Abstract
Alum is the only adjuvant approved for routine use in humans, although the basis for its adjuvanticity remains poorly understood. We have recently shown that Alum activates caspase-1 and induces secretion of mature IL-1β and IL-18. Here we show that in human and mice macrophages, alum-induced IL-1β, IL-18, and IL-33 secretion is mediated by the NLR protein NLRP3 and its adaptor ASC, but not by NLRC4. Other particulate adjuvants, such as QuilA and chitosan, induce inflammasome activation in a NLRP3-dependent fashion, suggesting that activation of the NLRP3-inflammasome may be a common mechanism of action of particulate adjuvants. Importantly, we demonstrate that antigen-specific antibody production elicited by vaccines that contain alum is significantly impaired in NLRP3-deficient mice. Our results demonstrate for the first time a role for the NLRP3-inflammasome during development of the immune response elicited by alum-enhanced vaccination. and suggest that therapeutic intervention aimed at NLRP3 may improve adjuvant efficacy.
Keywords: Monocytes/macrophages, Cytokines, Inflammation, Vaccination
INTRODUCTION
Aluminum hydroxide and aluminum phosphate adjuvants (herein referred to as Alum) have been used for decades and are the only adjuvants licensed for routine human vaccination. Alum has proven its efficacy in several vaccine formulations (reviewed in ref. 1), although its mode of action remains unclear. Alum is unable to act as a TLR agonist and does not promote DC maturation (2), hence whether its activity is mediated by Pattern Recognition Receptors (PRR) is unknown. It is generally accepted that adsorption of the antigen on the alum particle forms an antigen depot at the injection site. This is believed to maximize the interaction time between the antigen and the APC and would ensure that antigen and adjuvant are delivered to the same population of APC. This notion however has been challenged by several reports. It is likely that multiple mechanisms (reviewed in ref. 3) are responsible for the adjuvant effect of alum and of other particulate adjuvants, such as chitosan and QuilA/saponin. Alum has been shown to fix complement (4), to cause granulomas containing antibodies-producing plasma cells and neutrophils (5), and to induce the appearance in the spleen of Gr1+, IL-4-secreting eosinophils that prime B cells (6, 7). How alum achieves these effects and their role in alum adjuvanticity remains unknown.
We have recently shown that alum is capable of activating caspase-1 and inducing release of IL-1β and IL-18 (8) in a MyD88-independent fashion, thus suggesting a possible mechanism for alum adjuvant effect. IL-1β and IL-18 are prototypical proinflammatory cytokines with pleiotropic functions (reviewed in ref. 9), including adjuvant capacity (10, 11). Their biosynthesis is regulated at several levels with two key steps playing a prominent role. One is the transcription and translation of their mRNA into the immature proteins pro-IL-1β and pro-IL-18. This step is regulated by classical proinflammatory stimuli, most notably TLR agonists. The second step is required for their secretion and involves the proteolytic processing of pro-IL-1β and pro-IL-18, which is carried out by activated caspase-1. Caspase-1 activity is also required for secretion of IL-33, a recently identified member of the IL-1 family (12). Caspase-1 activation is not triggered by TLR signaling and occurs in the context of a multiprotein complex termed inflammasome. Key components of the inflammasome are cytoplasmic sensors of pathogen-associated molecules that belong to a subgroup of the NLR (nucleotide-binding domain leucine-rich repeat-containing) family (reviewed in ref.13). Two of the best characterized NLR molecules are NLRP3 (also known as Cryopyrin, NALP3, CIAS1, Pypaf1) and NLRC4 (also known as IPAF, CARD12). Both molecules have been shown to mediate caspase-1 activation in response to a growing number of intracellular bacteria, toxins, and danger signals. Inflammasome activation by both proteins depends on the adaptor molecule ASC (14, 15)-though the details of the interaction between NLRC4 and ASC are not clear. Our discovery that alum activates caspase-1 and triggers IL-1β and IL-18 secretion suggests that the activity of alum may be mediated by NLR molecules. Here we show that inflammasome activation by alum and alum’s adjuvanticity are mediated by NLRP3 and ASC but not NLRC4.
MATERIALS AND METHODS
Mice
NLRP3−/− mice were provided by Millenium Pharmaceuticals and backcrossed for nine generations to C57BL/6 mice. ASC−/−, and NLRC4−/− mice have been backcrossed for over nine generations to C57BL/6 mice and were kindly provided by Dr. Vishva Dixit, Genentech. Age and sex-matched C57BL/6 were purchased from the National Cancer Institute. All experiments using mice were approved by the University of Tennessee Health Science Center and the University of North Carolina Institutional Animal Care and Use Committee.
Reagents
Alhydrogel is an aluminum hydroxide gel (Sigma, St. Louis, MO). Imject Alum and Complete Freund Adjuvant (CFA) were from Pierce (Rockfor, IL). Tetanus and Diphtheria toxoids adsorbed for pediatric use was from Sanofi-Pasteur. Endotoxin-free chicken ovalbumin was from Profos (Regensburg, Germany). Chitosan was from Sigma. QuilA was from Brennetag (Fredericksund, Denmark). Caspase-1 inhibitor Z-YVAD-FMK was from Alexis Biochemicals (San Diego, CA) and was used at 10 µM. The following antibodies were used: rabbit anti-caspase-1 (Upstate Biotechnologies, Lake Placid, NY), goat anti-mIL-1β (R&D Systems), anti-hIL-1β 3ZD mAb (NCI BRB Preclinical repository), rabbit anti-IL-33 (Alexis Biochemicals, San Diego, CA).
PBMC and bone marrow-derived mononuclear cells (BMM) isolation
Human PBMC were isolated from Leukopacks by Ficoll-Hystopaque density gradient centrifugation. Mouse mononuclear cells were generated by incubating bone marrow in RPMI 1640-10% FCS supplemented with rmGM-CSF (20ng/ml) ( R&D Systems, Minneapolis, MN) for 8 days. This procedure routinely results in 60–80% CD11c+ cells.
Lentiviral shRNA constructs
Expression of ASC and NLRP3 was silenced using a derivative of the lentiviral vector pLL3.7 (Addgene, Cambridge, MA) where EGFP was replaced with the puromycin resistance gene. The following shRNA target sequences were selected: mASC-45, GTCAGGGGATGAACTCAAA; hASC-327, GCCAGGCCTGCACTTTATA; hNLRP3-52, GTGGACTTGAAGAAATTTA. To construct the shRNA-resistant form of human ASC, the following silent mutations (in bold, compared to the shRNA target sequence shown above) were introduced: GCCGGGTTTACATTTCATA. The resulting mutated ASC was cloned into the retroviral construct pLXIZ (a derivative of pLXIN from Clontech, where the Neomycin resistant marker is substituted with the Zeocin marker). The THP-1 macrophage cell line was infected with pLL3.7 lentiviral stocks and selected with puromycin. The efficiency of silencing was confirmed by RNase protection assay and immunoblotting. To rescue ASC expression, the shASC-THP-1 cell line was infected with the pLXIZ-mutASC stocks and selected with zeocin.
Cytokines measurements
Cytokines levels in conditioned supernatants were measured by ELISA using the following paired antibodies kits: hIL-1β and hIL-18(R&D Systems), mIL-1β, and mIL-6 (eBioscience), mIL-18 (MBL Nagoya, Japan).
Mice Vaccination
Mice were vaccinated intraperitoneally with 50 µg ovalbumin adsorbed to alum (50 µl) or CFA (100 µl), or one tenth of human dose of the DT/TT vaccine (50 µl, 0.67 Lf DT, 0.5 Lf TT) in a total volume of 200 µl saline. Boost injections had the same composition (except that Incomplete Freund adjuvant was used in place of CFA) and were administered i.p. two weeks after the first immunization. On day 14, 28, and 56 blood samples were collected by retroorbital puncture.
Determination of antibody titers
Ovalbumin-or DT-specific immunoglobulin levels in sera were measured by ELISA. Serial dilutions of sera were plated in 96 wells plates coated with ovalbumin (10 µg/ml) or diphtheria toxoid (2.5 Lf/ml). HRP-conjugated goat anti-mouse IgG1 (Southern Biotech Associates, Birmingham, AL) was added followed by TMB substrate. Antigen-specific serum IgG1 titers were expressed as the average±standard error of the mean (S.E.M.) of the reciprocal of the dilution at which absorbance was 0.15 units higher than the strongest negative control reading (preimmune sera). Total IgE levels were measured using a commercial ELISA kit (Bethyl Laboratories, Montgomery, TX).
Statistical analysis
All data were expressed as mean ±S.E.M. Student’s t test was used for statistical evaluation of the results. Significance was set at p<0.05.
RESULTS AND DISCUSSION
Alum activates the NLRP3-Inflammasome
In order to determine the mechanism through which alum induces caspase-1 activation and secretion of mature IL-1β, bone marrow derived mononuclear cells (BMM) from wild type mice or mice deficient in the NLR molecules NLRP3 and NLRC4 or the adaptor molecule ASC were stimulated with LPS in presence or absence of alum. Cells were also infected with L monocytogenes or S. typhimurium, which activate the inflammasome through NLRP3 and NLRC4, respectively (15–18). In agreement with our previous results (8), cells stimulated with LPS alone or alum alone secreted negligible amounts of IL-1β(Fig. 1A) while stimulation of wild type cells with LPS in presence of alum, or infection with L. monocytogenes or S. typhimurium, resulted in secretion of large amount of mature IL-1β. In contrast, secretion of IL-1β in response to LPS plus alum was abolished in NLRP3−/− and ASC−/− cells but not in NLRC4−/− cells. IL-1β secretion was induced to the same extent in wild type and Nod2−/− BMM stimulated with LPS plus alum (data not shown). IL-6, whose production does not depend on caspase-1 activation, was secreted at comparable level in cells stimulated with LPS alone or LPS plus alum and by wild type, NLRP3−/−, or NLRC4−/− cells (Fig. 1A), confirming that the observed defects are not due to potentiation of LPS signaling by alum or to generalized hyporesponsiveness of NLRP3−/− cells, and are specific for inflammasome-dependent cytokines. Interestingly, IL-6 secretion was compromised in ASC-deficient cells, a phenomenon we previously observed in a human macrophage cell line with knocked-down ASC expression (19), suggesting that ASC may be involved in signaling pathways other than inflammasome activation. Immunoblotting experiments (Fig. 1B) confirmed that the mature form of IL-1β could be detected in the supernatant of wild type and NLRC4−/− cells treated with LPS plus alum but not in supernatants of NLRP3−/− or ASC−/− cells. Importantly, LPS-stimulated BMM of all four genotypes expressed pro-IL-β at comparable level (figure 1B, middle panel), indicating that the absence of secreted mature IL-1β is not due to hyporesponsiveness of selected BMM but is specific for the inflammasome-dependent maturation step. Caspase-1 activation depends on an autocatalytic processing step of the 45 kDa caspase-1 that generates the p20 and p10 active subunits. As shown in Fig. 1B (lower panel), the p20 subunit of caspase-1 could be detected in the supernatant of wild type or NLRC4−/− cells treated with alum but not in the supernatant of NLRP3−/− or ASC−/− cells. Importantly, while IL-1β secretion was dependent on a concomitant LPS plus alum stimulation, caspase-1 activation was detected in response to alum alone, as we previously showed (8). Thus, NLRP3-mediated caspase-1 activation in response to alum does not require the presence of LPS while pro-IL1β expression is dependent on LPS. These results demonstrate that caspase-1 activation and processing and secretion of IL-1β in response to alum is mediated by the NLRP3-inflammasome and not the NLRC4-inflammasome.
Figure 1. Alum activates the NLRP3-Inflammasome.
Wild type, ASC−/−, NLRP3−/−, or NLRC4−/− BMM were stimulated for 9 hours with LPS (5 ng/ml) in presence or absence of aluminum hydroxide (AlHy, 130 µg/ml), or for 4 hours with L. monocytogenes or S. typhymurium (L.m., S.t.,, MOI=10). (A) IL-1β or IL-6 were measured in conditioned supernatants.(B) The presence of the immature and mature forms of IL-1β and caspase-1 in cell culture supernatants (Sup) or cell lysates (Lys) were analyzed by immunoblot. One experiment representative of five (WT and NLRP3−/−) or two (ASC−/−, NLRC4−/−) is shown. Values are mean ± SEM. Asterisks denote significant difference versus WT. p<0.001
ASC- and NLRP3-knockdown shRNA prevents inflammasome activation by alum in THP-1 macrophage cell lines
The involvement of NLRP3 and ASC in the inflammasome activation by alum was confirmed in the human macrophage cell line THP-1 through the use of shRNA. THP-1 cell lines with silenced or severely diminished ASC and NLRP3 expression were generated (Fig. 2A). A THP-1 cell line that expresses a shRNA specific for mouse ASC sequence not present in the human genome was used as control reference. As shown in Fig. 2B, IL-1 β secretion in response to alum (or ATP,L. monocytogenes, S typhimurium) was abolished in the ASC-shRNA cell line but not the control shRNA cell line. Importantly, expression of a shRNA-resistant form of ASC in the ASC-shRNA cell line rescued the IL-1β secretion in response to Alum (or the other agonists). Alum-induced IL-1β secretion was also drastically decreased in a THP-1 cell line in which NLRP3 expression was knocked-down through shRNA, confirming NLRP3 involvement in alum effect. Secretion of IL-1 β in response to L. monocytogenes or ATP was also abolished in this cell line while the response to S. typhimurium was minimally affected, showing the NLPRP3-specificity of the shRNA. The inhibition of IL-1β secretion in the knocked-down THP-1 cell lines occurred at the level of inflammasome activation and was not due to reduced responsiveness of the different cell lines to LPS as demonstrated by the fact that similar levels of pro-IL-1β were detected in the cytoplasmic extracts of all cell lines stimulated with LPS plus alum (data not shown). Remarkably, secretion of mature IL-33 was also triggered by alum treatment in THP-1 cells and depended on ASC and NLRP3 (fig 2C) Thus, inflammasome activation by alum is mediated by NLRP3 in both mouse and human cells.
Figure 2. ASC- and NLRP3-knockdown shRNA prevents inflammasome activation by alum in THP-1 cell lines.
(A) Expression of ASC or NLRP3 was measured in different THP-1 cell lines by RNase protection assay. NLRP3-a and NLRP3-b are two probes of different sizes both detecting NLRP3. (B) Control-, ASC-, and NLRP3-knockdown THP-1 cell lines were stimulated with LPS (10 ng/ml) in presence of 5 mM ATP, aluminum hydroxide (AlHy, 130 µg/ml), L. monocytogenes, or S typhymurium for 10 hours. IL-1β was measured in conditioned supernatants. One experiment representative of five is shown. Values are mean ± SEM. Asterisks denotes significant difference versus Control-shRNA cell line. p<0.001 (C) THP-1 knockdown cell lines were stimulated as indicated and IL-33 expression was analyzed by immunoblot in cell supernatants.
Inflammasome activation by particulate adjuvants is mediated by NLRP3
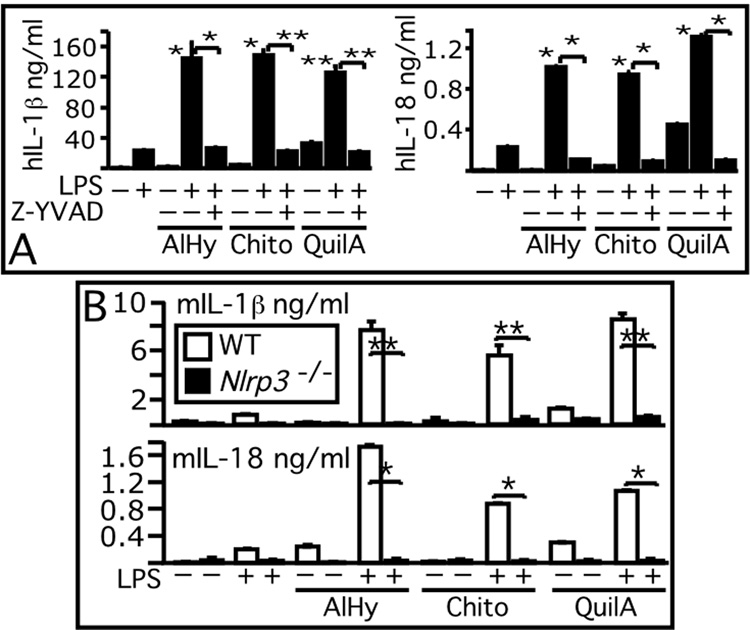
We next tested whether inflammasome activation is triggered by other particulate adjuvants (reviewed in ref. 20). QuilA, a saponin extracted from the bark of the Quillaja saponaria tree, is an adjuvant that is incorporated into liposome particles to form the immunostimulating complex “ISCOM”. Chitosan, a biodegradable polysaccharide derived from chitin, is another particulate material that is known to act as adjuvant The mechanism responsible for QuilA and chitosan adjuvant effect is unknown. As shown in Fig. 3A, QuilA and chitosan particles were able to induce IL-1β and IL-18 secretion in human PBMC. Secretion of both cytokines in response to Alum, QuilA, or chitosan reflected caspase-1 activation since it was inhibited by the caspase-1 inhibitor Z-YVAD. In mouse BMM, inflammasome activation by QuilA or chitosan appeared to occur with modalities similar to alum’s, since IL-1β and IL-18 secretion in response to both particles was abolished in NLRP3−/− cells (Fig. 3B). This result suggests that NLRP3-mediated inflammasome activation and IL-1 β and IL-18 secretion may be a common mechanism of action of particulate adjuvants. Interestingly, particulate adjuvants stimulated the release of low amount of IL-18 independently of LPS, suggesting that IL-18, which is constitutively express by several cell types and which can stimulate IL-1β transcription, may initiate a proinflammatory cascade during alum vaccination. In agreement with this notion, the level of IL-18 present in the peritoneal lavage (400 µl) of mice injected with alum (n=5) for 24 hours was significantly higher (p<0.05) in wild type mice (168±81 pg/ml)) than NLRP3−/− mice (83±36 pg/ml). IL-1β was not detected. It was recently demonstrated (21) that in vivo some of alum’s effects are mediated by uric acid, a NLRP3 activator (22). In our in vitro experiments, secretion of IL-1β by mouse or human cells stimulated with alum, QuilA, or chitosan was not affected by uricase treatment (data not shown), suggesting that in vitro NLRP3-inflammasome activation by particulate adjuvants is not mediated by uric acid. Interestingly, uric acid induces in vitro DC maturation while alum does not (23), although both activate NLRP3. However, in vivo alum does induce DC maturation (21) suggesting that uric acid may mediate this effect by stimulating additional pathways. An additional difference between uric acid and alum is that the former promotes Th1 responses (23) while the latter does not.
Figure 3. Inflammasome activation by particulate adjuvants is mediated by NLRP3.
Human PBMC (A) or wild type and NLRP3−/− BMM (B) were stimulated for 9 hours with LPS (5 ng/ml) in presence or absence of aluminum hydroxide (AlHy, 130 µg/ml), QuilA (5 µg/ml), or chitosan (200 µg/ml). IL-1β or IL-18 were measured in conditioned supernatants. Z,Z-YVAD caspase-1 inhibitor. One experiment representative of two is shown. Values are mean ± SEM. Asterisks denote significant difference versus treatment with LPS alone or treatment in presence of Z-YVAD, as indicated *, p<0.001. **,p<0.005
Alum adjuvant effect is mediated by NLRP3
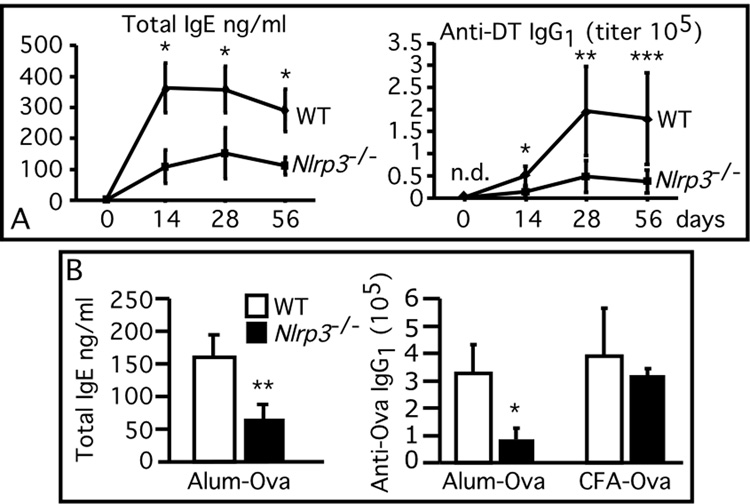
We next tested whether the adjuvanticity of alum depends on NLRP3. Wild type and NLRP3 deficient mice were vaccinated using a pediatric diphtheria/tetanus (DT/TT) toxoid vaccine that contains alum as adjuvant. Mice were also vaccinated with chicken ovalbumin mixed to Complete Freund adjuvant (CFA) or adsorbed to alum. As shown in Fig 4A, wild type mice vaccinated with the DT/TT vaccine developed a potent antibody response characterized by high IgE and DT-specific IgG1 titers (the characteristic immunoglobulin profile elicited by alum). In contrast, the titer of total IgE and DT-specific IgG1 was significantly reduced in NLRP3-deficient mice A similar reduction in total IgE and Ova-specific IgG1 titers was also observed between the wild type and NLRP3-deficient mice vaccinated with ovalbumin-alum (Fig. 4B). On the other hand, ova-specific IgG1 titers were not significantly different among wild type and NLRP3-deficient mice vaccinated with ovalbumin-CFA. As expected, since alum is known to bias the response toward Th2, the ovalbumin- or DT-specific IgG2a titers were modest and were not different between the wild type and NLRP3-deficient mice groups (not shown).
Figure 4. Alum adjuvant effect is mediated by NLRP3.
Wild type (n=5) and NLRP3-deficient mice (n=5) were vaccinated (A) with alum-adsorbed diphtheria toxoid (DT), or (B) with ovalbumin adsorbed to alum or Complete Freund adjuvant (CFA). Boost injections were administered at day 14 Total IgE or DT- and ovalbumin-specific IgG1 were measured in sera obtained at the indicated time points (A) or at 14 days (B). Values are mean ± SEM *, p<0.005. **, p<0.01. ***, p<0.05. N.D., not detectable.
In conclusion, our results demonstrate that the ability of alum and other particulate adjuvants to induce caspase-1 activation and trigger IL-1β, IL-18, and IL-33 secretion is mediated by the NLRP3 inflammasome. More importantly, we show that alum’s adjuvanticity is in good part dependent on NLRP3. The fact that the antibody response of NLRP3-deficient mice is not completely compromised suggests that other pathways activated by alum may contribute to its adjuvant effect. Although our results did not reveal a role for the NLRP3-inflammasome in CFA adjuvant effect, this possibility could not be ruled out. The powerful stimulation of TLR-mediated signaling by the mycobacterial products found in CFA may in fact be sufficient to elicit the immune response in absence of NLRP3. The analysis of CFA adjuvanticity in mice deficient in both TLR-signaling and inflammasome activation may shed light on the contribution of each pathway to the immune response during vaccination and may provide an explanation to the contrasting results that have been reported regarding the role of TLR signaling in CFA adjuvant effect (24,25). One important implication suggested by our results is that cytokines belonging to the IL-1 family may play an important role during alum-assisted vaccination. IL-1β is known to possess adjuvant capacity (10, 11) and to preferentially activate Th2 cells and enhance antibody production (26). IL-33 is particularly active on Th2 cells and mast cells and strongly promotes production of Th2-associated cytokines IL-4, IL-5, IL-13 (12), a pattern consistent with the type of immune response elicited by alum. Studies in mice have indicated a role, modest but not negligible, for IL-1β or IL-18 in alum assisted vaccination (27, 28). Since the IL-1-family cytokines share common signaling pathways and overlapping activities, more conclusive results may be obtained by the analysis of compounded ko mice. Our results identified for the first time NLRP3 as an important player in alum’s adjuvant effect and indicate an important role for the inflammasome in development of adaptive immunity. The ability to activate the inflammasome must be one of the properties to be considered during the development of new generation vaccines (3).
ACKNOLEDGMENTS
We are grateful to Vishva Dixit, Genentech, for ASC−/−, and NLRC4−/− mice, to Millenium Pharmaceutical for NLRP3−/− mice, to Peter Murray, St.Jude Children Hospital, for Nod2−/− bone marrow, to Bernard Metz, The Netherlands Vaccine Institute, for diphtheria toxoid, and Coy Allen for help with animals.
Footnotes
This work was supported by National Institutes of Health grants R21AI076835-01 (F.R.), and grants R01AI057157, R01AI063031, R01DE16326, and SERCEB A1-02-031 (J.P.-Y.T.).
Abbreviations used: AlHy, aluminum hydroxide; BMM, bone marrow derived mononuclear cells; DT, diphtheria toxoid; NLR, nucleotide-binding domain leucine-rich repeat-containing;
DISCLOSURES The authors have no financial conflict of interest.
Contributor Information
Hanfen Li, Department Molecular Sciences, University of Tennessee Health Science Center, Memphis, TN 38163, USA, and Lineberger Comprehensive Cancer Center, Curriculum in Genetics and Molecular Biology, Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, NC 27599, USA.
Stephen B. Willingham, Lineberger Comprehensive Cancer Center, Curriculum in Genetics and Molecular Biology, Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, NC 27599, USA
Jenny P-Y. Ting, Lineberger Comprehensive Cancer Center, Curriculum in Genetics and Molecular Biology, Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, NC 27599, USA
Fabio Re, Department Molecular Sciences, University of Tennessee Health Science Center, Memphis, TN 38163, USA, and Lineberger Comprehensive Cancer Center, Curriculum in Genetics and Molecular Biology, Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, NC 27599, USA.
REFERENCES
- 1.Gupta RK, Relyveld EH, Lindblad EB, Bizzini B, Ben-Efraim S, Gupta CK. Adjuvants--a balance between toxicity and adjuvanticity. Vaccine. 1993;11:293–306. doi: 10.1016/0264-410x(93)90190-9. [DOI] [PubMed] [Google Scholar]
- 2.Sun H, Pollock KG, Brewer JM. Analysis of the role of vaccine adjuvants in modulating dendritic cell activation and antigen presentation in vitro. Vaccine. 2003;21:849–855. doi: 10.1016/s0264-410x(02)00531-5. [DOI] [PubMed] [Google Scholar]
- 3.McKee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27:687–690. doi: 10.1016/j.immuni.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Ramanathan VD, Badenoch-Jones P, Turk JL. Complement activation by aluminium and zirconium compounds. Immunology. 1979;37:881–888. [PMC free article] [PubMed] [Google Scholar]
- 5.White RG, Coons AH, Connolly JM. Studies on antibody production. III. The alum granuloma. J Exp Med. 1955;102:73–82. doi: 10.1084/jem.102.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 2004;304:1808–1810. doi: 10.1126/science.1089926. [DOI] [PubMed] [Google Scholar]
- 7.Wang HB, Weller PF. Eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. J Leukoc Biol. 2008 doi: 10.1189/jlb.0607392. published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1 beta and IL-18 release. J Immunol. 2007:5271–5276. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83:447S–455S. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- 10.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 11.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591–598. [PubMed] [Google Scholar]
- 12.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1 beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 15.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 16.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 17.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 18.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 19.Taxman DJ, Zhang J, Champagne C, Bergstralh DT, Iocca HA, Lich JD, Ting JP. Cutting edge: ASC mediates the induction of multiple cytokines by Porphyromonas gingivalis via caspase-1-dependent and - independent pathways. J Immunol. 2006;177:4252–4256. doi: 10.4049/jimmunol.177.7.4252. [DOI] [PubMed] [Google Scholar]
- 20.Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, Plebanski M. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006;40:1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 24.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 25.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber M, Beuscher HU, Rohwer P, Kurrle R, Rollinghoff M, Lohoff M. Costimulation via TCR and IL-1 receptor reveals a novel IL-1alpha-mediated autocrine pathway of Th2 cell proliferation. J Immunol. 1998;160:4242–4247. [PubMed] [Google Scholar]
- 27.Nakae S, Komiyama Y, Yokoyama H, Nambu A, Umeda M, Iwase M, Homma I, Sudo K, Horai R, Asano M, Iwakura Y. IL-1 is required for allergen-specific Th2 cell activation and the development of airway hypersensitivity response. Int Immunol. 2003;15:483–490. doi: 10.1093/intimm/dxg054. [DOI] [PubMed] [Google Scholar]
- 28.Pollock KG, Conacher M, Wei XQ, Alexander J, Brewer JM. Interleukin-18 plays a role in both the alum-induced T helper 2 response and the T helper 1 response induced by alum-adsorbed interleukin-12. Immunology. 2003;108:137–143. doi: 10.1046/j.1365-2567.2003.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]