Summary
Previously formed memories are susceptible to disruption immediately after recall due to a necessity to be reconsolidated after retrieval. Protein translation mechanisms have been widely implicated as being necessary for memory reconsolidation, but gene transcription mechanisms have been much less extensively studied in this context. We found that retrieval of contextual conditioned fear memories activates the NF-κB pathway to regulate histone H3 phosphorylation and acetylation at specific gene promoters in hippocampus, specifically via IKKα and not the NF-κB DNA-binding complex. Behaviorally, we found that inhibition of IKKα regulation of either chromatin structure or NF-κB DNA-binding complex activity leads to impairments in fear memory reconsolidation, and that elevating histone acetylation rescues this memory deficit in the face of IKK blockade. These data provide novel insights into IKK-regulated transcriptional mechanisms in hippocampus that are necessary for memory reconsolidation.
Introduction
Formation of long-term memory involves activation of multiple signaling pathways and the regulation of a wide variety of transcription factors, which affects a highly coordinated pattern of gene transcription that is necessary for memory stabilization. The transcription factor nuclear-factor kappa B (NF-κB) has been implicated in the induction of synaptic plasticity and initial formation of long-term memory (Dash et al., 2005; Freudenthal et al., 2005; Levenson et al., 2004a; Liou and Hsia, 2003; Meffert et al., 2003; Yeh et al., 2004; Yeh et al., 2002). In addition, recent investigations into the role of NF-κB signaling in memory formation have identified this pathway in the process of long-term memory reconsolidation in the crab Chasmagnathus (Merlo et al., 2005). These findings suggest that specific mechanisms exist for activation of the NF-κB transcriptional pathway during various stages of memory formation. However, the regulatory mechanism and molecular targets through which the NF-κB pathway mediates transcriptional regulation to stabilize long-term memory have not been experimentally investigated.
Memories, when retrieved or recalled, can become labile and susceptible to disruption, which implies the necessity of a process for re-stabilizing previously formed memories. This process is commonly referred to as memory reconsolidation (Nader et al., 2000; Sara, 2000). For example, in a rodent contextual fear conditioning paradigm a novel context (training chamber) is paired with a footshock and after this training event a long-term memory for this association is formed. After memory formation, re-exposing the animal to the training chamber triggers memory retrieval and subsequent reconsolidation of the associative memory. Re-establishment of the contextual conditioned fear (CCF) memory is subject to disruption through inhibition of protein synthesis, or when signaling cascades such as the extracellular signal-regulated kinase-mitogen-activated protein kinase (ERK/MAPK) are inhibited (Duvarci and Nader, 2004; Duvarci et al., 2005; Suzuki et al., 2004). Using a similar training paradigm in the crab Chasmagnathus Merlo and colleagues (Merlo et al., 2005) demonstrated that NF-κB is activated by memory retrieval and that this activation is required for memory reconsolidation. The goal of the present study was to investigate the involvement of the NF-κB signaling cascade, and molecular targets of this pathway, during reconsolidation in a mammalian long-term memory paradigm, contextual fear conditioning.
The NF-κB/Rel transcription factors are highly regulated and require modification of Inhibitor kappa B (IκB) proteins for activation. In most cells, the binding of IκB to NF-κB causes cytoplasmic retention of the complex, blocking its capacity for transcriptional regulation. IκB proteins are marked for proteolytic degradation when they are phosphorylated by the IκB kinase (IKK) complex. The IKK complex consists of two kinase catalytic subunits, IKKα and IKKβ, and a regulatory subunit IKKγ (DiDonato et al., 1997; Zandi et al., 1998). Once released from IκB proteins by the action of the IKK complex, NF-κB translocates to the nucleus and binds to the promoter region of target genes by recognizing the κB consensus elements within DNA (reviewed in Albensi and Mattson, 2000).
Several mechanisms have been described for NF-κB transcriptional regulation in addition to the binding of the NF-κB complex to κB regulatory elements in DNA. For example, signaling components of the NF-κB pathway have been shown to be involved in the regulation of gene expression through modification of histone phosphorylation and acetylation in concert with histone deacetylases (HDAC) in non-neuronal cells (Ashburner et al., 2001; Ito et al., 2001; Kumar et al., 2005; Viatour et al., 2003; Yamamoto et al., 2003). The IκB protein isoform, IκBα, has been shown to regulate transcription independent of NF-κB DNA binding activity through interaction with HDAC1 and HDAC3 (Viatour et al., 2003). Moreover, the IKKα subunit has been shown to function independently of the IKK complex to regulate cytokine-induced gene expression through regulation of histone H3 phosphorylation in vitro (Anest et al., 2003; Park et al., 2006; Yamamoto et al., 2003). Overall, these studies identify new roles for signaling components of the NF-κB pathway, such as IκBα and IKKα, in regulating chromatin structure and controlling gene expression independent of NF-κB binding to regulatory elements in DNA.
These unique regulatory mechanisms have heretofore not been investigated in the nervous system. However, a number of studies suggest that altered chromatin structure allows for robust and lasting changes in gene expression, particularly in the nervous system (Alarcon et al., 2004; Battaglioli et al., 2002; Colvis et al., 2005; Huang et al., 2002; Korzus et al., 2004; Levenson et al., 2004b; Wood et al., 2006). Several studies have specifically suggested that epigenetic mechanisms, such as chromatin remodeling through histone regulation, are critical for normal synaptic plasticity and triggering long-term changes in neuronal function (reviewed in Colvis et al., 2005; reviewed in Levenson and Sweatt, 2005). In this study, we investigated the role of regulation of chromatin structure in memory and found that post-translational modification of the histone H3-tail occurs in the hippocampus after recall of CCF memories, and that this regulation required activation of the NF-κB pathway. Thus, triggering memory recall in animals trained in a fear conditioning paradigm elicited an increase in histone H3 phosphorylation and acetylation in hippocampus. These are the first data to implicate regulation of chromatin remodeling as a mechanism contributing to memory reconsolidation. Furthermore, the increase in histone H3 regulation was significantly attenuated with inhibition of IKK activity, but not inhibition of the NF-κB transcriptional complex, after recall of CCF memories. Blockade of IKK also attenuated memory reconsolidation and elevating histone acetylation pharmacologically rescued memory reconsolidation in the face of IKK blockade. Overall, these findings demonstrate that activation of the IKK/NF-κB pathway and regulation of chromatin structure in hippocampus are important in memory reconsolidation in the mammalian CNS.
Results
Inhibition of NF-κB signaling after memory recall impairs memory reconsolidation
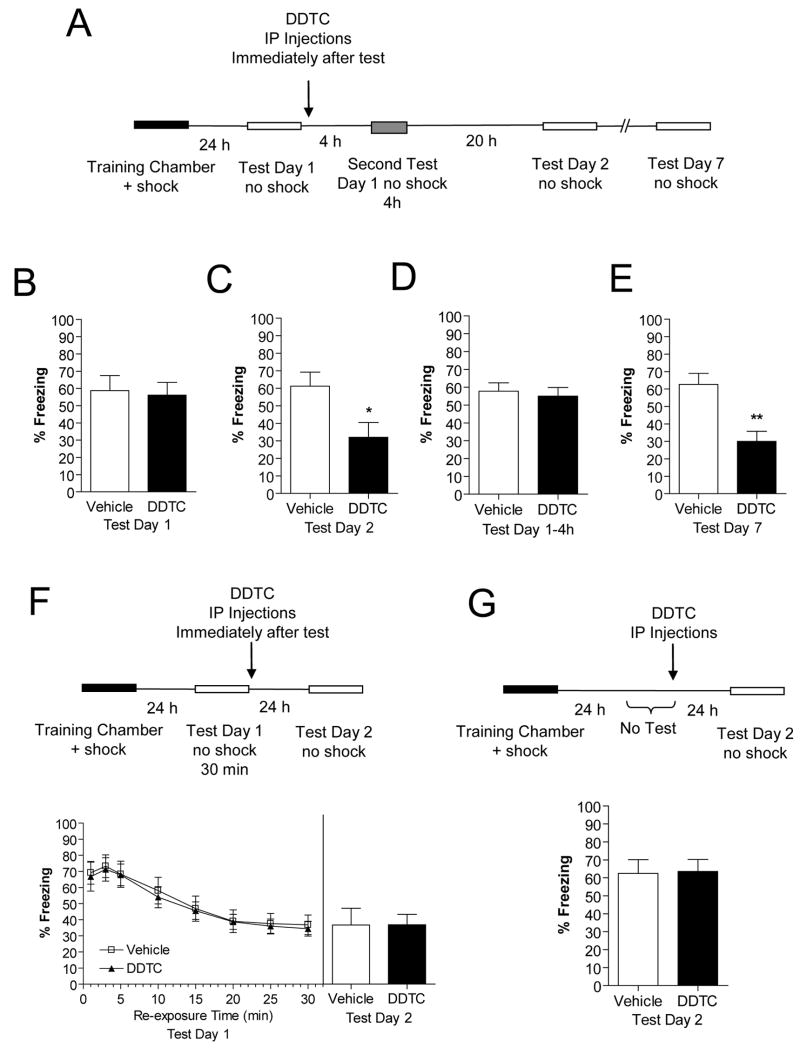
To assess the effect of inhibiting NF-κB signaling activity on fear-conditioned learned behavior after memory recall we used a well-known NF-κB inhibitor, diethyldithiocarbamate (DDTC) (Hayakawa et al., 2003; Miyajima et al., 2003; Morais et al., 2006; Schreck et al., 1992). In these experiments, DDTC was administered intraperitoneally (IP) which has been shown to be effective for inhibiting NF-κB signaling activity in the brain (Blondeau et al., 2001; Kis et al., 2003). Figure 1A depicts the timing of DDTC administration and the time points at which we determined its effects on freezing behavior after recall of contextual conditioned fear (CCF) memories.
Figure 1.
Effect of inhibition of the NF-κB signaling pathway on contextual fear conditioning after context re-exposure. (A) Diagram outlines the experimental design used with data presented below in panels B–E (vehicle, n=10; DDTC, n=9). (B) Freezing behavior on Test Day 1. (C) Freezing behavior during re-exposure on Test Day 2. (D) Short-term memory test, assessed 4 h after re-exposure to chamber. (E) Freezing behavior on Test Day 7. (F) Effect of context re-exposure for 30 min (Test Day 1) on freezing behavior assessed on Test Day 2. (G) Injection of DDTC at the same time interval as the test animals in Panel B but with the first test given 48 h later. Student’s t-test; *p<0.05, **p<0.01 compared to vehicle. Error bars are SEM.
Twenty-four hours after training, animals were re-exposed to the training chamber with no footshock (Test Day 1). Immediately following re-exposure to the training chamber animals were injected with saline (vehicle) or DDTC. Both vehicle and DDTC-treated animals displayed normal freezing behavior on Test Day 1 (Fig. 1B), as expected because their injections were given after context re-exposure. On Test Day 2, DDTC-treated animals exhibited a significant decrease in freezing behavior compared to vehicle-treated animals (t(14)=2.50, p<0.05; Fig. 1C). We also performed a short-term memory test 4 h after context re-exposure and found that vehicle and DDTC-treated animals displayed similar freezing behavior (t(10)=0.41, p>0.05; Fig. 1D). These results indicatethat the NF-κB signaling pathway participates in retrieval-induced reconsolidation of long-term CCF memories.
In order to ensure that the drug-induced interference of CCF memories did not fade at longer drug-retest time intervals, we also delayed re-test for 7 days. For these experiments, vehicle or DDTC was administered immediately after re-exposure (Test Day 1) and the animals were given a second test (Test Day 7). Although the freezing behavior of the vehicle-treated animals was still intact on Test Day 7, it was significantly reduced in the DDTC-treated animals (t(14)=3.80, p<0.05; Fig. 1D). These findings indicate that the disruption of CCF memories by inhibition of NF-κB signaling activity lasts at least 7 days.
A possible effect of inhibiting NF-κB signaling activity after memory recall could be an enhancement of memory extinction instead of a blockade of memory reconsolidation. For assessment of this possible effect, we used a longer re-exposure trial (30 min) on Test Day 1 to initiate memory extinction. At the end of the 30 min session freezing behavior decreased in both vehicle and DDTC-treated animals (Vehicle: F(1,8)=14.90, p<0.001; DDTC: F(1,8)=10.41, p<0.001; Fig. 1E). DDTC was then administered to animals immediately after the 30 min re-exposure trial and animals were given a second test (Test Day 2). Reassuringly, measurement of freezing behavior in DDTC-treated animals was similar to freezing behavior in vehicle-treated animals (Fig. 1E). These results suggest that inhibition of NF-κB signaling activity after memory retrieval does not result in an enhancement of memory extinction. While we performed this experiment as a control for reconsolidation studies, to our knowledge, this is the first study to address the potential role of the NF-κB signaling pathway in memory extinction as well.
Another possible effect of inhibiting NF-κB signaling activity with DDTC is that DDTC may have induced amnesia without reactivation of memory. To control for this possible effect, DDTC was administered at the same time interval as the test animals on Test Day 1 without re-exposure to the training chamber. On Test Day 2, we found that freezing behavior persisted in both vehicle and DDTC-treated animals (Fig. 1F). These findings indicate that the behavioral effects of DDTC are specifically related to inhibition of reconsolidation of CCF memories.
NF-κB signaling activity after memory recall is specific to context re-exposure
The hypothesis of an involvement of the NF-κB signaling pathway in memory reconsolidation predicts that NF-κB signaling activity should increase when memory recall is triggered. Therefore, we confirmed NF-κB signaling activity by evaluating two molecular markers of the pathway: IKK activation and NF-κB DNA-binding complex activity. Evaluation of IKK activity indicates whether recall triggers activation of the NF-κB signaling pathway at the IKK level of the cascade. An assessment of NF-κB-complex DNA binding activity indicates whether recall triggers active binding of NF-κB to κB regulatory elements in DNA.
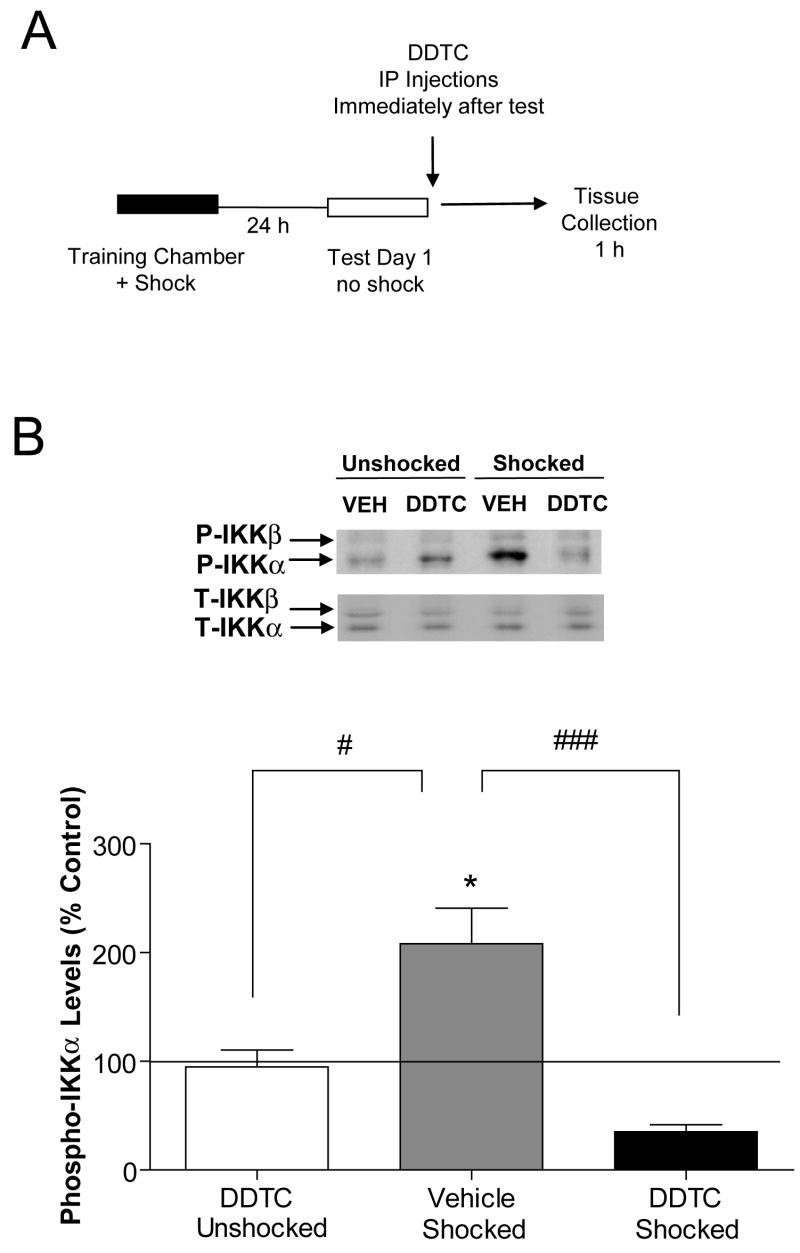
IKK activation can be directly assessed by measuring phosphorylation of IKKα at Ser-180 or IKKβ at Ser-181, an event that causes a conformational change that results in kinase activation (Delhase et al., 1999; DiDonato et al., 1997; Mercurio et al., 1997). To evaluate IKK activity after retrieval, protein extracts were prepared from area CA1 of hippocampus 1 h after context re-exposure and IKK phosphorylation assessed by western blotting with a phospho-selective antibody (Fig. 2A; Test Day 1). Western blotting analysis revealed significant increases in IKKα phosphorylation after re-exposure (F(3,14)=12.15, p<0.05; Fig. 2B) with no change in IKKβ phosphorylation. These results suggest that memory recall triggers IKKα, but not IKKβ, activation in hippocampus after re-exposure to the training chamber.
Figure 2.
Activation of IKKα in hippocampus after context re-exposure. (A) Experimental design used with data presented below. (B) Western blot densities for phosphorylated IKKα (P-IKKα)normalized to total IKKα (T-IKKα) levels from area CA1 (vehicle-unshocked, n=3; DDTC-unshocked, n=3; vehicle-shocked, n=6; DDTC-shocked, n=6). At the 1 h time point assessed, there were no changes in P-IKKα levels in area CA3 or dentate gyrus after context re-exposure (Data not shown). Two-way ANOVA with post-hoc test; *p<0.05, **p<0.01 compared with unshocked-DDTC, #p<0.05, ##p<0.01 compared with vehicle-shocked group. Error bars are SEM; Solid lines represent normalized vehicle control levels.
As a control, we confirmed that DDTC administered immediately after context re-exposure blocked IKKα activation in hippocampus. Similar to inhibition of CCF memories, recall-mediated increases in phosphorylated IKKα were significantly inhibited with DDTC treatment (F(3, 14)=12.55, p<0.001; Fig. 2B). Thus, these data confirm that administration of a single dose of DDTC significantly attenuates IKKα activation in hippocampus after recall of CCF memories.
It is possible that the up-regulation in IKKα activity in hippocampus was not specific to memory recall but rather was due simply to context exposure or to a persistent effect from the prior day’s training. To test this possibility, we evaluated IKKα activity in the following four groups of animals: 1) Group A; animals trained but not re-exposed to the training chamber, 2) Group B; animals trained and re-exposed to the training chamber (as described in previous section), 3) Group C; untrained animals that were exposed to the training chamber and 24 h later re-exposed without being shock, 4) Group D; untrained animals that were exposed to the training chamber once without being shocked. All groups were compared to naïve animals that were not exposed to the training chamber.
Western blotting analysis revealed no significant change in IKKα phosphorylation 25 h after training with no context re-exposure (Fig. 3B, Group A). Animals that were trained and re-exposed to the training chamber (24 h later) showed a significant increase in IKKα phosphorylation 1 h after context re-exposure (t(16)=2.41, p<0.05; Fig. 3B, Group B) as we described above. Re-exposure of untrained animals to the training chamber showed no change in IKKα phosphorylation (t(7)=0.13, p<0.05; Fig. 3B, Group C). One-hour after a 7-min exposure to the training chamber, which was similar to the duration of the training session, we found no significant change in IKKα phosphorylation (Fig. 3B, Group D). Together these results indicate that re-exposure to the training chamber, in previously trained animals, selectively triggered an increase in IKKα activity in hippocampus.
Figure 3.
Activation of the NF-κB signaling pathway is regulated after re-exposure to the context. (A) Outline of the experimental procedure performed. (B) Representative Western blots and densitometry analysis for phosphorylated IKKα (P-IKKα) normalized to total IKKα (T-IKKα) levels are shown (Group A, n=9; Group B, n=9; Group C, n=6; Group D, n=8). Student’s t-test; *p<0.05 compared with naive group. (C) Nuclear extracts were prepared from area CA1 from all groups in parallel. NF-κB DNA binding activity was measured using EMSA (Group A, n=5; Group B, n=10; Group C, n=8, Group D, n=5). Student’s t-test; *p<0.05 compared with naive group. (D) Nuclear extracts were prepared from area CA1 1 h after re-exposure (vehicle-shocked, n=6; DDTC-shocked, n=6). The specific band is indicated with an arrow. The relative optical density (R.O.D) values of the specific NF-κB shifted-band normalized to vehicle group (VEH) are shown. Student’s t-test; *p<0.05 compared with vehicle-shocked group. Error bars are SEM; Solid lines represent normalized naïve control levels.
Using an electrophoretic mobility shift assay (EMSA), we assessed NF-κB-complex activity in hippocampal nuclear extracts after context re-exposure. This assay is a technique for measurement of DNA binding activity of the NF-κB-complex: NF-κB DNA binding activity in vitro in this assay system is a reflection of nuclear translocation and activation of the NF-κB transcription factor. In nuclear extracts from area CA1, only one shifted band was detected with this assay (see Fig. 3C and D). This band corresponds to NF-κB-subunit-containing protein complexes that specifically bind to the κB consensus sequence, as we previously demonstrated using competition assays (Lubin et al., 2005).
One-hour after context re-exposure, a significant increase in NF-κB DNA binding activity was observed in area CA1 of hippocampus (t(12)=2.91, p<0.05; Fig. 3C). Similar to IKKα activity, treatment of animals with DDTC significantly attenuated NF-κB DNA binding activity after context re-exposure (t(10)=2.45, p<0.05; Fig. 3D). These results demonstrate that the NF-κB DNA-binding complex is activated in hippocampus after recall of CCF memories, and that DDTC blocks this effect. Overall, then, we observed hippocampal NF-κB pathway activation after memory recall using two independent biochemical assay procedures.
Like IKKα, NF-κB DNA binding activity in area CA1 of hippocampus showed no change 25 h after training with no context re-exposure (Fig. 3C, Group A). In animals that were trained and re-exposed to the same training chamber we found a significant increase in NF-κB DNA binding activity (t(16)=3.86, p<0.01; Fig. 3C, Group B), as described above. Animals that were exposed to the training chamber twice showed no change in NF-κB DNA binding activity (t(10)=0.26, p<0.01; Fig. 3C, Group C). At 1 h after a single exposure to the training chamber no significant change in NF-κB DNA binding activity was detected in area CA1 of hippocampus (Fig. 3C, Group D). These results indicate that activation of the NF-κB DNA-binding complex in hippocampus occurred selectively after re-exposure of trained animals to the context.
Results obtained in these experiments thus indicate that recall triggers NF-κB signaling at both the IKKα and the NF-κB DNA-binding complex level in hippocampus. Furthermore, these results are consistent with the hypothesis that increases in IKKα and NF-κB DNA-binding complex activity are part of the retrieval-induced memory reconsolidation process.
Regulation of Histone H3 modifications by IKKα signaling activity
In an effort to identify molecular targets of the IKKα/NF-κB pathway during memory reconsolidation, we determined whether chromatin remodeling is a potential transcription-regulating mechanism regulated by this pathway after memory recall. The binding of transcription factors to promoter regions within DNA can potentially trigger chromatin remodeling as a mechanism for regulating the transcription of the gene (for review see, Natoli et al., 2005) in addition to their effects to recruit RNA polymerase to the initiation site. With this in mind, we investigated whether activation of the NF-κB DNA-binding complex might trigger changes in chromatin structure 1 h after retrieval, the time point at which we observed NF-κB activation.
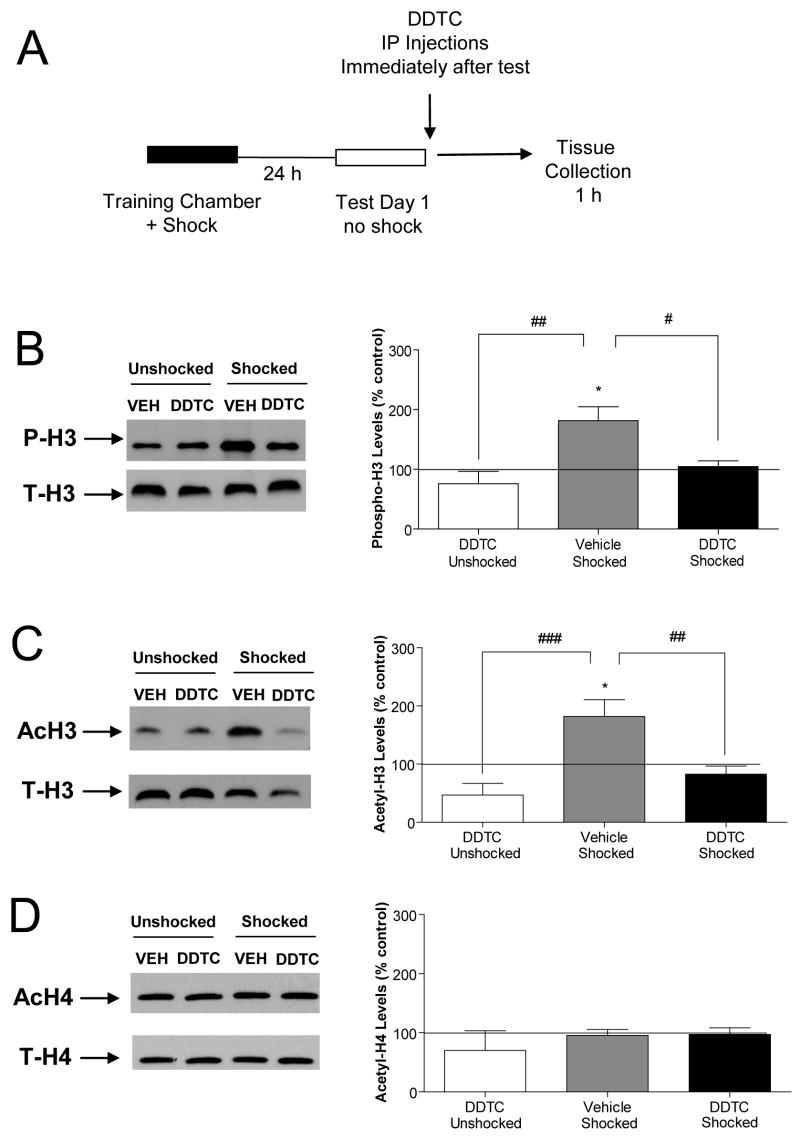
Thus, we assessed histone H3 posttranslational modifications in area CA1 of hippocampus 1 h after context re-exposure of trained animals. We observed significant increases in hippocampal histone H3 phosphorylation and acetylation levels after context re-exposure (P-H3; F(3, 25)=6.43, p<0.05, AcH3; F(3, 23)=8.14, p<0.05; Fig. 4A, B). These data suggest that chromatin remodeling occurs in response to memory recall as part of the reconsolidation process. To determine if these changes were dependent on NF-κB pathway activation, we assessed the effects of the IKK inhibitor DDTC on histone phosphorylation and acetylation. The increases in histone H3 phosphorylation and acetylation after re-exposure were significantly attenuated with DDTC treatment (P-H3; F(3, 25)=6.43, p<0.01, AcH3; F(3, 23)=8.14, p<0.01; Fig. 4A, B). These results indicate that IKKα/NF-κB signaling activity is required for histone H3 regulation in hippocampus following memory recall.
Figure 4.
Regulation of histone H3 phosphorylation and acetylation is associated with activation of the NF-κB signaling pathway after context re-exposure. (A) Histone extracts from area CA1 were prepared from animals 1 h after DDTC or saline (vehicle) treatment. (B) Phosphorylated histone H3 (P-H3) levels were normalized to total histone H3 (T-H3) protein levels from area CA1. There were no changes in histone H3 modifications in area CA3 or dentate gyrus at 1 h after recall (Data not shown) (vehicle-unshocked, n=5; DDTC-unshocked, n=6; vehicle-shocked, n=9; DDTC-shocked, n=9). (C) Acetylated histone H3 levels (AcH3) levels were normalized to total histone H3 (T-H3) protein levels (vehicle-unshocked, n=6; DDTC-unshocked, n=6; vehicle-shocked, n=6; DDTC-shocked, n=9). (D) Acetylated H4 (AcH4) normalized to total histone H4 (T-H4) levels (vehicle-unshocked, n=4; DDTC-unshocked, n=4; vehicle-shocked, n=6; DDTC-shocked, n=6). Two-way ANOVA with post-hoc test; **p<0.01, ***p<0.001 compared with unshocked-DDTC, #p<0.05, ##p<0.01 compared with vehicle-shocked group. Error bars are SEM; Solid lines represent normalized vehicle-unshocked control levels.
To assess whether the effect of inhibiting IKKα/NF-κB signaling activity after recall was specific to histone H3 or might have been a more general phenomenon, we also investigated histone H4 acetylation. We found no change in histone H4 acetylation at 1 h after re-exposure (F(3, 16)=0.60, p>0.05; Fig. 4C). Based on these data we hypothesize that a candidate mechanistic target locus for transcriptional regulation by IKKα/NF-κB signaling is regulation of histone H3 phosphorylation and acetylation in hippocampus, after recall of CCF memories.
Histone H3 phosphorylation and acetylation is specific to context re-exposure
To determine whether histone H3 regulation in hippocampus was specific to recall of the learned context-plus-shock association or whether it was induced by context exposure alone, we evaluated histone regulation in the four groups of animals previously described (Fig. S1A). We found no significant change in histone H3 phosphorylation and acetylation 25 h after training with no context re-exposure (Fig. S1A, B; Group A). Histone H3 phosphorylation and acetylation significantly increased 1 h after context re-exposure of previously trained animals (P-H3; t(17)=3.90, p<0.01, AcH3; t(17)=3.72, p<0.01; Fig. S1A, B; Group B). Re-exposure of untrained animals to the training chamber 24 h later elicited no change in histone H3 phosphorylation and acetylation (P-H3; t(17)=3.904, p<0.01, AcH3; t(17)=3.723, p<0.01; Fig. S1A, B; Group C). Exposure to the training chamber alone elicited no change in histone H3 phosphorylation and acetylation (Fig. S1A, B; Group D). In all groups we observed no change in histone H4 acetylation (Fig. S1C). Together these results suggest that histone H3 regulation in hippocampus was specific to re-exposure to the context used for conditioned training. These are the first findings implicating the regulation of chromatin structure as a mechanism contributing to memory reconsolidation.
IKK activity regulates histone H3 phosphorylation and acetylation after recall
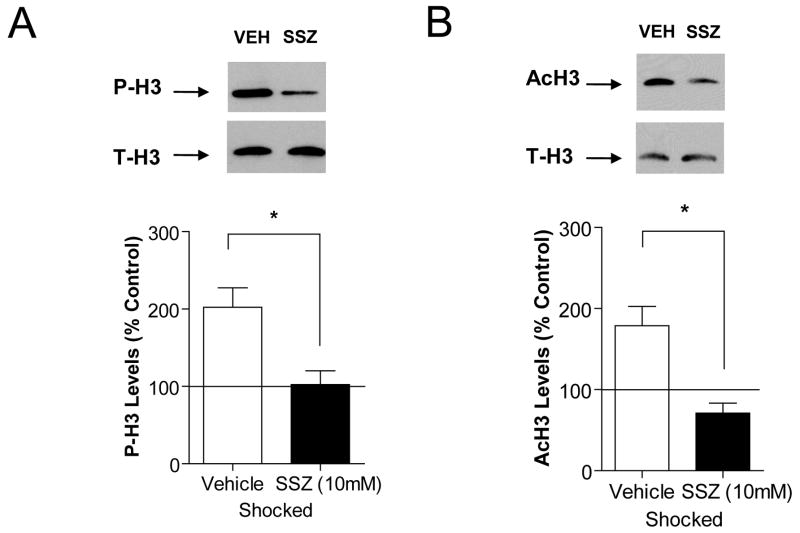
Next, we examined the effects of directly inhibiting IKKα activity on recall-mediated histone H3 regulation. For these experiments we used sulfasalazine (SSZ), which is a direct pharmacologic inhibitor of IKK (Wahl et al., 1998; Weber et al., 2000). We first determined the effects of inhibiting IKKα activity on memory reconsolidation assessed behaviorally. Two different doses of SSZ, 5 mM and 10 mM in 5 μl of vehicle, were administered intracerebroventricularly (i.c.v.) and their effects were assessed on CCF memory reconsolidation (Fig. 5A). On Test Day 1, no significant differences in freezing behavior were observed between the groups of animals used for subsequent vehicle and SSZ treatment (Fig. 5B). Upon re-testing (Test Day 2) animals infused with the lower dose of SSZ immediately after context re-exposure showed a trend but no significant difference in freezing behavior when compared to the vehicle-treated animals (Fig. 5C). However, animals infused with the higher dose of SSZ showed significantly less freezing behavior compared to animals injected with vehicle (t(9)=3.28, p<0.01; Fig. 5C). We found no effect of the higher dose of SSZ on short term memory (t(9)=0.61, p>0.05; Fig. 5D) which confirms the selective actions of SSZ for long-term memory after memory recall (24 h). These results demonstrate that inhibition of IKKα activity with SSZ after recall impairs reconsolidation of CCF long-term memories.
Figure 5.
Inhibition of IKKα effects contextual fear conditioned memories after re-exposure. (A) The experimental design used is shown with data presented below in panels B–E. (B) Freezing behavior of animals infused with either vehicle or SSZ immediately following re-exposure on Test Day 1 (Vehicle, n=6; SSZ, n=10). (C) Freezing behavior of animals during re-exposure on Test Day 2 (Vehicle, n=6; 5 mM SSZ n=5, 10 mM SSZ, n=5). (D) Assessment of freezing behavior 4 h after re-exposure (10 mM SSZ, n=6). (E) Western blots and graph summary of data for phosphorylated IKKα (P-IKKα) normalized to total IKKα (T-IKKα) levels are shown (Vehicle, n=5; 10 mM SSZ, n=5). (F) The binding activity for NF-κB measured 1 h after 10 mM SSZ treatment is shown. The specific band is indicated by an arrow. The specific NF-κB retarded band from the 10 mM SSZ treated group (n=4) was normalized to the Vehicle treated group (n=4). Two-way ANOVA with post-hoc test; *p<0.05, compared with Shocked-vehicle. Error bars are SEM; Solid lines represent normalized vehicle control levels. R.O.D. = relative optical density.
As a control, we confirmed the efficacy of the higher dose of SSZ to inhibit IKKα/NF-κB signaling after recall. Western blotting analysis show that increases in IKKα phosphorylation 1 h after re-exposure to the training chamber was significantly attenuated with SSZ treatment (F(2, 12)=12.13, p<0.01; Fig. 5D). Recall-mediated increases in NF-κB DNA binding activity were also blocked with SSZ treatment (t(6)=3.03, p<0.05; Fig. 5E). These results indicate that administration of SSZ effectively inhibited recall-mediated IKKα activity and subsequent generation of the active NF-κB DNA-binding complex in hippocampus.
Having established a requirement for IKKα activity in memory reconsolidation, we next investigated the effect of inhibiting IKKα on histone H3 regulation in hippocampus after memory recall. We found that recall-mediated increases in histone H3 phosphorylation and acetylation levels were blocked with SSZ treatment (P-H3; F(2, 9)=9.32, p<0.05, AcH3; F(2, 8)=8.76, p<0.05; Fig. 6A, B). These results suggest a new mechanism for post-translational modifications of the histone H3-tail during memory reconsolidation; specifically that IKKα regulates histone H3 phosphorylation and acetylation. Furthermore, these results confirm using another inhibitor of the IKKα/NF-κB pathway that this signaling cascade is involved in chromatin regulation in hippocampus after retrieval of CCF memories.
Figure 6.
IKKα contributes to increases in histone H3 phosphorylation and acetylation during reconsolidation. (A) Quantitative analysis of phosphorylated histone H3 (P-H3) levels after re-exposure to the training chamber are shown. (unshocked, n=4; shocked-vehicle, n=4; shocked-SSZ, n=4). (B) Western blot analysis of acetylated histone H3 (AcH3) levels from area CA1 after 10 mM SSZ treatment. (naïve, n=4; shocked-vehicle, n=4; shocked-SSZ, n=4). One-way ANOVA; *p<0.05, compared with unshocked-vehicle. Error bars are SEM; Solid lines represent normalized unshocked-vehicle control levels.
Effect of NF-κB inhibition on histone H3 modifications after memory recall
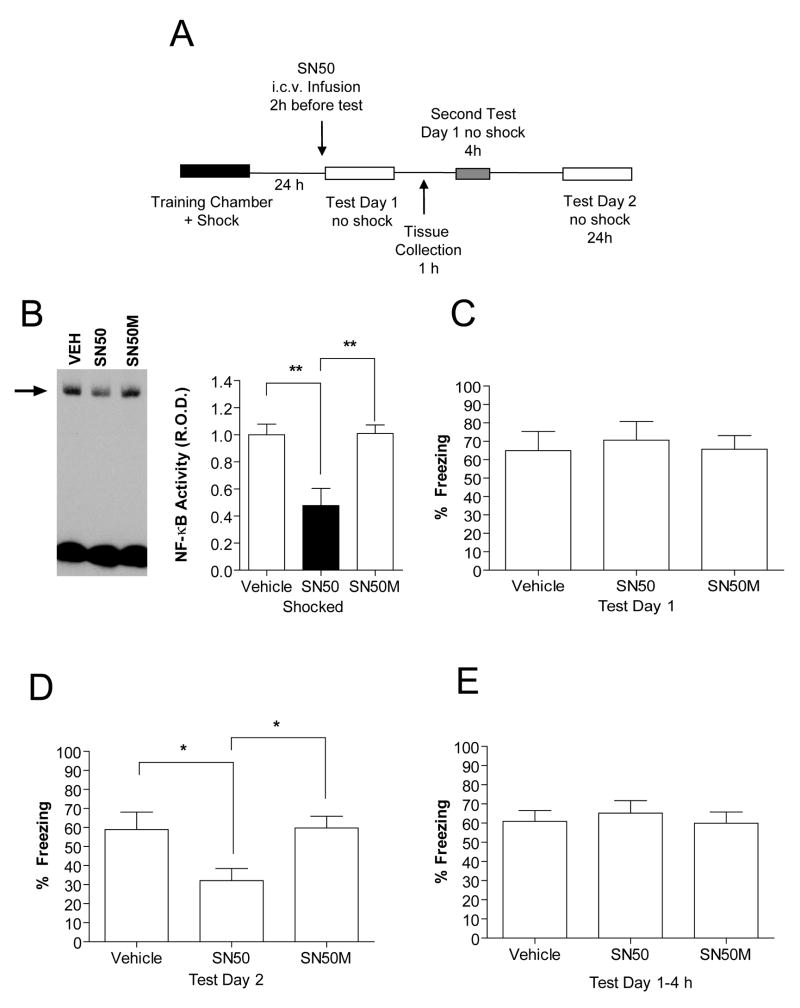
Next, we considered that IKKα regulation of histone H3 in hippocampus after recall might have occurred as a result of two possible scenarios: (1) IKKα recruiting the NF-κB-complex to mediate regulation of histone H3; or (2) IKKα directly mediating regulation of histone H3. We distinguished between these two scenarios by using a direct inhibitor of the NF-κB DNA-binding complex, SN50. The SN50 active peptide prevents the NF-κB transcriptional complex from interacting with its normal DNA binding sites and is known to be effective in neurons even when applied outside of the cell (Kubota et al., 2000; Lee and Rivier, 2005). We administered the SN50 active peptide i.c.v. to animals two hours prior to re-exposure to the training chamber (Fig. 7A).
Figure 7.
Effect of direct inhibition of the NF-κB transcriptional complex during reconsolidation. (A) Outline of the experimental procedure used. (B) Representative EMSA showing NF-κB binding activity [Relative optical density (R.O.D.)] in hippocampal nuclear extracts from animals injected with vehicle (VEH), SN50 or SN50M. Student’s t-test; **p<0.01. (C) On Test Day 1 performance of animals infused with either vehicle (n=8), the active peptide, SN50 (n=8) or the inactive peptide, SN50M (n=7). (D) Freezing behavior of SN50 treated animals (n=8) compared to vehicle (n=8) or SN50M (n=7) treated animals on Test Day 2. (E) Freezing behavior of vehicle, SN50M, or SN50-treated animals (n=5) 4 h after re-exposure. One-way ANOVA; **p<0.01, compared with Shocked-vehicle. Error bars are SEM.
As a positive control for the effectiveness of SN50, we examined nuclear extracts prepared from area CA1 1 h after context re-exposure using EMSA. As expected, EMSA analysis showed a significant decrease in NF-κB DNA binding activity with SN50 treatment, as compared to vehicle or SN50M treatment (F(2, 9)=10.58, p<0.01; Fig. 7B). These results indicate that SN50 significantly attenuated NF-κB DNA binding activity in hippocampus after memory recall.
We next determined whether direct inhibition of the NF-κB-complex by SN50 could impair CCF memories (freezing) after retrieval. On Test Day 1, all animals showed similar freezing behavior (Fig. 7C) indicating that administration of SN50 2 h prior to re-exposure did not prevent the retrieval of CCF memories. On Test Day 2, animals infused with SN50 showed a significant decrease in freezing behavior when compared to vehicle or SN50M-treated animals (F(2, 21)=4.19, p<0.05; Fig. 7D). We also confirmed that the effect of SN50 was specific for long-term CCF memory by showing that SN50 did not effect short-term memory, assessed 4 h later, relative to vehicle or SN50M-treated animal controls (F(2, 13)=0.08, p>0.05; Fig. 7E). These results demonstrate that disruption of CCF memories after recall requires activation of the NF-κB transcriptional complex. These experiments are also a third independent line of evidence indicating that the NF-κB transcriptional pathway is required for memory reconsolidation.
Once the amnestic effects of SN50 were determined, we next examined the effect of inhibiting NF-κB DNA binding activity on histone H3 regulation in area CA1 of hippocampus after memory recall. SN50 treatment had no significant effect on recall-induced increases in histone H3 phosphorylation and acetylation (P-H3; F(3, 12)=3.47, p<0.05, AcH3; F(3, 12)=3.45, p<0.05; Fig. S2A, B). This indicates that NF-κB DNA binding is not involved in regulating histone H3 phosphorylation and acetylation after memory retrieval. However, it is possible that blocking NF-κB DNA binding might trigger alternative modifications of histones that are not normally seen. To assess this possibility we also measured histone H4 acetylation 1 h after re-exposure to the training chamber. No change in histone H4 acetylation levels was observed with SN50 treatment as compared to either vehicle or SN50M treatment (Fig. S2C).
In summary, the results in this section suggest that the mechanism of regulation of histone H3 phosphorylation and acetylation during memory reconsolidation is not at the level of the NF-κB DNA-binding complex, but rather at the level of upstream IKKα activity in the NF-κB signaling pathway. These surprising findings indicate an unexpected divergence of IKKα/NF-κB signaling upstream of the genome. Thus, IKKα appears to independently regulate chromatin structure in addition to triggering NF-κB-complex activation competent to bind their cognate DNA regulatory elements.
IKK signaling activity regulates histone H3 modifications at gene promoter regions
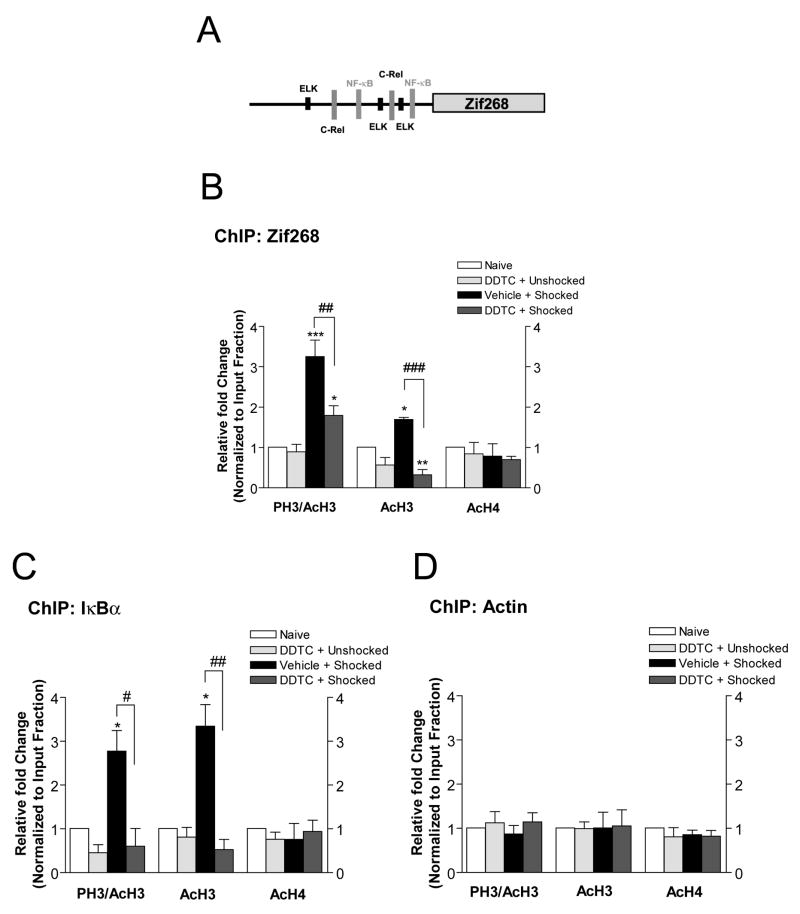
In prior Affymetrix microarray studies, we identified 35 memory-associated genes expressed in area CA1 of hippocampus after fear conditioning (Levenson et al., 2004a). We found that 7 of these genes contained NF-κB regulatory elements in their upstream regions. Specifically, we found NF-κB regulatory elements in the upstream regions of the immediate-early gene Zif268 (Fig. 8A; also known as EGR-1, NGFI-A, Krox 24, TIS 8, ZENK) that is upregulated in hippocampus after memory reactivation (Bozon et al., 2003; Hall et al., 2001; Lee et al., 2004). This is consistent with recent studies that have identified Zif268 as a bona-fide NF-κB pathway gene target (Carayol et al., 2006). These findings suggested the intriguing possibility that IKKα activity might be directly involved in chromatin remodeling across the Zif268 promoter to mediate memory reconsolidation. To this end, we investigated whether or not recall triggered changes in histone H3 phosphorylation and acetylation specifically at the level of the Zif268 gene promoter and examined whether or not IKKα inhibition can alter histone modifications at this gene promoter.
Figure 8.
Inhibition of IKKα effects histone modifications around specific gene promoters. (A) NF-κB binding sites, including sites for the c-Rel NF-κB subunit and ELK, identified within 1 kbp promoter upstream sequences of the Zif268 gene (GenBank accession number M18416). (B) Histone modifications [H3 phosphoacetylation (PH3/AcH3), acetylation (AcH3), and histone H4 acetylation (AcH4)] at the Zif268 promoter. (C) Histone modifications at the IκBα promoter. (D) Histone modifications around the β-Actin promoter. One-way ANOVA; *p<0.05, ** p<0.01 compared with Naïve controls, #p<0.05, ##p<0.01, ###p<0.001 compared to shocked-DDTC, n=4. Error bars are SEM.
Using ChIP assay combined with quantitative real-time PCR, we found that recall triggered a significant increase in histone H3 phosphoacetylation and acetylation levels at the Zif268 promoter that were significantly attenuated with DDTC treatment relative to naïve controls (PH3/AcH3; F(3, 12)=18.49, p<0.01, AcH3; F(3,11)=25.18, p<0.001; Fig. 8B). There were no significant changes in histone H4 acetylation after memory recall (AcH4; F(3, 12)=0.37, p>0.05; Fig. 8B). These findings indicate that IKKα regulates histone H3 phosphorylation and acetylation at the Zif268 promoter in response to memory recall.
As a positive control for our ChIP assay, we also tested the effect of IKKα inhibition on histone modifications around the IκBα promoter, a well-defined gene target of the NF-κB signaling pathway (Baldwin, 1996). Re-exposure of animals to the training chamber triggered a significant increase in histone H3 phosphorylation and acetylation at the IκBα promoter that was blocked with IKKα inhibition with DDTC compared to naïve controls (PH3/AcH3; F(3, 12)=10.95, p<0.05, AcH3; F(3,12)=9.49, p<0.01; Fig. 8C). We found no changes in histone H4 acetylation at the IκBα promoter after memory recall (AcH4; F(3, 12)=0.28, p>0.05; Fig. 8C). As an additional control, we also included analysis of histone modifications around the β-Actin promoter during memory reconsolidation. There were no changes in recall-mediated histone modifications at promoter for β-Actin (Fig. 8D). Together these results provide further evidence for IKKα-mediated changes in hippocampal histone H3 phosphorylation and acetylation occuring at individual gene promoters during memory reconsolidation, and identify Zif268 as a target of chromatin modification in memory reconsolidation.
Effect of inhibiting HDAC and IKKα/NF-κB signaling after memory retrieval
The IKKα/NF-κB inhibitor studies suggested that regulation of histone H3 phosphorylation and acetylation after memory recall required IKKα activity and was necessary for memory reconsolidation (Figs 4 and 6). We therefore sought to determine whether augmenting chromatin remodeling through triggering increased histone acetylation could affect memory reconsolidation. Thus, we used the HDAC inhibitor, NaB to enhance hippocampal histone acetylation and determined if this manipulation could rescue memory reconsolidation in the presence of IKK inhibition. We speculated that inhibition of HDAC activity would enhance histone acetylation and mimic the capacity of IKKα to alter chromatin structure, and thus overcome the deficits in memory reconsolidation triggered by IKKα inhibition.
Thus, to test whether IKKα-mediated histone H3 regulation is functionally relevant to memory reconsolidation, we performed the following experiment using NaB and DDTC. Animals were trained as previously described and on Test Day 1, animals were injected with vehicle or NaB 1 h prior to re-exposure to the training chamber (Fig. 9A). At the end of the re-exposure trial on Test Day 1, no differences in the freezing behavior were observed in vehicle-treated animals compared to NaB-treated animals (t(31)=0.24, p>0.05; Fig. 9B). These results indicate that HDAC inhibition did not disrupt retrieval of CCF memories.
Figure 9.
Effects of inhibition of the NF-κB signaling pathway on enhanced acetylation activity during memory reconsolidation. (A) Experimental design is outlined. (B) Freezing behavior following vehicle or sodium butyrate (NaB) treatment on Test Day 1 (vehicle, n=16; NaB, n=17). (C) Freezing behavior on Test Day 2 during 1 min re-exposure (vehicle, n=7; NaB, n=7; DDTC, n=8; NaB+DDTC, n=6). One-way ANOVA; *p<0.05, compared with shocked-vehicle, **p<0.05, compared with shocked-NaB, #p<0.05 compared to shocked-DDTC. Error bars are SEM.
Immediately after re-exposure to the training chamber on Test Day 1 the vehicle and NaB-treated animals were separated into two groups and each group received either vehicle or DDTC injections. On Test Day 2, no differences in freezing behavior were observed with NaB treatment compared to vehicle treatment. As expected, the conditioned freezing in animals was significantly disrupted with DDTC treatment (F(3,40)=6.02, p<0.01; Fig. 9C). However, no impairment of memory (freezing) was observed with NaB plus DDTC treatments, indicating that enhancing histone acetylation was sufficient to ameliorate the effect of inhibiting IKKα/NF-κB activity on CCF memory reconsolidation. This finding is consistent with the hypothesis that regulation of chromatin structure by IKKα is functionally relevant in memory reconsolidation.
Discussion
In the present study, we demonstrate that the NF-κB transcriptional-regulating pathway contributes to memory reconsolidation in a mammalian CCF paradigm. We observed an up-regulation in hippocampal NF-κB signaling activity that was associated with increases in histone H3 phosphorylation and acetylation, selectively triggered after retrieval of CCF memories. We found that inhibition of the NF-κB signaling cascade at the level of the IKKα protein kinase, blocked both CCF memory reconsolidation and histone H3 posttranslational modifications in hippocampus after retrieval. This is the first evidence to link the IKKα protein kinase and its regulation of histone H3 modifications in vivo to the process of memory reconsolidation. These findings provide new insights into roles for both NF-κB pathway-mediated transcriptional and chromatin structure regulation in the process of memory reconsolidation.
Recent investigations into the molecular mechanisms underlying formation of long-term memory have implicated the NF-κB signaling pathway in the process of initial memory consolidation (Freudenthal et al., 2005; Levenson et al., 2004a; Merlo et al., 2005; Yeh et al., 2002). Here, we found that inhibition of NF-κB signaling significantly impaired memory reconsolidation after retrieval of CCF memories in rats. This effect extended for at least 7 days. This lasting effect is particularly relevant because experiments using protein synthesis inhibition to interrupt the reconsolidation of CCF memories suggests that under some circumstances the memory deficit may fade at longer re-test intervals (Lattal and Abel, 2004).
Thus far, the molecular targets of the NF-κB cascade in memory formation are not known and we were interested in identifying these molecular marks in hippocampus during memory reconsolidation. Recent work has shown that IKKα translocates into the nucleus to mediate NF-κB-dependent and independent gene expression (Birbach et al., 2002; Ear et al., 2005; Massa et al., 2005). Investigations into the nuclear role of IKKα have demonstrated a co-recruitment of IKKα and the histone acetyltransferase CREB-binding protein (CBP) onto gene promoter sites (Anest et al., 2003; Yamamoto et al., 2003). Moreover, the association between IKKα and CBP has been shown to induce IKKα-mediated phosphorylation of histone H3 at Ser-10 in vitro (Anest et al., 2003; Yamamoto et al., 2003). Thus, IKKα regulation of CBP is an appealing potential mechanism for regulating phosphorylation and acetylation of histone H3 in hippocampus in our fear conditioning reconsolidation model.
These observations prompted us to hypothesize that histone H3 regulation is a mechanism by which NF-κB signaling exerts its effects in the process of memory reconsolidation. To shed light on this question, we extended our studies to investigate chromatin regulation. After recall of CCF memories, we observed a considerable increase in histone H3 phosphorylation and acetylation in area CA1 of the hippocampus. Like CCF memory reconsolidation itself, the increases in phosphorylation and acetylation of histone H3 were blocked with inhibition of IKK complex activity. Thus, our results suggest that there are two possible mechanisms for NF-κB pathway-mediated transcriptional regulation during memory reconsolidation. One mechanism is that recall triggers IKKα activation to mediate release of the NF-κB complex for binding to κB regulatory elements within DNA and subsequently alter chromatin structure. The second mechanism is that recall triggers IKKα activation for direct regulation of chromatin structure independent of NF-κB binding to DNA. To evaluate the specific mechanism of IKKα regulation of histone H3 during memory reconsolidation, we used two distinct inhibitors of the NF-κB pathway, sulfasalazine (SSZ) and SN50. SSZ, specifically targets and interferes with the ATP binding site of IKKα and IKKβ, blocking kinase activity (Weber et al., 2000) while the SN50 peptide inhibitor produces direct inhibition of the NF-κB transcriptional DNA-binding complex (Lin et al., 1995). SSZ treatment significantly reduced histone H3 phosphorylation and acetylation in area CA1 of the hippocampus after context re-exposure. Surprisingly, treatment with the SN50 peptide inhibitor failed to inhibit histone H3 phosphorylation and acetylation after recall of CCF memories. These results suggest that posttranslational modifications of the histone H3-tail (phosphorylation and acetylation) are directly regulated at the IKKα level of the NF-κB pathway, independent of activation of the NF-κB transcriptional DNA-binding complex.
Still, the SN50 inhibitor studies indicate that NF-κB complex activity induced by retrieval is required for memory reconsolidation, because SN50 blocked reconsolidation assessed behaviorally. Together with previous studies in which the inhibition of the CREB and NF-κB transcription factors impaired memory reconsolidation (Kida et al., 2002; Merlo et al., 2005), our results further support a requirement for direct transcription factor DNA binding and transcriptional activation in the re-establishment of reactivated memories.
Despite a large body of literature establishing a role for signaling components of the NF-κB pathway, such as IκBα and IKKα, in NF-κB-independent gene regulation, the NF-κB complex has often been thought of as the final target for activation of the NF-κB signaling cascade. Our data suggest a revision to this idea, as we found that IKKα activation regulates histone H3 modifications, a molecular event involved in gene transcription, but in our studies regulated independently of NF-κB DNA binding. Thus, we expose a different mechanism for transcriptional regulation by the NF-κB pathway during memory reconsolidation: regulation of chromatin remodeling by IKKα. These studies provide insight into the role in vivo of IKKα in the regulation of histone modifications, and complement prior investigations using cell culture and construct transfection (Anest et al., 2004; Yamamoto et al., 2003).
Importantly, we found changes in histone H3 modifications across individual gene promoter regions, including Zif268, which were induced during memory reconsolidation through the IKKα/NF-κB signaling pathway. Although functional roles of some transcription factors are unclear in the context of memory formation, activation of Zif268 has been found to play an important role in memory reconsolidation (Bozon et al., 2003; Hall et al., 2001; Izquierdo and Cammarota, 2004; Lee et al., 2004). Since the inhibition of IKKα/NF-κB blocked recall-induced histone H3 phosphorylation and acetylation at the Zif268 promoter, it is possible that recall triggers IKKα/NF-κB signaling and subsequently induces Zif268 to further modulate memory reconsolidation. Thus, our studies support previous findings that Zif268 is involved in memory formation and expand on the mechanisms responsible for regulation of this gene in hippocampus during memory reconsolidation.
An interesting finding from these studies is that IKKα mediates both histone H3 phosphorylation and acetylation in hippocampus during memory reconsolidation. One potential outcome of histone H3 phosphorylation is the recruitment and activation of histone acetyltransferases (HATs) (Cheung et al., 2000). The CREB binding protein (CBP), which contains HAT activity, has been shown to interact with chromatin-bound IKKα at gene promoter sites (Hoberg et al., 2006; Yamamoto et al., 2003). Therefore, we can conclude that a possible mechanism for IKKα regulation of histone H3 acetylation is through the interaction of IKKα with CBP. Interestingly, CBP has also been shown to be involved in memory formation and consolidation as well (Alarcon et al., 2004; Korzus et al., 2004; Wood et al., 2006).
Since inhibition of IKKα activity blocks histone H3 acetylation during memory reconsolidation, we hypothesized that enhancing histone acetylation would rescue memory reconsolidation in the face of IKKα inhibition. Indeed, results obtained using the histone deacetylase (HDAC) inhibitor, NaB, showed that enhancing histone acetylation blocks the effect of DDTC on freezing behavior. This demonstrates that IKKα inhibition produces freezing behavior deficits after memory recall that can be rescued by enhancing histone acetylation. This is consistent with our hypothesis that histone acetylation is one target for IKKα activation in hippocampus during the re-establishment of fear memories.
An interesting complexity to our HDAC inhibitor studies is that enhancing histone acetylation rescues the effect of inhibiting IKKα, even though our results show that IKK inhibition blocks regulation of both chromatin structure and NF-κB DNA binding during memory reconsolidation. Because NF-κB DNA binding is necessary for memory reconsolidation one might expect that IKK inhibition would still lead to loss of memory reconsolidation even if histone acetylation was still intact. A likely explanation for this result is that increasing protein acetylation with HDAC inhibition rescues the NF-κB DNA-binding limb of the pathway as well. This would be consistent with the known capacity of acetylation of NF-κB subunits to promote DNA regulatory element binding. For example, recent investigations into post-nuclear translocation of the NF-κB DNA-binding complex indicate that NF-κB subunits are acetylated in order to promote a maximal transcriptional response (Greene and Chen, 2004; Quivy and Van Lint, 2004). Indeed, acetylation of lysines on NF-κB subunits regulate different functions of the NF-κB DNA-binding complex, including transcriptional activation, DNA binding affinity, IκB assembly, and subcellular localization (review in, Perkins, 2006). For example, acetylated RelA/p65, like the histone proteins, is subject to deacetylation by HDACs, specifically HDAC3 which is important for RelA/p65 deacetylation both in vivo and in vitro (Chen et al., 2001; Kiernan et al., 2003). Thus, a plausible effect of HDAC inhibition with NaB is not only an enhancement of histone acetylation but also an enhancement of NF-κB acetylation and enhanced NF-κB DNA binding activity, thereby leading to a rescue of the effect of IKKα inhibition on memory reconsolidation. Thus, while our study with the HDAC inhibitor rescuing memory reconsolidation is consistent with our hypothesis that increased histone acetylation is involved in memory reconsolidation, these results may also be indicative of a more general role for protein acetylation in memory formation as well (Swank and Sweatt, 2001; Yeh et al., 2004).
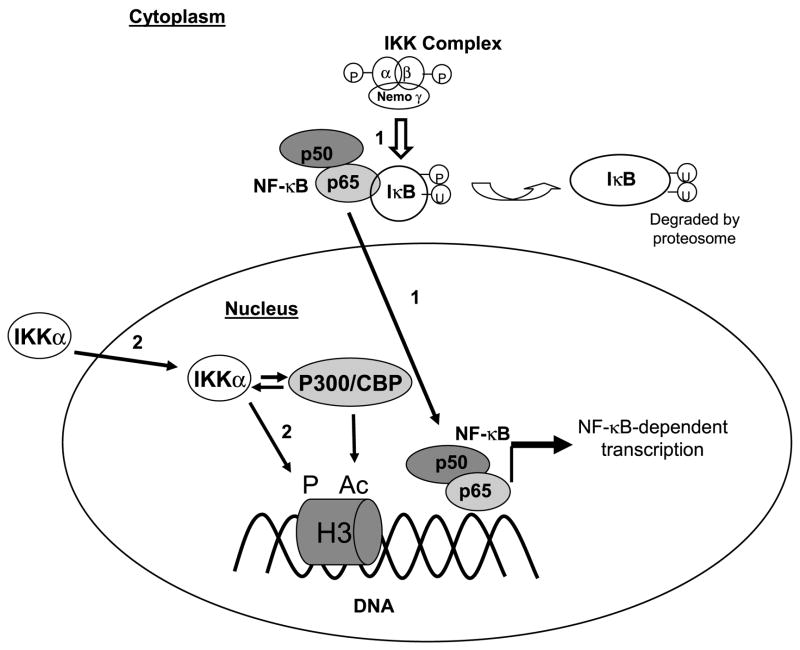
In conclusion, our results suggest that the NF-κB signaling pathway regulates transcription in the hippocampus during memory reconsolidation (Fig. 10). Our working model is that the NF-κB signaling pathway accomplishes this through two transcriptional control mechanisms: IKK complex-mediated activation of the NF-κB complex for direct binding to κB regulatory sites within DNA, and IKKα regulation of histone modifications (Fig. 10). One interesting possibility is that the IKKα regulation of chromatin is permissive for transcriptional control, affecting transcription triggered not only by NF-κB but also by a variety of transcription factors including CREB. Regardless of this specific idea, the model illustrates the dynamic and complex nature of NF-κB signaling in memory processing after retrieval. In addition, there are several mechanisms involved in the regulation of chromatin structure during memory formation that also might be involved in memory reconsolidation. For example, our previous studies have also implicated the ERK/MAPK pathway in targeting histone H3 phosphorylation during memory consolidation (Chwang et al., 2006). Thus, it is possible that IKKα and ERK might target histone H3 independently during different stages of the process of memory formation and re-formation. We anticipate that a major challenge for molecular behaviorists will be to determine how the IKKα/NF-κB and ERK signaling pathways integrate signals to regulate chromatin structure in memory formation processes. While there is still much to be understood about the role of IKKα/NF-κB signaling activity in memory formation, the results presented here provide the first evidence that IKKα activity regulates gene transcription and chromatin structure in hippocampus to enable memory reconsolidation.
Figure 10.
Model for the role of the IKKα kinase protein in the regulation of chromatin structure during long-term memory reconsolidation. Upon activation after memory recall, the IKKα kinase protein initiates two pathways for transcriptional regulation of genes: (1) The IKKα kinase protein at the IKK complex level functions to increase the DNA binding activity of the NF-κB complex for modulation of gene transcripts; (2) Additionally, the IKKα kinase protein acts independently from the IKK complex to mediate changes in chromatin structure that are apparent as an increase in phosphorylation of histone H3 and subsequent acetylation of histone H3 through its interaction with CBP. The changes in chromatin structure ultimately lead to changes in transcriptional regulation of genes relevant for re-stabilization of memory after retrieval.
Experimental Procedure
See supplemental data for detailed procedures.
Fear Conditioning
Animals were handled for 5 days and on the day of experiments they were transported to the laboratory at least 2 h prior to fear conditioning. Animals were placed into the training chamber and allowed to explore for 2 min, after which they received an electric shock (1 s, 0.5 mA). The 2 min/1 s shock paradigm was repeated for a total of three shocks. After the last shock, animals were allowed to explore the context for an additional 1 min prior to removal from the training chamber. See supplemental data.
Isolation of Area CA1
Whole brains were immersed in oxygenated (95%/5% O2/CO2) ice-cold cutting saline (CS [in mM]: 110 Sucrose, 60 NaCl, 3 KCl, 1.25 NaH2PO4, 28 NaHCO3, 0.5 CaCl2, 7 MgCl2, 5 Glucose, 0.6 Ascorbate) prior to isolation of CA1 subfields. Area CA1 of hippocampus were microdissected and immediately frozen dry ice and stored at −80 °C until protein extracts were prepared.
Chromatin Immunoprecipitation (ChIP) Assay
Immunoprecipitated DNA was subjected to quantitative real-time PCR using primers specific for 150–200 bp segments corresponding to promoters upstream of the rat Zif268, IκBα, or β-actin (used as a negative control): Zif268 sense, 5′-ATGGGCTGTTAGGGACAGTG -3′; antisense, 5′-TTGGGGATTTAGCTCAGTGG-3′; IκBα sense, 5′-CGCTAAGAGGAACAGCCTAG-3′; antisense, 5′-CAGCTGGTCGAAACATGGC-3′; β-actin sense, 5′-CTCTCTCCCAGGAGTTGTGC -3′; antisense, 5′-GCTACAGCAGGGGATCAGAG-3′. The cumulative fluorescence for each amplicon was normalized to input amplification. See supplemental data for additional procedures.
Supplementary Material
Acknowledgments
The authors thank Aswin Sundarakrishnan and Dr. Hyung Jin Ahn for technical assistance. This work was supported by the NIH (NS048811, MH57014, and NS37444), the Epilepsy Foundation, the American Health Assistance Foundation, and the Evelyn F. McKnight Brain Research Foundation.
Abbreviations
- CCF
contextual conditioned fear
- DDTC
diethyldithiocarbamate
- P-H3
Phospho-histone H3
- AcH3
acetylated-histone H3
- AcH4
acetylated-histone H4
- NF-κB
nuclear-factor kappa B
- IKK
IκB kinase
- SSZ
sulfasalazine
- IκB
inhibitor kappa B
- HDAC
histone deacetylase
- HAT
histone acetyltransferase
- NaB
sodium butyrate
- CBP
CREB binding protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Anest V, Cogswell PC, Baldwin AS., Jr IkappaB kinase alpha and p65/RelA contribute to optimal epidermal growth factor-induced c-fos gene expression independent of IkappaBalpha degradation. J Biol Chem. 2004;279:31183–31189. doi: 10.1074/jbc.M404380200. [DOI] [PubMed] [Google Scholar]
- Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- Ashburner BP, Westerheide SD, Baldwin AS., Jr The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol. 2001;21:7065–7077. doi: 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Battaglioli E, Andres ME, Rose DW, Chenoweth JG, Rosenfeld MG, Anderson ME, Mandel G. REST repression of neuronal genes requires components of the hSWI. SNF complex. J Biol Chem. 2002;277:41038–41045. doi: 10.1074/jbc.M205691200. [DOI] [PubMed] [Google Scholar]
- Birbach A, Gold P, Binder BR, Hofer E, de Martin R, Schmid JA. Signaling molecules of the NF-kappa B pathway shuttle constitutively between cytoplasm and nucleus. J Biol Chem. 2002;277:10842–10851. doi: 10.1074/jbc.M112475200. [DOI] [PubMed] [Google Scholar]
- Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Activation of the nuclear factor-kappaB is a key event in brain tolerance. J Neurosci. 2001;21:4668–4677. doi: 10.1523/JNEUROSCI.21-13-04668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozon B, Davis S, Laroche S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron. 2003;40:695–701. doi: 10.1016/s0896-6273(03)00674-3. [DOI] [PubMed] [Google Scholar]
- Carayol N, Chen J, Yang F, Jin T, Jin L, States D, Wang CY. A dominant function of IKK/NF-kappaB signaling in global lipopolysaccharide-induced gene expression. J Biol Chem. 2006;281:31142–31151. doi: 10.1074/jbc.M603417200. [DOI] [PubMed] [Google Scholar]
- Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvis CM, Pollock JD, Goodman RH, Impey S, Dunn J, Mandel G, Champagne FA, Mayford M, Korzus E, Kumar A, et al. Epigenetic mechanisms and gene networks in the nervous system. J Neurosci. 2005;25:10379–10389. doi: 10.1523/JNEUROSCI.4119-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moore AN. Sequestration of serum response factor in the hippocampus impairs long-term spatial memory. J Neurochem. 2005;93:269–278. doi: 10.1111/j.1471-4159.2004.03016.x. [DOI] [PubMed] [Google Scholar]
- Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K. Characterization of fear memory reconsolidation. J Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- Ear T, Cloutier A, McDonald PP. Constitutive nuclear expression of the I kappa B kinase complex and its activation in human neutrophils. J Immunol. 2005;175:1834–1842. doi: 10.4049/jimmunol.175.3.1834. [DOI] [PubMed] [Google Scholar]
- Freudenthal R, Boccia MM, Acosta GB, Blake MG, Merlo E, Baratti CM, Romano A. NF-kappaB transcription factor is required for inhibitory avoidance long-term memory in mice. Eur J Neurosci. 2005;21:2845–2852. doi: 10.1111/j.1460-9568.2005.04126.x. [DOI] [PubMed] [Google Scholar]
- Greene WC, Chen LF. Regulation of NF-kappaB action by reversible acetylation. Novartis Found Symp. 2004;259:208–217. 218–225. [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa M, Miyashita H, Sakamoto I, Kitagawa M, Tanaka H, Yasuda H, Karin M, Kikugawa K. Evidence that reactive oxygen species do not mediate NF-kappaB activation. Embo J. 2003;22:3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoberg JE, Popko AE, Ramsey CS, Mayo MW. IkappaB kinase alpha-mediated derepression of SMRT potentiates acetylation of RelA/p65 by p300. Mol Cell Biol. 2006;26:457–471. doi: 10.1128/MCB.26.2.457-471.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Doherty JJ, Dingledine R. Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J Neurosci. 2002;22:8422–8428. doi: 10.1523/JNEUROSCI.22-19-08422.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Jazrawi E, Cosio B, Barnes PJ, Adcock IM. p65-activated histone acetyltransferase activity is repressed by glucocorticoids: mifepristone fails to recruit HDAC2 to the p65-HAT complex. J Biol Chem. 2001;276:30208–30215. doi: 10.1074/jbc.M103604200. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Cammarota M. Neuroscience. Zif and the survival of memory. Science. 2004;304:829–830. doi: 10.1126/science.1098139. [DOI] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, Silva AJ. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Kiernan R, Bres V, Ng RW, Coudart MP, El Messaoudi S, Sardet C, Jin DY, Emiliani S, Benkirane M. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J Biol Chem. 2003;278:2758–2766. doi: 10.1074/jbc.M209572200. [DOI] [PubMed] [Google Scholar]
- Kis A, Yellon DM, Baxter GF. Role of nuclear factor-kappa B activation in acute ischaemia-reperfusion injury in myocardium. Br J Pharmacol. 2003;138:894–900. doi: 10.1038/sj.bjp.0705108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Kushikata T, Fang J, Krueger JM. Nuclear factor-kappaB inhibitor peptide inhibits spontaneous and interleukin-1beta-induced sleep. Am J Physiol Regul Integr Comp Physiol. 2000;279:R404–413. doi: 10.1152/ajpregu.2000.279.2.R404. [DOI] [PubMed] [Google Scholar]
- Kumar A, Lin Z, SenBanerjee S, Jain MK. Tumor necrosis factor alpha-mediated reduction of KLF2 is due to inhibition of MEF2 by NF-kappaB and histone deacetylases. Mol Cell Biol. 2005;25:5893–5903. doi: 10.1128/MCB.25.14.5893-5903.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Abel T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc Natl Acad Sci U S A. 2004;101:4667–4672. doi: 10.1073/pnas.0306546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- Lee S, Rivier C. Role played by hypothalamic nuclear factor-{kappa}B in alcohol-mediated activation of the rat hypothalamic-pituitary-adrenal axis. Endocrinology. 2005;146:2006–2014. doi: 10.1210/en.2004-1268. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Choi S, Lee SY, Cao YA, Ahn HJ, Worley KC, Pizzi M, Liou HC, Sweatt JD. A bioinformatics analysis of memory consolidation reveals involvement of the transcription factor c-rel. J Neurosci. 2004a;24:3933–3943. doi: 10.1523/JNEUROSCI.5646-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004b;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- Liou HC, Hsia CY. Distinctions between c-Rel and other NF-kappaB proteins in immunity and disease. Bioessays. 2003;25:767–780. doi: 10.1002/bies.10306. [DOI] [PubMed] [Google Scholar]
- Lubin FD, Johnston LD, Sweatt JD, Anderson AE. Kainate mediates nuclear factor-kappa B activation in hippocampus via phosphatidylinositol-3 kinase and extracellular signal-regulated protein kinase. Neuroscience. 2005;133:969–981. doi: 10.1016/j.neuroscience.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Massa PE, Li X, Hanidu A, Siamas J, Pariali M, Pareja J, Savitt AG, Catron KM, Li J, Marcu KB. Gene expression profiling in conjunction with physiological rescues of IKKalpha-null cells with wild type or mutant IKKalpha reveals distinct classes of IKKalpha/NF-kappaB-dependent genes. J Biol Chem. 2005;280:14057–14069. doi: 10.1074/jbc.M414401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- Merlo E, Freudenthal R, Maldonado H, Romano A. Activation of the transcription factor NF-kappaB by retrieval is required for long-term memory reconsolidation. Learn Mem. 2005;12:23–29. doi: 10.1101/lm.82705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A, Kosaka T, Seta K, Asano T, Umezawa K, Hayakawa M. Novel nuclear factor kappa B activation inhibitor prevents inflammatory injury in unilateral ureteral obstruction. J Urol. 2003;169:1559–1563. doi: 10.1097/01.ju.0000045686.21766.c1. [DOI] [PubMed] [Google Scholar]
- Morais C, Pat B, Gobe G, Johnson DW, Healy H. Pyrrolidine dithiocarbamate exerts anti-proliferative and pro-apoptotic effects in renal cell carcinoma cell lines. Nephrol Dial Transplant. 2006;21:3377–3388. doi: 10.1093/ndt/gfl543. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Natoli G, Saccani S, Bosisio D, Marazzi I. Interactions of NF-kappaB with chromatin: the art of being at the right place at the right time. Nat Immunol. 2005;6:439–445. doi: 10.1038/ni1196. [DOI] [PubMed] [Google Scholar]
- Park GY, Wang X, Hu N, Pedchenko TV, Blackwell TS, Christman JW. NIK Is Involved in Nucleosomal Regulation by Enhancing Histone H3 Phosphorylation by IKK{alpha} J Biol Chem. 2006;281:18684–18690. doi: 10.1074/jbc.M600733200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene. 2006;25:6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- Quivy V, Van Lint C. Regulation at multiple levels of NF-kappaB-mediated transactivation by protein acetylation. Biochem Pharmacol. 2004;68:1221–1229. doi: 10.1016/j.bcp.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank MW, Sweatt JD. Increased histone acetyltransferase and lysine acetyltransferase activity and biphasic activation of the ERK/RSK cascade in insular cortex during novel taste learning. J Neurosci. 2001;21:3383–3391. doi: 10.1523/JNEUROSCI.21-10-03383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viatour P, Legrand-Poels S, van Lint C, Warnier M, Merville MP, Gielen J, Piette J, Bours V, Chariot A. Cytoplasmic IkappaBalpha increases NF-kappaB-independent transcription through binding to histone deacetylase (HDAC) 1 and HDAC3. J Biol Chem. 2003;278:46541–46548. doi: 10.1074/jbc.M306381200. [DOI] [PubMed] [Google Scholar]
- Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CK, Liptay S, Wirth T, Adler G, Schmid RM. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology. 2000;119:1209–1218. doi: 10.1053/gast.2000.19458. [DOI] [PubMed] [Google Scholar]
- Wood MA, Attner MA, Oliveira AM, Brindle PK, Abel T. A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn Mem. 2006;13:609–617. doi: 10.1101/lm.213906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- Yeh SH, Lin CH, Gean PW. Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Mol Pharmacol. 2004;65:1286–1292. doi: 10.1124/mol.65.5.1286. [DOI] [PubMed] [Google Scholar]
- Yeh SH, Lin CH, Lee CF, Gean PW. A requirement of nuclear factor-kappaB activation in fear-potentiated startle. J Biol Chem. 2002;277:46720–46729. doi: 10.1074/jbc.M206258200. [DOI] [PubMed] [Google Scholar]
- Zandi E, Chen Y, Karin M. Direct phosphorylation of IkappaB by IKKalpha and IKKbeta: discrimination between free and NF-kappaB-bound substrate. Science. 1998;281:1360–1363. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.