Abstract
A randomized, within-subject, double blind, inpatient study of the physiological and subjective effects of oral 3,4-methylenedioxymethamphetamine (MDMA) was conducted in human volunteers with previous MDMA experience. Placebo, low (1.0 mg/kg), and high (1.6 mg/kg) doses of oral MDMA were administered in a controlled inpatient setting at least 7 days apart to six African-American (4 male, 2 female) and two Caucasian (both male) volunteers, mean (SE) age 21.1 (0.8) years and weight 77.2 (7.7) kg. MDMA doses were 46–150 mg, in the range of typical recreational doses. Participants completed all sessions without clinically significant adverse events. MDMA produced significant, dose-dependent increases in heart rate (highest 132 bpm), systolic (highest 171 mm Hg) and diastolic (highest 102 mm Hg) blood pressure, and subjective responses for energy level, closeness to others, mind racing, heightened senses, and high (evaluated by visual analog scales). Peak effects occurred 1–2 h post-dose, with no secondary peak. There were no significant effects on body temperature (measured at tympanic membrane), respiratory rate, or blood oxygen saturation (by pulse oximetry). Although most physiological and subjective parameters were significantly correlated to MDMA plasma concentrations, correlation coefficients were low and clinically insignificant, eliminating the ability to predict effects from single plasma concentrations. These findings suggest that oral MDMA in typical recreational doses produces short-term effects on cardiovascular function and subjective state, but that temperature effects may result from interaction with environmental and subject factors.
Keywords: MDMA, Controlled dosing, cardiovascular, subjective effects, Ecstasy
3,4-methylenedioxymethamphetamine (MDMA, ecstasy), a ring substituted phenethylamine structurally similar to methamphetamine and mescaline, has gained worldwide popularity as a drug of abuse. The S-MDMA enantiomer is primarily responsible for stimulation and R-MDMA for hallucinogenic effects.1 Therapeutic use of MDMA in conjunction with psychotherapy is based on its entactogenic (“touching within”) properties to lower psychological defenses, increase insightfulness, and enhance communication 2, 3. Usual recreational doses are 30 – 150 mg/pill, although purity of the street drug is notoriously poor 4.
Previous human laboratory studies of controlled MDMA administration have demonstrated marked increases in cardiovascular parameters, pupil dilation, dry mouth, and loss of appetite due to sympathetic nervous system stimulation 5–8. Common subjective effects reported in multiple studies include euphoric/loving feelings, greater self-confidence, self-acceptance, and enhanced empathy and understanding 3, 6, 8–12. Commonly reported acute adverse effects include jaw clenching, grinding of the teeth, nausea, tremor, and feelings of tension. Previous studies generally included predominantly male Caucasian subjects and monitored subjective and physiologic effects for less than 24 hours. Documentation of drug abstinence prior to MDMA administration varied from requesting subjects to abstain, to housing subjects on a research unit prior to dosing.
Following oral administration, MDMA is absorbed rapidly into the bloodstream 13–19. Maximum plasma MDMA concentrations are achieved at approximately 2 h; the terminal elimination half-life is 7 – 9 h 1, 8, 15, 18. The major metabolic route involves conversion by CYP2D6, and to a lesser extent CYP1A2 and CYP3A4 14, 20 to 3,4-dihydroxymethamphetamine (HHMA), an unstable intermediate, and then by catechol-O-methyltransferase (COMT) 16 to 4- hydroxy-3-methoxymethamphetamine (HMMA). In the minor metabolic pathway, MDMA is N-demethylated by CYP1A2, and to a lesser extent CYP2D6, to 3,4-methylenedioxyamphetamine (MDA).
The primary objective of this controlled oral MDMA administration study was evaluation of physiological and subjective effects after typical recreational doses in a gender and racially varied subject population using rigorous design features, e.g., objective confirmation of recent MDMA use, subjects not under the acute influence of MDMA or other drugs (urine testing and monitored 12 h abstinence), and assessment in a controlled environment for up to 167 hours. We hypothesized that MDMA would produce dose-dependent changes in physiological and subjective variables with this study design similar to those reported in prior less rigorously controlled studies. A secondary objective was elucidating the relationship between MDMA plasma concentrations and physiological and subjective effects.
METHODS
Human Participants
The protocol was approved by the National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP) Institutional Review Board (IRB). Individuals were recruited by television, radio and newspaper advertisements, flyers, and word of mouth. All participants provided written informed consent and were paid for their participation. Inclusion criteria required individuals to be 18 to 40 years of age, with lifetime consumption of at least five tablets of ecstasy and at least one in the 90 days prior to screening. History of MDMA use was confirmed by a positive urine amphetamines (cross reacts with MDMA, methamphetamine, and amphetamine) or hair MDMA test within 90 days prior to study entrance. Female subjects were required to use a reliable method of birth control or abstain from sexual intercourse throughout study participation. Serum and urine pregnancy tests were administered at the screening visit and on the morning of each dosing session, respectively.
All potential subjects received a comprehensive medical and psychological evaluation, including medical and drug use history and physical examination, clinical laboratory tests, 12-lead ECG with 3-minute rhythm strip, SCL-90R, and computer-administered version of the Structured Clinical Interview for the Diagnostic & Statistical Manual of Mental Disorders IV (DSM-IV). Individuals meeting any of the following criteria were excluded: nursing or pregnant women; current medical condition or history of neurological illness; axis I psychiatric diagnosis other than abuse or dependence on nicotine, cannabis or MDMA; recent (within 30 days of MDMA administration) prescription of a CYP2D6 or CYP3A4 inhibitor or CYP3A4 inducer (with reconsideration 30 days after the individual voluntarily stopped use); SBP > 135, DBP > 85 or heart rate > 100 bpm after 5 min rest; total cholesterol > 250 mg/dL if older than 30 years; hemoglobin < 12.5 g/100 mL (male) or < 12 g/100 mL (female); clinically significant abnormal ECG; serum transaminase levels > three times normal.
Eight healthy volunteers (four African-American males, two African-American females and two Caucasian males) met study eligibility criteria. Participants’ mean ± standard error (SE) age was 21.1 ± 0.8 years (range: 18 – 24) and weight 77.2 ± 7.7 kg (range: 46.4 – 103.5). Current self-reported MDMA use ranged from 3 pills/week to 2 pills/month for < 1 to 5 years. Pupil measurements, participant dose identification and duration of effect data included one additional participant (African-American male, 18 years old, 73.3 kg). Other physiological and subjective data from this participant were collected within a functional magnetic resonance imaging (fMRI) scanner environment, and thus could not be combined with other subjects’ data.
Study design
All participants resided on the closed research unit of the NIDA IRP. One participant completed all 3 drug administration sessions in one stay, with 7 days between sessions. Seven participants were discharged after each session and readmitted later for the other two sessions. The interval (mean ± SE; range) between unit discharge and readmission was 15.8 ± 3.5; 3 – 46 days; all three sessions were completed within 42.9 ± 8.4 (14 –81) days.
Separate stay participants were reevaluated at each re-admission to confirm continued study eligibility. Urine was screened for benzodiazepines, cocaine, amphetamines (cross reacting with MDMA, methamphetamine, and amphetamine), cannabis, opiates, PCP, and barbiturates with a Triage® 7 Drugs of Abuse panel (Biosite, Inc., San Diego, CA). Negative results were required for all drug classes except amphetamines (includes cross-reaction with MDMA) and cannabis (many MDMA users also use cannabis) for the dosing session to proceed. Participants entered the inpatient unit at least 12 h prior to controlled dosing to ensure dissipation of effects from any previously self-administered drug. Participants ate a light breakfast about 2 h prior to MDMA dosing. Females were administered a urine pregnancy test. After obtaining baseline measures, biological specimens and a 12-lead ECG, participants ingested one of three doses: 0 (placebo), 1.0 mg/kg (low) or 1.6 mg/kg (high) MDMA (Lipomed, Arlesheim, Switzerland), while seated in a quiet room maintained at approximately 21°C. Active drug was prepared as the hydrochloride salt; placebo contained only lactose. For safety purposes, there was a maximum absolute dose limit of 150 mg MDMA. One male participant whose weight exceeded 93.75 kg received this maximum dose. Participants remained on the unit for three to seven days after dosing.
Physiological monitoring
After double-blind MDMA or placebo administration, participants remained seated during monitoring by medical staff for 3 hours or until SBP, DBP and heart rate returned to within 20% of pre-dose levels (or heart rate to < 95 bpm), which ever occurred later. Physiological measurements and biological specimen collection continued throughout the stay on the unit. Heart rate, SBP, DBP, blood oxygen saturation, and respiratory rate were recorded at −0.25, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0, 2.25, 2.5, 2.75, 3.0, 3.5, 4.0, 5.0, 6.0, 7.0, 8.0, 13, 23, 29, 47, 71, 95, 119, 143 and 167 h, using a Datascope-Passport Model El (Datascope Corporation, Paramus, NJ). Tympanic temperature was measured at these same times using a ThermoScan Instant Thermometer HM2 (Braun, Lynnfield, MA). Pupil diameter was measured prior to drug administration and 1.25 and 6 h post dose using a Coolpix 3200 digital camera (Nikon Corporation, Tokyo, Japan). Participants held a ruler under their eye during the photograph to provide a scale for later caliper measurement of pupil diameter.
Subjective evaluations
Participants completed VAS for subjective response for 29 h after dosing. Six subjective effects were evaluated: high, energy level, feelings of closeness to others, mind racing, heightened senses, and ability to concentrate. Participants were seated at a computer monitor while VAS were individually presented on the screen as 265 mm lines with anchors at each end: “not at all” to “most ever” (high, mind racing, heightened senses) or “least ever”to “most ever” (feelings of closeness to others, energy level, ability to concentrate). Subjects used a joystick to move the cursor on the screen to indicate which point on the scale best described how they felt in the past five minutes. Responses were scored as distance (in mm) from the left end of the line.
Participants were asked by a research nurse on the day after dosing if they believed they received a placebo, low or high MDMA dose, and also indicated on a questionnaire the duration (in h) of their MDMA experience.
Analysis of MDMA in plasma
MDMA was quantified in human plasma according to a previously published method 21. Briefly, MDMA-d5 as internal standard was added to 1 mL participant plasma, calibrator or quality control sample. Acidic hydrolysis was performed to release conjugated metabolites for assay as part of a detailed pharmacokinetic study (data to be presented elsewhere). MDMA concentrations were unaffected by acidic hydrolysis 21. Derivatization was accomplished with 10 µL heptafluorobutyric acid anhydride and heating at 60°C for 20 min. Two-dimensional gas chromatography/electron impact mass spectrometry (2D-GC/EI-MS) operated in selected ion monitoring mode was used to quantify MDMA from 2.5 – 400 ng/mL. Method accuracy was > 80%. The greatest coefficient of variation (CV) for an intra-assay batch was 8.4%, while CV for inter-assay imprecision were ≤ 6.7%. Plasma concentration data are presented as mean ± standard error.
Statistical analysis
The primary statistical analysis evaluated the joint effects of MDMA dose and time after MDMA administration on a battery of physiological and subjective parameters. Raw data were used for analysis. For each MDMA response, repeated measures regression (SAS Proc Mixed) was used to assess the main effects of dose and time and dose-by-time interaction. If the main effect of dose was significant, post-hoc paired comparisons were performed to determine which doses differed from each other. Tukey’s test was used to constrain the overall type I error rate to < 0.05. If the main effect of time was significant, a Dunnett’s test was performed separately by dose to determine if effects at specific times differed from baseline and to constrain the overall type I error rate to < 0.05. The time frame evaluated was limited to times for which an effect was apparent in time course graphs: 0 – 4 h post-administration for physiological measures (Figure 1) and 0 – 5 h post-administration for all subjective measures except heightened senses and high (0 – 9 h) (Figure 2).
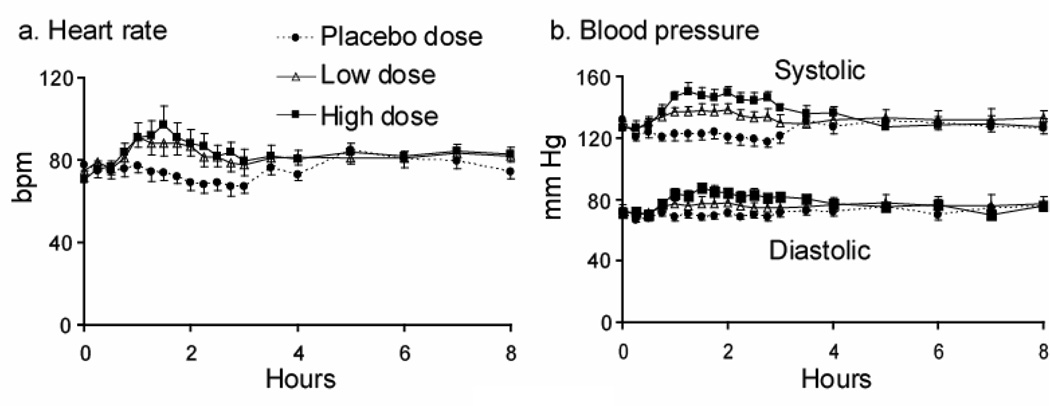
FIGURE 1.
Heart rate (panel a) and systolic and diastolic blood pressure (panel b) responses after double blind, randomized, controlled oral administration of placebo, 1.0 mg/kg (low), and 1.6 mg/kg (high) 3,4-methylenedioxymethamphetamine (MDMA) (N = 8). Data presented as mean ± standard error.
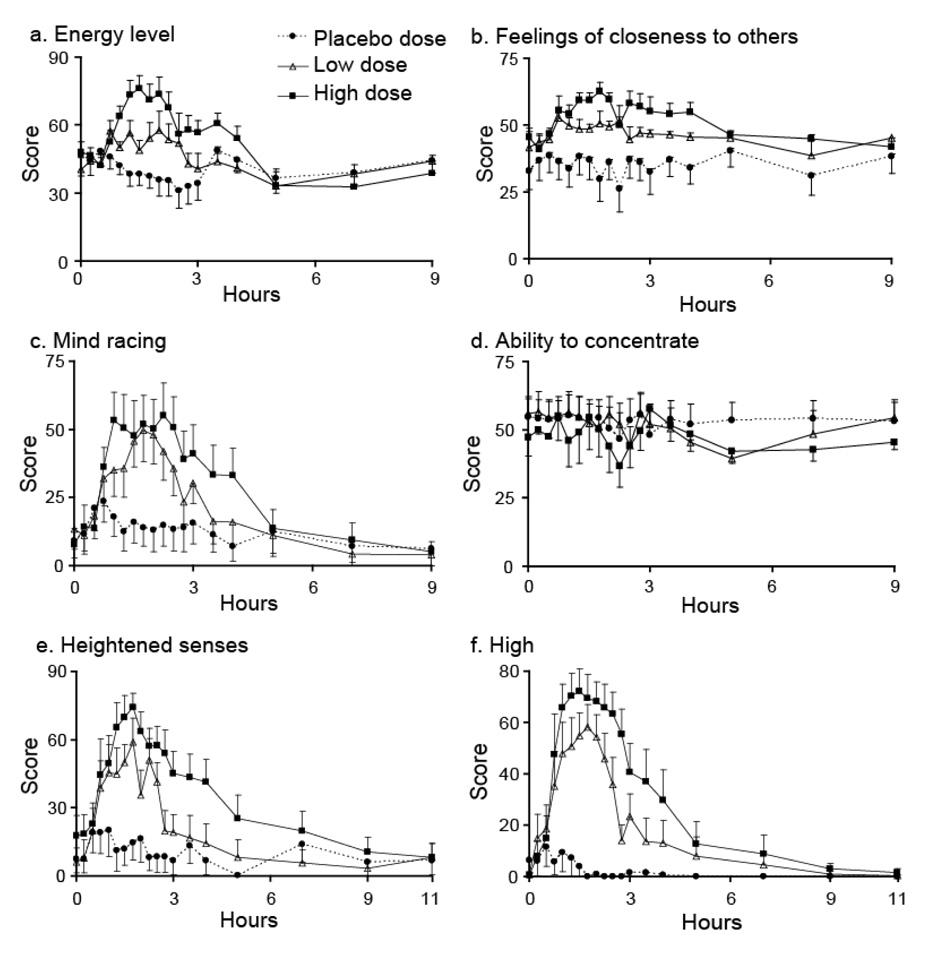
FIGURE 2.
Subjective responses after double blind, randomized controlled oral administration of placebo, 1.0 mg/kg (low) and 1.6 mg/kg (high) 3,4-methylenedioxymethamphetamine (MDMA) (N = 8). Data presented as mean ± standard error. Subjective responses measured by 265-mm visual analog scales.
A secondary analysis examined the effects of MDMA dose on measures that summarized the time course for each dose within-subject: the effect’s maximum increase from baseline (decrease for blood oxygen saturation and ability to concentrate), the time corresponding to the maximum effect (Tmax) and the area under the time-effect curve (AUC). Change scores were used to control for inter- and intra-subject variability between sessions. If the maximum response occurred at multiple time points, the first such time point was considered Tmax. The same parameters and time frame were used as for the primary analysis. Repeated-measures linear regression (SAS Proc Mixed) was used to assess the effect of dose. If this was significant, post-hoc paired comparisons were done to determine which doses differed from each other; Tukey’s test was used to constrain the overall type I error rate to < 0.05.
Pearson correlation coefficient, coefficient of determination and the equation for the line (using least-squares linear regression) assessed the degree of association between MDMA plasma concentrations (achieved by both active doses) and each physiological or subjective effect over the entire time course. Correlation with pupil diameter was evaluated at 1.25 h post-dose.
All analyses employed SAS version 9.1 (SAS Institute Inc., Cary, NC) with a two-tailed p value < 0.05 indicating statistical significance. Descriptive statistics are mean ± SE unless otherwise noted.
RESULTS
Physiological response
MDMA had a significant dose-dependent stimulatory effect on heart rate and blood pressure. Peak effects occurred 1 – 2 h after administration, with no secondary or delayed peaks (Figure 1, Tables 1 and 2). The response to high-dose MDMA often lasted significantly longer than after the low dose (Figure 1). MDMA had no significant overall effect on tympanic temperature (maximum increase of 1.9°C in a single participant after the high dose), respiratory rate, or blood oxygen saturation (Tables 1 and 2). Participants completed all dosing sessions with no clinically significant adverse events.
Only high-dose MDMA produced a maximum heart rate increase from baseline significantly greater than placebo (p = 0.0009) (Table 2). There was no significant dose effect on AUC0–4h or Tmax (Table 2). The fastest individual heart rate was 132 bpm, occurring after a high MDMA dose. Increases from baseline ranged from 11 – 58 and 15 – 59 bpm after the 1.0 and 1.6 mg/kg doses, respectively.
Both MDMA doses produced maximum increases in SBP significantly different from placebo and each other (Table 2): low > placebo (p = 0.0016), high > placebo (p = 0.0001) and high > low (p = 0.0066). Maximum increases from baseline ranged from 13 – 27 mm Hg after the low and 13 – 43 mm Hg after the high dose. The highest recorded SBP was 171 mm Hg. Mean AUC0–4h showed a similar dose response to maximum increase: low > placebo (p = 0.0002), high > placebo (p < 0.0001) and high > low (p = 0.0021). There was no significant dose effect on Tmax, which ranged from 1 – 4 h.
High dose MDMA produced significantly greater maximum increases from baseline in DBP compared to placebo (p = 0.0014) and low dose (p = 0.0127) (Table 2). Peak increases from baseline of 0 – 28 and 17 – 31 mm Hg were recorded after the low and high doses, respectively. The highest observed DBP of 102 mm Hg occurred in two subjects after the 1.6 mg/kg dose. There was a significant dose effect on AUC0–4h: low > placebo (p = 0.023), high > placebo (p < 0.0001) and high > low doses (p = 0.0053). Tmax occurred 1 – 2 h post-dose.
MDMA significantly increased pupil diameter 1.25 h post-dose (Tables 1 and 2), with diameters increased by up to 4 mm.
High-dose MDMA significantly increased the maximum change from baseline in respiratory rate (p = 0.0223) and shortened the Tmax (p = 0.0495), but had no effect on AUC0–4h (Table 2). Tmax ranged from 0.25 – 2.25. All peak respiratory rates were between 17 and 28 breaths/min.
MDMA had no significant effect on maximum decrease from baseline in blood oxygen saturation, Tmax or AUC0–4h (Table 1). All measurements were between 95 and 100%.
Subjective effects
MDMA produced significant dose-dependent subjective responses (Figure 2, Table 1) in all variables except ability to concentrate (Figure 2, Tables 1 and 2). Significant increases from baseline in subjective responses always were observed after the high dose (Table 1). After the low dose, only increases in mind racing, heightened senses, and high significantly differed from baseline. All significant increases from baseline occurred between 0.75 and 3 h after dosing (Figure 2).
Subjective responses showing the strongest effects, i.e. high and heightened senses, also had the most robust increases from baseline and AUC0–9h (Table 2). For high, maximum increase from baseline comparisons were low dose > placebo (p = 0.008), high dose > placebo (p = 0.0002) and high dose > low dose (p = 0.03); AUC0–9h comparisons were low dose > placebo (p = 0.05), high dose > placebo (p = 0.006) and high dose > low dose (p = 0.05). For heightened senses, maximum increase from baseline comparison was high dose > placebo (p = 0.002); AUC0–9h comparisons were high dose > placebo (p = 0.03) and high dose > low dose (p = 0.04).
Four of nine participants correctly guessed their dosing. Three participants identified both the 1.0 and 1.6 mg/kg MDMA doses as low doses. One participant identified both placebo and low dose as placebo and considered the high dose to be low. The ninth participant identified placebo as a low dose and both low and high doses as high doses. Subjects reported MDMA effects as lasting 0 – 1 h after placebo, 0 – 4 h after the low, and 1.5 – 7 h after the high dose. Duration of low dose experience was always ≤ duration of high dose experience, regardless of how participants identified a dose.
Correlation with plasma MDMA concentrations
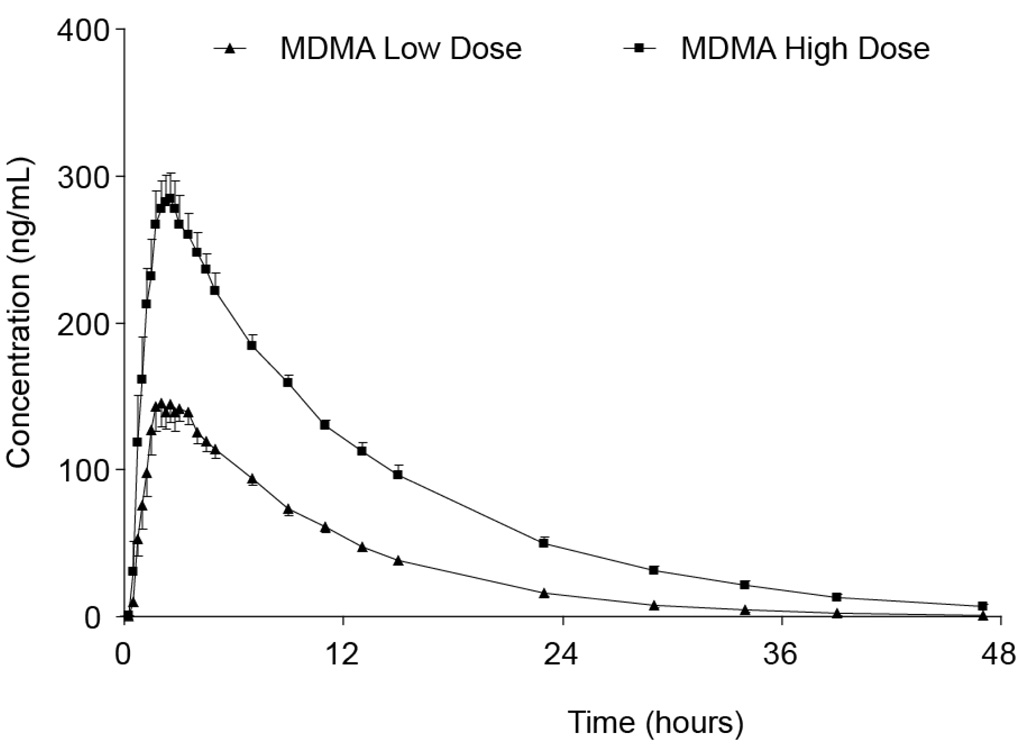
Mean MDMA plasma concentrations (n = 8) following administration of 1.0 and 1.6 mg/kg MDMA are shown in Figure 3. The time course is displayed for 47 h following dosing, the period when most participants’ plasma remained MDMA positive. Mean peak MDMA plasma concentrations of 161.4 ± 11.5 and 305.7 ± 16.9 ng/mL were achieved after the low and high doses, respectively. Mean plasma MDMA concentrations (n = 8) at 47 h were 0.6 ± 0.6 ng/mL after the low and 6.8 ± 1.7 ng/mL after the high dose; although only one participant’s plasma remained MDMA positive 47 h after the low dose, seven participants’ plasma specimens were positive at this time following the high dose. Statistically significant correlations were observed between plasma concentrations of MDMA and physiological parameters of heart rate, SBP, DBP, and temperature and subjective responses of energy level, feelings of closeness to others, mind racing, heightened senses, and high (correlation coefficients 0.23–0.50, all p < 0.0001). The highest correlations were observed between MDMA plasma concentration and high (r = 0.50), heightened senses (r = 0.48) and SBP (r = 0.45). There were no significant correlations with respiratory rate, blood oxygen concentration, pupil diameter, or ability to concentrate.
FIGURE 3.
Mean 3,4-methylenedioxymethamphetamine (MDMA) plasma concentrations (N = 8) for 47 hours following double blind, randomized, controlled oral administration of 1.0 (low) and 1.6 (high) mg/kg MDMA. Data presented as mean ± standard error.
DISCUSSION
This double blind, placebo-controlled study evaluated subjective effects for 29 h and physiological effects for 167 h after 1.0 and 1.6 mg/kg oral MDMA administration in eight healthy men and women with prior MDMA experience. Doses were chosen to reflect typical recreational doses taken in the community; all but one participant identified both doses as active MDMA.
MDMA produced dose-dependent increases in heart rate and blood pressure, similar to those previously reported 8, 22, 23. Peak effects occurred within the first 1 – 2 h after drug administration, with no delayed or secondary peaks (Figure 1). These effects are presumably due to MDMA’s activation of the sympathetic nervous system 24. No acute cardiotoxic effects were observed in this controlled laboratory setting, similar to previous reports 5, 6, 18, 25.
This finding cannot necessarily be generalized to the community setting. Participants were rigorously screened to meet stringent medical eligibility criteria. MDMA could have more pronounced effects in individuals with compromised health, genetic predisposition, or other unknown vulnerability factors. This study administered pure MDMA alone. In the community, MDMA is of unknown purity and may be taken with other substances. In this study, subjects were seated calmly in a cool room and kept well hydrated throughout the period of MDMA administration. It is likely that vigorous exercise, high ambient temperatures, and dehydration, such as often occur at raves 26, are contributory factors in any acute cardiotoxic response experienced after recreational MDMA use.
Hyperthermia has been observed in hospital admissions following recreational MDMA ingestion 24, 27–29. In the present study, MDMA had no significant effect on tympanic temperature; temperatures were always ≤ 38.2°C and individual increases never exceeded 1.9°C. In prior studies, comparable doses produced significant increases in mean oral or core body temperature of ≤ 0.6°C in the first 4 h after dosing 8, 25, 30. One study noted that temperatures never exceeded 38°C 11. Different sites of measurement or different ambient temperatures could partially explain differences between studies. A higher ambient temperature of 30°C was associated with greater temperature response to controlled MDMA administration 30. Research participants do not experience the increased motor activity experienced by many recreational users. In addition, inadequate hydration is not an issue in a laboratory setting. These findings point to the effects of high environmental temperature, decreased fluid intake, increased physical activity, and/or co-ingestion of other illicit and licit substances, often experienced by recreational users at dance clubs and raves, as possible factors in MDMA-associated hyperthermia. Another possibility is that MDMA does not directly cause hyperthermia, but re-regulates the hypothalamus, responsible for intrinsic temperature control 31, to prevent heat dissipation and maintenance of temperature when challenged by external factors.
Mydriasis was observed in the current study, with pupil diameter increases of up to 4 mm. Dilated pupils can negatively affect driving ability in daylight 32 by increasing glare sensitivity and decreasing contrast sensitivity, and at night, when, despite improved light sensitivity, headlight glare can be distracting. When these deficits are combined with MDMA’s subjective effects, including mind racing and high, it is likely that driving skills could be further impaired. Pupil size is routinely evaluated by drug recognition experts (DRE), including police officers specially trained in recognizing signs and symptoms of drugs in drivers. Our data support increased pupil size as a marker for ingestion of MDMA 33.
Ability to concentrate was the only subjective variable not affected by MDMA in this study. The literature presents mixed results on the effects of MDMA on concentration that may be due to differences in dosing environment, prior illicit drug use, or method of assessment. Our finding of no effect using VAS is consistent with other VAS results noted after 75 and 125 mg MDMA 10. However, studies using the Subjective Drug Effects Questionnaire 7, List of Complaints 6, and Vegetative Lability Scale 25 reported impaired concentration following MDMA doses of 1.5 – 1.7 mg/kg. Two studies that observed significant responses included subjects with limited or no experience with recreational drugs 6, 25, while the current and previous studies administering VAS included experienced illicit drug users 10. It is possible that, compared to past self-administered illicit drug experiences, the laboratory setting produced less of an effect on concentration.
The stimulatory effect of MDMA (increased energy level and mind racing) is consistent with previous studies administering 1.1 – 2.1 mg/kg MDMA 10, 11, 22, 23, 34–36. Although the current study was the first to explicitly measure mind racing, this parameter previously was reported by a single subject as an undesirable side effect of use 3. Subjective high also is consistent with other investigations after 0.9 – 2.1 mg/kg MDMA 7, 10, 22, 23, 34–36; a lower dose (0.5 mg/kg) did not produce a significant high 7.
The increased feeling of closeness to others observed in this study is consistent with the putative entactogenic effect of MDMA. In contrast, a previous investigation found no significant change in this variable (also assessed with VAS) after 0.5 and 1.5 mg/kg, although there was a trend toward significance after the higher dose 7. Participants’ comfort in the dosing environment and familiarity with research staff could contribute to this difference.
The predominantly positive subjective effects of MDMA observed in this study are consistent with its appeal to recreational users. Prolonged effects of high, heightened senses, and feelings of closeness to others presumably contribute to MDMA’s abuse potential. Motivation for treatment may be hindered by the relatively few immediately undesirable sequelae 25.
MDMA’s psychoactive effects had a longer duration than its physiological effects. This is consistent with the longer elimination half-life of R-MDMA 17, thought to be responsible for its subjective effects, as compared to S-MDMA, which reportedly is more amphetamine-like 37 and, therefore, likely the cause of the physiological effects.
Dose also influenced duration of effects. Some physiological (Figure 1) and subjective (Figure 2) parameters returned to pre-dose levels earlier after the low dose than after the high (Table 1). Further support for the influence of dose comes from participants’ responses regarding the duration of their MDMA experience. More than 75% of participants reported that effects lasted longer after the high dose, even in situations where they reported both the 1.0 and 1.6 mg/kg doses as high doses.
Although MDMA plasma concentrations were significantly correlated with heart rate, SBP, DBP, temperature, energy level, feelings of closeness to others, mind racing, heightened senses, and high, correlation coefficients were low and clinically insignificant, eliminating the ability to predict effects from single plasma concentrations.
This study has several strengths compared to previously published human experimental studies of MDMA administration. First, our protocol included African-American and female participants, groups not well represented in prior studies. Although the sample sizes were not large enough to allow subgroup analyses, their inclusion does improve the external validity (generalizability) of the study. Second, all participants were abstinent from MDMA and other psychoactive substances for at least 12 hours prior to dosing, ensuring that there were no residual drug effects during study sessions. Many prior studies either did not report abstinence status of subjects or relied on self-report. Third, all subjects were required to have a positive biological test documenting MDMA use prior to inclusion. Fourth, data collection continued for 29 h (subjective effects) and 167 h (physiological effects) after dosing, allowing evaluation for possible delayed or secondary effects. Finally, dosing sessions occurred with subjects in a calm, quiet environment with controlled ambient temperature and limits on motor activity, permitting determination of MDMA’s influence alone on sympathomimetic and hyperthermic effects. Although previous studies have included some of these design features, this study incorporated all of these features to provide maximum scientific rigor.
In conclusion, this placebo-controlled, double-blind human laboratory study found that oral MDMA, when administered in typical recreational doses (1.0 and 1.6 mg/kg) to experienced MDMA users free of acute MDMA or other drug effects (due to ≥ 12 h abstinence), produced dose-dependent increases in heart rate and systolic and diastolic blood pressure and subjective responses of high, heightened senses, and mind racing. All effects resolved within 4–6 hours, with no delayed or secondary peaks. There were no significant effects on tympanic temperature, respiratory rate, or blood oxygenation. These findings suggest that oral MDMA produces short-term effects on cardiovascular function and psychological state, but that its temperature effects may depend on environmental and subject factors such as ambient temperature and levels of hydration and motor activity. This rigorous characterization of the physiological and subjective responses to MDMA provides data on possible mechanisms involved in its toxicity and reasons for abuse, and may contribute to drug abuse prevention and treatment efforts.
ACKNOWLEDGEMENTS
The authors thank Dr. Jennifer Schroeder for her statistical advice and Kathleen Demuth, Susan Baskin, Cecile Shindell, Janeen Nichels, and John Etter for assistance in experimental sessions.
This research was supported by the National Institute on Drug Abuse Intramural Research Program, National Institutes of Health.
REFERENCES
- 1.de la Torre R, Farre M, Roset PN, et al. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26:137–144. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Ropero-Miller JD, Goldberger BA. Recreational drugs: current trends in the 90s. Toxicology. 1998;18:727–746. [PubMed] [Google Scholar]
- 3.Greer G, Tolbert R. Subjective reports of the effects of MDMA in a clinical setting. Journal of Psychoactive Drugs. 1986;18:319–327. doi: 10.1080/02791072.1986.10472364. [DOI] [PubMed] [Google Scholar]
- 4.Hall AP, Henry JA. Acute toxic effects of 'Ecstasy' (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth. 2006;96:678–685. doi: 10.1093/bja/ael078. [DOI] [PubMed] [Google Scholar]
- 5.Lester SJ, Baggott M, Welm S, et al. Cardiovascular effects of 3,4-methylenedioxymethamphetamine. A double-blind, placebo-controlled trial. Ann Intern Med. 2000;133:969–973. doi: 10.7326/0003-4819-133-12-200012190-00012. [DOI] [PubMed] [Google Scholar]
- 6.Vollenweider FX, Gamma A, Liechti M, et al. Psychological and cardiovascular effects and short-term sequelae of MDMA ("Ecstasy") in MDMA-naive healthy volunteers. Neuropsychopharmacol. 1998;19:241–251. doi: 10.1016/S0893-133X(98)00013-X. [DOI] [PubMed] [Google Scholar]
- 7.Harris DS, Baggott M, Mendelson JH, et al. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2002;162:396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- 8.de la Torre R, Farre M, Roset PN, et al. Pharmacology of MDMA in humans. Ann NY Acad Sci. 2000;914:225–237. doi: 10.1111/j.1749-6632.2000.tb05199.x. [DOI] [PubMed] [Google Scholar]
- 9.Downing J. The psychological and physiological effects of MDMA on normal volunteers. J Psychoactive Drugs. 1986;18:335–340. doi: 10.1080/02791072.1986.10472366. [DOI] [PubMed] [Google Scholar]
- 10.Cami J, Farre M, Mas M, et al. Human pharmacology of 3,4-methylenedioxymethamphetamine ("Ecstasy"): psychomotor performance and subjective effects. J Clin Psychopharmacol. 2000;20(4):455–466. doi: 10.1097/00004714-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Liechti ME, Gamma A, Vollenweider FX. Gender differences in the subjective effects of MDMA. Psychopharmacology. 2001;154:161–168. doi: 10.1007/s002130000648. [DOI] [PubMed] [Google Scholar]
- 12.Siegel RK. MDMA. Nonmedical use and intoxication. J Psychoactive Drugs. 1986;18:349–354. doi: 10.1080/02791072.1986.10472368. [DOI] [PubMed] [Google Scholar]
- 13.Kraemer T, Maurer HH. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24:277–289. doi: 10.1097/00007691-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Segura M, Ortuno J, Farre M, et al. 3,4-Dihydroxymethamphetamine (HHMA). A major in vivo 3,4-methylenedioxymethamphetamine (MDMA) metabolite in humans. Chem Res Toxicol. 2001;14:1203–1208. doi: 10.1021/tx010051p. [DOI] [PubMed] [Google Scholar]
- 15.de la Torre R, Farre M, Ortuno J, et al. Non-linear pharmacokinetics of MDMA ('ecstasy') in humans. J Clin Pharmacol. 2000;49:104–109. doi: 10.1046/j.1365-2125.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortuno J, Pizarro N, Farre M, et al. Quantification of 3,4-methylenedioxymetamphetamine and its metabolites in plasma and urine by gas chromatography with nitrogen-phosphorus detection. J Chromatogr B Biomed Sci Appl. 1999;723:221–232. doi: 10.1016/s0378-4347(98)00506-4. [DOI] [PubMed] [Google Scholar]
- 17.Fallon JK, Kicman AT, Henry JA, et al. Stereospecific analysis and enantiomeric disposition of 3,4-methylenedioxymethamphetamine (ecstasy) in humans. Clin Chem. 1999;45:1058–1069. [PubMed] [Google Scholar]
- 18.Mas M, Farre M, de la Torre R, et al. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3,4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther. 1999;290:136–145. [PubMed] [Google Scholar]
- 19.Navarro M, Pichini S, Farre M, et al. Usefulness of saliva for measurement of 3,4-methylenedioxymehamphetamine and its metabolites: correlation with plasma drug concentrations and effect of salivary pH. Clin Chem. 2001;47:1788–1795. [PubMed] [Google Scholar]
- 20.Maurer HH, Bickeboeller-Friedrich J, Kraemer T, et al. Toxicokinetics and analytical toxicology of amphetamine-derived designer drugs ('Ecstasy') Toxicol Lett. 2000;112–113:133–142. doi: 10.1016/s0378-4274(99)00207-6. [DOI] [PubMed] [Google Scholar]
- 21.Kolbrich EA, Lowe RH, Huestis MA. Two-dimensional gas chromatography/electron impact-mass spectrometry with cryofocusing for the sensitive, specific and simultaneous quantification of 3,4-methylenedioxymethamphetamine (MDMA, 3,4-methylenedioxyamphetamine (MDA), 4-hydroxy-3-methoxymethamphetamine (HMMA), 4-hydroxy-3-methoxyamphetamine (HMA), and 3,4-methylenedioxyethylamphetamine (MDEA) in human plasma. Clin Chem. 2008;54:379–387. doi: 10.1373/clinchem.2007.096800. [DOI] [PubMed] [Google Scholar]
- 22.Farre M, Abanades S, Roset PN, et al. Pharmacological interaction between 3,4-methylenedioxymethamphetamine (ecstasy) and paroxetine: pharmacological effects and pharmacokinetics. J Pharmacol Exp Ther. 2007;323:954–962. doi: 10.1124/jpet.107.129056. [DOI] [PubMed] [Google Scholar]
- 23.Farre M, de la Torre R, Mathuna BO, et al. Repeated doses administration of MDMA in humans: pharmacological effects and pharmacokinetics. Psychopharmacology (Berlin) 2004;173:364–375. doi: 10.1007/s00213-004-1789-7. [DOI] [PubMed] [Google Scholar]
- 24.Patel MM, Belson MG, Wright D, et al. Methylenedioxymethamphetamine (ecstasy)-related myocardial hypertrophy: an autopsy study. Resuscitation. 2005;66:197–202. doi: 10.1016/j.resuscitation.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Liechti ME, Vollenweider FX. The serotonin uptake inhibitor citalopram reduces acute cardiovascular and vegetative effects of 3,4-methylenedioxymethamphetamine ("Ecstasy") in healthy volunteers. J Psychopharmacol. 2000;14:269–274. doi: 10.1177/026988110001400313. [DOI] [PubMed] [Google Scholar]
- 26.Parrott AC. MDMA in humans: factors which affect the neuropsychobiological profiles of recreational ecstasy users, the integrative role of bioenergetic stress. J Psychopharmacol. 2006;20:147–163. doi: 10.1177/0269881106063268. [DOI] [PubMed] [Google Scholar]
- 27.Bordo DJ, Dorfman MA. Ecstasy overdose: rapid cooling leads to successful outcome. Am J Emerg Med. 2004;22:326–327. doi: 10.1016/j.ajem.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Gill JR, Hayes JA, deSouza IS, et al. Ecstasy (MDMA) deaths in new york city: a case series and review of the literature. J Forensic Sci. 2002;47:121–126. [PubMed] [Google Scholar]
- 29.Henry JA, Jeffreys KJ, Dawling S. Toxicity and deaths from 3,4-methylenedioxymethamphetamine ("ecstasy") Lancet. 1992;340:384–387. doi: 10.1016/0140-6736(92)91469-o. [DOI] [PubMed] [Google Scholar]
- 30.Freedman RR, Johanson CE, Tancer ME. Thermoregulatory effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2005;183:248–256. doi: 10.1007/s00213-005-0149-6. [DOI] [PubMed] [Google Scholar]
- 31.Boulant JA. Neuronal basis of Hammel's model for set-point thermoregulation. J Appl Physiol. 2006;100:1347–1354. doi: 10.1152/japplphysiol.01064.2005. [DOI] [PubMed] [Google Scholar]
- 32.Wood JM, Garth D, Grounds G, et al. Pupil dilatation does affect some aspects of daytime driving performance. Br J Ophthalmol. 2003;87:1387–1390. doi: 10.1136/bjo.87.11.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunsman GW. Human performance toxicology. In: Levine B, editor. Principles of Forensic Toxicology. USA: American Association for Clinical Chemistry, Inc.; 1999. pp. 13–30. [Google Scholar]
- 34.Hernandez-Lopez C, Farre M, Roset PN, et al. 3,4-methylenedioxymethamphetamine (Ecstasy) and alcohol interactions in humans: psychomotor performance, subjective effects, and pharmacokinetics. J Pharmacol Exp Ther. 2002;300:236–244. doi: 10.1124/jpet.300.1.236. [DOI] [PubMed] [Google Scholar]
- 35.Tancer ME, Johanson CE. The subjective effects of MDMA and mCPP in moderate MDMA users. Drug Alcohol Depend. 2001;65:97–101. doi: 10.1016/s0376-8716(01)00146-6. [DOI] [PubMed] [Google Scholar]
- 36.Tancer M, Johanson CE. The effects of fluoxetine on the subjective and physiological effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2007;189:565–573. doi: 10.1007/s00213-006-0576-z. [DOI] [PubMed] [Google Scholar]
- 37.Moore KA, Mozayani A, Fierro MF, et al. Distribution of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) stereoisomers in a fatal poisoning. Forensic Sci Int. 1996;83:111–119. doi: 10.1016/s0379-0738(96)02025-7. [DOI] [PubMed] [Google Scholar]