Abstract
Cancer immunosurveillance failure is largely attributed to insufficient activation signals and dominant inhibitory stimuli for tumor-antigen (TAg) specific CD8 T cells. CD4 T cells have been shown to license dendritic cells (DC), thereby having the potential for converting CD8 T cell responses from tolerance to activation. To understand the potential cooperation of TAg specific CD4 and CD8 T cells, we have characterized the responses of naïve TCR transgenic CD8 and CD4 T cells to poorly immunogenic murine tumors. We found that while CD8 T cells sensed TAg and were tolerized, the CD4 T cells remained ignorant throughout tumor growth and did not provide help. This disparity in responses was due to normal TAg MHC class-I cross-presentation by immature CD8α+ DC in the draining LN, but poor MHC class-II presentation on all DC subsets due to inhibition by the tumor microenvironment. Thus, these results reveal a novel mechanism of cancer immunosubversion, where inhibition of MHC-II TAg presentation on DC prevents CD4 T cell priming, thereby blocking any potential for licensing CD8α+ DC and helping tolerized CD8 T cells.
Keywords: Tumor Immunity, T cells, Dendritic Cells, Antigen Presentation/Processing, Cell activation
Introduction
CD8 T cells require three signals for optimal programming of the immune response, which include an obligate cognate MHC-I peptide complex as signal one, co-stimulation as signal two, and either IL-12, type-I IFN or IL-21 as signal three (1-3). It is thought that the levels of signals that the CD8 T cell sees during priming determine whether the cell will undergo programming for induction of tolerance, or activation that is maintained in both primary and memory responses (4, 5). Depending on the level of maturation, DC provide varying levels of all three signals to T cells during priming, and therefore are the critical determinant for the outcome of T cell responses (6).
CD4 T cells have been shown to promote DC maturation through CD40 ligation, causing increased DC longevity, surface stabilization of MHC-peptide complexes, increased expression of co-stimulatory molecules and production of IL-12 (7-10). In fact, CD4 T cells are required for maximal clonal expansion and acquisition of effector function for some CD8 T cell primary responses and most memory responses examined to date (11-16). Through cognate interactions with the same DC, CD4 T cells thereby have the capacity to determine whether the responding CD8 T cells will undergo induction of tolerance or activation (11, 12, 17).
It has become clear that different DC subsets in the spleen and LNs specialize in presentation to CD8 and CD4 T cells, and that not all DC subsets can simultaneously present exogenous proteins on MHC-I and MHC-II. Only the CD8α+ DC subset can efficiently cross-present Ag for CD8 T cell priming, as well as directly present proteins on MHC-II for CD4 T cell activation (18, 19). Interestingly, the same DC subset is known to produce the highest levels of third signal cytokines in response to CD40 ligation (20, 21). While not formally proven, the CD8α+ DC subset appears to be the critical link in the CD4 T cell help for CD8 T cell responses.
Examinations of TAg specific CD8 T cell responses against poorly immunogenic tumors have shown that while the CD8 T cells do respond, they undergo poor clonal expansion, are functionally impaired and fail to affect tumor progression (22-24). Through various immunization protocols, these responses can be converted to immunogenic ones that lead to effective anti-tumor therapies (22, 25-28). Interestingly, treatment success in some studies shows critical dependence on CD4 T cells for development of CD8 T cell effector function, maximal clonal expansion, and control of tumor growth (23, 25, 29-32). This emphasizes the idea that the level of CD8 T cell activation, governed by CD4 T cell help and DC maturation state, can dictate the outcome of tumor progression.
Considering the importance of CD4 T cell help for anti-tumor CD8 T cell responses, there is a lack of detailed information on how naïve CD4 T cells respond to a cognate TAg and whether they can cause DC licensing in tumor-bearing animals. Additionally, while cross-presentation of TAg to CD8 T cells has been shown to be mediated by DC, it is not currently known which DC subset presents TAg to CD8 and CD4 T cells (24, 28). We therefore set out to examine the behavior of adoptively transferred, OVA323-339-specific OT-II CD4 T cells, and their ability to help naïve, OVA257-264-specific OT-I or melanocyte gp100 protein specific Pmel CD8 T cells in a response to a subcutaneous challenge with either B16.OVA melanoma or E.G7 thymoma tumor, which grow as solid tumors and secrete whole OVA protein as the TAg. We found that CD8 T cells sense cognate TAg cross-presented on MHC-I by CD8α+ DC subset in the DLN, but undergo overall tolerogenic activation. In contrast, OT-II T cells remain ignorant and do not provide help to CD8 T cells due to lack of TAg MHC-II presentation by DC. A similar discrepancy was observed in the endogenous, polyclonal T cell populations. This introduces the novel possibility that the tumor microenvironment selectively inhibits DC mediated MHC-II presentation, thereby limiting CD4 T cell help and causing tolerogenic anti-tumor CD8 T cell responses.
Materials and Methods
Mice, Cell Lines, and Reagents
OVA257-264/H-2Kb specific, TCR transgenic OT-I mice and OVA323-339/I-Ab specific, TCR transgenic OT-II mice were a gift from Dr. Francis Carbone, U. of Melbourne, Australia. OT-I.PL and OT-II.PL mice were generated through crossing OT-I or OT-II mice (respectively) with Thy1-congenic B6.PL-Thy1a/Cy (Thy1.1) mice (Jackson Laboratory, Bar Harbor, ME) and breeding to homozygosity. OT-II Rag 1-/- were generated by crossing OT-II.PL to C57BL/6J-recombinase-activating gene 1 (Rag1)tm1Mom (Rag1-deficient, 2216) mice (Jackson Laboratory). Pmel mice (B6.Cg-Thy1a/CyTg(TcraTcrb)8Rest/JC57BL/6NCr) were purchased from Jackson Laboratory. C57BL/6NCr and CD45.1-congenic B6 (B6.Ly5.2) mice and were purchased from the National Cancer Institute. Experiments were conducted under specific pathogen-free conditions at the University of Minnesota, and were performed in compliance with relevant laws and institutional guidelines and with the approval of the Institutional Animal Care and Use Committee at the University of Minnesota (Minneapolis, MN). EL4 and E.G7 (EL-4 transfected with a secreted form of OVA cells were maintained by in vitro culture in complete RPMI-1640 medium (1) without or with 250 g/ml G418 (respectively) (Mediatech, Herndon, VA). B16.F10 and B16.OVA (OVA transfected B16.F10) cells were maintained in complete RPMI-1640 medium with 800 g/ml G418. All antibodies were purchased from Biolegend, BD Biosciences, eBioscience, or Invitrogen.
Adoptive Transfer and Tumor challenge
95% pure, naïve CD8+ T cells were obtained from OT-I.PL or Pmel mice as previously described (1). OT-II T cells were purified using a CD4+ T cell enrichment kit (Miltenyi Biotech) to >70% purity. Cells were then labeled with carboxyfluoroscein succinimidyl ester (CFSE), and 1×106 cells were i.v. injected into recipient mice. 24-48 hours later, recipients were s.c. injected with either 2×105 B16.F10 or B16.OVA cells in HBSS, or 2×106 EL4 or E.G7 cells in PBS. Tumor area was analyzed by taking the product of perpendicular size measurements using calipers. In some experiments 50 μg of OVA323-339 and LPS were injected i.v. into day 7 E.G7-bearing or day-10 B16.OVA bearing animals. In some experiments, 107 freeze/thawed or irradiated B16.OVA cells were injected, and T cells were analyzed for CFSE dilution 4 days later. In certain experiments, B16.F10 cells were injected first, followed by adoptive transfer of T cells on day 8 or 9 post injection, followed by intra-tumoral injection of indicated doses of LPS-free OVA protein (Sigma-Aldrich, St. Louis, MO) on day 10. As control, tumor-free mice were injected with OVA protein into heavy musculature of the upper thigh (intramuscularly), into plantar surface of the hind foot (footpad), or subcutaneously. Alternatively, 50μg LPS-free EαRFP or EαGFP (kind gift of Dr. Marc K. Jenkins) were injected without prior T cell transfer. In certain experiments, animals were first perfused, followed by isolation of LNs, spleen, lungs, liver, bone marrow, and tumor tissues.
Flow Cytometric and Statistical Analysis
Mice were sacrificed at indicated times and single cell suspensions from tissues were obtained through homogenization. Transferred cells were identified using, CD8α or CD4 specific staining and congenic markers for Thy1.1 and/or CD45.2, and sometimes with Vα2 TCR-specific antibody. Cell counts were obtained using PKH26 reference microbeads (Sigma-Aldrich, St. Louis, MO). Detection of Granzyme B or IFN-γ production was done as described previously (1). Foxp3 staining was done using the Foxp3 Staining Buffer Set and anti-Foxp-APC antibody according to manufacturers specifications (eBioscience). All stained cells were collected on either a FACSCalibur flow cytometer or LSR-II flow cytometer (BD Biosciences), and analyzed using FlowJo software (Treestar, Inc., Ashland, OR). Statistical significance was calculated by using two-tailed T-tests, or 1-way ANOVA. P values representation: less than 0.05 are represented by one asterisk; less than 0.01 by two asterisks; less than 0.001 by three asterisks.
DC purification and in vitro proliferation
DLNs or tumors of B16.OVA bearing mice were harvested and treated with 400 U/ml Collagenase D (Roche Applied Science) solution, and incubated for 25 min at 37° C. EDTA was added to yield a 10 mM solution for 1 minute, followed by a PBS wash. In some experiments, CD11c+ cells were isolated by incubating with anti-CD11c beads followed by positive enrichment (Myllteniy Biotech). For intracellular IL-12p40 staining, DC were incubated in Brefeldin A (1μg/ml) during colagenase digestion and for an additional 4 hrs in complete RPMI-1640 medium with Brefelin A at 37° C and 5% CO2 (BD Biosciences). To separate CD8α+ DC, cell suspensions were first depleted of T, NK, and B cells using Thy1.2, DX-5, and B220 specific Abs and anti-FITC beads. Remaining cells were then stained with anti-CD8α-FITC, incubated with anti-FITC beads and separated into CD8α -/+ fractions by LS column separation. 2×104 DC were then incubated with 3×104 CFSE labeled OT-I and OT-II T cells in wells (96 well plates) for 4 days at 37° C.
Results
TAg specific CD4 T cells do not alter anti-tumor CD8 T cell responses
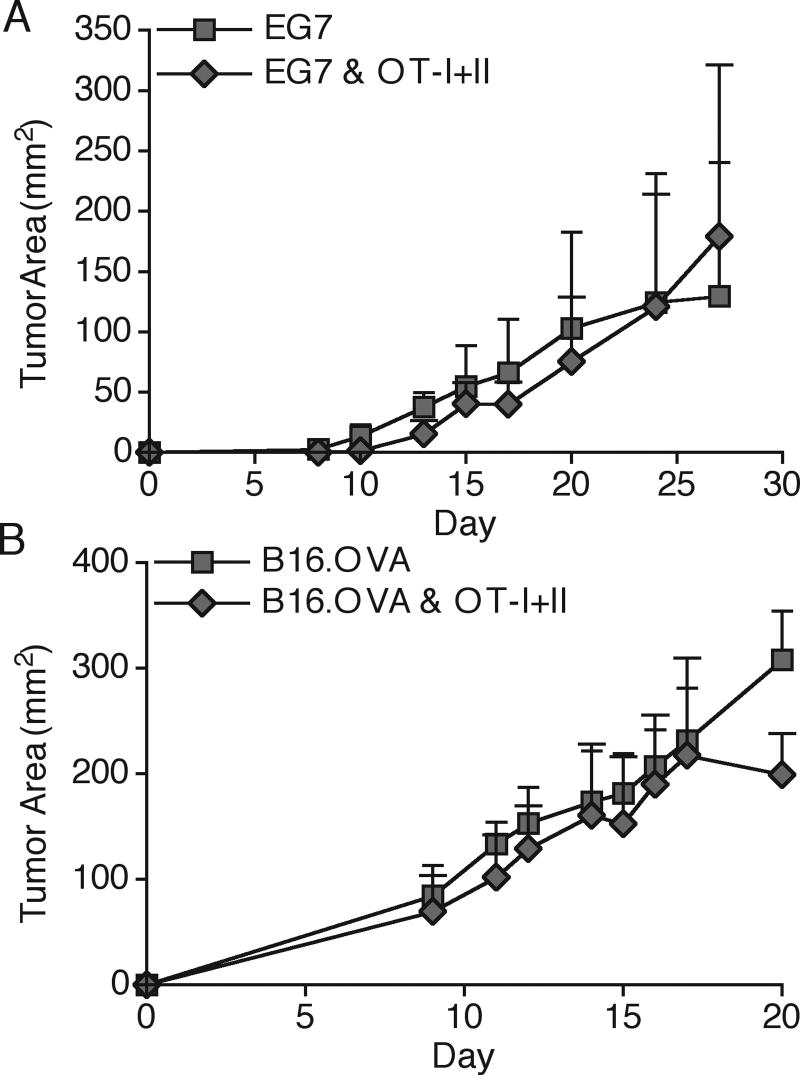
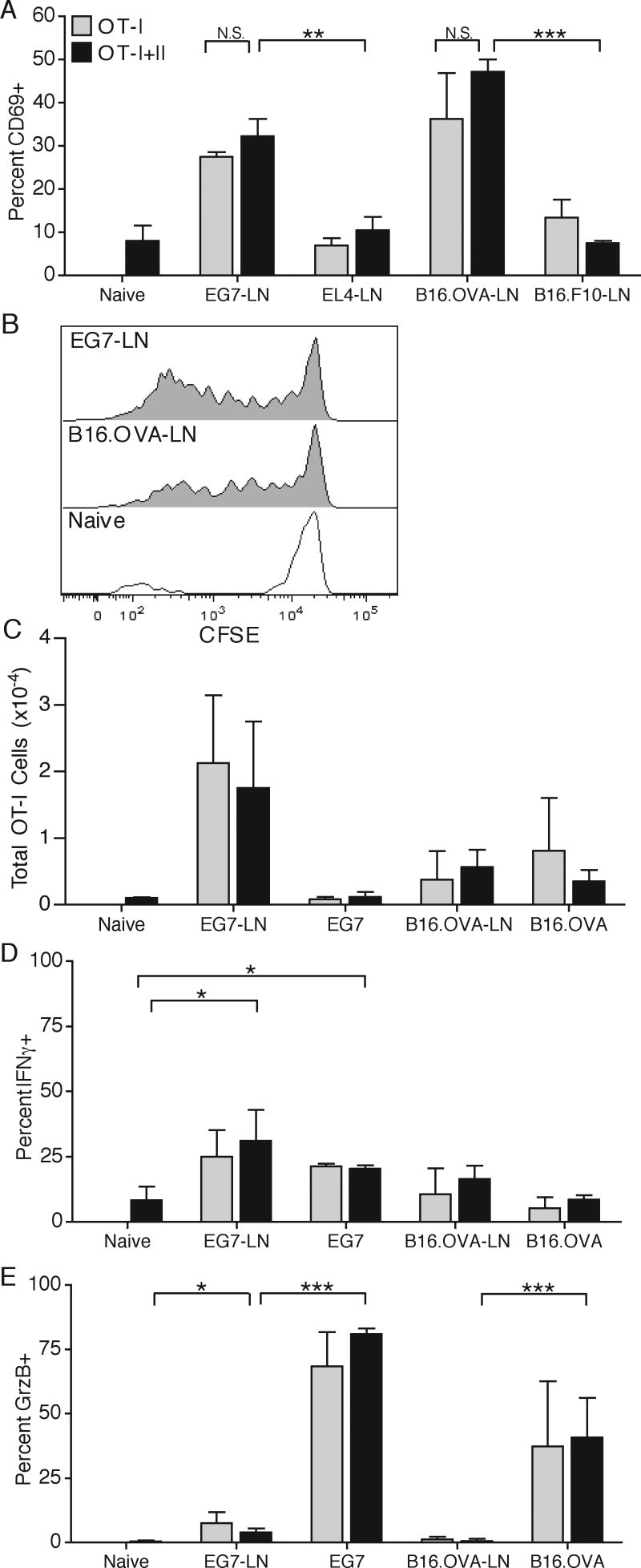
We have recently shown that adoptive transfer of 1.5×106 naïve OT-I T cells does not alter tumor progression due to poor expansion and weak activation, caused by insufficient provision of a third signal cytokine during priming (22) (Popescu and Mescher, Manuscript in preparation). Since CD4 T cells have the ability to enhance production of third signal cytokines and costimulatory molecules by DC, and therefore can enhance CD8 T cell activation, we adoptively co-transferred 1×106 purified, naive OT-I T cells and OT-II T cells and s.c. challenged the mice with E.G7 or B16.OVA tumor a day later. Surprisingly, tumor growth was not significantly altered in either tumor model when both OT-I and OT-II T cells were co-transferred (Fig. 1). The small difference in B16 growth at day 20 was not reproducible in other experiments. Examination of the OT-I response in the DLN at the earliest time of visible tumor detection revealed that OT-I T cells upregulated surface CD69 expression in a antigen specific manner, since no upregulation was seen in the DLN of EL.4 and B16.F10 tumors that did not express OVA (Fig. 2A). OT-I T cells underwent substantial CFSE dilution, but failed to accumulate to large numbers in either DLN or tumor (Fig. 2B,C). Additionally, a small portion of the cells did acquire an ability to make IFNγ at both DLN and the tumor site, and a majority of OT-I cells at the tumor site produced granzyme B (GrzB) (Fig. 2D,E). Importantly, no differences in CD69 expression, clonal expansion or cytokine production were seen when OT-II T cells were co-transferred (Fig. 2A,C-E), indicating that OT-II T cells did not help OT-I T cell responses. Similar results were obtained throughout the course of tumor growth (data not shown).
Figure 1. TAg-specific CD4 and CD8 T cells do not alter tumor growth.
1×106 CFSE labeled OT-I and OT-II T cells were adoptively transferred into C57Bl/6 recipients. One day later, animals were s.c. challenged in the right flank with either 2×106 E.G7 (A) or 2×105 B16.OVA cells (B). Tumor area was monitored by taking the product of perpendicular tumor size measurements. Results are represented as mean ± SD. Data is representative of at least three independent experiments.
Figure 2. OT-I T cell anti-tumor responses.
1×106 OT-I T cells were either adoptively transferred alone or in combination with OT-II T cells. B16.OVA and B16.F10 cells were injected one day later in opposite flanks. E.G7 and EL4 were injected three days post adoptive transfer in opposite flanks. On day 7 post E.G7/EL4 injection and day 9 post B16.OVA/B16.F10 injection, draining lymph nodes (DLN) were harvested and OT-I T cells were analyzed for surface CD69 expression (A) and CFSE dilution (B). DLN and tumor samples were analyzed for total OT-I T cells numbers (C). OT-I T cells were examined for intracellular IFNγ (D) and granzyme B (GrzB) protein levels (E) after a four hour in vitro restimulation with OVA257-264 and GolgiStop. Results are represented as mean ± SD. Data is representative of at least three independent experiments.
CD8 T cell respond similarly to secreted OVA and cytoplasmic endogenous TAgs
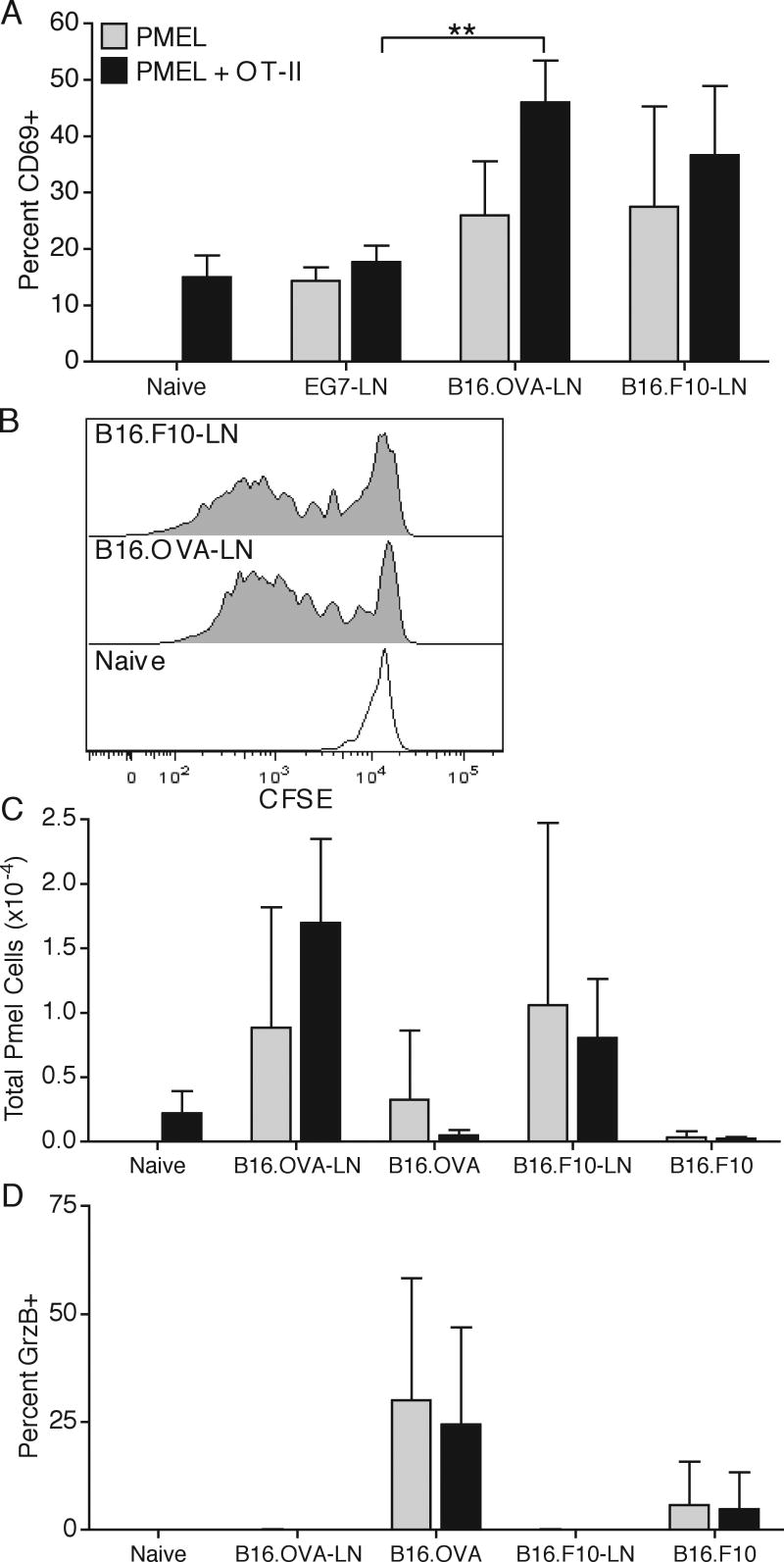
Since the majority of TAgs are derived from cytoplasmic and not secreted proteins, we compared the responses of OT-I T cells to Pmel transgenic CD8 T cells specific for a melanocyte-specific intracellular protein gp100, which is expressed in the B16 melanoma. As seen with the OT-I T cells, at the earliest time of visible tumor growth, DLN Pmel T cells upregulated surface CD69 expression and proliferated, as assessed by CFSE dilution in response to both B16.F10 and B16.OVA tumors, but not to the E.G7 tumor (Fig. 3A,B). Additionally, Pmel T cells produced GrzB at the tumor site, indicating at least partial activation, but did not clonally expand by more than 2-3 fold and did not affect tumor growth (Fig. 3C,D, and data not shown), suggesting that the level of third signal cytokines available at the DLN is insufficient for supporting survival and full differentiation (22). Importantly, as with OT-I T cells, Pmel responses were not significantly altered by a co-transfer of OT-II T cells (Fig. 3A,C,D). Similar results were obtained throughout the course of tumor growth (data not shown). These results indicated that cytoplasmic and secreted TAgs are efficiently cross-presented in tumor DLN on MHC-I but lead to CD8 T cells tolerance, and cannot be helped by even a relatively large population of TAg-specific CD4 T cells. Additionally, the similarity of Pmel and OT-I T cell responses validated OVA as a relevant artificial TAg model.
Figure 3. Pmel T cell anti-tumor responses.
1×106 CFSE labeled Pmel T cells were adoptively transferred alone or in combination with OT-II T cells, followed by a subcutaneous E.G7/EL4 or B16.OVA/B16.F10 opposite-flank tumor inoculation. On day 7 post E.G7/EL4 injection and day 9 post B16.OVA/B16.F10 injection, DLN and tumor samples were harvested and Pmel T cells were analyzed for surface CD69 expression (A), CFSE dilution (B), total cellular recovery (C), and intracellular expression of GrzB without peptide restimulation (D). Results are represented as mean ± SD. Data is representative of two independent experiments.
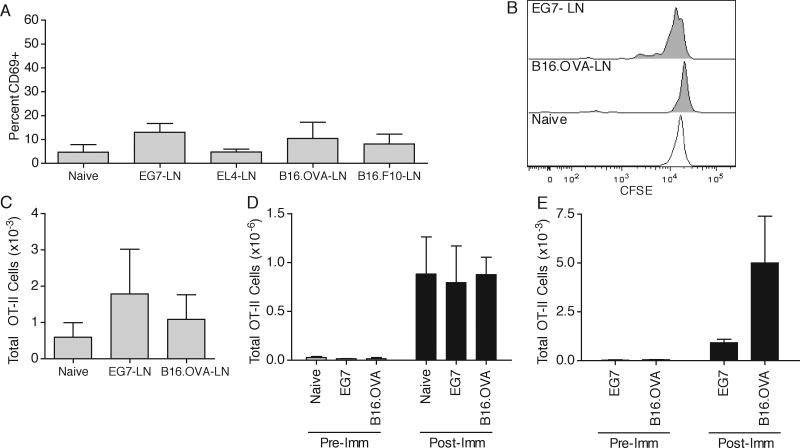
OT-II T cells do not respond to a cognate tumor challenge
To investigate the reason for lack of helper activity of CD4 T cells, we analyzed the responses of OT-II T cells in the DLN, spleen, and tumor. In contrast to the OT-I and Pmel responses, OT-II T cells did not upregulate surface CD69 expression in the tumor DLN, did not dilute CFSE, did not undergo clonal expansion, and did not infiltrate the tumor, indicating a complete lack of a response to tumor-secreted OVA protein (Fig. 4A-C,E). Similar results were obtained at later time points (data not shown). To examine whether OT-II T cells were defective in responding to Ag in tumor bearing animals, we immunized tumor-bearing or tumor-free animals by i.v. injection of OVA323-339 and LPS and analyzed the clonal expansion of cells on day 4 post immunization. As expected, substantial OT-II expansion occurred in tumor-free animals (Fig. 4D). Importantly, OT-II T cells expanded identically in the spleens of both E.G7 and B16.OVA tumor-bearing animals compared to tumor-free controls, and infiltrated the tumor site, showing that OT-II T cells remain naïve throughout tumor growth and are not tolerized (Fig. 4D,E). This suggested that the reason for poor OT-II T cell responses was due to a lack of sufficient OVA presentation on MHC-II in the tumor DLN.
Figure 4. OT-II T cell anti-tumor responses.
(A-C) 1×106 CFSE labeled OT-II T cells were adoptively transferred, followed by a subcutaneous E.G7/EL4 or B16.OVA/B16.F10 opposite-flank tumor inoculation. On day 7 post E.G7/EL4 and day 9 post B16.OVA/B16.F10 inoculation, DLN were harvested and OT-II T cells were analyzed for surface CD69 expression (A), CFSE dilution (B), and total cellular recovery (C). (D,E) Naïve, day 7 E.G7-bearing, or day 10 B16.OVA-bearing were i.v. injected with 50μg OVA323-339 and LPS. Total OT-II cellular recovery was analyzed four days later (Post-Imm) in the spleen (D) and tumor (E) samples and compared to pre-immunization levels (Pre-Imm). Results are represented as mean ± SD. Data is representative of at least three independent experiments.
T cell sensitivity does not govern differential responsiveness to TAg
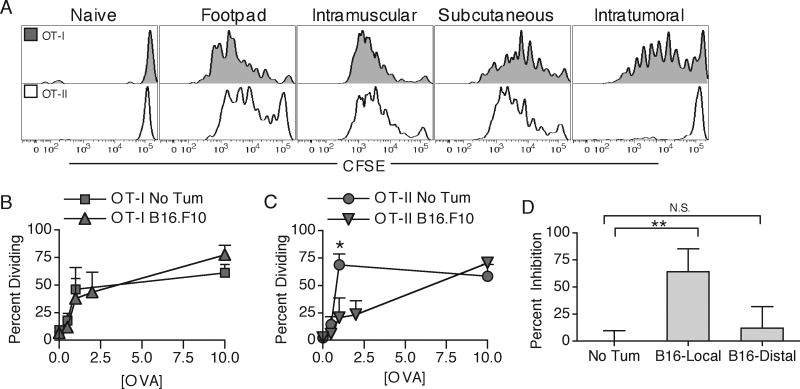
OT-I and OT-II T cells have different TCR affinities and therefore differing abilities to respond to low levels of MHC-peptide complex (33). It was therefore possible that the differential anti-tumor responses were simply due to low levels of MHC-I and MHC-II presented OVA protein. We therefore compared the ability of OT-I and OT-II T cells to respond to different doses of OVA protein injected into various solid tissues and subcutaneous sites in naïve mice or intratumorally (i.t.) into B16.F10-bearing animals. We found that in naïve hosts, OT-II T cells divided to a similar or greater extent than OT-I T cells to low doses of footpad, intramuscularly, or subcutaneously injected OVA protein (Fig. 5A-C), demonstrating efficient presentation of soluble proteins on MHC class-II for all routes of administration (34). In contrast, the OT-II responses were dramatically decreased at low Ag concentrations when OVA was injected directly i.t. compared to the same amount in naïve hosts, while the OT-I responses remained largely unaltered (Fig. 5A-C). Interestingly, the inhibition of OT-II responses in tumor-bearing animals was restricted only to the local tumor site (B16-local) and was not observed in the contra-lateral side, distal subcutaneous injection (Fig. 5D). This evidence suggests that the differing responses of OT-I and OT-II T cells are not due to intrinsic cell differences, but rather due to specific inhibition of MHC-II presentation of TAg in the DLN by the local tumor microenvironment.
Figure 5. T cell sensitivity does not govern differential responsiveness to TAg.
OT-I and OT-II T cells were transferred into tumor-free or B16.F10 tumor-bearing hosts. On day 10 of tumor growth, the animals were injected with 0.5μg OVA protein into the footpad, intramuscularly, subcutaneously (s.c.), or intratumorally (i.t.). Four days later, DLN were harvested and analyzed for OT-I (grey) and OT-II (white) CFSE dilution profiles (A). Percent of cells undergoing division to indicated amounts of OVA protein was quantified and compared between s.c. injected tumor-free and i.t. injected tumor-bearing animals for OT-I (B) and OT-II (C) T cells. 1μg OVA was injected s.c. into tumor-free, i.t., or s.c. into contraleteral side of tumor-bearing animals. Inhibition of proliferation was measured as percent decrease in CFSE diluted cells as compared to tumor-free responses. Results are represented as mean ± SD. Data is representative of at least two independent experiments.
Polyclonal CD4 and CD8 T cells recapitulate transgenic T cell responses
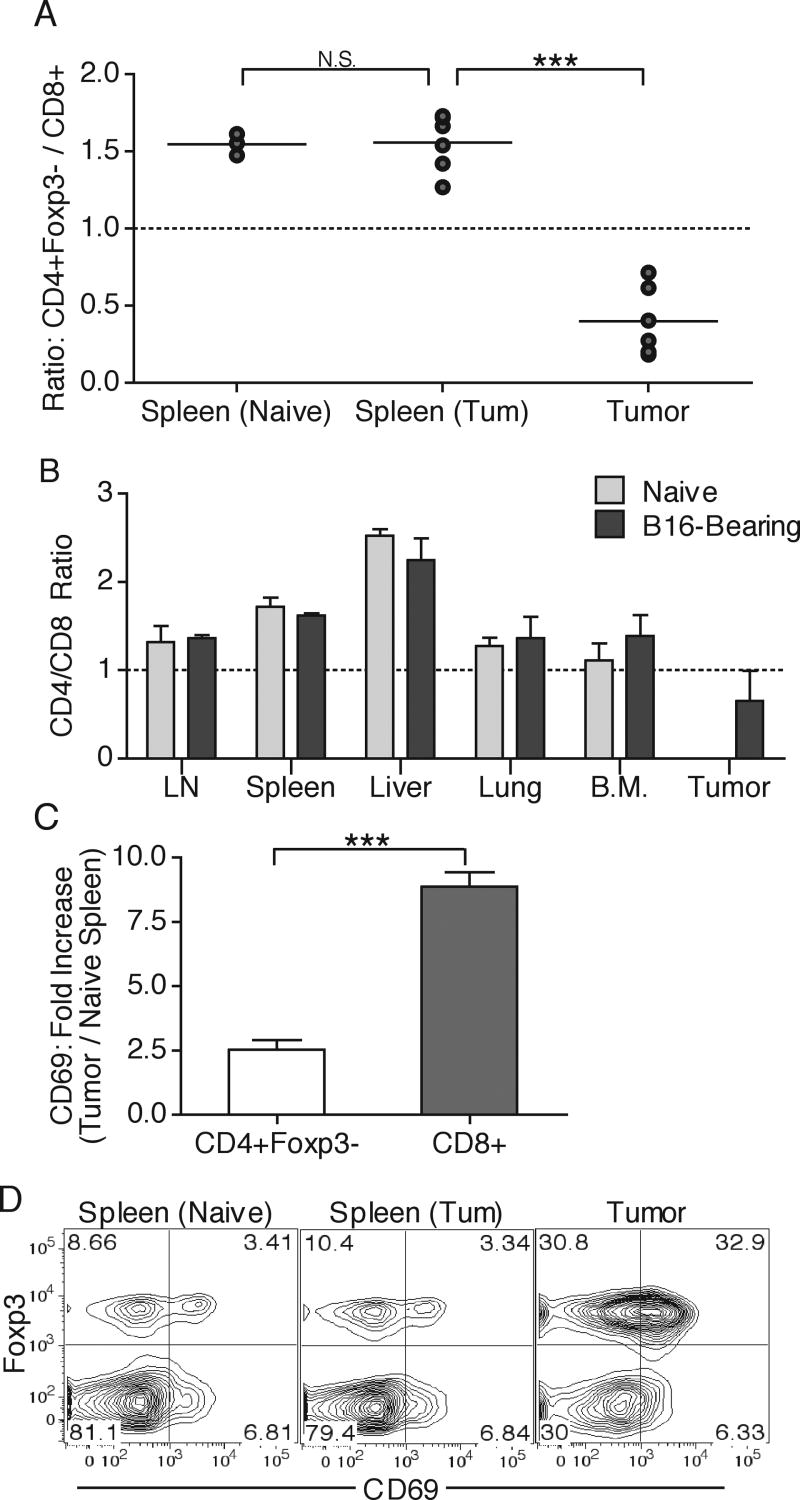
Adoptive transfer allows in vivo detection of early responses by CD8 and CD4 T cell prior to extensive clonal expansion. However, transfer of high numbers of T cells introduces the variable of intraclonal competition, which can change the kinetics, activation requirements and outcome of responses to highly immunogenic antigens (23, 35-37). In the case of the anti-tumor responses, we do not observe large expansion and activation of CD8 T cells at any time point during tumor growth, indicating that decreasing the transfer cell numbers would only decrease the ability to detect sufficient cell numbers for statistical analysis. Nevertheless, we examined the endogenous polyclonal T cell populations for evidence of preferential CD8 T cell activation that would indicate that the observed differential OT-I and OT-II T cell responses are not unique to the transgenic T cells. We first separated the CD4 lymphocytes into T regulatory (Treg) and potential TAg-specific T helper (non-Treg) cells by means of intracellular Foxp3 staining (38), and compared the total numbers of endogenous non-Treg CD4 T cells and CD8 T cells in the spleens and tumors of naive or tumor-bearing animals. To minimize variability we expressed the total cell numbers as a ratio of non-Treg CD4 T cells to CD8 T cells. We found no difference in the relative numbers of these cells in spleens of tumor-bearing animals when compared to naive animals, with the ratio being about 1.5, indicating that non-Treg CD4 T cells numerically dominate in this tissue. However, at the tumor site the ratio dropped to approximately 0.5, i.e. twice as many CD8 T cells compared to non-Treg CD4 T cells were present in the tumor (Fig. 6A). In addition, CD4 T cells clearly numerically dominated over CD8 T cells in other lymphoid and non-lymphoid peripheral tissues, such as: lymph nodes, liver, lungs, and bone marrow, indicating that the specific decrease in CD4 T cell tumor-infiltration is not an artifact of solid tissue infiltration (Fig. 6B). Interstingly, the ratio of Treg CD4 T cells to CD8 T cells did not dramatically change between tumor-free and tumor-bearing animals in spleen or tumor sites, indicating that only the non-Treg CD4 T cell population is inhibited from responding to the tumor (data not shown). Additionally, examination of surface CD69 expression revealed that a much higher percentage of CD8 T cells upregulated CD69 expression than non-Treg CD4 T cells when normalized to basal CD69 expression in naïve spleens (Fig. 6C). Since CD4 T cells require MHC-II to sense presented Ag, it would not be surprising that CD4 T cells would see less Ag at the tumor, where the majority of cells are MHC-II negative tumor cells. However, in contrast to the non-Treg CD4 T cells, the Foxp3+ Treg population was predominantly CD69+ at the tumor site, arguing that enough MHC-II-peptide complexes exist at the tumor site for upregulation of CD69 expression (Fig. 6D). These results indicate that the endogenous polyclonal T cells behave similarly to the transgenic T cells, with the CD8 T cells responding to a much greater extent compared to non-Treg CD4 T cells.
Figure 6. Endogenous T cell anti-tumor responses.
Naïve or day 14 B16.OVA-bearing animal spleens and tumor tissues were harvested and analyzed for the ratio of total numbers of endogenous CD4+Foxp3- to CD8+ T cells (A). Tumor-free or B16.F10-bearing animals were perfused and LNs, spleen, liver, lungs, bone marrow, and tumor tissues were isolated and examined for the ratio of infiltrating CD4 and CD8 T cells (B). CD69 staining was used to calculate the fold increase in CD69 expression of tumor-infiltrating compared to spleen-derived naïve CD4+Foxp3- and CD8+ T cells (C). CD4+ T cells from tumors and spleens of naïve and tumor-bearing (Tum) animals were analyzed surface expression of CD69 and intracellular expression of Foxp3 (D). Numbers represent percent of total cells in each quadrant. Results are represented as mean ± SD. Data is representative of at least three independent experiments.
While DC do not present TAg to CD4 T cells, DLN CD8α+ DC efficiently cross-present TAg to CD8 T cells
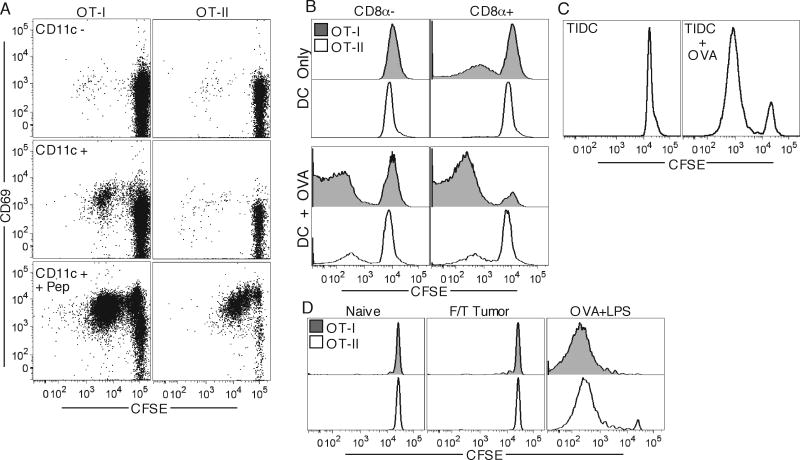
To investigate the presentation capacity of DLN DC to CD4 and CD8 T cells, we isolated CD11c+ and CD11c- cells from B16.OVA DLN and incubated them ex vivo with CFSE labeled OT-I and OT-II T cells for four days. Only the CD11c+ fraction elicited proliferative responses in OT-I T cells. However, neither fraction was capable of eliciting detectable OT-II proliferation. Both subsets were capable of eliciting OT-I and OT-II responses when OVA323-339 and OVA257-264 peptides were added to the culture, indicating that there was no intrinsic T cell defect and that sufficient MHC class-I/II was present to elicit proliferation (Fig. 7A). DC that migrate from the inflamed tissue and resident LN DC have different capacities to prime CD8 and CD4 T cells (18, 19). While CD8α+ DC have been implicated to be the principal DC subset responsible for efficient cross-presentation of exogenous soluble proteins on MHC-I, the migratory interstitial DC are thought to be responsible for sustained presentation to CD4 T cells in skin DLN (39, 40). We therefore separated CD11c+ cells of B16.OVA DLNs into CD8α+ and CD8α- fractions and performed the in vitro proliferation assay. We found that while the CD8α+ DC subset caused robust OT-I proliferation, neither DC subset was capable of causing proliferation of OT-II T cells unless OVA protein was added to the culture media (Fig. 7B). Since it was possible that tumor infiltrating DC (TIDC) may be able to present Ag on MHC-II but are unable to migrate to DLN, or that they process Ag too quickly for presentation in DLN to CD4 T cells, we examined whether TIDC were capable of MHC-II OVA presentation directly ex vivo. We found that TIDC were unable to cause OT-II proliferation unless additional OVA protein was added to the culture media (Fig. 7C).
Figure 7. MHC-I but not MHC-II presentation of TAg by DC.
OT-I and OT-II T cells were in vitro incubated with CD11c– (top) and CD11c+ (middle, bottom) purified cell fractions from DLN of day 12 B16.OVA-bearing animals having OVA257-264, 323-339 peptides added to some wells (bottom), and were analyzed for CFSE dilution and CD69 expression four days later (A). OT-I and OT-II T cells were incubated with CD8- (left) and CD8+ (right) purified DC fractions from DLN of day 13 B16.OVA-bearing animals with OVA protein added to some wells (bottom), and were analyzed for CFSE dilution four days later (B). OT-II T cells were incubated with tumor-derived purified CD11c+ cells (TIDC) with (right) or without (left) addition of OVA protein (C). OT-I and OT-II T cells were adoptively transferred into recipients which were challenged a day later with 107 freeze/thaw killed tumor cells or 100μg OVA protein and LPS. DLN were harvested four days later and analyzed for T cell CFSE dilution (D).
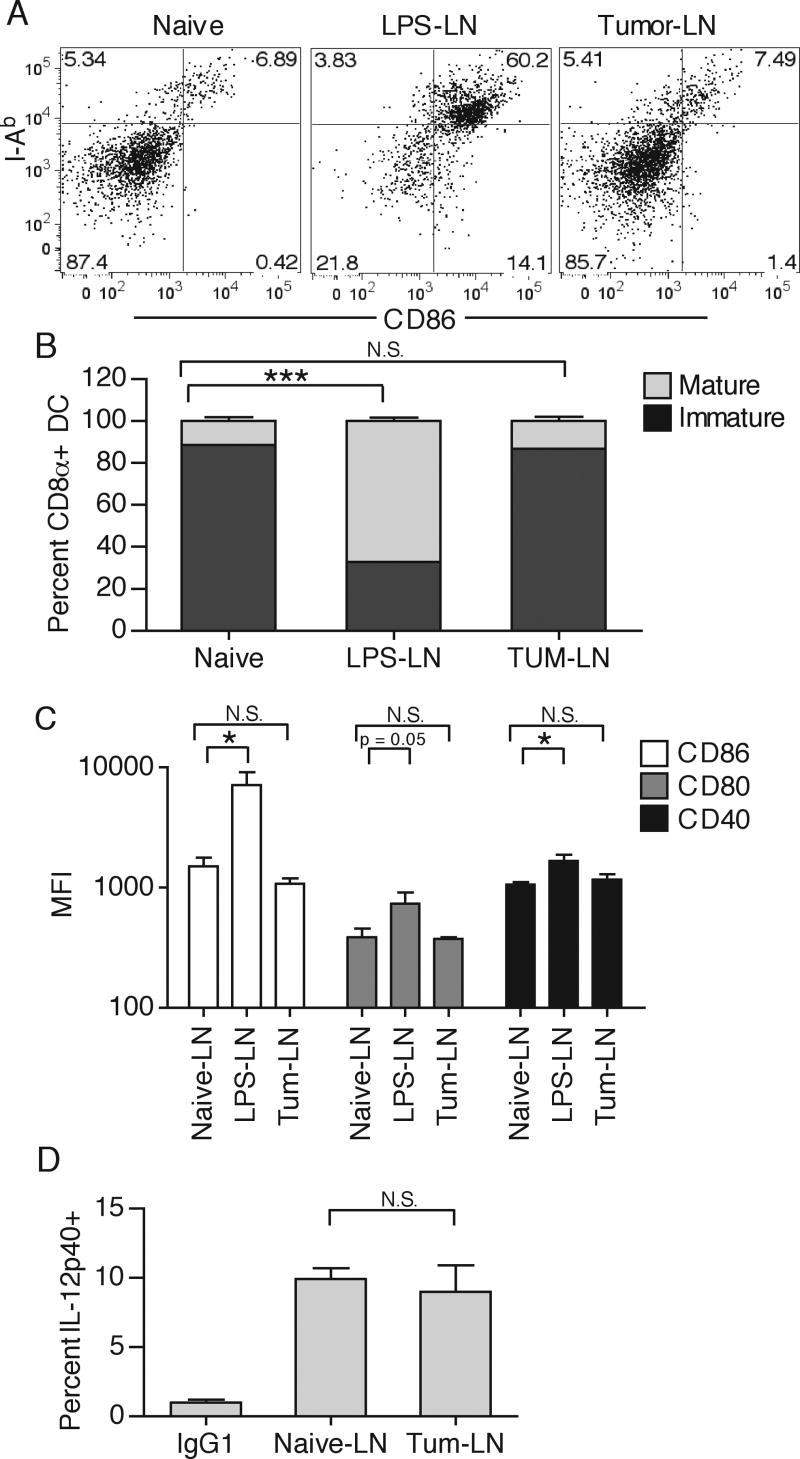
Since cellular associated proteins have been shown to be specifically targeted for cross-presentation, it was possible that normally secreted OVA was taken up in association with dying tumor cells, or that DC were cross-dressing with MHC class-I-peptide complexes directly from dying tumor cell membranes (33, 41, 42). These antigen acquisition pathways could then lead to the observed phenotype of CD8 but not CD4 T cell priming. However, injection of up to 1×107 freeze/thaw killed or irradiated dying tumor cells failed to elicit responses in either OT-I or OT-II T cells, indicating that cell-associated OVA protein and DC cross-dressing do not substantially contribute to CD8 T cell priming (Fig. 7D, and data not shown). Even though enough Ag was available to stimulate OT-I responses, as seen in vivo and in vitro, DLN CD8α+ DC were predominantly phenotypically immature, as assessed by MHC-II expression, CD86, CD80, CD40 levels, and IL-12 production (Fig. 8A-D), which could explain why the in vivo response is tolerogenic and does not control tumor progression. Together these results indicate that while the immature CD8α+ DLN DC competently cross-present TAg on MHC-I, neither TIDC, migratory interstitial DC, nor resident CD8α+ DCs are able to efficiently present the same antigen on MHC-II in tumor-bearing animals.
Figure 8.
Inguinal DLN from either naïve, LPS-treated (50μg subcutaneously injected 16 hours prior), or day 12 B16.F10-bearing mice were collected and CD8α+ DC were examined for expression of I-Ab and CD86 by gating on CD3-, CD19-, CD11c+, CD11b-, CD8α+ cells (A). Immature and mature CD8α+ DC were quantified by counting percent I-Ab positive/dim or I-Ab positive/bright, respectively (B). CD8α+ DC mean fluorescence intensity (MFI) for CD86, CD80, and CD40 was quantified (C). Percent IL-12p40+ of total CD8α+ DC was quantified after a 4hr Brefeldin A in vitro incubation. No enhancement in IL-12p40 production was detected in the LPS-treated group. (D). Results are represented as mean ± SD. Data is representative of at least two independent experiments.
Inhibition of MHC-II presentation in migratory DC in tumor DLN
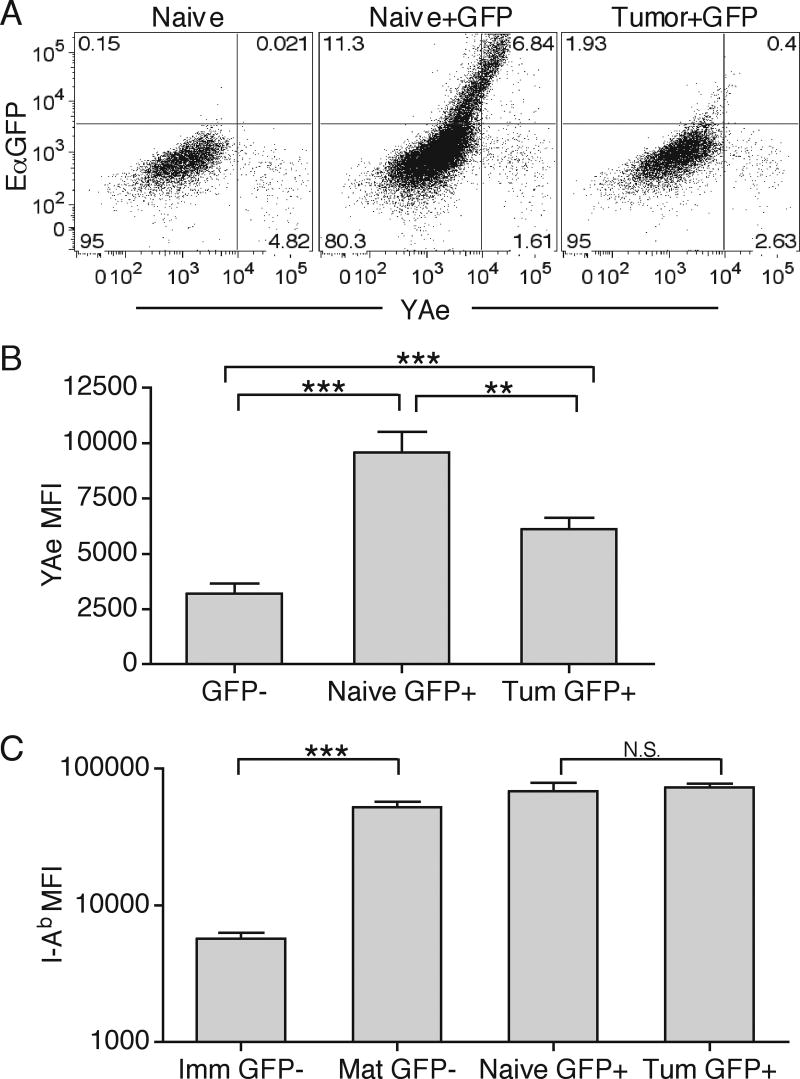
To directly visualize differences in MHC-II presentation in naïve and tumor-bearing hosts, we utilized the EαGFP fluorescent protein system that allows detection of cells with internalized soluble protein, and where the MHC-II presentation can be assessed using the YAe antibody staining to detect I-Ab - Eα52-68 peptide complex (39, 43). We injected EαGFP either s.c. into naïve or i.t. into B16.F10 bearing animals and examined DC complex formation in DLN 24 hours later by isolating CD11c+ DC and staining with the YAe antibody. Naive DLN had a population of migratory DC (CD11c+, CD11b+, CD8α-, CD4-), but not resident DC (not shown), that had high levels of internalized fluorescent protein and stained brightly by the YAe antibody, indicating that normal antigen processing for MHC-II presentation took place (39). In contrast, there was a significant reduction in GFP+ migratory DC in tumor DLN (Fig. 9A). The few GFP+ cells that were detected displayed significantly lower amounts of MHC-II-peptide complex, as well as total intracellular protein when compared to GFP+ cells from naïve animals (Fig. 9A and 9B). Interestingly, GFP+ cells in both naïve and tumor-bearing DLN displayed similarly high levels of MHC-II, which were directly comparable to mature and not immature (based on MHC-II staining) migratory DC (Fig. 9C). These experiments show that the migratory DC phenotype appears to be different from the resident CD8α+ DC in tumor DLN (compare Fig. 8A and 9C). This potentially suggests that while the migratory DC antigen delivery and MHC-II presentation machinery, but not the overall surface MHC-II levels, is directly inhibited in tumor-bearing animals, the immature CD8α+ DC presentation machinery remains largely intact to cause CD8 T cell tolerance.
Figure 9. Inhibition of migratory DC MHC-II presentation in DLN.
Naïve or day 10 B16.F10 tumor-bearing animals were s.c. or i.t. (respectively) injected with 50μg EαGFP. 24 hours later, CD11c+ DC were isolated from DLN and separated into resident and migratory DC subsets. Migratory DC (gate: CD3-, CD19-, I-Ab+, CD11c+, CD11b+, CD8α-, CD4-) were then analyzed for EαGFP fluorescence and YAe staining (A). GFP- and GFP+ migratory DC from naïve or tumor-bearing (Tum) DLN were analyzed for total YAe MFI, mean fluorescence intensity (B). Numbers represent percent of total cells in each quadrant. GFP- CD11b+ DC were separated into immature I-Ab positive/dim (GFP- Imm) and mature I-Ab positive/bright (GFP-Mat) subsets and compared to GFP+ migratory DC from naïve or tumor-bearing DLN for MFI of I-Ab (C). Results are represented as mean ± SD. Data are representative of two independent experiments
Discussion
Evasion of the immune system is currently regarded as the seventh hallmark of cancer progression, with some of the more recently discovered strategies affecting DC differentiation and maturation (44, 45). Considering that the steady-state tumor microenvironment does not contain strong stimuli for DC maturation, it becomes heavily dependant on CD4 T cell help, ablation of which would lead to tolerant anti-tumor CD8 T cell responses (44, 45). It is therefore not surprising that the progressing tumor would evolve mechanisms to inhibit this last safeguard against CD8 T cell tolerance induction.
By examining TAg-specific naïve CD4 and CD8 T cell responses to two tumor cell lines we found that the CD8 T cells rapidly responded to a subcutaneous challenge by recognizing cross-presented cytoplasmic and secreted TAg, but were tolerogenic in nature and did not alter tumor growth. We have previously shown that this is in part due to low levels of signal three cytokines available for CD8 T cells during priming in DLN (22). In contrast to the CD8 responses, OT-II TAg-specfic CD4 T cells remained completely ignorant and naïve throughout tumor progression. Even though OT-II CD4 T cells have been reported to have poor responsiveness to low levels of MHC-II-peptide complexes (33), we found that they responded equivalently or better than OT-I CD8 T cells to immunization with soluble OVA into various solid and subcutaneous sites, but their response was dramatically decreased to i.t. OVA administration. Interestingly, OT-II responses remained intact in tumor-bearing animals if OVA was administered at a distal from the tumor site. These results indicated that T cell response differences were due to a localized tumor-specific mechanism for selective inhibition of MHC-II-restricted TAg presentation, and not due to T cell intrinsic differences, a mutation of tumor OVA protein, or DC cross-dressing themselves with tumor membrane-derived MHC-I complexes (42).
Examination of polyclonal endogenous T cells indicated that they respond similarly to transgenic T cells, with CD8 T cells infiltrating the tumor site in greater magnitude and specificity than CD4 T cells. We additionally found that Treg CD4 T cells are enriched in the tumor with greater than 50% of the population sensing antigen. We hypothesize that the Treg population senses self-antigen MHC class-II-peptide complexes, that are derived from the intracellular autophagy-dependent pathway and not from external phagocytosis/pinocytosis dependent pathways of tumor-antigen processing. It has been recently shown that nutrient starvation, most likely found at the tumor site, causes an increase in presentation of intracellular self-proteins on MHC class-II due to increased autophagy. This enhanced presentation of self by DC could thereby be responsible for the observed defect in presentation of soluble OVA or EaGFP on MHC-II, and increased presentation to the self-specific Treg population (38, 46). Interestingly, TIDC did not have sufficient MHC-II-peptide complexes to cause priming of OT-II T cells directly ex vivo. This indicates that while presentation of self-Ag by TIDC can occur, exogenous proteins are strongly inhibited from being presented on MHC-II.
We found that the tumor DLN CD8α+ DC subset was responsible for cross-presenting TAg for CD8 T cell priming, with neither the CD8α+ nor the CD8α- DC subset being able to cause CD4 T cell activation. Interestingly, CD8 T cell responses to both cytoplasmic and secreted tumor proteins were fundamentally identical, indicating that cross-presentation of different TAgs by DLN DC is a relatively efficient process. Moreover, we were able to characterize the CD8α+ DC subset in tumor DLN to be functionally immature, explaining the tolerogenic nature of the CD8 T cell response. Since MHC-II presentation by the CD8α+ DC subset is not as efficient as cross-presentation, its possible that CD4 T cells are not primed by this subset due to limiting TAg levels and overall low MHC-II levels (18, 19). By utilizing soluble EαGFP protein, we were able to directly visualize MHC-II presentation inhibition in tumor DLN in comparison with tumor-free controls. We found both a decreased frequency of migratory DC carrying the fluorescent protein, as well as decreased levels of intracellular protein and surface MHC-II-peptide complex on DC in tumor DLN. Interestingly, the migratory DC displayed functionally high levels of total MHC-II, potentially displaying self-antigens and evoking Treg responses, as witnessed at the tumor site. Put together, selective inhibition of the migratory DC MHC-II presentation machinery coupled with CD8α+ DC limited MHC-II presentation capacity could lead to a blockade in anti-tumor CD4 T cell responses. However, it is also possible that MHC-II presentation machinery in both migratory and resident LN DC is affected by the tumor microenvironment.
While the majority of studies agree that adoptively transferred CD8 T cells undergo tolerance and do not control tumor growth, there are some examples in the literature of naïve CD8 T cells controlling tumor progression without immunization. Recently Boissonnas and colleagues have shown that 1×107 transferred naïve OT-I CD8 T cells undergo efficient activation and can control E.G7 tumor growth (47). However, such a high transfer number has been shown to cause a CD4 T cell help independent activation of CD8 T cells in both non-tumor and tumor models (23, 36). Additionally, several reports show that naïve CD4 T cells respond to a cognate tumor challenge and help CD8 T cell responses without immunization (23, 48, 49). However, these same studies also show clear dependence of the effective anti-tumor responses on the high transfer number of CD4 and CD8 T cells. In our hands, adoptive transfer of 1×106 transgenic T cells does not result in CD8 T cell clonal expansion and control of tumor growth, indicating that the transfer numbers are insufficient for effective CD8 T cell activation to occur independently of CD4 T cell help. This is additionally supported by the fact that the low precursor frequency tumor-specific endogenous T cells appear to mirror the transgenic T cell response.
In addition, certain leukemia and prostate tumor models show relatively robust tolerogenic CD4 T cell activation to TAg (50, 51). One difference between the systems is that CD4 T cell responses described in our model represent responses to tumor-specific and not tumor-associated antigens, as described in the prostate model (50). More importantly, it is likely that in liquid tumors, antigen levels in the blood and lymph are higher than if they were derived from a solid tumor, thereby having greater access to resident spleen and lymph node DCs. In agreement with this, transgenic and endogenous CD4 T cells undergo a tolerogenic response to the E.G7 thymoma when it is injected i.p. and grows in ascities fluid (52). This tolerance can be reversed through CTLA-4 blockade, which allows for CD4 T cell help-dependent control of the tumor by CD8 T cells. This further supports the notion that provision of CD4 T cell help critically depends on CD4 T cell activation by antigen presenting cells. It is also important to note that these particular studies did not examine both the CD8 and CD4 T cell response kinetics, which introduces the possibility that unsynchronized CD8 and CD4 T cell responses may occur (50, 51). Thereby CD4 T cells could respond much later than CD8 T cells and not be able to provide help during priming, causing tolerance in CD8 T cell responses. Moreover, the majority of the aforementioned studies utilize either fairly immunogenic tumor cell lines, or poorly characterized spontaneous tumor models where immunogenicity is difficult to control (23, 49, 51, 53). Thus, while clearly not universal to all tumor models, our experimental system describes the behavior of low T cell precursor frequencies in response to certain poorly immunogenic, solid tumors.
Overall, these results point to a novel mechanism of tumor immune evasion, where the tumor microenvironment causes aberrant DC functionality by selectively restricting MHC-II presentation by activated interstitial DC that migrate from the tumor site to the draining lymph nodes, while leaving cross-presentation by immature, lymph node resident CD8α+ DC virtually intact. The decrease in the ability of DC to present TAg to CD4 T cells thereby prevents them from providing help for effective CD8 T cell priming. However, it is possible that even if CD4 T cells were activated, CD4 T cell help would not be sufficient to rescue CD8 T cell tolerance induction, as more than one dominant inhibitory mechanism might be in place in tumor-bearing animals. Nevertheless, lack of MHC class-II presentation on DC does represent the earliest block in the helper pathway and hence is at least in part responsible for tolerance induction in CD8 T cells.
While the mechanisms of impairment of DC presentation capacity are currently unresolved, the tumor microenvironment is known to harbor a milieu of suppressive cytokines derived from tumor cells themselves, or produced by infiltrating Treg CD4 T cells or M2 macrophages. Possible mediators include but are not limited to, VEGF, IL-6, M-CSF, TGFβ, IL-10, COX2, PGE2, and gangliosides, and multiple factors may be involved (44, 45). Additionally, nutrient deprivation, which is most likely found at the tumor site, has been shown to alter MHC-II presentation machinery (46). Functionally, a number of steps in DC MHC-II processing and presentation could be altered to cause the observed phenotype, including: DC pinocytosis of extracellular proteins at the tumor site, lysosome acidification, cathepsin activity, H2M and H2-O function, DC migration to DLN, and others (34). It will be important to understand the basis for the selective inhibition of DC MHC-II presentation demonstrated here, and determine whether this phenotype is observed in human cancer patients and, if so, its association with clinical outcome. In addition, a means of reversing the MHC-II defect would have obvious potential for improving T cell based anti-tumor therapies.
Acknowledgments
We would like to thank Dr. Stephen C. Jameson, Dr. Marc K. Jenkins and Dr. Christopher A. Pennell for critical review of the document. We appreciate the help and advice of Dr. Elizabeth Ingulli. We would also like to thank Dr. Drew M. Catron and Dr. Lori Rusch for help with EαGFP and EαRFP protein experiments. Additionally, we thank Dr. Lyse A. Norian for advice on DC purification methodology.
Nonstandard Abbreviations
- TAg
Tumor antigen
- DLN
Draining lymph node
- TIDC
Tumor infiltrating DC
- i.t.
Intratumoral
Footnotes
This research was supported by National Institutes of Health grants AI34824 (M.F.M.), CA82596 (M.F.M.), and NCI 2 T32 CA009138-31 (M.Y.G.).
References
- 1.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 3.Casey KA, Mescher MF. IL-21 Promotes Differentiation of Naive CD8 T Cells to a Unique Effector Phenotype. J Immunol. 2007;178:7640–7648. doi: 10.4049/jimmunol.178.12.7640. [DOI] [PubMed] [Google Scholar]
- 4.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 5.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 7.Miga AJ, Masters SR, Durell BG, Gonzalez M, Jenkins MK, Maliszewski C, Kikutani H, Wade WF, Noelle RJ. Dendritic cell longevity and T cell persistence is controlled by CD154-CD40 interactions. Eur J Immunol. 2001;31:959–965. doi: 10.1002/1521-4141(200103)31:3<959::aid-immu959>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Mueller SN, Jones CM, Stock AT, Suter M, Heath WR, Carbone FR. CD4+ T cells can protect APC from CTL-mediated elimination. J Immunol. 2006;176:7379–7384. doi: 10.4049/jimmunol.176.12.7379. [DOI] [PubMed] [Google Scholar]
- 9.Delamarre L, Holcombe H, Mellman I. Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation. J Exp Med. 2003;198:111–122. doi: 10.1084/jem.20021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filatenkov AA, Jacovetty EL, Fischer UB, Curtsinger JM, Mescher MF, Ingulli E. CD4 T cell-dependent conditioning of dendritic cells to produce IL-12 results in CD8-mediated graft rejection and avoidance of tolerance. J Immunol. 2005;174:6909–6917. doi: 10.4049/jimmunol.174.11.6909. [DOI] [PubMed] [Google Scholar]
- 12.Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR, Belz GT. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 13.Wang JC, Livingstone AM. Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J Immunol. 2003;171:6339–6343. doi: 10.4049/jimmunol.171.12.6339. [DOI] [PubMed] [Google Scholar]
- 14.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 15.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 16.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 18.Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8- dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J Immunol. 2001;166:5327–5330. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 19.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O'Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 22.Curtsinger JM, Gerner MY, Lins DC, Mescher MF. Signal 3 availability limits the CD8 T cell response to a solid tumor. J Immunol. 2007;178:6752–6760. doi: 10.4049/jimmunol.178.11.6752. [DOI] [PubMed] [Google Scholar]
- 23.Lyman MA, Aung S, Biggs JA, Sherman LA. A spontaneously arising pancreatic tumor does not promote the differentiation of naive CD8+ T lymphocytes into effector CTL. J Immunol. 2004;172:6558–6567. doi: 10.4049/jimmunol.172.11.6558. [DOI] [PubMed] [Google Scholar]
- 24.Anderson MJ, Shafer-Weaver K, Greenberg NM, Hurwitz AA. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J Immunol. 2007;178:1268–1276. doi: 10.4049/jimmunol.178.3.1268. [DOI] [PubMed] [Google Scholar]
- 25.Tatsumi T, Takehara T, Kanto T, Miyagi T, Kuzushita N, Sugimoto Y, Jinushi M, Kasahara A, Sasaki Y, Hori M, Hayashi N. Administration of interleukin-12 enhances the therapeutic efficacy of dendritic cell-based tumor vaccines in mouse hepatocellular carcinoma. Cancer Res. 2001;61:7563–7567. [PubMed] [Google Scholar]
- 26.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilinc MO, Aulakh KS, Nair RE, Jones SA, Alard P, Kosiewicz MM, Egilmez NK. Reversing tumor immune suppression with intratumoral IL-12: activation of tumor-associated T effector/memory cells, induction of T suppressor apoptosis, and infiltration of CD8+ T effectors. J Immunol. 2006;177:6962–6973. doi: 10.4049/jimmunol.177.10.6962. [DOI] [PubMed] [Google Scholar]
- 28.van Mierlo GJ, Boonman ZF, Dumortier HM, den Boer AT, Fransen MF, Nouta J, van der Voort EI, Offringa R, Toes RE, Melief CJ. Activation of dendritic cells that cross-present tumor-derived antigen licenses CD8+ CTL to cause tumor eradication. J Immunol. 2004;173:6753–6759. doi: 10.4049/jimmunol.173.11.6753. [DOI] [PubMed] [Google Scholar]
- 29.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kikuchi T, Uehara S, Ariga H, Tokunaga T, Kariyone A, Tamura T, Takatsu K. Augmented induction of CD8+ cytotoxic T-cell response and antitumour resistance by T helper type 1-inducing peptide. Immunology. 2006;117:47–58. doi: 10.1111/j.1365-2567.2005.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labeur MS, Roters B, Pers B, Mehling A, Luger TA, Schwarz T, Grabbe S. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J Immunol. 1999;162:168–175. [PubMed] [Google Scholar]
- 33.Li M, Davey GM, Sutherland RM, Kurts C, Lew AM, Hirst C, Carbone FR, Heath WR. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol. 2001;166:6099–6103. doi: 10.4049/jimmunol.166.10.6099. [DOI] [PubMed] [Google Scholar]
- 34.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 35.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mintern JD, Davey GM, Belz GT, Carbone FR, Heath WR. Cutting edge: precursor frequency affects the helper dependence of cytotoxic T cells. J Immunol. 2002;168:977–980. doi: 10.4049/jimmunol.168.3.977. [DOI] [PubMed] [Google Scholar]
- 37.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 40.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-Derived Dendritic Cells Formed at the Infection Site Control the Induction of Protective T Helper 1 Responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Shen L, Rock KL. Cellular protein is the source of cross-priming antigen in vivo. Proc Natl Acad Sci U S A. 2004;101:3035–3040. doi: 10.1073/pnas.0308345101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol. 2006;177:6018–6024. doi: 10.4049/jimmunol.177.9.6018. [DOI] [PubMed] [Google Scholar]
- 43.Pape KA, Catron DM, Itano AA, Jenkins MK. The Humoral Immune Response Is Initiated in Lymph Nodes by B Cells that Acquire Soluble Antigen Directly in the Follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 45.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 46.Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Muller M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, Brock R, Driessen C, Rammensee HG, Stevanovic S. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci U S A. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, Scott B. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J Immunol. 2000;165:6047–6055. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 49.Zhou G, Lu Z, McCadden JD, Levitsky HI, Marson AL. Reciprocal changes in tumor antigenicity and antigen-specific T cell function during tumor progression. J Exp Med. 2004;200:1581–1592. doi: 10.1084/jem.20041240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sotomayor EM, Borrello I, Rattis FM, Cuenca AG, Abrams J, Staveley-O'Carroll K, Levitsky HI. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood. 2001;98:1070–1077. doi: 10.1182/blood.v98.4.1070. [DOI] [PubMed] [Google Scholar]
- 51.Mihalyo MA, Hagymasi AT, Slaiby AM, Nevius EE, Adler AJ. Dendritic cells program non-immunogenic prostate-specific T cell responses beginning at early stages of prostate tumorigenesis. Prostate. 2007;67:536–546. doi: 10.1002/pros.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11:483–493. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 53.Krup OC, Kroll I, Bose G, Falkenberg FW. Cytokine depot formulations as adjuvants for tumor vaccines. I. Liposome-encapsulated IL-2 as a depot formulation. J Immunother. 1999;22:525–538. doi: 10.1097/00002371-199911000-00007. [DOI] [PubMed] [Google Scholar]