Abstract
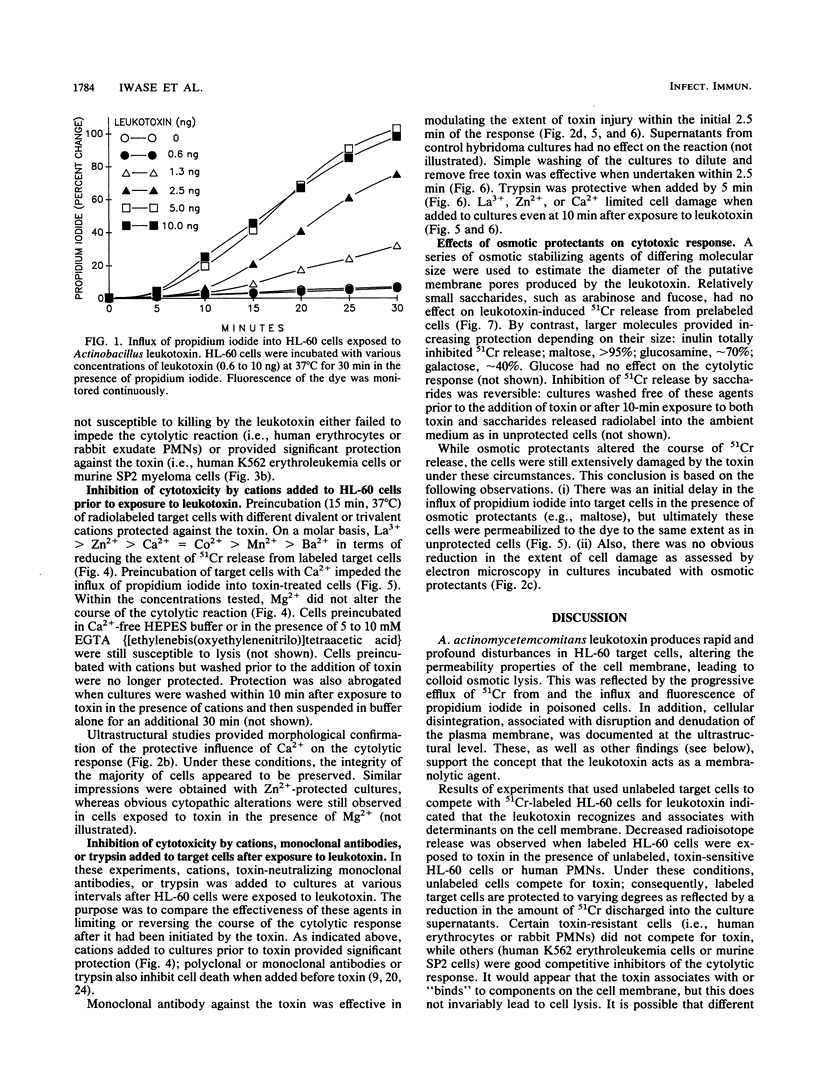
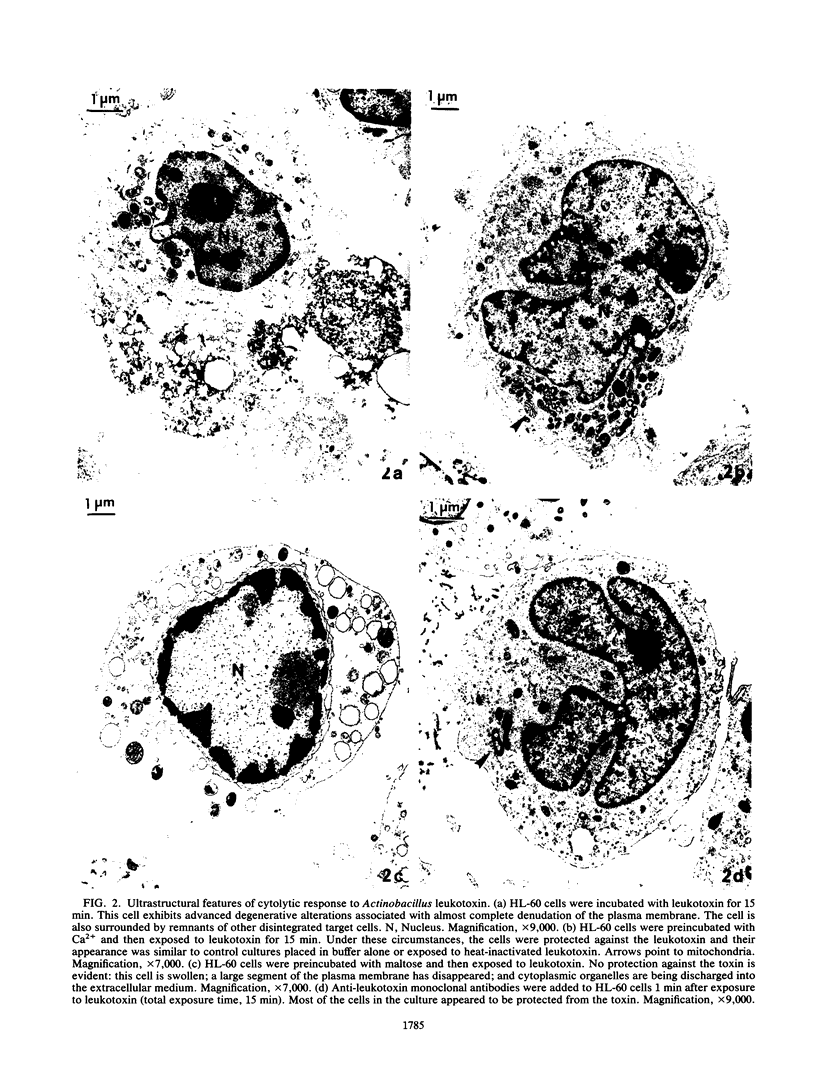
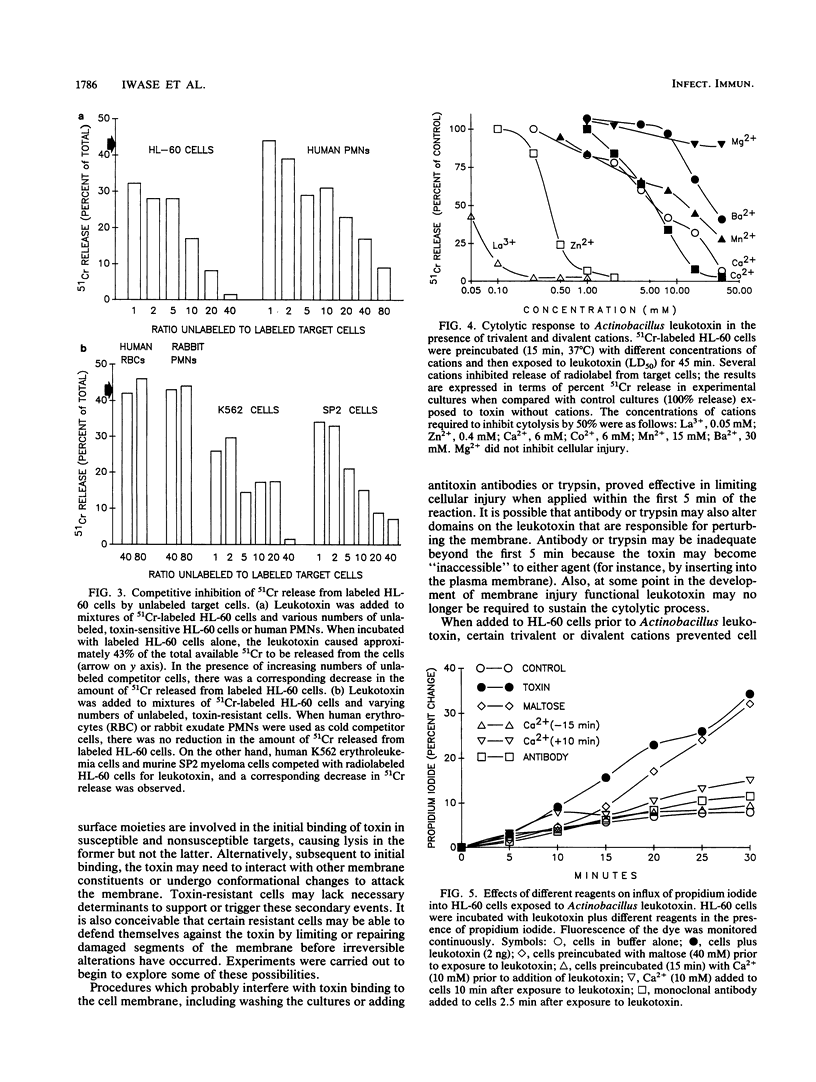
Actinobacillus actinomycetemcomitans leukotoxin permeabilized the plasma membrane of HL-60 promyelocytic leukemia cells, resulting in colloid osmotic lysis. These events were associated with efflux of 51chromium (from prelabeled cells), influx of propidium iodide, and ultrastructural evidence of cellular damage. Target cell lysis was inhibited by procedures which may interfere with the initial interaction of the toxin with the plasma membrane. For example, washing cultures (to dilute and remove toxin) or the addition of monoclonal antibodies (to neutralize toxin) or trypsin (to inactivate toxin) limited lysis when undertaken within the first 5 min of the reaction. The extent of injury was also diminished when radiolabeled HL-60 cells were exposed to toxin in the presence of unlabeled, toxin-sensitive cells (e.g., HL-60 cells or human neutrophils) or certain toxin-resistant target cells (e.g., human K562 erythroleukemia cells). This suggests that the association of the toxin with the cell membrane may not be sufficient to cause lysis without activation of additional effector mechanisms. The addition of specific trivalent (e.g., La3+) or divalent (e.g., Ca2+ and Zn2+) cations to toxin-treated cells appeared to enhance their capacity to repair or minimize the extent of toxin-mediated membrane damage. Depending on size, certain saccharides served as osmotic protectants: maltose almost completely inhibited radiolabel release, while smaller molecules provided correspondingly less protection. The results imply that the leukotoxin has membranolytic activity, producing pores in target cells with a functional diameter approximately the size of maltose (0.96 nm).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avigad L. S., Bernheimer A. W. Inhibition by zinc of hemolysis induced by bacterial and other cytolytic agents. Infect Immun. 1976 May;13(5):1378–1381. doi: 10.1128/iai.13.5.1378-1381.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashford C. L., Alder G. M., Menestrina G., Micklem K. J., Murphy J. J., Pasternak C. A. Membrane damage by hemolytic viruses, toxins, complement, and other cytotoxic agents. A common mechanism blocked by divalent cations. J Biol Chem. 1986 Jul 15;261(20):9300–9308. [PubMed] [Google Scholar]
- Bashford C. L., Menestrina G., Henkart P. A., Pasternak C. A. Cell damage by cytolysin. Spontaneous recovery and reversible inhibition by divalent cations. J Immunol. 1988 Dec 1;141(11):3965–3974. [PubMed] [Google Scholar]
- Bhakdi S., Greulich S., Muhly M., Eberspächer B., Becker H., Thiele A., Hugo F. Potent leukocidal action of Escherichia coli hemolysin mediated by permeabilization of target cell membranes. J Exp Med. 1989 Mar 1;169(3):737–754. doi: 10.1084/jem.169.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clinkenbeard K. D., Mosier D. A., Confer A. W. Transmembrane pore size and role of cell swelling in cytotoxicity caused by Pasteurella haemolytica leukotoxin. Infect Immun. 1989 Feb;57(2):420–425. doi: 10.1128/iai.57.2.420-425.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Tsai C. C., Shenker B. J., Taichman N. S., Lally E. T. Monoclonal antibodies to leukotoxin of Actinobacillus actinomycetemcomitans. Infect Immun. 1985 Jan;47(1):31–36. doi: 10.1128/iai.47.1.31-36.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Lee E. Y., Welch R. A. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol. 1985 Jul;163(1):88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodrubetz D., Dailey T., Ebersole J., Kraig E. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect Immun. 1989 May;57(5):1465–1469. doi: 10.1128/iai.57.5.1465-1469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally E. T., Kieba I. R., Demuth D. R., Rosenbloom J., Golub E. E., Taichman N. S., Gibson C. W. Identification and expression of the Actinobacillus actinomycetemcomitans leukotoxin gene. Biochem Biophys Res Commun. 1989 Feb 28;159(1):256–262. doi: 10.1016/0006-291x(89)92431-5. [DOI] [PubMed] [Google Scholar]
- Lo R. Y., Strathdee C. A., Shewen P. E. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica A1. Infect Immun. 1987 Sep;55(9):1987–1996. doi: 10.1128/iai.55.9.1987-1996.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake M., Honda T., Miwatani T. Effects of divalent cations and saccharides on Vibrio metschnikovii cytolysin-induced hemolysis of rabbit erythrocytes. Infect Immun. 1989 Jan;57(1):158–163. doi: 10.1128/iai.57.1.158-163.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer R., Gerhardt P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol. 1971 Sep;107(3):718–735. doi: 10.1128/jb.107.3.718-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. L., Berthold P., Taichman N. S. Killing of human myelomonocytic leukemia and lymphocytic cell lines by Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun. 1988 May;56(5):1162–1166. doi: 10.1128/iai.56.5.1162-1166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Extensive homology between the leukotoxin of Pasteurella haemolytica A1 and the alpha-hemolysin of Escherichia coli. Infect Immun. 1987 Dec;55(12):3233–3236. doi: 10.1128/iai.55.12.3233-3236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman N. S., Dean R. T., Sanderson C. J. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infect Immun. 1980 Apr;28(1):258–268. doi: 10.1128/iai.28.1.258-268.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman N. S., Simpson D. L., Sakurada S., Cranfield M., DiRienzo J., Slots J. Comparative studies on the biology of Actinobacillus actinomycetemcomitans leukotoxin in primates. Oral Microbiol Immunol. 1987 Sep;2(3):97–104. doi: 10.1111/j.1399-302x.1987.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., Shenker B. J., DiRienzo J. M., Malamud D., Taichman N. S. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect Immun. 1984 Feb;43(2):700–705. doi: 10.1128/iai.43.2.700-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]