–84. doi: 10.1097/00004872-199715120-00002. [DOI] [PubMed] [Google Scholar]
- 15.Kronmal RA, Rutan GH, Manolio TA, et al. Properties of the random zero sphygmomanometer. Hypertension. 1993;21:632–7. doi: 10.1161/01.hyp.21.5.632. [DOI] [PubMed] [Google Scholar]
- 16.Mackie A, Whincup P, McKinnon M. Does the Hawksley random zero sphygmomanometer underestimate blood pressure, and by how much. J Hum Hypertens. 1995;9:571–3. [PubMed] [Google Scholar]
- 17.De Gaudemaris R, Folsom AR, Prineas RJ, et al. The random-zero versus the standard mercury sphygmomanometer: a systematic blood pressure difference. Am J Epidemiol. 1985;121:282–90. doi: 10.1093/oxfordjournals.aje.a113998. [DOI] [PubMed] [Google Scholar]
- 18.Brown WCB, Kennedy S, Inglis GC, et al. Mechanisms by which the Hawksley random zero sphygmomanometer underestimates blood pressure and produces a non-random distribution of RZ values. J Hum Hypertens. 1997;11:73–93. doi: 10.1038/sj.jhh.1000405. [DOI] [PubMed] [Google Scholar]
- 19.Conroy RM, O’Brien E, O’Malley K, et al. Measurement error in the Hawksley random zero sphygmomanometer: what damage has been done and what can we learn. BMJ. 1993;306:1319–22. doi: 10.1136/bmj.306.6888.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gensalt Collaborative Research Group. Genetic epidemiology network of salt sensitivity (GenSalt): Rationale, design, methods, and baseline characteristics of study participants. J Hum Hypertens. 2007;21:639–646. doi: 10.1038/sj.jhh.1002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometer. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 23.Kearney P, Whelton M, Reynolds K, Muntner P, Whelton P, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 24.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 25.van Popele NM, Bos WJ, de Beer NA, et al. Arterial stiffness as underlying mechanism of disagreement between an oscillometric blood pressure monitor and a sphygmomanometer. Hypertension. 2000;36:484–8. doi: 10.1161/01.hyp.36.4.484. [DOI] [PubMed] [Google Scholar]
- 26.Miall WE. Instrument is accurate if used correctly. BMJ. 1993;307:124. doi: 10.1136/bmj.307.6896.124-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canner PL, Borhani NO, Oberman A, et al. The hypertension prevention trial: assessment of the quality of blood pressure measurements. Am J Epidemiol. 1991;134:379–92. doi: 10.1093/oxfordjournals.aje.a116100. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien E, Waeber B, Parati G, Staessen J, Myers MG. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ. 2001;322:531–6. doi: 10.1136/bmj.322.7285.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waugh JJ, Gupta M, Rushbrook J, Halligan A, Shennan AH. Hidden errors of aneroid sphygmomanometers. Blood Pressure Monitoring. 2002;7:309–12. doi: 10.1097/00126097-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Canzanello VJ, Jensen PL, Schwartz GL. Are aneroid sphygmomanometers accurate in hospital and clinic settings. Arch Intern Med. 2001;161:729–31. doi: 10.1001/archinte.161.5.729. [DOI] [PubMed] [Google Scholar]
- 31.Beales D. How accurate are automated blood pressure monitors. Brit J Community Nursing. 2005;10:334–8. doi: 10.12968/bjcn.2005.10.7.18331. [DOI] [PubMed] [Google Scholar]
- 32.Omboni S, Riva I, Giglio A, Caldara G, Groppelli A, Parati G. Validation of the Omron M5-I, R5-I and HEM-907 automated blood pressure monitors in elderly individuals according to the International Protocol of the European Society of Hypertension. Blood Pressure Monitoring. 2007;12:233–42. doi: 10.1097/MBP.0b013e32813fa386. [DOI] [PubMed] [Google Scholar]
- 33.Pini C, Pastori M, Baccheschi J, Omboni S, Parati G. Validation of the Artsana CSI 610 automated blood pressure monitor in adults according to the International Protocol of the European Society of Hypertension. Blood Pressure Monitoring. 2007;12:179–84. doi: 10.1097/MBP.0b013e3280b08394. [DOI] [PubMed] [Google Scholar]
- 34.de Greeff A, Reggiori F, Shennan AH. Clinical assessment of the DINAMAP ProCare monitor in an adult population according to the British Hypertension Society Protocol. Blood Pressure Monitoring. 2007;12:51–5. doi: 10.1097/MBP.0b013e3280858b73. [DOI] [PubMed] [Google Scholar]