Abstract
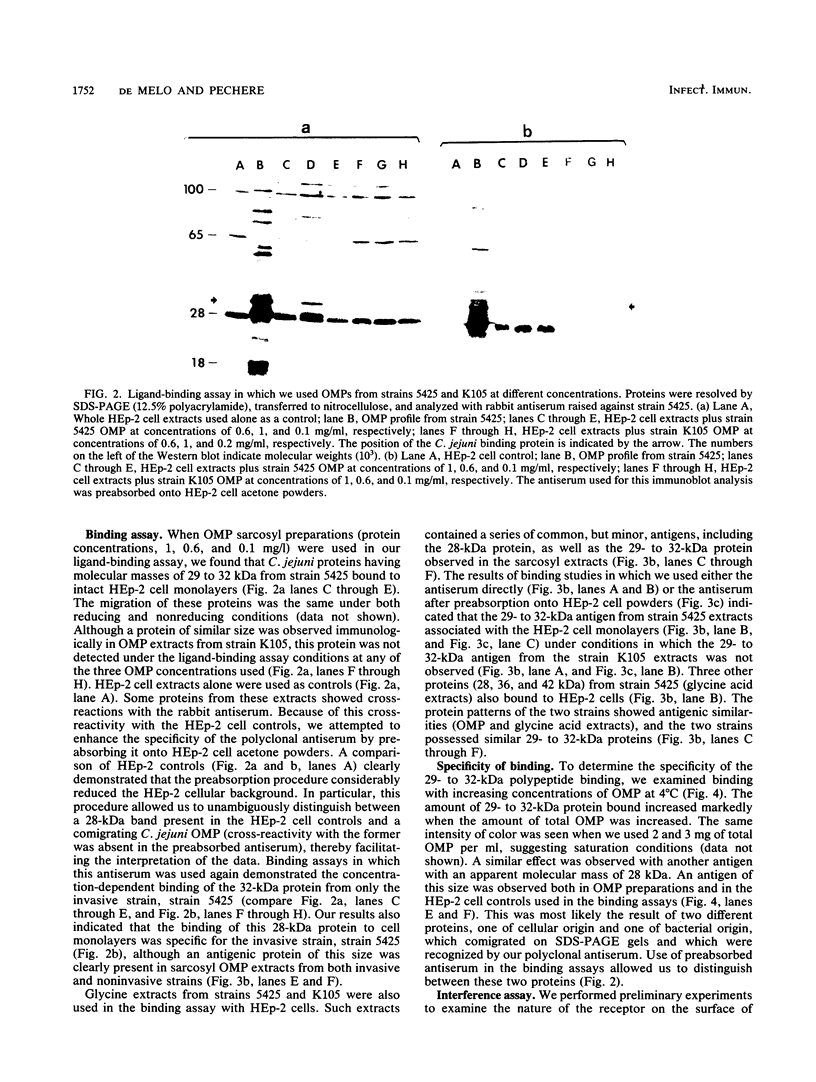
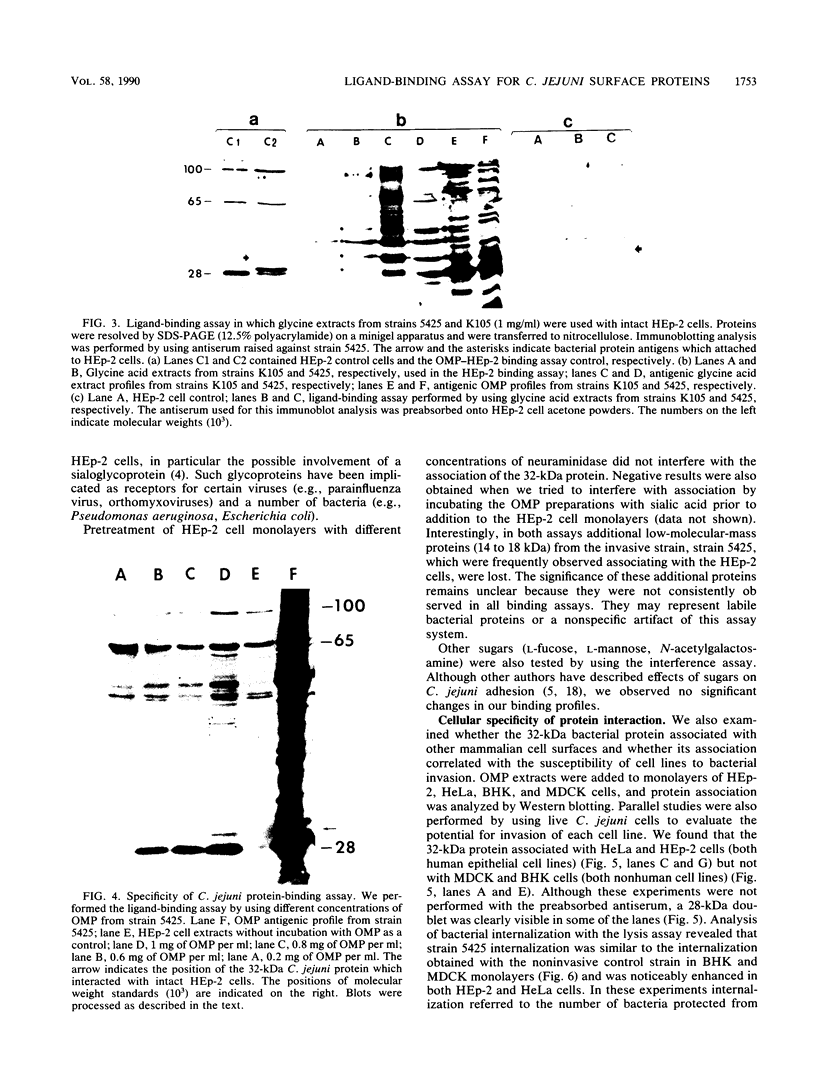
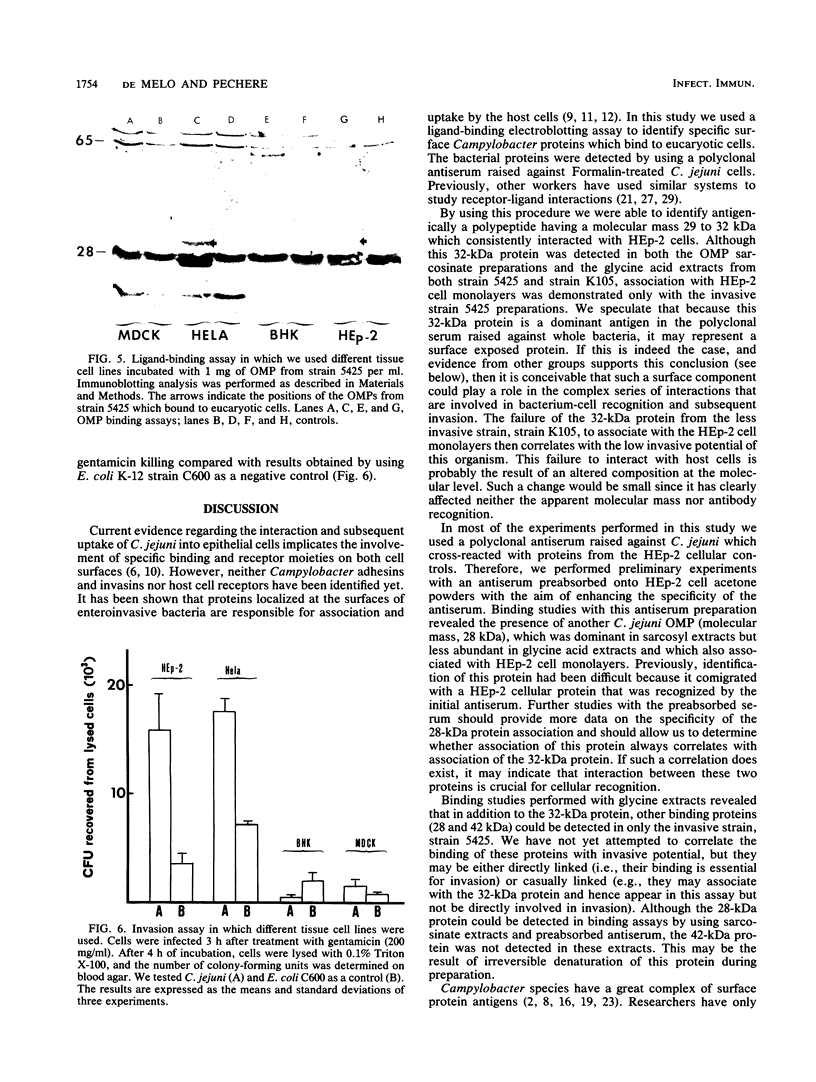
To understand the role of Campylobacter jejuni surface proteins in the interaction of C. jejuni with cultured mammalian cell lines in vitro, we developed a ligand-binding assay. This procedure allowed us to antigenically identify C. jejuni outer membrane proteins (OMPs) that attach to intact host cell membranes. OMPs isolated from an invasive strain and a less invasive strain were antigenically indistinguishable. However, we found that proteins with molecular masses of 28 and 32 kilodaltons (kDa) from just the invasive strain bound to HEp-2 cell monolayers. Binding of the 32-kDa OMP was cell line specific and correlated directly with the ability of the invasive C. jejuni strain to penetrate. Such a correlation was probably also true for the 28-kDa OMP. We also investigated the binding of glycine acid extracts with cell line HEp-2. We identified four proteins with apparent molecular masses of 28, 32, 36, and 42 kDa in the invasive strain extracts that bound to HEp-2 cells. In contrast, only the 36-kDa protein from the less invasive strain bound to HEp-2 cells. Our data suggest that binding of these surface exposed proteins may play a key role in C. jejuni-host cell interactions and ultimate invasion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J., Hopkins J. A., Berka R. M., Vasil M. L., Wang W. L. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect Immun. 1983 Oct;42(1):276–284. doi: 10.1128/iai.42.1.276-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Vasil M. L. Campylobacter jejuni outer membrane proteins are antigenic for humans. Infect Immun. 1984 Mar;43(3):986–993. doi: 10.1128/iai.43.3.986-993.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Melo M. A., Gabbiani G., Pechère J. C. Cellular events and intracellular survival of Campylobacter jejuni during infection of HEp-2 cells. Infect Immun. 1989 Jul;57(7):2214–2222. doi: 10.1128/iai.57.7.2214-2222.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B. E., Blaser M. J., Snyder E. L. Two-dimensional gel electrophoresis and immunoblotting of Campylobacter outer membrane proteins. Infect Immun. 1987 Jul;55(7):1564–1572. doi: 10.21236/ada265461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauchere J. L., Rosenau A., Veron M., Moyen E. N., Richard S., Pfister A. Association with HeLa cells of Campylobacter jejuni and Campylobacter coli isolated from human feces. Infect Immun. 1986 Nov;54(2):283–287. doi: 10.1128/iai.54.2.283-287.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Heffron F., Falkow S. Epithelial cell surfaces induce Salmonella proteins required for bacterial adherence and invasion. Science. 1989 Feb 17;243(4893):940–943. doi: 10.1126/science.2919285. [DOI] [PubMed] [Google Scholar]
- Formal S. B., Hale T. L., Sansonetti P. J. Invasive enteric pathogens. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S702–S707. doi: 10.1093/clinids/5.supplement_4.s702. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Sansonetti P. J., Schad P. A., Austin S., Formal S. B. Characterization of virulence plasmids and plasmid-associated outer membrane proteins in Shigella flexneri, Shigella sonnei, and Escherichia coli. Infect Immun. 1983 Apr;40(1):340–350. doi: 10.1128/iai.40.1.340-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Grayston J. T. Effect of polycations, polyanions and neuraminidase on the infectivity of trachoma-inclusin conjunctivitis and lymphogranuloma venereum organisms HeLa cells: sialic acid residues as possible receptors for trachoma-inclusion conjunction. Infect Immun. 1973 Jul;8(1):74–79. doi: 10.1128/iai.8.1.74-79.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee A., O'Rourke J. L., Barrington P. J., Trust T. J. Mucus colonization as a determinant of pathogenicity in intestinal infection by Campylobacter jejuni: a mouse cecal model. Infect Immun. 1986 Feb;51(2):536–546. doi: 10.1128/iai.51.2.536-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983 Nov;42(2):675–682. doi: 10.1128/iai.42.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Structural and antigenic heterogeneity of lipopolysaccharides of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1984 Jul;45(1):210–216. doi: 10.1128/iai.45.1.210-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweegan E., Walker R. I. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun. 1986 Jul;53(1):141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. D., Bradbury W. C. Human antibody response to outer membrane proteins of Campylobacter jejuni during infection. Infect Immun. 1984 Feb;43(2):739–743. doi: 10.1128/iai.43.2.739-743.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser I., Hellmann E. In vitro binding of Campylobacter jejuni surface proteins to murine small intestinal cell membranes. Med Microbiol Immunol. 1989;178(4):217–228. doi: 10.1007/BF00202555. [DOI] [PubMed] [Google Scholar]
- Oblas B., Boyd N. D., Singer R. H. Analysis of receptor-ligand interactions using nitrocellulose gel transfer: application to Torpedo acetylcholine receptor and alpha-bungarotoxin. Anal Biochem. 1983 Apr 1;130(1):1–8. doi: 10.1016/0003-2697(83)90641-3. [DOI] [PubMed] [Google Scholar]
- Preston M. A., Penner J. L. Structural and antigenic properties of lipopolysaccharides from serotype reference strains of Campylobacter jejuni. Infect Immun. 1987 Aug;55(8):1806–1812. doi: 10.1128/iai.55.8.1806-1812.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautelin H., Kosunen T. U. An acid extract as a common antigen in Campylobacter coli and Campylobacter jejuni strains. J Clin Microbiol. 1983 Apr;17(4):700–701. doi: 10.1128/jcm.17.4.700-701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. G., Blaser M. J., Sarmiento J. I., Fox J. Experimental Campylobacter jejuni infection in Macaca nemestrina. Infect Immun. 1989 May;57(5):1438–1444. doi: 10.1128/iai.57.5.1438-1444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E. M., King G. L., Maratos-Flier E. Characterization of a common high-affinity receptor for reovirus serotypes 1 and 3 on endothelial cells. J Virol. 1989 Mar;63(3):1318–1325. doi: 10.1128/jvi.63.3.1318-1325.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. I., Caldwell M. B., Lee E. C., Guerry P., Trust T. J., Ruiz-Palacios G. M. Pathophysiology of Campylobacter enteritis. Microbiol Rev. 1986 Mar;50(1):81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenman W. M., Meuser R. U. Chlamydia trachomatis elementary bodies possess proteins which bind to eucaryotic cell membranes. J Bacteriol. 1986 Feb;165(2):602–607. doi: 10.1128/jb.165.2.602-607.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A. J., McCoy E. C., Fullmer C. S., Burda K., Bier P. J. Microcapsule of Campylobacter fetus: chemical and physical characterization. Infect Immun. 1978 Dec;22(3):963–971. doi: 10.1128/iai.22.3.963-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo M. A., Pechère J. C. Effect of mucin on Campylobacter jejuni association and invasion on HEp-2 cells. Microb Pathog. 1988 Jul;5(1):71–76. doi: 10.1016/0882-4010(88)90083-6. [DOI] [PubMed] [Google Scholar]