Abstract
The onset of autoimmunity in experimental rodent models and patients frequently correlates with a lymphopenic state. In this condition, the immune system has evolved compensatory homeostatic mechanisms that induce quiescent naive T cells to proliferate and differentiate into memory-like lymphocytes even in the apparent absence of antigenic stimulation. Because memory T cells have less stringent requirements for activation than naive cells, we hypothesized that autoreactive T cells that arrive to secondary lymphoid organs in a lymphopenic environment could differentiate and bypass the mechanisms of peripheral tolerance such as those mediated by self-antigen cross-presentation. Here, we show that lymphopenia-driven proliferation and differentiation of potentially autoreactive CD8+ T cells into memory-like cells is not sufficient to induce self-reactivity against a pancreatic antigen. Induction of an organ-specific autoimmunity required antigen-specific CD4+ T cell help. Notably, we found that this function could be accomplished by memory-like CD4+ T cells generated in vivo through lymphopenia-induced proliferation. These helper cells promoted the further differentiation of memory-like CD8+ T cells into effectors in response to antigen cross-presentation, resulting in their migration to the tissue of antigen expression where autoimmunity ensued. Thus, the cooperation of self-reactive memory-like CD4+ and CD8+ T cells under lymphopenic conditions overcomes cross-tolerance resulting in autoimmunity.
Keywords: autoimmunity, T cell help, T cell homeostasis
Autoimmunity has been paradoxically associated with lymphopenia, a state in which reduced numbers of circulating lymphocytes are present, in a number of experimental rodent models and patients (1, 2). In humans, the association of T cell lymphopenia with autoimmunity has been described in patients with Sjogren's syndrome, rheumatoid arthritis, systemic lupus erithematosus, and Crohn's disease, among others (2). Lymphopenia may also occur after viral infections, as has been described in the case of HIV infection. In AIDS patients who undergo highly active antiretroviral therapy, the frequency of HIV-associated immune reconstitution inflammatory syndrome, a phenomenon that shares many characteristics with autoimmune processes, and rheumatoid arthritis and type 1 diabetes, is increased (3, 4). Importantly, the association between lymphopenia and the exacerbation of anti-self responses has recently been exploited to augment the responsiveness of T lymphocytes against cancer. Specifically, adoptive T cell immunotherapy targeting self-tumor antigens has been enhanced by prior lymphodepletion of melanoma patients (5).
Studies in murine models have been instrumental in furthering our understanding of the link between lymphopenia and the onset of autoimmunity. Indeed, thymectomy in neonatal mice and the transfer of low numbers of lymphocytes into irradiated, SCID or RAG−/− mice result in autoimmune processes (1). This type of studies served to unveil the role of regulatory lymphocytes in peripheral tolerance (6, 7). However, an absence of regulatory T cells is not the sole factor triggering autoimmunity (8). Under lymphopenic conditions, the expansion of residual conventional T cells, in the apparent absence of antigenic stimulation, is well established (9). In the case of naive T cells, T cell antigen receptor (TCR) interactions with MHC/self-peptide complexes (those that mediate positive selection) and the IL-7 cytokine appear to be required for this expansion (10–15). Recently, an IL-7 independent form of lymphopenia-driven proliferation has also been described (16). However, in all these cases, proliferating cells differentiate and acquire a memory-like phenotype and the ability to rapidly secrete effector cytokines (12, 17–19). Although memory-like T cells have never been activated by cognate antigen, do not pass through an effector phase, and, as such, cannot be considered to be “true” memory cells, recent reports demonstrate that they are functionally indistinguishable from memory cells (20). Therefore, it has been hypothesized that the proliferation and differentiation of potentially autoreactive T cells may result in the induction of autoimmunity in a lymphopenic environment (21). Indeed, the group of N. Sarvetnik elegantly demonstrated that nonobese diabetic (NOD) mice are mildly lymphopenic and that there is a strong correlation between lymphopenia-induced proliferation of autoreactive T cells and the onset of diabetes (22). Autoreactive CD8+ and CD4+ T cells displayed a memory phenotype, with CD8+ T cells having a major role in the pathogenesis of the disease (22).
The ensemble of these observations points to lymphopenia as a trigger of autoimmunity and to memory-like T cells, generated through homeostatic mechanisms, as pathogenic effectors. However, in complex models such as NOD mice, multiple genetic and environmental factors contribute to disease. Therefore, it is not known whether potentially autoreactive memory-like T cells are in themselves sufficient to induce organ-specific autoimmunity under lymphopenic conditions and whether CD8+ and CD4+ T cells cooperate in this task. Also, the mechanisms by means of which lymphopenia-induced proliferation and differentiation of potentially autoreactive T cells interfere with the normal mechanisms of peripheral self-tolerance have not been determined. To address these issues, we have used a well-characterized transgenic murine system consisting of 3 different mouse lines: InsHA mice express the hemagglutinin (HA) of the influenza virus under the control of the rat insulin promoter, driving its expression in the beta cells of the pancreas (23). Clone 4 TCR and HNT TCR transgenics express HA-specific MHC class I and class II-restricted TCRs, respectively (24, 25). After transfer into InsHA mice, naive Clone 4 CD8+ T cells recirculate through secondary lymphoid organs, but are unable to reach peripheral tissues and, hence, cannot directly enter in contact with pancreatic islets (26, 27). Under these conditions, the transferred CD8+ lymphocytes encounter HA antigen only in the draining lymph nodes (LNs) of the pancreas cross-presented by antigen presenting cells (APCs) (26, 27). Cross-presentation is a process by means of which professional APCs, in particular dendritic cells, acquire exogenous antigens, transport them to the lymphoid organs, and present them to T cells (28). In InsHA mice, the encounter between cross-presenting APCs and HA-specific T cells results in tolerance, a process that has been termed cross-tolerance, characterized by the abortive activation of the Clone 4 CD8+ T cells and their subsequent apoptosis (26).
Here, we show that the cotransfer of naive Clone 4 CD8+ T cells together with naive HNT CD4+ T helper cells into lympho-replete InsHA mice does not result in autoimmunity. This absence of reactivity is because the CD8+ T cells are tolerized through antigen cross-presentation even in the presence of helper cells (29). Notably, when InsHA mice are rendered lymphopenic the same potentially autoreactive CD8+ and CD4+ T cells differentiate into memory-like cells and cooperate to induce autoimmune diabetes overcoming cross-tolerance.
Results
Memory-Like CD8+ T Cells Are Not Sufficient to Induce Autoimmune Diabetes Under Lymphopenic Conditions.
To assess whether HA-specific Clone 4 CD8+ T cells undergo lymphopenia-induced proliferation, these cells were adoptively transferred into sublethally irradiated BALB/c mice. Naive Clone 4 CD8+ T cells proliferated under lymphopenic conditions in syngeneic recipients at a rate similar to that described for polyclonal naive CD8+ T cells [supporting information (SI) Fig. S1] (10). Proliferating cells acquired a phenotype resembling that of central memory T cells, CD44hi CD122+ CD25− CD62Lhi, but not that of effector cells as they failed to up-regulate CD25 (Fig. S1). However, proliferating CD8+ T cells did display effector function, as demonstrated by their ability to produce IFN-γ, albeit to a lesser extent than effector cells (Fig. S1). These results demonstrate that naive Clone 4 CD8+ T cells proliferate under lymphopenic conditions and differentiate into memory-like T cells.
Next, we sought to determine whether potentially autoreactive memory-like CD8+ T cells, generated under lymphopenic conditions, are able to induce autoimmune diabetes. To this end, we transferred naive Clone 4 CD8+ T cells into sublethally irradiated InsHA transgenic mice, in which the target HA antigen of Clone 4 cells is expressed at low levels in the beta cells of the pancreas. Our results show that the transfer of varying numbers of naive Clone 4 CD8+ T cells (range, 2 to 10 × 106) into irradiated InsHA mice was not sufficient to induce autoimmune diabetes during a 1-month period (Table 1). These results strongly suggest that memory-like CD8+ T cells are not in themselves sufficient to induce disease.
Table 1.
CD8+ and CD4+ T cells cooperate to induce autoimmunity under lymphopenic conditions
| Cell transfer* | Number of cells | Diabetes incidence† | Diabetes onset‡ |
|---|---|---|---|
| Recipient, irradiated InsHA | |||
| Clone 4 CD8+ | 2 × 106 | 0/11 | — |
| 3.5 × 106 | 0/6 | — | |
| 5 × 106 | 1/21 | d 12 | |
| 7 × 106 | 0/5 | — | |
| 10 × 106 | 0/6 | — | |
| HNT CD4+ | 2 × 106 | 0/10 | — |
| 3.5 × 106 | 1/8 | d 12 | |
| 5 × 106 | 0/18 | — | |
| 7 × 106 | 0/5 | — | |
| 10 × 106 | 2/10 | d 21 | |
| Clone 4 CD8+ + HNT CD4+ | 2 × 106 + 2 × 106 | 7/7 | d 14 ± 4 |
| 3.5 × 106 + 3.5 × 106 | 13/15 | d 14 ± 3 | |
| 5 × 106 + 5 × 106 | 14/14 | d 11 ± 1 | |
| Clone 4 CD8+ + DO11.10 CD4+ | 3.5 × 106 + 3.5 × 106 | 0/11 | — |
| Clone 4 CD8+ + InsHA CD4+ | 3.5 × 106 + 3.5 × 106 | 0/8 | — |
| Recipient, nonirradiated InsHA | |||
| Clone 4 CD8+ + HNT CD4+ | 5 × 106 + 5 × 106 | 0/8 | — |
*Purified transgenic CD8+ and/or CD4+ T cells, as indicated, were injected into either irradiated or nonirradiated InsHA mice.
†The onset of autoimmunity was evaluated by measuring blood glucose levels. Mice were followed over a 1 month period and were considered diabetic when levels were above 300 mg/dL in 2 consecutive measurements.
‡The day of disease onset (d) is indicated when applicable.
Memory-Like CD8+ and CD4+ T Cells Cooperate to Induce Autoimmune Diabetes Under Lymphopenic Conditions.
The important role of CD4 help for CD8+ T cell responses, including responses to self-antigens, has been well established (29–31). Therefore, it was important to determine whether CD8+ T cell responsiveness to HA under lymphopenic conditions would be modulated by the presence of antigen-specific CD4+ T cells. Notably, coinjection of HA-specific HNT CD4+ T cells together with Clone 4 CD8+ T cells does not result in autoimmunity in lympho-replete InsHA mice (see Table 1 and ref. 29). In marked contrast, cotransfer of naive Clone 4 CD8+ T cells with HNT CD4+ T cells, which also undergo lymphopenia-induced proliferation and differentiation into memory-like cells (Fig. S2), results in the onset of diabetes in the vast majority of mice between day 11 and 20 after transfer (34 of 36 mice, Table 1). This effect was not due to increased total T cell numbers, because even at the lowest dose tested, 2 × 106 Clone 4 + 2 × 106 HNT T cells, 100% of mice became diabetic (Table 1). None of the mice that received 10 × 106 Clone 4 CD8+ cells developed disease, and only 2 of 10 mice that received 10 × 106 HNT CD4+ cells showed any symptoms (Table 1). These results indicate that memory-like CD8+ T cells require the participation of memory-like CD4+ T cells to induce autoimmunity.
CD4+ T Helper Cells Promote the Accumulation of Autoreactive CD8+ T Cells Under Lymphopenic Conditions.
To shed light on the processes by means of which CD8+ and CD4+ T cells cooperate to induce autoimmune diabetes under lymphopenic conditions, we assessed proliferation, phenotype, and gain of effector function of donor T cells in lymphoid organs. The proliferation profiles, phenotype, and functionality of HNT CD4+ T cells were not significantly different when injected alone or together with Clone 4 CD8+ T cells (Fig. S3). In contrast, the in vivo fate of Clone 4 CD8+ T cells was modulated by the presence of HNT CD4+ T cells as described below.
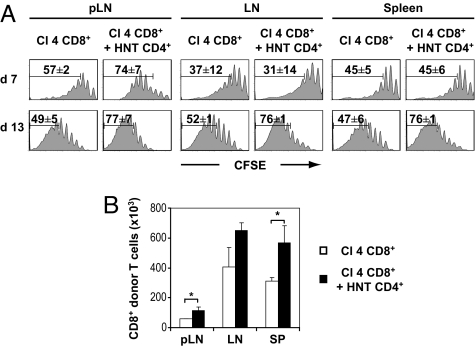
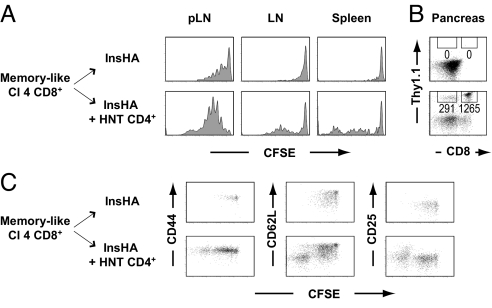
The 5- and 6-carboxy-fluorescein succinimidyl ester (CFSE) profiles of the donor CD8+ T cell population revealed an extensive proliferation in spleen, LNs, and pancreatic (p)LN on transfer into irradiated hosts (Fig. 1A). These results, which very much resembled those obtained in wild type mice (Fig. S1), strongly suggest that most of the observed proliferation is due to the condition of lymphopenia itself and not to the expression of the HA self-antigen in the pancreas. In the presence of CD4+ T helper cells, there was an increased proliferation of CD8+ T cells, translating into a significantly enhanced accumulation in the spleen and pLNs at day 13 (Fig. 1). In the draining LNs of the pancreas, the HNT-promoted enhanced proliferation of CD8+ T cells was already prominent at day 7 (Fig. 1A), indicating that this effect may be related to the presence of cross-presented HA antigen.
Fig. 1.
Proliferation of Clone 4 CD8+ T cells in irradiated InsHA mice is enhanced by CD4 help. (A) Irradiated InsHA mice were injected with either 5 × 106 CFSE-labeled naive Clone 4 Thy1.1+ CD8+ T cells (Cl 4 CD8+) or with a combination of 5 × 106 Clone 4 Thy1.1+ CD8+ T cells and 5 × 106 HNT Thy1.1+ CD4+ T cells (HNT CD4+). Mice were killed on days 7 or 13 after transfer and the levels of CFSE fluorescence on gated CD8+ Thy1.1+ donor lymphocytes in pLNs, LNs, and spleen are shown. The mean percentages (± SD, n = 3) of proliferating CD8+ donor T cells are indicated. (B) Total numbers of CD8+ donor T cells in these lymphoid organs were enumerated at day 13 posttransfer after the injection of Clone 4 CD8+ T cells alone (□) or together with HNT CD4+ T cells (■). Results are presented as means ± SD (n = 3). Data of 1 representative experiment out of 4 are presented.
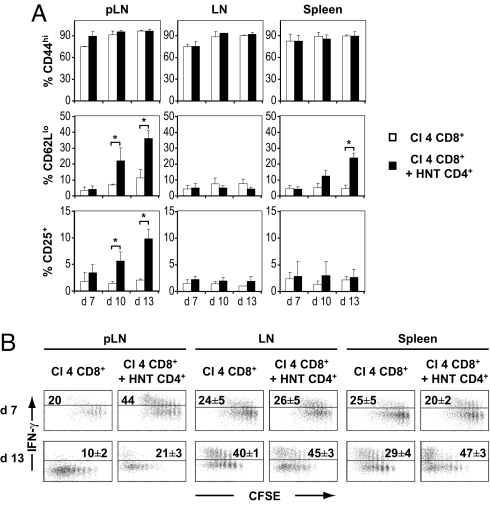
Because a modest increase in autoreactive CD8+ T cell proliferation was not in itself likely to be the cause of disease, we analyzed the phenotype and functionality of proliferating Clone 4 cells. The majority of un-helped CD8+ T cells displayed a typical central memory phenotype (CD44hi CD25− CD62Lhi) in LNs, pLNs, and spleen at all time points tested (7, 10, and 13 days after transfer; Fig. 2 and Fig. S4). In marked contrast, in the presence of HNT CD4+ T cells, there was an important subpopulation of highly proliferating CD62Llo CD25+ Clone 4 cells in the pLNs at days 10 and 13, but not in other LNs (Fig. 2 and Fig. S4). The generation of this effector phenotype subpopulation was associated with a 2-fold increase in effector function, as assessed by IFN-γ production (Fig. 2B). Intriguingly, a subpopulation of highly proliferating CD62Llo Clone 4 cells was also observed in the spleen of lymphopenic InsHA mice in the presence of helper cells, correlating with a 40% enhancement in effector function (Fig. 2 and Fig. S4). However, these cells were detected only at day 13 after transfer, a time point at which mice were already sick for a few days (Fig. 2). Thus, these cells are likely a consequence of the autoreactive attack rather than the cause, whereas the earlier accumulation of CD62Llo cells in the pLNs probably reflects the generation of effector cells after antigen encounter in the presence of help.
Fig. 2.
Proliferating Clone 4 CD8+ T cells differentiate into effectors in irradiated InsHA mice in the presence of help. (A) Seven, 10, and 13 days after transfer, expression of CD44, CD62L, and CD25 on CD8+ donor T cells was assessed in pLN, LN, and spleen of mice from the groups described in Fig. 1 by flow cytometry. The percentages of CD44hi, CD62Llo, and CD25+ CD8+ donor T cells in InsHA mice injected with Clone 4 CD8+ T cells alone (□) or together with HNT CD4+ T cells (■) are presented as means ± SD (n = 3–11). (B) On day 7 or 13 after transfer, cells from lymphoid organs were stimulated with Kd HA peptide. IFN-γ production by donor Clone 4 cells was assessed by intracellular staining and plots represent CFSE fluorescence versus IFN-γ on gated CD8+ Thy1.1+ lymphocytes. The percentages of IFN-γ-producing CD8+ donor T cells are indicated as means ± SD (n = 3–5 per group). Background in nonstimulated controls was <1%. Data from 1 representative experiment out of 4 are presented.
CD4+ T Helper Cells Promote the Migration of Autoreactive CD8+ T Cells to the Pancreas Under Lymphopenic Conditions.
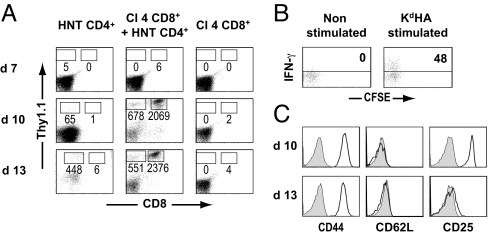
Collectively, the results presented above point to the adoptively-transferred CD8+ T cells as pathogenic effectors in our model. To corroborate this hypothesis, we assessed the presence of donor T cells in the pancreas at different time points after transfer. Although we were not able to detect any infiltrating donor CD8+ T cells in the pancreas of InsHA mice that had received only Clone 4 T cells, they were easily detected in diabetic and even prediabetic InsHA mice, when administered together with HNT CD4+ T cells (Fig. 3A). Analyses of these infiltrating CD8+ T cells showed that only the most highly proliferating cells had migrated to the site of antigen expression, and these cells also had high effector potential (Fig. 3B). Phenotypic analyses of the infiltrating Clone 4 cells in prediabetic or recently diagnosed diabetic mice (approximately day 10 post T cell transfer) revealed that they were CD44hi CD62Llo CD25+, a phenotype typical of effector cells. However, in mice that were hyperglycemic for 3 or 4 days and appeared sick (approximately day 13), the infiltrating Clone 4 CD8+ T cells were CD25− with a phenotype resembling that of effector memory cells (Fig. 3C). In contrast to CD8+ T cells, HNT CD4+ T cells infiltrated the pancreas of InsHA mice even when injected alone (Fig. 3A). This infiltration was expected based on our previous observation that proliferating HNT cells had a mixed effector-memory (CD62Llo)/central-memory (CD62Lhi) phenotype in lymphoid organs (Fig. S2 and Fig. S3). No significant difference was observed in the phenotype or functionality of infiltrating donor CD4+ T cells in the 2 conditions (data not shown). Together, our results indicate that CD4+ T cells promote the differentiation of Clone 4 CD8+ T cells into effector cells and the subsequent migration of these effectors into the pancreas mediates diabetes in InsHA mice.
Fig. 3.
Migration of Clone 4 CD8+ T cells to the pancreas of irradiated InsHA mice depends on CD4 help. (A) Irradiated InsHA mice were injected with either 5 × 106 CFSE-labeled naive HNT Thy1.1+ CD4+ T cells, 5 × 106 CFSE-labeled naive Clone 4 Thy1.1+ CD8+ T cells, or a combination of 5 × 106 Clone 4 CD8+ T cells and 5 × 106 HNT CD4+ T cells. Mice were killed on days 7, 10, or 13 after transfer and the presence of CD8+ Thy1.1+ and CD4+ Thy1.1+ donor T cells in the pancreas was evaluated by FACS. Numbers indicate FACS event counts in the depicted gates. (B) Cells from the pancreas were either stimulated with Kd HA peptide or left untreated and IFN-γ production was assessed by intracellular staining. Plots represent CFSE fluorescence versus IFN-γ on gated CD8+ Thy1.1+ lymphocytes. The percentages of IFN-γ secreting CD8+ donor T cells are indicated. (C) Expression of CD44, CD62L, and CD25 markers on gated CD8+ Thy1.1+ lymphocytes in the pancreas is shown at days 10 and 13. Shaded histograms show isotype controls. Data from 1 representative experiment of 5 are presented.
Antigen Specificity of CD4+ T Helper Cells.
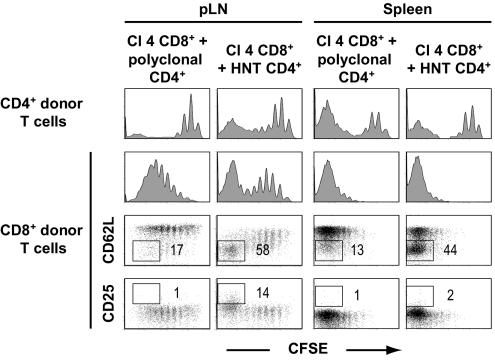
To determine whether the CD4+ T cell help necessary for the induction of autoimmunity functions in an antigen-specific fashion, we adoptively transferred CD4+ T cells with distinct TCR specificities. Specifically, CD4+ T cells from DO11.10 transgenic mice, which express a TCR specific for an I-Ad-restricted ovalbumin (OVA) epitope (32), and polyclonal CD4+ T cells from InsHA transgenic mice, which should be devoid of high-avidity HA-specific CD4+ T cells (23), were cotransferred with Clone 4 CD8+ T cells into lymphopenic InsHA mice. Importantly, none of the mice coinjected with DO11.10 cells or polyclonal CD4+ T cells from InsHA mice developed disease (Table 1). Phenotypic analyses of Clone 4 CD8+ T cells transferred with polyclonal CD4+ T cells revealed an absence of the CD62Llo and CD25+ subpopulations that characterized the diabetic mice (Fig. 4). Also, Clone 4 CD8+ cells that proliferated in the presence of nonspecific CD4+ T cells did not migrate to the pancreas (data not shown). The lack of helper activity by DO11.10 or InsHA CD4+ T cells was not due to their inability to become activated, because they underwent lymphopenia-induced proliferation and differentiation in the irradiated InsHA recipient mice (Fig. 4 and data not shown). These results demonstrate that the effect of helper T cells on the responsiveness of memory-like CD8+ T cells is antigen dependent.
Fig. 4.
Clone 4 CD8+ T cell-mediated autoimmunity in lymphopenic InsHA mice depends on antigen-specific CD4 help. Irradiated InsHA mice were injected with 3.5 × 106 Thy1.1+ Clone 4 CD8+ T cells together with either 3.5 × 106 InsHA Thy1.1+ CD4+ T cells or 3.5 × 106 HNT Thy1.1+ CD4+ T cells. Mice were killed on day 20 after transfer and proliferation of gated CD4+ Thy1.1+ and CD8+ Thy1.1+ lymphocytes cells in pLN, and spleen was assessed as a function of CFSE fluorescence (Top). Expression of CD62L and CD25 on gated CD8+ Thy1.1+ lymphocytes was assessed and the percentages of highly proliferating donor CD62Llo cells and CD25+ cells are indicated in each condition. Data from 1 representative experiment of 4 are presented.
CD4+ T Helper Cells Promote the Generation of Effector CD8+ T Cells Through Antigen Cross-Presentation and Their Migration to the Pancreas.
In InsHA mice, HA is cross-presented by professional APCs in the draining LNs of the pancreas (27). In the experiments described above (see Fig. 1), the CD4-enhanced proliferation of Clone 4 CD8+ T cells was preferentially observed in pLNs. This effect suggested that APCs may act as bridge between memory-like CD4+ and CD8+ T cells. However, in those experiments, it was difficult to specifically assess antigen-driven proliferation, because lymphopenia-induced proliferation was extensive in all lymphoid organs. To precisely investigate the role of CD4 help on the recognition of the HA antigen by memory-like CD8+ T cells, we designed double transfer experiments, in which homeostatic proliferation/differentiation was uncoupled from antigen recognition. InsHA mice were irradiated and half were injected with naive HNT CD4+ T cells. After 10 days, all mice were injected with lymphocytes from naive nonirradiated InsHA mice to “fill” the space and prevent any subsequent lymphopenia-induced proliferation. After resting for an additional 3 days, InsHA mice from both groups received CFSE labeled memory-like Clone 4 CD8+ T cells that had been generated by their prior transfer into irradiated BALB/c mice. At the time of secondary transfer, Clone 4 cells isolated from irradiated BALB/c mice had a CD44hi CD25− CD62Lhi phenotype. Five days later, InsHA mice were killed and proliferation of donor Clone 4 cells was assessed in the lymphoid organs. In the absence of help, memory-like Clone 4 CD8+ T cells proliferated exclusively in the pLNs (Fig. 5A). Proliferation was slow and only a small percentage of donor T cells had been activated after 5 days, likely reflecting the low amount of cross-presented antigen. Proliferating T cells did not recirculate to other lymphoid organs or migrate to the pancreas (Fig. 5). Thus, memory-like Clone 4 cells behaved very much as naive cells undergoing deletional tolerance in the pLNs (26). In marked contrast, CD4 help dramatically enhanced the proliferation of memory-like Clone 4 CD8+ T cells in the pLNs of irradiated/“refilled” InsHA mice (Fig. 5A). Also, CD4 help induced the differentiation of highly proliferating memory-like CD8+ T cells into effectors, as demonstrated by the dow-regulation of CD62L and the up-regulation of CD25 (Fig. 5C). Most importantly, proliferating Clone 4 cells, in the presence of CD4+ T cells, migrated to the pancreas and were also found in other lymphoid organs (Fig. 5). Therefore, antigen-specific CD4+ T helper cells enhanced the proliferation of memory-like CD8+ T cells in response to antigen cross-presentation and promoted their differentiation into effector cells capable of migrating to the site of antigen expression.
Fig. 5.
CD4 help enhances proliferation of memory-like Clone 4 CD8+ T cells in the pLNs of InsHA mice. (A) Irradiated InsHA mice were either injected with 5 × 106 CFSE-labeled naive HNT Thy1.1+ CD4+ T cells or PBS. On day 10, both groups of mice were refilled with 100 × 106 spleen and LN cells from unmanipulated InsHA mice. Three days later, the refilled InsHA mice received enriched CFSE-labeled memory-like Clone 4 Thy1.1+ CD8+ T cells, generated by injection of naive cells into irradiated BALB/c mice 13 days earlier. Five days after secondary transfer, InsHA mice were killed, and donor CD8+ Thy1.1+ lymphocytes from pLN, LN, and spleen were analyzed by FACS. Histograms represent CFSE fluorescence on gated CD8+ Thy1.1+ lymphocytes. (B) The presence of CD8+ Thy1.1+ and CD8− Thy1.1+ donor T cells in the pancreas was evaluated by FACS. FACS event counts in the depicted gates are indicated. (C) Plots represent expression of CD44, CD62L, and CD25 as a function of CFSE fluorescence on gated CD8+ Thy1.1+ lymphocytes in the pLN. Data from 1 representative experiment of 3 are presented.
Discussion
Understanding the checkpoints that prevent potentially autoreactive T cells from mounting efficient anti-self responses is essential for the rational design of new immunotherapeutic strategies against autoimmune diseases and cancer. Lymphopenia has been proposed as a putative trigger of autoimmunity and, conversely, as a promising tool for the development of anti-self tumor antigen T cell responses (1, 2, 33). It has long been known that the reduced numbers of regulatory cells associated with lymphopenia may promote autoimmunity (6, 7). The results that we present here demonstrate that, in this condition, additional mechanisms of peripheral tolerance, such as those mediated by self-antigen cross-presentation, are overcome.
Collectively, our data point to the following sequence of events in disease progression in InsHA mice. Potentially autoreactive naive CD8+ T cells that reach secondary lymphoid organs in a lymphopenic environment proliferate and differentiate into central memory-like T cells. In the absence of help, these memory-like CD8+ T cells do not gain access to the pancreas, as shown by our inability to detect them in this organ at all tested time points. However, as they recirculate to the draining LNs of the pancreas, they do encounter HA β cell antigen that is cross-presented by APCs. In the presence of memory-like CD4+ T helper cells, this encounter results in further proliferation and differentiation of the CD8+ T cells into effector cytotoxic T lymphocytes (CTL) that are then able to migrate to the pancreas, inducing autoimmune diabetes. It is important to note that the coinjection of HA-specific naive CD8+ and CD4+ T cells into lymphoreplete InsHA mice does not result in autoimmune diabetes, and naive CD8+ T cells do not differentiate into effectors after antigen encounter (29). This absence of reactivity is most likely due to the tolerization of both cell types through antigen cross-presentation (26, 29, 34). Therefore, under conditions of lymphopenia, the cooperation between CD4+ and CD8+ T cells bypasses one important checkpoint for autoreactive T cells in the periphery, namely cross-tolerization.
Memory-like Clone 4 CD8+ T cells, although they displayed considerable effector function, were not sufficient to induce autoimmunity under lymphopenic conditions on their own. This observation is in agreement with a previous report, showing that conventional antigen-driven memory Clone 4 CD8+ T cells are not able to induce disease in InsHA mice because they undergo deletional tolerance through antigen cross-presentation (35). Memory-like Clone 4 CD8+ T cells likely follow the same fate in InsHA mice. Our double transfer experiments showed that although these cells did undergo a few rounds of division, they did not accumulate as they proliferated and did not migrate elsewhere in the lymphoid tissue or pancreas, suggesting that they died in situ after activation.
Memory-like CD8+ T cells generated under lymphopenic conditions were modulated by memory-like CD4+ T helper cells, resulting in the generation of an efficient response against a self-antigen. Although previous studies determined that help for CD8+ T cells responding to self-antigens could be provided by in vitro activated CD4+ T cells (29, 30), our data show that the lymphopenic state itself drives the differentiation of CD4+ T cells into memory-like cells fully capable of providing help. This observation illustrates that autoimmunity can be induced without evoking a requirement for external activation events. Initially, CD4 help was thought to be required for the generation of primary CTL responses (36). Today, it appears that this is the case only as regards responses generated under noninflammatory conditions, such as for peptide immunization regimes or after cross-presentation of tumor or self-antigens (29, 37, 38). More recently, it has been shown that even under inflammatory conditions, such as those generated after viral infection, CD4+ T cells have an essential role in the generation of functional memory CD8+ T cells (38–41). Interestingly, in a recent report, Hamilton et al. (20) have shown that the generation of protective memory-like CD8+ T cells also requires the presence of polyclonal CD4+ T cells during homeostatic proliferation in the absence of cognate antigen. Here, we show that memory-like CD8+ T cells require antigen-specific CD4 help at the time of antigen encounter to differentiate into CTL and generate an efficient anti-self antigen response.
HNT CD4+ T cells proliferating under lymphopenic conditions displayed a heterogeneous phenotype with the generation of both central and effector memory-like cells. Thus, it was not surprising that they were able to migrate to the pancreas of InsHA mice even when injected alone. We had shown that activated HNT cells migrate to the pancreas, inducing peri-insulitis, but are not diabetogenic in themselves (29). Indeed, NHT CD4+ T cells activated under lymphopenic conditions also appear to be nondiabetogenic even if they are able to migrate to the pancreas. Although memory HNT CD4+ T cells do not induce diabetes, they could potentially induce limited damage in the islets resulting in the release of HA antigen. Larger amounts of available antigen could be the underlying mechanism by means of which they provide help and enhance the proliferation of CD8+ T cells. However, our data indicate that antigen release is neither the only nor the main mechanism involved. We had shown that increasing amounts of antigen presented by quiescent APCs enhances Clone 4 CD8+ T cell proliferation but not their differentiation into effectors (26). Notably, we find that the main effect of helper cells on CD8+ T cells occurs in the pLNs and is antigen dependent. Also, we show that CD4 help promotes the conversion of a tolerogenic signal to a priming signal for CD8+ T cells, inducing not only enhanced proliferation but also their differentiation into effectors, in the pLNs. This observation strongly suggests an implication of the cross-presenting APCs on this effect. These APCs may be activated by helper cells after encounter (42–44) or mediate a direct effect of CD4+ on CD8+ T cells responding to the same antigen (45–47).
The molecular pathways by means of which CD4+ T cells provide help for CD8+ T cell responses remain elusive. A major breakthrough was made when the role of the CD40/CD40L pathway in the licensing of cross-presenting dendritic cells by CD4+ T cells was shown (42–44). However, in certain models CD40 engagement cannot replace help and account for the full differentiation of CD8+ T cells into effectors (29, 48). In agreement with these latter observations, we found no major role for CD40L in the help provided by HNT cells to memory-like Clone 4 CD8+ T cells (C.L. and J.H., unpublished results). The finding that CD4+ help is partially or fully independent of CD40 engagement indicates the existence of additional, yet unknown, activation pathways.
Our results emphasize the importance of memory-phenotype T cells as targets for therapeutic intervention in autoimmunity and their potential implication in cancer immunotherapy. Cancer immunotherapy relies on the potential ability of CD8+ and CD4+ T cells to eliminate tumor cells (37). Because most identified tumor-associated antigens are self-proteins, responses against these antigens are subject to the same constraints. Therefore, taking advantage of the lymphopenic environment ensuing after currently used conditioning therapies to optimize antitumor T cell responses is a promising strategy (33). Conditions favoring the generation and cooperation of memory-like CD8+ and CD4+ T cells may help to overcome tolerance allowing the generation of efficient antitumor responses.
Materials and Methods
Mice.
BALB/c mice were purchased from Charles River and then bred at the Institut de Génétique Moléculaire de Montpellier. InsHA (23), Clone 4 TCR (24), HNT TCR (25) (kindly provided by Linda A. Sherman, The Scripps Research Institute), and DO11.10 (a gift from Pascale Plence and Christian Jorgensen, Institut National de la Santé et de la Recherche Médicale, Montpellier, France) (32), transgenic mice lines had been backcrossed with BALB/c mice for at least 10 generations. Clone 4 and HNT mice were then crossed with BALB/c Thy1.1+/+ for 2 generations to achieve homozygosity for Thy1.1. All mice used in these studies were between 8 and 16 weeks of age. Mice were propagated and maintained under specific pathogen-free conditions at the animal facility of the Institut de Génétique Moléculaire de Montpellier. All experimental procedures were approved by the local animal facility Institutional Review Board.
Preparation of CFSE Labeled Naive TCR Transgenic T Cells.
CD8+ T cells from Clone 4 TCR Thy1.1 transgenic mice were prepared from single cell LN suspensions by magnetic depletion of non CD8+ T cells by using the T cell isolation kit from Dynal, supplemented with an anti-CD4 mAb (clone 191.1), according to the manufacturer's instructions. CD4+ T cells from HNT Thy1.1, InsHA Thy1.1, and DO11.10 transgenic mice were similarly prepared by using an anti-CD8 mAb (clone 169.4) for the depletion of CD8+ T cells. T cell purity was >90%. Purified T cells (2 × 107 cells per mL) were labeled with 5 μM CFSE (Molecular Probes) in PBS for 10 min at 37 °C.
Induction of Lymphopenia by Irradiation.
BALB/c or InsHA mice were sublethaly irradiated (600 rads) by using a 60Cobalt source. Under these conditions, depletion of host T cells was ≈90% at 24 h after irradiation and 70% at 7 days. Mice were used for adoptive transfer experiments 24 h after irradiation, unless otherwise noted.
Adoptive Transfer.
Irradiated or nonirradiated InsHA and BALB/c mice were injected i.v. with either CFSE-labeled Clone 4 TCR Thy 1.1 CD8+ T cells or CFSE-labeled HNT TCR Thy 1.1 CD4+ T cells in PBS. In other experiments, mice were coinjected with equal numbers of CFSE-labeled Clone 4 TCR Thy 1.1 CD8+ T cells and CFSE-labeled HNT TCR Thy1.1, InsHA Thy1.1 or DO11.10 TCR CD4+ T cells. The numbers of injected cells are indicated for each experiment.
For double transfer experiments, InsHA mice were irradiated, and 24h later half of the mice received HNT TCR Thy1.1 CD4+T cells. All mice were refilled with a mixture of 100 × 106 spleen and LN cells from nonirradiated InsHA mice 10 days later. At day 13, the InsHA mice received secondary i.v. transfers of CFSE-labeled, enriched memory-like Clone 4 TCR Thy1.1 CD8+ T cells. These latter cells were obtained from the LNs and spleen of irradiated BALB/c mice that had been injected 13 days before with naive Clone 4 TCR Thy1.1 CD8+ T cells. Cells recovered from 1 single primary host were transferred into 1 secondary recipient mouse.
Diabetes Monitoring.
Mice were monitored for diabetes by measuring blood glucose every 4 days for a maximum period of 30 days with a Glucomatic ESPRIT Apparatus (Bayer). In some instances, daily measurements were performed on urine samples with Multistix 8 SG strips (Bayer). Animals were considered diabetic when glucose levels were >300 mg/dL during 2 consecutive measurements.
Flow Cytometry.
Flow cytometry was performed essentially as described in ref. 26. For details, see SI Methods.
Statistical Analyses.
Statistical significance was determined by using a Student's t test with a 1-tailed distribution and 2-sample equal variance. Data were considered to be statistically different (*) for P < 0.05.
Supplementary Material
Acknowledgments.
We thank Dr. Linda A. Sherman (The Scripps Research Institute, La Jolla, CA) for providing us with the HA transgenic mouse system and critically reading the manuscript, Drs. Pascale Plence and Christian Jorgensen (Institut National de la Santé et de la Recherche Médicale U844, Montipellier, France) for additional valuable mice, Dr. Thomas Stratmann for valuable discussions, Drs. Alfred Singer and Valérie Zimmermann for constructive comments on the manuscript, the MRI-RIO imaging platform (GIS-IBISA, Languedoc-Roussillon) for flow cytometry experiments, the T&TA core facilities for animal experiments, and in particular Chantal Jacquet for excellent technical assistance with mice. C.L. was supported by the Ligue Contre le Cancer-Region Languedoc-Roussillon. N.T. is supported by Institut National de la Santé et de la Recherche Médicale and a Contrat d'interface with the Centre Hospitalier Universitaire de Amiens. J.H. is supported by Institut National de la Santé et de la Recherche Médicale and a Contrat d'interface with the Centre Hospitalier Universitaire de Montellier. This work was supported by grants from the European Community, Association pour la Recherche contre le Cancer, the Association de langue française pour l'étude du diabète et des maladies métaboliques (J.H.), and the European Community Contract LSHC-CT-2005-018914 “ATTACK” (to N.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807743105/DCSupplemental.
References
- 1.Gleeson PA, Toh BH, van Driel IR. Organ-specific autoimmunity induced by lymphopenia. Immunol Rev. 1996;149:97–125. doi: 10.1111/j.1600-065x.1996.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 2.Khoruts A, Fraser JM. A causal link between lymphopenia and autoimmunity. Immunol Lett. 2005;98:23–31. doi: 10.1016/j.imlet.2004.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shelburne SA, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. Aids. 2005;19:399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 4.Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329–337. doi: 10.1016/s1568-9972(02)00086-1. [DOI] [PubMed] [Google Scholar]
- 5.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knoechel B, Lohr J, Kahn E, Abbas AK. The link between lymphocyte deficiency and autoimmunity: Roles of endogenous T and B lymphocytes in tolerance. J Immunol. 2005;175:21–26. doi: 10.4049/jimmunol.175.1.21. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 8.McHugh RS, Shevach EM. Depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002;168:5979–5983. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- 9.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 10.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 11.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kieper WC, Jameson SC. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc Natl Acad Sci USA. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 14.Tan JT, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viret C, Wong FS, Janeway CA., Jr Designing and maintaining the mature TCR repertoire: The continuum of self-peptide:self-MHC complex recognition. Immunity. 1999;10:559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- 16.Min B, Yamane H, Hu-Li J, Paul WE. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J Immunol. 2005;174:6039–6044. doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- 17.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murali-Krishna K, Ahmed R. Naive T cells masquerading as memory cells. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 21.Theofilopoulos AN, Dummer W, Kono DH. T cell homeostasis and systemic autoimmunity. J Clin Invest. 2001;108:335–340. doi: 10.1172/JCI12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 23.Lo D, et al. Peripheral tolerance to an islet cell-specific hemagglutinin transgene affects both CD4+ and CD8+ T cells. Eur J Immunol. 1992;22:1013–1022. doi: 10.1002/eji.1830220421. [DOI] [PubMed] [Google Scholar]
- 24.Morgan DJ, et al. CD8(+) T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–983. [PubMed] [Google Scholar]
- 25.Scott B, et al. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994;1:73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez J, Aung S, Redmond WL, Sherman LA. Phenotypic and functional analysis of CD8(+) T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J Exp Med. 2001;194:707–717. doi: 10.1084/jem.194.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan DJ, et al. Ontogeny of T cell tolerance to peripherally expressed antigens. Proc Natl Acad Sci USA. 1999;96:3854–3858. doi: 10.1073/pnas.96.7.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belz GT, Carbone FR, Heath WR. Cross-presentation of antigens by dendritic cells. Crit Rev Immunol. 2002;22:439–448. [PubMed] [Google Scholar]
- 29.Hernandez J, Aung S, Marquardt K, Sherman LA. Uncoupling of proliferative potential and gain of effector function by CD8(+) T cells responding to self-antigens. J Exp Med. 2002;196:323–333. doi: 10.1084/jem.20011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calzascia T, et al. CD4 T cells, lymphopenia, and IL-7 in a multistep pathway to autoimmunity. Proc Natl Acad Sci USA. 2008;105:2999–3004. doi: 10.1073/pnas.0712135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurts C, et al. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J Exp Med. 1997;186:2057–2062. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 33.Baccala R, Gonzalez-Quintial R, Dummer W, Theofilopoulos AN. Tumor immunity via homeostatic T cell proliferation: Mechanistic aspects and clinical perspectives. Springer Semin Immunopathol. 2005;27:75–85. doi: 10.1007/s00281-004-0196-9. [DOI] [PubMed] [Google Scholar]
- 34.Adler AJ, et al. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J Exp Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreuwel HT, Aung S, Silao C, Sherman LA. Memory CD8(+) T cells undergo peripheral tolerance. Immunity. 2002;17:73–81. doi: 10.1016/s1074-7613(02)00337-0. [DOI] [PubMed] [Google Scholar]
- 36.Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang JC, Livingstone AM. CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J Immunol. 2003;171:6339–6343. doi: 10.4049/jimmunol.171.12.6339. [DOI] [PubMed] [Google Scholar]
- 39.Janssen EM, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 40.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett SR, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 43.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 44.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 45.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 46.Castellino F, et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 47.Wong SB, Bos R, Sherman LA. Tumor-Specific CD4+ T Cells Render the Tumor Environment Permissive for Infiltration by Low-Avidity CD8+ T Cells. J Immunol. 2008;180:3122–3131. doi: 10.4049/jimmunol.180.5.3122. [DOI] [PubMed] [Google Scholar]
- 48.Lu Z, et al. CD40-independent pathways of T cell help for priming of CD8(+) cytotoxic T lymphocytes. J Exp Med. 2000;191:541–550. doi: 10.1084/jem.191.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.