Abstract
Rationale
Orbital/insular areas of the prefrontal cortex (PFC) are implicated in cocaine addiction. However, the role of dopamine D1 receptors in mediating cocaine self-administration in these sub-regions remains unknown.
Objectives
To define the role of the dorsal agranular insular (AId) sub-region of the PFC, we investigated the effects of D1 receptor manipulation on self-administration behavior maintained by cocaine and cocaine-related stimuli.
Methods
Rats were trained to lever press for cocaine (1 mg/kg) under a fixed-interval 5-min (fixed-ratio 5:S) second-order schedule of reinforcement in the presence of conditioned light cues and contextual sound cues. Intra-AId infusions of vehicle, the D1-like receptor agonist SKF 81297 (0.1, 0.2, 0.4 μg/side) or the D1-like receptor antagonist SCH 23390 (1.0, 2.0, 4.0 μg/side) were administered prior to 1-hr self-administration test sessions. Food-maintained responding under a second-order schedule was examined in separate rats to determine if pretreatment with D1 ligands produced general impairments in responding.
Results
Infusion of SKF 81297 (0.2 and 0.4 μg/side) reduced active lever responses during the first 30min of 1-hr test sessions, but did not influence cocaine intake. Infusion of 4.0 μg/side SCH 23390 reduced active lever responses and cocaine intake throughout 1-hr test sessions. Additionally, this dose of SCH 23390 disrupted food-maintained responding and intake.
Conclusions
D1 receptor agonists and antagonists in the AId have diverse consequences and time courses of action. D1 receptor stimulation in the AId may reduce the motivating influence of cocaine-related stimuli on responding whereas D1 receptor blockade in this PFC sub-region produces global disruptions in behavior.
Keywords: Cocaine, D1 receptor, Dorsal agranular insular cortex, Prefrontal cortex, SCH 23390, Self-Administration, SKF 81297
Introduction
Several studies using animal models of drug addiction have reported changes in responding elicited by cocaine and/or cocaine-related stimuli following systemic administration of dopamine receptor agonists and antagonists (Self et al. 1996; Weissenborn et al. 1996; Caine et al. 1999; Khroyan et al. 2000; Alleweireldt et al. 2002; Crombag et al. 2002; Alleweireldt et al. 2003; Khroyan et al. 2003; Olsen and Duvauchelle 2006). Interestingly, systemic injections of D1-like receptor agonists or antagonists produce similar, rather than opposing, dose-dependent reductions in responding maintained by cocaine during cocaine self-administration sessions (Caine et al. 2000; Platt et al. 2001; Mutschler and Bergman 2002; Barett et al. 2004), suggesting an important inhibitory role for D1 receptors in mediating cocaine self-administration. However, studies involving intra-cranial infusions of D1 ligands indicate that the effects of D1 receptor manipulations during cocaine self-administration are more complex. For instance, D1 receptor antagonism in the nucleus accumbens (NAc) shell with SCH 23390 (1.0 μg/side) attenuates cocaine-induced reinstatement (Anderson et al. 2003), whereas administration of the D1-like receptor agonist SKF 81297 (1.0 μg/side) reinstates drug-seeking behavior (Bachtell et al, 2005; Schmidt et al. 2006). Moreover, infusion of SCH 23390 (2.0 μg/side) into the caudal basolateral amygdala (BLA) has been shown to increase the number of infusions earned during cocaine self-administration sessions, whereas infusion into the rostral BLA (2.0 μg/side) fails to alter this behavior, but attenuates cue- and cocaine-induced reinstatement of drug-seeking behavior (Alleweireldt et al. 2006).
Given these contrasting findings, it is clear that D1 receptors in the amygdala and NAc mediate cocaine consumption and the motivating influence of cocaine-related stimuli in different ways and in manners that are dependent on specific sub-regions within these brain sites. Another important brain area implicated in drug-seeking behavior is the prefrontal cortex (PFC) (Weissenborn et al. 1997; McFarland and Kalivas 2001; McLaughlin and See 2003; Fuchs et al. 2004; Fuchs et al. 2005). Previous studies have shown that rats will intra-cranially self-administer cocaine into the medial PFC (Goeders and Smith 1983; Goeders et al. 1986) and that DA manipulation in this brain region via 6-hydroxydopamine lesions or D2 receptor antagonism prevents intra-cranial cocaine-maintained responding (Goeders and Smith 1986). Moreover, similar to systemic administration of D1 ligands, D1-like receptor antagonism in the medial PFC with low to intermediate doses of SCH 23390 (0.1–2.0 μg/side) is sufficient to block the reinstatement of cocaine-induced drug-seeking behavior (Sun and Rebec 2005) and cocaine conditioned place preference (Sanchez et al. 2003). Lastly, stimulation of medial PFC D1 receptors with 0.1μg/side SKF 81297 has been demonstrated to block the expression of cocaine sensitization (Sorg et al. 2001). Thus, similar to D1 receptors in the shell region of the NAc and in the rostral portion of the amygdala, these findings suggest that D1 receptors in the medial PFC are also critical for mediating drug-seeking behavior induced by cocaine priming injections or by presentations of cocaine-conditioned cues.
However, given that the PFC is comprised of numerous anatomically and functionally distinct sub-regions, the role of D1 receptors located in other prefrontal sub-regions, such as in orbital and insular cortices, in mediating cocaine self-administration remains unknown and must be investigated as well. Recent work from our laboratory has shown that the working memory functions of rodent orbital/agranular insular cortex are particularly sensitive to the effects of self-administered cocaine (Kantak et al. 2005). Therefore, the current study examined the effects of infusing the D1-like receptor antagonist SCH 23390 or the full D1-like receptor agonist SKF 81297 into the dorsal agranular insular (AId) sub-region of the PFC in rats trained to self-administer cocaine under a second-order schedule of reinforcement (see Figure 1 for a timeline of the cocaine self-administration protocol). The second-order schedule was chosen because it is particularly useful for ascertaining the neural systems important for maintaining cocaine self-administration and the motivating influence of cocaine-related stimuli (Weissenborn et al. 1997; Arroyo et al. 1998; Everitt and Robbins 2000; Kantak et al. 2002a; Schindler et al. 2002).
Figure 1.

Diagram depicting the timeline for the cocaine self-administration protocol.
Materials and Methods
Subjects and apparatus
Twenty Crl(WI)BR rats (Wistar strain, Charles River Breeding Labs, Portage, MI), weighing approximately 275g upon arrival, were maintained at 85% of an adjusted ad libitum body weight throughout the duration of the study by restricting food to approximately 16g per day. Between experimental sessions, the rats had unlimited access to water in their home cages. Rats were housed in individual clear plastic cages (24cm × 22cm × 20cm) in a temperature- (21–23°C) and light- (08:00h on, 20:00h off) controlled vivarium. Eight experimental chambers (model ENV-008CT; Med Associates, Georgia, VT), as previously described in detail (Kantak et al. 2002), were used. Motor driven syringe pumps (Model PHM-100, Med Associates, Georgia, VT) located outside of each experimental chamber were used for drug delivery. A different model syringe pump (Orion/Sage, Model 341A, Boston, MA) was used for intracerebral infusions. The policies and procedures set forth in the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003) were followed, as well as specific national laws. The Boston University Institutional Animal Care and Use Committee approved these studies.
Drugs
The drugs used in this study were cocaine hydrochloride (gift from NIDA, Bethesda, MD), (+)-SKF 81297 hydrobromide and (+)-SCH 23390 hydrochloride. The D1 ligands were both purchased from Sigma (St. Louis, MO). Cocaine was dissolved in sterile 0.9% saline solution containing 3IU heparin/ml up to a final concentration of 2.68 mg/ml. For all self-administration experiments, a 1.0 mg/kg unit infusion dose of cocaine was used and delivered intravenously at a rate of 1.8 ml/min. To attain a dose of 1.0 mg/kg, infusion volume was adjusted for body weight, resulting in drug delivery times of 1.2 sec/100 g body weight in individual rats. During saline self-administration sessions, heparinized saline solution was substituted for cocaine. SCH-23390 was dissolved in sterile 0.9% saline and the solution was made fresh on testing days immediately before intracranial infusions took place. A stock solution was made using 5 mg SKF 81297 dissolved in 3.125ml of sterile 0.9% saline (0.8 μg/0.5 μl, salt weight). Lowerconcentrations were made via serial dilution. Sterile 0.9% saline was used as the control vehicle for SKF 81297 and SCH 23390. The pH of all solutions, including the sterile 0.9% saline solution used as the vehicle control, was 4.0.
Surgery and Histology Procedures
Guide cannulae were stereotaxically implanted into the agranular insular area (AP+2.8, L±4.5, DV−5.8) of the PFC as detailed in Di Pietro et al. (2004). For intravenous delivery of cocaine or saline during self-administration sessions, surgical implant of jugular vein catheters was performed as previously described in detail (Kantak et al. 2000). Children’s Tylenol in the drinking water (5 ml/250 ml water) and subcutaneously administered Buprenorphine (0.05 mg/kg) were provided to manage post-surgical pain and discomfort for 48 hr. Rats were also injected subcutaneously with the antibiotic Baytril (15 mg/kg) for 5 days post-surgery to reduce the risk of infection. Catheters were checked for function weekly by infusing a 0.1 ml solution containing 1mg methohexital sodium (Brevital; King Pharmaceuticals, Inc, Bristol, TN, USA) and noting the presence or absence of sedation. A new catheter was implanted into either the left jugular vein or right femoral vein as needed to replace leaking or non-functional catheters. All rats were allowed to recover from surgery for 1 week before initiation of the experiments. Wounds were treated daily with nitrofurazone powder until healed. Catheters were maintained by flushing them daily (Monday through Friday) with 0.1 ml of a 0.9% saline solution containing 0.3 IU heparin (LymphoMed, Rosemont, IL, USA) and 6.7 mg timentin (Smith Kline Beecham Pharmaceuticals, Philadelphia, PA, USA). On Fridays, a locking solution consisting of glycerol and undiluted heparin (3:1) was used to fill the catheter dead space. This solution remained in the catheters over the weekend and it was removed and replaced with the diluted saline/heparin solution on Mondays prior to the start of the behavioral sessions.
Upon completion of the experiments, rats were given an overdose of sodium pentobarbital and then intracardially perfused with saline and 10% formalin solution. Brains were removed, post-fixed in 10% formalin for 4hrs, and then stored in 30% sucrose at 4°C overnight. Coronal sections that were 50 μm thick were collected using a freezing microtome. Sections were then mounted on gelatin-coated slides, stained with thionin to verify cannula placements, dried and dehydrated before cover slipping.
Microinfusion procedure
Rats received bilateral infusions of drug or vehicle into the AId immediately before each test session. Prior to intracranial infusions, dummy cannulae were removed and infusions of 0.5μl/side were administered through 28-gauge injection cannulae connected to a 5-μl syringe via PE 20 tubing. Injection cannulae extended 1 mm beyond the tip of the guide and infusions took place over a 90-s period. Infusion of the drug was confirmed by movement of a small air bubble in the PE 20 tubing a fixed distance. Injection cannulae were left in place for 60 s to allow for diffusion into brain tissue before removal, and then dummy cannulae were replaced. Each intra-AId infusion was separated by a minimum of 2 days of training to reestablish baseline performance. Animals received a maximum of eight intracranial infusions to avoid tissue damage at the injection site. Previous studies demonstrated that the number of sessions to acquire a non-spatial working memory task requiring the intact functioning of the AId did not differ between control rats receiving daily infusions of vehicle into the AId (11 sessions; Di Pietro et al. 2004) vs. no treatment (10 sessions; Kantak et al. 2005). Thus, it is highly likely that the functional integrity of the AId was preserved following the eight AId infusions used in the present study.
Self-administration procedures
Training for Drug-Maintained Responding
Before surgery, rats (n=12) were trained to respond on a lever for food reinforcement under a fixed-ratio 1 (FR 1) schedule of food pellet delivery. Once rats learned to press for 100 food pellets in less than 30 min, right jugular vein catheters and guide cannulae were implanted. One week later, self-administration training began. Initial sessions were 2hr in duration. Rats were incrementally trained to self-administer 1.0 mg/kg cocaine under a terminal fixed-interval (FI)-based second-order schedule of drug delivery that incorporated contextual sound and conditioned light stimuli. For half of the rats in each group, the right lever was designated as the active lever, and for the other half, the left lever was designated as the active lever. Delivery of cocaine was contingent on the completion of five responses on the active lever after the 5-min FI elapsed. The light stimulus over the active lever remained illuminated for the duration of the infusion and during a 20-s timeout (TO) period that followed each infusion. The TO period was signaled by extinguishing the house light. During the FI, a 2-s brief stimulus light was presented after every fifth response on the active lever. For half the rats, the contextual sound cue consisted of an intermittent tone (70 db; 7 kHz; 0.5sec duration every sec) and for the other half a continuous white noise (70 db). The contextual cue was presented throughout the duration of 2 hr training sessions. Training sessions were conducted 5 days a week during the light phase and continued until cocaine intake was stable (number of infusions did not deviate by more than 20%) and the number of responses on the inactive lever was no greater than 25 per session for a 5-day period.
Training for Food-Maintained Responding
A separate group of animals (n=8) were bilaterally implanted with cannulae into the AId and trained to lever press for 45 mg chocolate-flavored purified food pellets (Bio-Serv, Frenchtown, NJ) under the FI5(FR5:S) second-order schedule during daily (Monday-Friday) 1-hr sessions. Second-order schedule training procedures for these rats were the same as described in the section above with the exception that food pellets rather than cocaine were used as the reinforcer. A single 45 mg food pellet was delivered upon completing the FR5 response requirement after each 5-min FI elapsed.
Experimental Design
Drug Maintenance Testing
To ensure that the cocaine cues were highly salient, against which the effects of SCH 23390 and SFK 81297 were tested, rats were given daily saline self-administration sessions with a unique set of light and sound cues in addition to daily cocaine self-administration sessions. Discrimination training procedures were implemented according to the methods outlined in Kantak et al. (2002a, 2002b) after baseline cocaine self-administration under the second-order schedule was acquired. Briefly, rats were given 10 discrimination sessions consisting of two (saline and cocaine) 1-hr sessions, separated by 1-hr. The order in which saline and cocaine sessions were conducted was counterbalanced for each rat. During cocaine and saline sessions, a unique discriminative contextual auditory stimulus (intermittent tone or continuous white noise) was presented and counterbalanced for each condition. In addition, to provide a unique conditioned light stimulus for saline sessions, the stimulus light present over the active lever flashed, instead of remaining constant as in cocaine sessions, during presentation of the brief stimulus, drug infusion, and TO period. Discrimination training took place 5 days per week.
Rats were then tested for the effects of intracranial infusions of SCH 23390 or SKF 81297 within the AId of the PFC on lever responding and cocaine intake maintained by an FI 5-min (FR5:S) schedule that utilized the cocaine-associated sound and light cues. One subset of rats was tested first with SCH 23390 and then with SKF 81297, and another subset of rats was tested first with SKF 81297 and then with SCH 23390. Bilateral infusions of vehicle (0 μg control) or the D1-like receptor antagonist SCH 23390 (1.0, 2.0, and 4.0 μg/side, salt weight) or the D1-like receptor agonist SFK 81297 (0.1, 0.2, 0.4 μg/side, salt weight) were given into the AId of rats immediately prior to maintenance testing sessions. Once maintenance testing with the D1 antagonist or agonist was complete, the same group of animals was given three days to re-establish baseline responding before maintenance testing with vehicle and the other D1-like ligand began. One hour saline self-administration sessions were not conducted on cocaine maintenance test days. The doses of SCH 23390 and SKF 81297 that were chosen for this study were infused in an irregular order in individual animals and were based on the range of doses used in other studies (Zahrt et al. 1997; Floresco and Phillips 2001; See et al. 2001; Anderson et al. 2003; Schmidt et al. 2006). Throughout the study, testing was conducted every third day, with discrimination sessions on intervening days.
Food Maintenance Testing
It has been shown that following a systemic injection of cocaine (10 mg/kg i.p.) and an intra-cranial infusion of 1.0 μg/side SCH 23390 into the accumbens or amygdala, motorinhibition is produced in rats (McGregor and Roberts 1993). Therefore, to determine if reductions in lever responding following pretreatment with a D1-like receptor agonist or antagonist were caused by a general impairment in motor responding, food-maintained responding was investigated. Only the highest doses of SCH 23390 and SKF 81297 that produced the most significant reductions in cocaine-maintained responding were tested. Following second-order schedule training and baseline measurements, vehicle was infused into the AId 5min before the start of the 1-hr food self-administration session (control test session). Infusions of SCH 23390 (4.0 μg/side) or SKF 81297 (0.4 μg/side) were similarly administered 5min prior to testing in a counter-balanced order. Testing was conducted every 2 to 3 days with 1-hr food self-administration sessions given on intervening days.
Data Analyses
Some rats died or had to be excluded from the study because of short catheter life spans or improper cannulae placements. Therefore, statistical analyses were performed on data from nine of 12 rats in the cocaine self-administration experiment and from six of eight rats in the food self-administration experiment. For cocaine and food self-administration experiments, three dependent measures were recorded over the course of 1-hr maintenance test sessions: (1) the number of active lever responses made; (2) the number of inactive lever responses made (non-specific motor effects); (3) the number of reinforcers earned (cocaine infusions or food pellets). Data over the last five cocaine and saline discrimination sessions and the last five food self-administration sessions were averaged for individual rats and used as a measure of baseline responding. Averaging the last five sessions captures the day-today variation in responding and provides a better representation of an individual animal’s baseline rate of responding. For these data, paired t-tests were used to determine if rats discriminated between cocaine and saline during drug self-administration baseline sessions and if the baseline rate of responding maintained by food was altered by the vehicle control infusion into the AId. For cocaine maintenance and food maintenance testing, a series of one-way ANOVAs with repeated measures were used to analyze each dependent variable, with each ANOVA comparing vehicle infusions with SCH 23390 and/or SKF 81297 infusions. In addition, given that the time course of the effects of D1 receptor manipulation has been shown to be greatest at the onset of cocaine self-administration test sessions (Alleweireldt et al. 2006), the number of active lever responses emitted and infusions earned during the first and last 30min of each cocaine session was calculated from these dependent measures and similarly analyzed using single factor ANOVAs with repeated measures. Tukey tests were used for all post-hoc comparisons.
Results
Histology
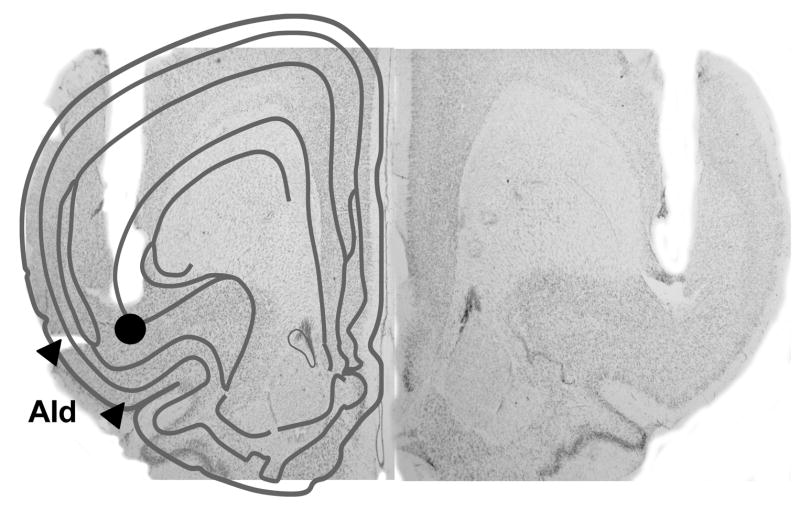
Histological verification of bilateral AId cannulae placements is depicted in Figure 2. For the fifteen rats used in the data analyses, cannulae were within 0.5 mm of the intended placement in the anterior-posterior plane, within 0.6 mm in the lateral plane, and within 0.4 mm in the dorsal-ventral plane.
Figure 2.
Representative photomicrograph of the extent of damage made by 22 gauge guide cannulae and the tip location of the 28 gauge infusion cannulae. A schematic representing a coronal section of the rat brain is superimposed on the left hemisphere to indicate the locations of the dorsal agranular insular area (AId). The circle indicates the location of D1 ligand or vehicle infusions. Guide cannulae were bilaterally positioned 1 mm above the infusion site at a level 2.8 mm anterior to bregma.
Drug Maintenance Testing
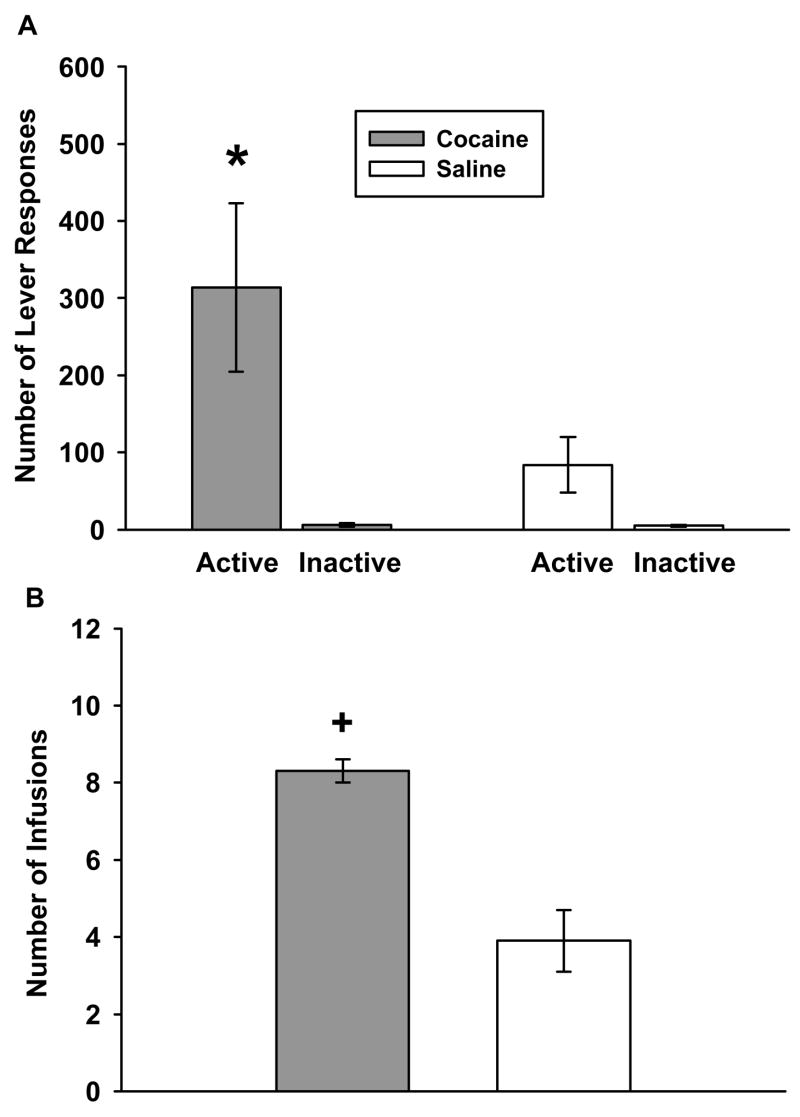
During baseline sessions, cocaine maintained significantly higher levels of active lever responding (Figure 3a) than saline (t(8)=3.067, p≤0.015). Inactive lever responding (Figure 3a) averaged fewer than 10 responses and was not significantly different under saline and cocaine baseline conditions. The number of infusions earned (Figure 3b) during baseline cocaine sessions was significantly greater than the number earned during baseline saline sessions (t(8)=7.438; p≤0.001). These data indicate that rats were able to successfully discriminate cocaine from saline and the active lever from the inactive lever prior to maintenance test sessions.
Figure 3.
Lever responding and cocaine consumption during 1-hr baseline self-administration sessions with cocaine or saline availability. Values are the mean ± SEM number of active and inactive lever responses (A) and number of infusions earned (B). * p < 0.05 compared to saline for active lever responses and + p < 0.05 compared to saline for infusions earned.
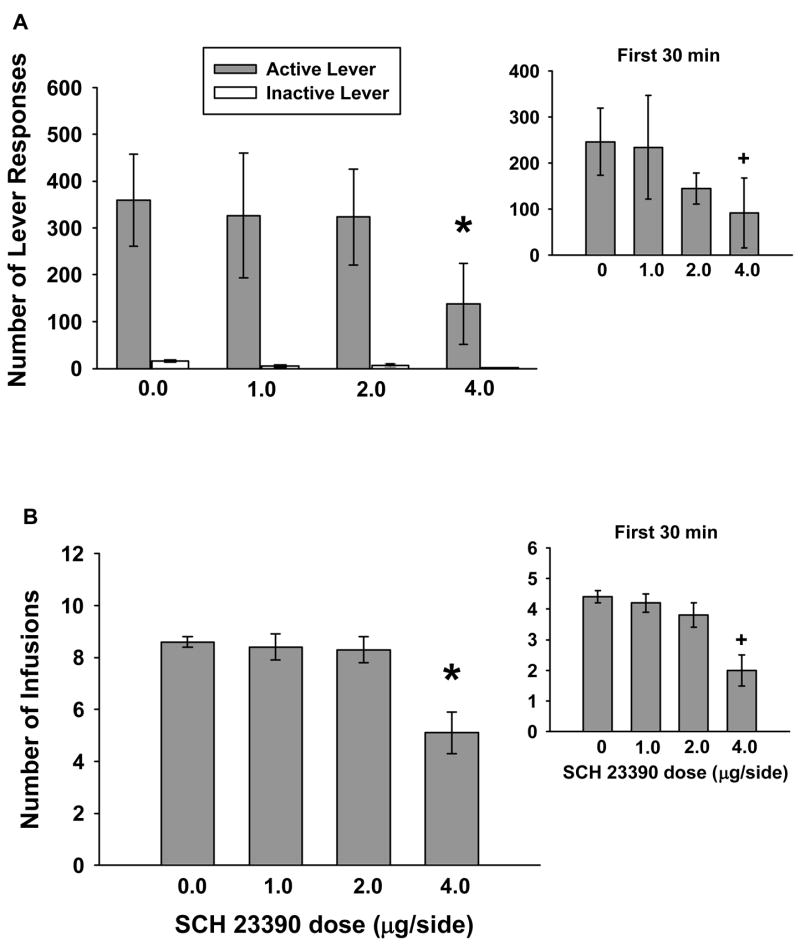
During maintenance test sessions, one-factor repeated measures ANOVA revealed that the number of active lever responses emitted over the course of 1-hr, was significantly altered (F(3,24)=14.646, p≤0.001) following infusion of the D1-like receptor antagonist SCH 23390 into the AId (Figure 4a). Tukey tests showed that active lever responding was significantly less (p≤0.05) following infusion with the highest dose of SCH 23390 (4.0 μg/side) compared to 0.0, 1.0 and 2.0 μg/side SCH 23390. In addition, the number of cocaine infusions earned during 1-hr test sessions (Figure 4b) was significantly altered as well (F(3,24)=15.929, p≤0.001). Post-test analyses indicated that cocaine infusions were significantly reduced (p≤0.05) following infusion with the 4.0 μg/side dose of SCH 23390 compared to 0.0 – 2.0 μg/side treatments. Inactive lever responding was not significantly altered following SCH 23390 infusions.
Figure 4.
Lever responding and cocaine consumption after 0.0–4.0 μg/side infusions of SCH 23390 within the AId during 1-hr drug maintenance test sessions. Values are the mean ± SEM number of active and inactive lever responses (A) and number of infusions earned (B). * p < 0.05 compared to 0.0 μg/side SCH 23390. Insets: Mean (± SEM) number of active lever responses (A) or infusions earned (B) during the first 30min of the 1-hr sessions. + p< 0.05 compared to 0.0 μg/side SCH 23390.
To determine the time course of action of the effects of SCH 23390 on active lever responding, one-factor repeated measures ANOVA during the first and last 30min of the 1-hr test sessions was carried out. These analyses revealed that SCH 23390 reduced active lever responses during the first (F(3,24)=3.723, p≤0.025) and last (F(3,24)=3.141, p≤0.044) 30min of the session, suggesting that the overall reductions in active lever responding occurred throughout the 1-hr test sessions. Post-hoc tests revealed that active lever responses during the first 30min (Figure 4a, inset) were attenuated following 4.0 mg/side SCH 23390 as compare to vehicle (p ≤ 0.05). During the last 30mins, infusion with the highest dose of SCH 23390 attenuated active lever responding as compared to infusion with 2.0 mg/side SCH 23390 (p ≤ 0.05). Similar examination of the number of cocaine infusions earned revealed that SCH 23390 significantly altered cocaine intake during the first 30min (F(3,24)= 9.925, p≤0.001) and last 30min (F(3,24)=5.733, p≤0.004). The highest dose of SCH 23309 produced a significant (p ≤ 0.05) reduction in the number of infusions earned compared to 0.0 – 2.0 μg/side treatments during the first (Figure 4b, inset) and last 30min of 1-hr test sessions.
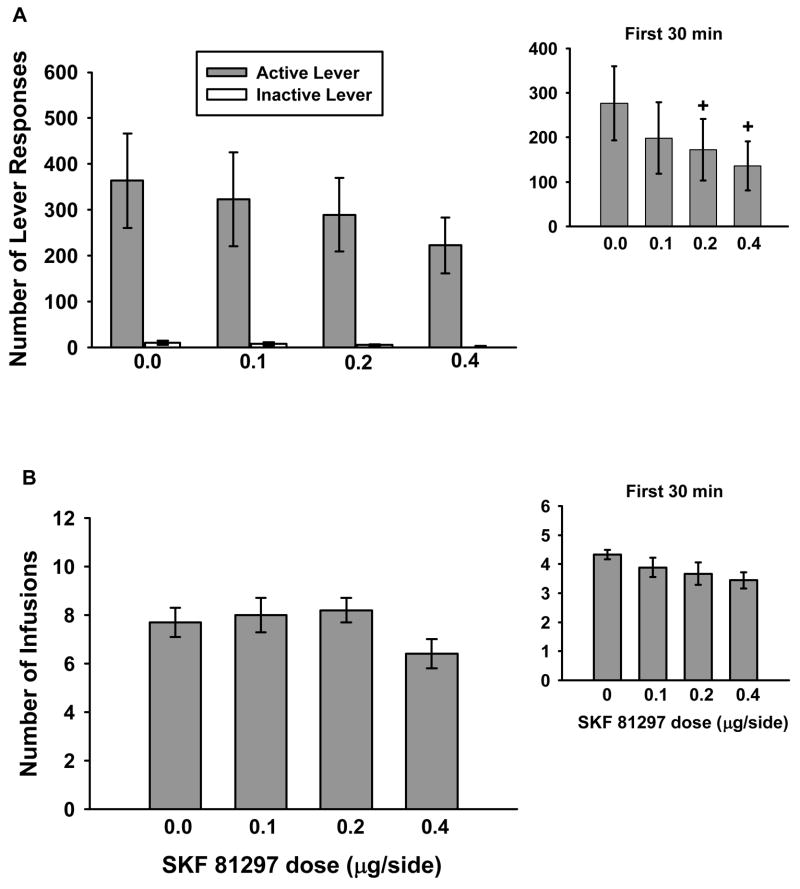
Infusion with the D1-like receptor agonist SKF 81297 into the AId did not significantly reduce active lever responding or cocaine intake over the course of entire 1-hr test sessions (Figures 5a and 5b), nor were inactive lever responses significantly altered following SKF 81297 infusions. However, one-factor repeated measures ANOVA analysis of the first and last 30min of the 1-hr session revealed that SKF 81297 did significantly alter active lever responding during the first 30min (Figure 5a, inset) of the session (F(3,24)=6.339, p≤0.003). Post-test analyses indicated that active lever responding was significantly attenuated following infusion with 0.2 μg/side (p≤ 0.05) and 0.4 μg/side (p≤ 0.05) doses of SKF 81297 as compared to vehicle (0.0 μg/side) during the first 30min. No significant differences were obtained for active lever responding during the last 30min. Lastly, similar examination of the number of cocaine infusions earned revealed that cocaine intake was not significantly altered within the first (Figure 5b, inset) or last 30min.
Figure 5.
Lever responding and cocaine consumption after 0.0–0.4 μg/side infusions of SKF 81297 within the AId during 1-hr drug maintenance test sessions. Values are the mean ±SEM number of active and inactive lever responses (A) and number of infusions earned (B). Insets: Mean (± SEM) number of activelever responses (A) or infusions earned (B) during the first 30min of the 1-hr sessions. + p< 0.05 compared to 0.0 μg/side SKF 81297.
Food Maintenance Testing
Paired t-tests revealed that baseline levels of active lever responses (477 ± 96), inactive lever responses (39 ± 8) and pellets earned (10 ± 0.1) did not significantly differ from levels measured after vehicle treatment within the AId. One-factor repeated measures ANOVA comparing vehicle to drug treatments revealed that active (F(2, 10)=14.6, p≤0.001) and inactive (F(2, 10)=10.9, p≤0.003) lever responding maintained by food was significantly altered as was the number of food pellets earned (F(2, 10)=20.05, p≤0.001). Tukey post-hoc tests indicated that active lever responses (118 ± 70), inactive lever responses (6 ± 1) and pellets earned (5 ± 1) following infusion with 4.0 μg/side SCH 23390 were significantly attenuated (p<0.05) as compared to vehicle and 0.4 μg/side SKF 81297 treatments, which did not differ statistically. Given that differences in active lever responding were greatest during the first 30mins of 1-hr cocaine self-administration sessions after SKF 81297, changes during 1-hr food-self-administration sessions were also examined during the first and last 30mins. One-factor repeated measures ANOVA comparing vehicle to drug treatments revealed that active lever responding was significantly altered during the first (F(2, 14)=8.3, p≤0.004) and last (F(2, 14)=9.7, p≤0.002) 30mins of food maintenance test sessions. Additionally, the number of food pellets earned during the first (F(2, 14)=14.6, p≤0.001) and last (F(2, 14)=17.3, p≤0.001) 30mins was significantly altered. Tukey post-hoc tests indicated that both measures were significantly attenuated (p<0.02) following infusion with 4.0 μg/side SCH 23390, but not 0.4 μg/side SKF 81297, as compared to vehicle treatment during the first and last 30min of the 1-hr sessions (Figure 6 a and b).
Figure 6.
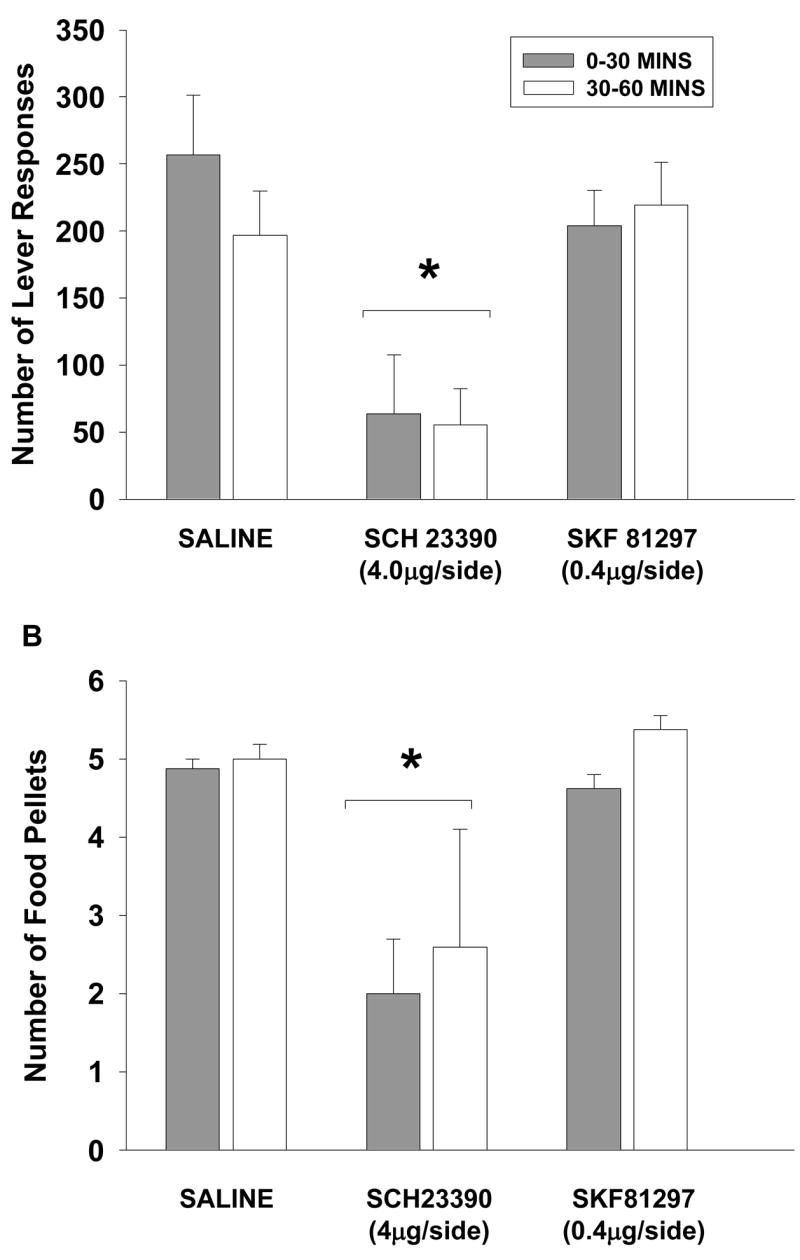
Lever responding and food consumption after vehicle, 4.0 μg/side SCH 23390 or 0.4 μg/side SKF 81297 within the AId during 1-hr food maintenance test sessions. Values are the mean (±SEM) number of active lever responses (A) and food pellets earned (B) during the first (left) and last (right) 30mins of the 1-hr sessions. * p < 0.05 compared to vehicle.
Discussion
Imaging and lesion/inactivation studies (Volkow and Fowler 2000; Porrino et al. 2002; Di Pietro et al. 2004; Fuchs et al. 2004) have indicated that orbital/insular areas of the PFC might be involved in cocaine addiction. However, the role of DA D1 receptors in these prefrontal sub-regions in mediating cocaine intake and responding maintained by cocaine and cocaine-related stimuli is unknown. Therefore, intra-AId infusions of the full D1-like receptor agonist SKF 81297 or the D1-like receptor antagonist SCH 23390 were administered to rats trained to self-administer cocaine under a second-order schedule. Our results showed that while cocaine intake remained unaffected, intra-AId infusions of 0.2 and 0.4 μg/side SKF 81297 reduced active lever responding during the first 30min of cocaine maintenance test sessions. Moreover, these effects were specific for responding maintained by cocaine and cocaine-related stimuli as infusion of the highest dose of SKF 81297 failed to alter lever responding maintained by food pellets or food consumption that was studied under an identical schedule of reinforcement.
These finding are interesting in light of recent evidence showing that infusions of 0.3 and 3.0 μg/side SKF 81297 into the NAc core and shell reinstated extinguished cocaine-seeking behavior but did not influence stabilized cocaine intake (Bachtell et al. 2005). Moreover, the effects of SKF 81297 in the NAc core were more transient than in the shell for facilitating lever responding, peaking at approximately 30 min after the start of reinstatement testing. Together, these data suggest that D1 receptor activation in the NAc core vs. the AId during test sessions may transiently alter responding in opposing manners while leaving cocaine consumption and food-maintained responding and consumption intact. Consistent with this idea, several studies report opposing influences of DA in the PFC and NAc whereby low extracellular DA levels in the PFC produce hyper-dopaminergic states in the NAc (Thompson and Moss 1995; King et al. 1997) and vice versa (Jackson et al. 2001; Jackson and Moghaddam 2001). Thus, activation of prefrontal AId DA D1 receptors may lead to a transient decrease in dopaminergic activity in the NAc core, a site believed necessary for drug-seeking behavior (McFarland and Kalivas, 2001). Anatomically, the AId sub-region has been shown to project to the NAc core while avoiding innervation of the shell (Wright and Groenewegen, 1996). Interestingly, stimuli paired with cocaine under a second-order schedule have been shown to increase extracellular levels of DA in the NAc core, but not the shell, whereas elevation of DA in the NAc shell is greater than in the core during cocaine self-administration (Ito et al. 2000). This suggests that the action of D1 receptor stimulation in the AId may be to reduce the motivating influence of cocaine-related stimuli on responding, particularly in light of the fact that SKF 81297 infusion into the AId did not alter cocaine intake in the present study.
Previous studies have reported reductions in cocaine-maintained responding following systemic injections of D1-like receptor agonists (Caine et al. 2000; Platt et al. 2001; Mutschler and Bergman 2002; Barett et al. 2004). The present findings therefore suggest that D1 receptors in the AId may be an important substrate for mediating the inhibitory effects of systemic administration of D1 agonists on cocaine-maintained responding. It remains uncertain, however, why the inhibitory effects of SKF 81297 are greatest at the onset of cocaine self-administration and then dissipate after 30mins. Further research is needed to elucidate the time course of action of SKF 81297 in the presence of cocaine and/or elevated levels of dopamine in the cortex.
Despite a disruption in lever responding maintained by cocaine and cocaine-related stimuli, cocaine intake was not altered following intra-AId infusions of SKF 81297. Again, these results are consistent with those of Bachtell et al (2005) showing no disruptions in cocaine intake following infusions with either D1 or D2 agonists in the NAc core or shell. Given that rats are self-administering cocaine throughout 1-hr test sessions, it is possible that the effects of cocaine on extracellular dopamine levels may override any additional effects of D1 agonist stimulation in the AId. Thus, activation of D1 receptors in additional brain sites may be necessary for changes in cocaine intake to occur. Alternatively, blockade of D1 receptors in the AId may be required to counteract the effects of cocaine-induced increases in extracellular dopamine.
Accordingly, D1 receptor antagonism in the AId with 4.0 μg/side SCH 23390 was sufficient to reduce the overall magnitude of active lever responding and cocaine intake within the same group of animals. However, given that food-maintained responding and consumption studied under an identical schedule of reinforcement were also significantly attenuated following infusion of 4.0 μg/side SCH 23390, it is likely that these behavioral changes reflect non-specific disruptions in motor responding similar to those reported by McGregor and Roberts (1993). Many studies have reported reduced responding maintained by food in monkeys and rats following systemic administrations of D1-like receptor agonists and antagonists (Woolverton and Virus 1989; Glowa and Wojnicki 1996; Weissenborn et al. 1996; Caine et al. 2000; Platt et al. 2001), indicating that the suppression of cocaine self-administration by systemically administered D1 receptor ligands is non-selective. However, in the study by Bachtell et al. (2005) it was shown that intra-NAc infusions of SCH 23390 (3 mg/side) failed to alter sucrose pellet self-administration in rats, suggesting that intra-cranial infusions of a D1-like antagonist could overcome global disruptions in motor responding. Unfortunately, intra-cranial infusion with 4 mg/side SCH 23390 in the current study produced significant disruptions in food-maintained responding and consumption. The disparity between our results and those of Bachtell et al (2005) for food self-administration likely reflect differences in the type of operant schedule used (FI5(FR5:S) versus FR1) and the brain region targeted. Moreover, it should be noted that the doses of SCH 23390 used in the current study are quite high and, as pointed out by others (Alleweireldt et al. 2006), any changes in behavior may reflect interactions with additional receptors in the AId such as 5-HT2C receptors (Millan et al. 2001) and G protein-coupled, inwardly rectifying potassium channels (Kuzhikandathil and Oxford 2002) for which SCH-23390 has an affinity. Given that SCH 23390 is a potent agonist at 5-HT2C receptors (Millan et al. 2001) and that 5-HT2C agonists have been shown to reduce food-maintained responding in squirrel monkeys (Brady and Barrett, 1985; McKearney, 1990), this may be why infusion of SCH 23390 but not SKF 81297 led to global disruptions in operant responding.
Others have shown that intra-cranial infusion of SCH 23390 within the medial PFC (0.3 μg/side), NAc shell (1.0 μg/side), or BLA (2.0 μg/side) reduces drug-seeking behavior induced by cocaine-associated cues or a priming injection of cocaine (See et al. 2001; Bachtell et al. 2005; Sun and Rebec 2005; Berglind et al. 2006), whereas infusion into the caudal BLA (2.0 μg/side) or NAc shell (1.0 μg/side) significantly increases the number of cocaine infusions earned during maintenance testing (Alleweireldt et al., 2006; Bachtell et al. 2005). In the present study however, intra-AId infusions with similar doses of SCH 23390 (i.e., 1.0 and 2.0 mg/side) did not yield any significant findings. Together with the food-maintenance data, it appears that D1 receptor inactivation within the AId with behaviorally selective doses (< 4.0 mg/side) is insufficient to selectively alter cocaine intake or lever responding under a second-order schedule.
Damage to orbital and insular cortices has been shown to block cue-induced reinstatement of drug-seeking behavior (Fuchs et al. 2004) and to disrupt the incentive value of anticipated reinforcers (Gallagher et al. 1999). The current data extend these findings to show that D1 receptors in the AId may help mediate the motivating influence of cocaine-related stimuli used to maintain responding at times when cocaine is not immediately available for self-administration. However, the finding that lever responding was selectively reduced following D1 receptor stimulation, but not D1 receptor inactivation, suggests dissociation between the effects of SCH 23390 and SKF 81297 within the AId. Recently, we reported that AId inactivation with the sodium channel blocker lidocaine did not alter responding maintained by cocaine and cocaine-related stimuli or its consumption in rats during 1-hr maintenance test sessions, nor did it alter drug-seeking behavior during cocaine prime-induced reinstatement test sessions (Di Pietro et al. 2006). However, in that report, intra-AId lidocaine infusions did attenuate reinstatement of drug-seeking behavior following presentation of cocaine-related stimuli alone. Thus, our findings suggest that in the presence of systemic cocaine, AId regulation of responding maintained by cocaine-related stimuli may be particularly sensitive to the effects of increased extracellular dopamine levels and subsequent dopamine receptor activity. Future studies examining the effects of D1 receptor manipulation on cue- and/or drug-induced reinstatement of drug-seeking behavior are needed to further explore this issue. Taken together, the current study adds to the rapidly growing body of evidence that lateral/orbital PFC sub-regions, in addition to the medial PFC, are key contributors to mediating behaviors related to cocaine addiction.
Acknowledgments
Grant Number R01DA011716 from the National Institute on Drug Abuse supported the project described. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Drug Abuse or the National Institutes of Health.
References
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology. 2006;31:363–374. doi: 10.1038/sj.npp.1300794. [DOI] [PubMed] [Google Scholar]
- Alleweireldt AT, Kirschner KF, Blake CB, Neisewander JL. D1-receptor drugs and cocaine-seeking behavior: investigation of receptor mediation and behavioral disruption in rats. Psychopharmacology. 2003;168:109–117. doi: 10.1007/s00213-002-1305-x. [DOI] [PubMed] [Google Scholar]
- Alleweireldt AT, Weber SM, Kirschner K, Bullock B, Neisewander JL. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2002;159:284–293. doi: 10.1007/s002130100904. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology. 2003;168:132–8. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology. 1998;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology. 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47:256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE. Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience. 2006;137:699–706. doi: 10.1016/j.neuroscience.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Brady LS, Barrett JE. Effects of serotonin receptor agonists and antagonists on schedule-controlled behavior of squirrel monkeys. J Pharmacol Exp Ther. 1985;235(2):436–41. [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK. Effects of dopamine D(1-like) and D(2-like) agonists on cocaine self-administration in rhesus monkeys: rapid assessment of cocaine dose-effect functions. Psychopharmacology. 2000;148:41–51. doi: 10.1007/s002130050023. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D(1-like) and D(2-like) agonists in rats that self-administer cocaine. J Pharmacol Exp Ther. 1999;291:353–360. [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1006–1015. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Complementary tasks to measure working memory in distinct prefrontal cortex subregions in rats. Behav Neurosci. 2004;118:1042–1051. doi: 10.1037/0735-7044.118.5.1042. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology. 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav Neurosci. 2001;115:934–939. [PubMed] [Google Scholar]
- Fuchs R, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowa JR, Wojnicki FH. Effects of drugs on food- and cocaine-maintained responding, III: Dopaminergic antagonists. Psychopharmacology. 1996;128:351–358. doi: 10.1007/s002130050144. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Dworkin SI, Smith JE. Neuropharmacological assessment of cocaine self-administration into the medial prefrontal cortex. Pharmacol Biochem Behav. 1986;24:1429–1440. doi: 10.1016/0091-3057(86)90206-6. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Reinforcing properties of cocaine in the medical prefrontal cortex: primary action on presynaptic dopaminergic terminals. Pharmacol Biochem Behav. 1986;25:191–199. doi: 10.1016/0091-3057(86)90252-2. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20(19):7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Moghaddam B. Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J Neurosci. 2001;21:676–681. doi: 10.1523/JNEUROSCI.21-02-00676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Frost AS, Moghaddam B. Stimulation of prefrontal cortex at physiologically relevant frequencies inhibits dopamine release in the nucleus accumbens. J Neurochem. 2001;78:920–923. doi: 10.1046/j.1471-4159.2001.00499.x. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance andreinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002a;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Stimulus-response functions of the lateral dorsal striatum and regulation of behavior studied in a cocaine maintenance/cue reinstatement model in rats. Psychopharmacology. 2002b;161:278–287. doi: 10.1007/s00213-002-1036-z. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology. 2000;148:251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Udo T, Ugalde F, Luzzo C, Di Pietro N, Eichenbaum HB. Influence of cocaine self-administration on learning related to prefrontal cortex or hippocampus functioning in rats. Psychopharmacology. 2005;181:227–236. doi: 10.1007/s00213-005-2243-1. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- Khroyan TV, Platt DM, Rowlett JK, Spealman RD. Attenuation of relapse to cocaine seeking by dopamine D1 receptor agonists and antagonists in non-human primates. Psychopharmacology. 2003;168:124–131. doi: 10.1007/s00213-002-1365-y. [DOI] [PubMed] [Google Scholar]
- King D, Zigmond MJ, Finlay JM. Effects of dopamine depletion in the medial prefrontal cortex on the stress-induced increase in extracellular dopamine in the nucleus accumbens core and shell. Neuroscience. 1997;77:141–153. doi: 10.1016/s0306-4522(96)00421-6. [DOI] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Oxford GS. Classic D1 dopamine receptor antagonist R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl- 2,3,4,5-tetrahydro-1H-3-benzaze pine hydrochloride (SCH23390) directly inhibits G protein-coupled inwardly rectifying potassium channels. Mol Pharmacol. 2002;62:119–126. doi: 10.1124/mol.62.1.119. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The Circuitry Mediating Cocaine-Induced Reinstatement of Drug-Seeking Behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Roberts DC. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Res. 1993;624:245–252. doi: 10.1016/0006-8993(93)90084-z. [DOI] [PubMed] [Google Scholar]
- McKearney JW. Effects of serotonin agonists on operant behavior in the squirrel monkey: quipazine, MK-212, trifluoromethylphenylpiperazine, and chlorophenylpiperazine. Pharmacol Biochem Behav. 1990;35(1):181–185. doi: 10.1016/0091-3057(90)90224-6. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Quentric Y, Cussac D. The ‘selective’ dopamine D1 receptor antagonist, SCH23390, is a potent and high efficacy agonist at cloned human serotonin2C receptors. Psychopharmacology. 2001;156:58–62. doi: 10.1007/s002130100742. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Bergman J. Effects of chronic administration of the D1 receptor partial agonist SKF 77434 on cocaine self-administration in rhesus monkeys. Psychopharmacology. 2002;160:362–370. doi: 10.1007/s00213-001-0976-z. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Duvauchelle CL. Prefrontal cortex D1 modulation of the reinforcing properties of cocaine. Brain Res. 2006;1075:229–235. doi: 10.1016/j.brainres.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Modulation of cocaine and food self-administration by low-and high-efficacy D1 agonists in squirrel monkeys. Psychopharmacology. 2001;157:208–216. doi: 10.1007/s002130100779. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22:7687–7694. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez CJ, Bailie TM, Wu WR, Li N, Sorg BA. Manipulation of dopamine D1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience. 2003;119:497–505. doi: 10.1016/s0306-4522(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology. 2002;163:327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci. 2006;23:219–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology. 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Li N, Wu WR. Dopamine D1 receptor activation in the medial prefrontal cortex prevents the expression of cocaine sensitization. J Pharmacol Exp Ther. 2001;297:501–508. [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology. 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- Thompson TL, Moss RL. In vivo stimulated dopamine release in the nucleus accumbens: modulation by the prefrontal cortex. Brain Res. 1995;686:93–98. doi: 10.1016/0006-8993(95)00429-t. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Deroche V, Koob GF, Weiss F. Effects of dopamine agonists and antagonists on cocaine-induced operant responding for a cocaine-associated stimulus. Psychopharmacology. 1996;126:311–322. doi: 10.1007/BF02247382. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Robbins TW, Everitt BJ. Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacology. 1997;134:242–257. doi: 10.1007/s002130050447. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Virus RM. The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacol Biochem Behav. 1989;32:691–697. doi: 10.1016/0091-3057(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Wright CI, Groenewegen HJ. Patterns of overlap and segregation between insular cortical, intermediodorsal thalamic and basal amygdaloid afferents in the nucleus accumbens of the rat. Neuroscience. 1996;73(2):359373. doi: 10.1016/0306-4522(95)00592-7. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]