Abstract
In four trace-conditioning experiments with rats, the influence on the blocking of differences between the blocking cue–unconditioned stimulus (US) and the blocked cue–US trace intervals was explored. Experiment 1 demonstrated blocking despite the blocked cue’s having a shorter trace interval than the blocking cue in both elemental (Phase 1) and compound (Phase 2) training. In Experiment 2, blocking was attenuated when the blocked cue had a longer trace interval than did the blocking cue in both elemental and compound training. In Experiments 3 and 4, the trace intervals of the two cues during compound training were matched (i.e., unlike in Experiments 1 and 2, neither had temporal priority). Blocking was attenuated when the blocking cue trace interval in the elemental phase was shorter (Experiment 3) or longer (Experiment 4) than the compound cue trace during compound training. The findings indicate that subjects encode interstimulus intervals, and they further suggest that cue competition is greatest when the competing cues have the same temporal information as the US.
The acquisition and expression of an association involves the memory representation of many conventional sensory attributes of the associated stimuli (e.g., shape, color, and size), as well as temporal information, such as stimulus duration. Similarly, associations between events encode not only that an event will occur, but also when and where it will occur. The representation of these attributes is important because they often influence stimulus control of behavior. The notion that organisms encode temporal relationships is not new. Pavlov (1927) observed that his dogs not only emitted a conditioned response to the conditioned stimulus (CS), but also—at asymptote—performed that response near the expected time of delivery of the unconditioned stimulus (US; i.e., inhibition of delay). This result suggests that animals learn the temporal characteristics of environmental events during Pavlovian conditioning. Moreover, such timing is the basis for understanding the temporal patterning of instrumental behavior supported by fixed interval schedules of reinforcement. However, most contemporary theories of associative learning do not acknowledge the importance of encoded temporal information (see, e.g., Mackintosh, 1975; Miller & Matzel, 1988; Pearce, 1987; Pearce & Hall, 1980; Rescorla & Wagner, 1972). Nevertheless, there are some notable exceptions (e.g., Church & Broadbent, 1991; Gallistel & Gibbon, 2000; Gibbon & Balsam, 1981; Killeen & Fetterman, 1988; Machado, 1997; Savastano & Miller, 1998).
If temporal information is an important component of a learned association, then why have the more popular theories of learning not seriously addressed the role of encoded temporal information? One potential explanation is that interest in the role of temporal factors was diverted by the widely cited findings of Kamin (1969) concerning cue competition effects. The term cue competition refers to the attenuation of behavioral control by a target cue at test due to the presence of a nontarget cue during training. This attenuation is seen in a number of different experimental designs, including blocking (Kamin, 1969), overshadowing (Pavlov, 1927), and the relative stimulus validity effect (Wagner, Logan, Haberlandt, & Price, 1968). In a prototypical blocking preparation, the blocking cue is paired with the US in an initial phase of treatment (i.e., blocking cue–US trials in Phase 1). The blocking cue is then compounded with the blocked cue and paired with the US in Phase 2. Training of the target (blocked cue) in compound with the pretrained blocking cue permits the blocking cue to effectively compete with the blocked cue. Thus, at the time of test, weaker responding to the blocked cue is observed relative to a control condition that had not received prior training with the blocking cue but that had received the same reinforced compound cue trials in Phase 2—an effect that generalizes across several paradigms (e.g., in autoshaping—see Rescorla & Durlach, 1981; in taste aversion—see Gillan & Domjan, 1977; in eyelid conditioning—see Wagner, 1969).
The discovery of blocking posed problems for contiguity theory and led many theorists to focus on cue interactions resulting from the surprise of the US (Kamin, 1969). The view that the US is less surprising because of the prior training of the blocking cue has been formalized in models such as that of Rescorla and Wagner (1972). Other models, such as those of Mackintosh (1975), Miller and Matzel (1988), Pearce (1987), Pearce and Hall (1980), and Wagner (1981), can also account for such cue competition, albeit through different mechanisms. However, all of these accounts emphasize whether the US is predicted rather than when it is predicted.
Despite reduced interest in temporal factors (such as contiguity) between the 1960s and 1980s due to the emergence of models that focused on whether the US is expected (e.g., cue competition), interest in the role of encoded temporal representations in the expression of behavior has recently regained momentum. Some modern so-called timing models suggest that temporal information concerning an association is encoded and contributes significantly to the expression of the learned association (see, e.g., Gallistel & Gibbon, 2000; Gibbon & Balsam, 1981; Savastano & Miller, 1998). Data supportive of this view have been reported from a large number of different laboratories (e.g., Arcediano, Escobar, & Miller, 2003; Balsam, Drew, & Yang, 2002; Goddard & Jenkins, 1988; Kirkpatrick & Church, 2003, 2004). However, very few studies have attempted to integrate topics such as cue competition and temporal encoding and to examine consequent issues such as the role of temporal specificity in blocking. Moreover, the data from this earlier work are mixed in regard to the effect on blocking of differences between the blocking and blocked cues in their temporal relations with the US. A potential explanation for these different findings is that most prior experiments investigated the role of the interstimulus interval (ISI) in cue competition by using a combination of delay, simultaneous, and backward preparations (e.g., Blaisdell, Denniston, & Miller, 1998) or delay and trace conditioning (e.g., Rick, 2005; Schreurs & Westbrook, 1982). That is, quantitative differences in the CS–US intervals had been confounded by either trace versus delay differences or forward, simultaneous, and backward conditioning procedural differences—all of which are qualitative. Thus, one might consider any observed differences in cue competition resulting from such experimental designs to be a function of procedural differences in assessing responding to a stimulus trained with a simultaneous relationship and a stimulus trained with a delay relationship (Arcediano & Miller, 2002; Savastano & Miller, 1998), or a stimulus trained with a delay relationship and trace relationship. Moreover, simultaneous and delay conditioning are thought to involve qualitatively different types of learning (i.e., stimulus–stimulus vs. stimulus–response learning, respectively; Rescorla, 1982) and switching between trace and delay conditioning between Phases 1 and 2 of a blocking procedure may attenuate blocking because of qualitative differences in the underlying neural structures that are engaged (e.g., Shors et al., 2001).
Using pigeons in an autoshaping procedure, Schreurs and Westbrook (1982) found evidence for the encoding of temporal information when they tested the notion that the expected time of US delivery is central to the blocking effect. Specifically, they changed the CS–US ISI between elemental training and compound training. During the elemental training in their Experiment 1, one group was presented with the 5-sec blocking cue followed by a trace interval (i.e., 7.5-sec interval between CS termination and US onset). Subsequently, the group trained on a 5-sec simultaneous compound composed of the blocking and blocked cues using a delay procedure (i.e., no trace interval but a 5-sec ISI [CS onset to US onset]), with CS duration constant between phases. In other words, in the elemental phase, these subjects were given food after a 7.5-sec interval, and, in the compound phase, the food was given immediately after the 5-sec CS terminated. Thus, exposure to two different ISIs between the two phases occurred. The subjects who were trained in this manner showed attenuated blocking as compared with a group that was trained in both phases with a delay procedure. The results suggest that decreasing the ISI between the elemental and compound training phases can attenuate blocking. Although these results appear to provide evidence that temporal information is encoded as part of the learned association, one alternative explanation is that the qualitative change from trace to delay or delay to trace allowed the target (i.e., blocked) cue to provide nonredundant information concerning the US, thereby attenuating blocking. A third account is that delay conditioning in their Phase 1 simply resulted in a stronger association than did trace conditioning. Thus, the change of the quantitative CS–US temporal interval per se may have been of little consequence.
Barnet, Grahame, and Miller (1993) altered the temporal relationship between the elemental and compound training phases in a blocking procedure by using rats in a conditioned suppression preparation. They observed stronger blocking effects when the temporal relationship between the elemental phase and the compound phase was the same as compared with when the temporal relationship between the two phases was different. Specifically, Barnet et al. (1993) trained subjects with the blocking stimulus alone using either a delay or a simultaneous procedure; then, they trained the compound with either a delay or a simultaneous procedure. They found greater blocking when the elemental and compound training both used either a delay or a simultaneous preparation, and weaker blocking when the elemental training was conducted using a delay procedure and the compound training was conducted using a simultaneous procedure, or vice versa. Consistent with this observation, Blaisdell et al. (1998) found overshadowing to be greater when the temporal relationships both between an overshadowed cue and the US and between its overshadowing cue and the US were qualitatively similar. In contrast, Kohler and Ayres (1982) examined the influence of temporal priority and different CS–US intervals on blocking, but failed to detect any influence of these factors on blocking (also see Maleske & Frey, 1979). However, the differences between phases in the CS–US intervals in Kohler and Ayres’s study were small as compared with the absolute CS–US intervals. Consequently, they may not have had adequate sensitivity.
These and related findings encouraged the formulation of the temporal coding hypothesis (Barnet, Arnold, & Miller, 1991; Matzel, Held, & Miller, 1988; Miller & Barnet, 1993; Savastano & Miller, 1998). Briefly stated, the tenets of the temporal coding hypothesis state that: (1) Contiguity alone is necessary for the formation of an association; (2) the temporal relationship between the associated events is automatically encoded as part of the association (i.e., temporal maps that link event representations are formed); and (3) temporal information plays a role in the nature, magnitude, and timing of the conditioned response. Furthermore, Tenet 4a states that temporal maps can be superimposed when two maps include a common element—thereby creating a temporal relationship between two elements that have never actually been paired (i.e., temporal information from different training situations can be integrated)—and Tenet 4b states that cues tested together will most strongly interact when their respective temporal maps predict the occurrence of USs or the nonoccurrence of USs at the same future moment in time. According to Tenet 4a of the temporal coding hypothesis, in conjunction with any model that anticipates blocking (see, e.g., Mackintosh, 1975; Miller & Matzel, 1988; Pearce & Hall, 1980; Rescorla & Wagner, 1972; Wagner, 1981), groups trained with compound CSs that share a common temporal relationship to a US should show maximal blocking because this should increase the redundancy of the competing cues. If organisms encode temporal information as part of the CS–US association and the temporal relationship were altered between phases, then they should be able to detect a difference in the temporal anticipation of the US on the basis of the blocking and blocked CSs, and, consequently, they should show an attenuation of blocking.
In order to assess whether temporal information per se encoded during training affects blocking, one should test changes solely within either a trace-conditioning or a delay-conditioning preparation. If animals are not only encoding temporal order during training but also encoding the specific interval between CS and US presentations, then changes in the length of the trace interval within a trace procedure should also attenuate blocking. Importantly, the dissertation work of Rick (2005) inspired our interest in this question.
EXPERIMENT 1
Experiment 1 was designed to test for temporally specific blocking in a procedure in which the trace interval between the blocking cue and the US remained constant across the elemental and compound training phases, but for the critical experimental group, the blocked cue had a shorter trace interval during compound training. Specifically, subjects were pseudorandomly assigned to one of four groups: 3–3 Block, 3–3 No Block, 15–3 Block, and 15–3 No Block. The experimental design is depicted in Table 1. Groups 3–3 No Block and 15–3 No Block controlled for possible overshadowing (which was minimized by making the blocked cue more salient than the blocking cue); no relevant Phase 1 treatment was administered to these animals. During Phase 1, these subjects received pairings of an irrelevant cue (B) with the US in order to equate experience with the US and the excitatory status of the training context. Group 3–3 Block was expected to exhibit maximal blocking because the trace intervals for CS A (the blocking cue) in Phases 1 and 2 were the same as those for CS X (the blocked cue) in Phase 2. Group 15–3 Block was expected to display a reduction in blocking due to the trace interval being different for CS X in Phase 2 relative to the trace interval for CS A in Phases 1 and 2. As mentioned previously, a difficulty in interpreting previous findings regarding temporal specificity in blocking is that most studies used a design that switched between delay to trace conditioning procedures or between simultaneous and forward procedures. The design of Experiment 1 controlled for such a change by using a forward trace procedure in which trace relationships existed between the blocking cue and the US during both phases of training, and between the blocked cue and the US during the compound training phase. If an attenuation of blocking occurs when the trace interval of the blocked cue and the US is different from the blocking cue—as predicted by the temporal coding hypothesis—then one might infer that effective blocking depends on a similarity in the temporal relationship between the blocked cue and the US in comparison with that between the blocking cue and the US.
Table 1.
Design of Experiment 1
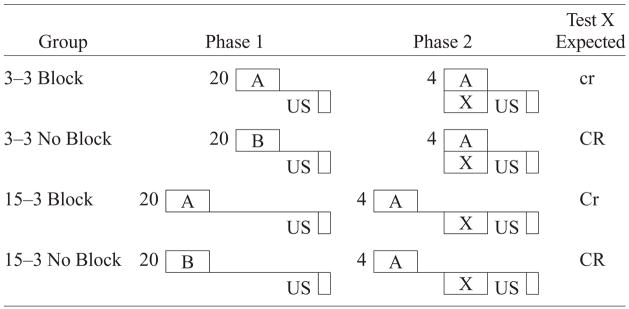
|
Note—The numbers 3 and 15 denote in seconds the time from CS termination to US onset (i.e., the trace interval). 3–3 indicates that CS A was trained with the same 3-sec trace interval in both phases, and 15–3 indicates that the CS X–US trace interval was shorter (3 sec) than the CS A–US trace interval (15 sec) in Phase 2. The number preceding the letters in Phase 1 and Phase 2 denotes the number of trials. CS X was the target (blocked) cue; CS A was the blocking cue; CS B was distinctly different from CS A. The expected magnitude of conditioned suppression to CS X is indicated in the right-hand column in the degree of capitalization: CR > Cr > cr.
Method
Subjects
The subjects were 24 male (257–315 g) and 24 female (180–210 g) adult, experimentally naive Sprague–Dawley rats (N = 48). They were individually housed in wire-mesh cages in a vivarium maintained on a 16:8-h light:dark cycle. From the time of weaning until the start of the experiment, all subjects were handled for 30 sec, three times per week. The experimental manipulations occurred approximately midway through the light portion of the diurnal cycle. A progressive water deprivation schedule was imposed over the week prior to the beginning of the experiment until water availability was limited to 30 min per day 1–4 h following treatment.
Apparatus
The apparatus consisted of 12 operant chambers, each measuring 30 × 30 × 27 cm (l × w × h). The sidewalls of each chamber were made of stainless steel sheet metal, and the front wall, back wall, and ceiling of the chamber were made of clear Plexiglas. On one metal wall of each chamber, there was an operant lever. Horizontally adjacent to that, a niche (4.5 × 4.0 × 4.5 cm) was centered 3.3 cm above the floor. Through the top of the niche, a solenoid-driven valve could deliver 0.04 ml of water into a cup at the bottom. Chamber floors were stainless steel grids that were 4 mm in diameter, spaced 1.7 cm apart center-to-center, and connected with NE-2 neon bulbs. This allowed delivery of a constant-current 0.65-mA footshock US by means of a high-voltage AC circuit in series with a 1.0-MΩ resistor. Each chamber was housed in its own sound- and light-attenuating environmental chest. Ventilation fans in each enclosure provided a constant 74-dB background noise. Background illumination was provided by a #1820 bulb positioned on the ceiling of the chambers. Each environmental chest was also equipped with three 45-Ω speakers that were mounted and widely separated on the inside walls of the environmental chest. One speaker mounted on the back sidewall of the environmental enclosure was used to deliver a click stimulus (6/sec) that was 6 dB (C-scale) above background. The click served as Cue X—the target (i.e., blocked cue). A second speaker mounted on the left sidewall of the enclosure was used to deliver a white noise stimulus that was 6 dB above background. A third speaker mounted on the right sidewall of the chamber was used to deliver a complex tone stimulus (400 and 1000 Hz) that was 6 dB above background. The tone and the white noise served as Cues A and B, counterbalanced within groups. A 0.5-sec visual stimulus consisting of a 75-W light (nominal at 120 VAC but driven at 100 VAC), mounted on the interior back side of each environmental chest approximately 30 cm from the center of the experimental chamber, served as a simultaneous signal for water delivery. Because treatment could have resulted in the training context differing between treatment groups with respect to ultimate associative strength, testing was conducted in a different context; that is, we trained each subject in a different context than that in which it was tested. Context 1—which always smelled of mint, always had a Plexiglas floor, and never had the houselight turned off—was used for leverpress shaping and testing. Context 2—which had no discrete odor cue, had a grid floor, and always had the houselight on—was used for conditioning. In Context 2, water was unavailable. In Context 1, the water delivery system was functional.
Procedure
Acclimation and shaping (Days 1–6)
On Days 1–5, all subjects were successively shaped to leverpress for water (0.04 ml) on a variable-interval 20-sec schedule during daily 60-min sessions in Context 1. In order to facilitate magazine training and lever-pressing, the onset of the water delivery was accompanied by the onset of a 0.5-sec visual stimulus: the 75-W light. On Days 1 and 2, a fixed-time 2-min schedule of noncontingent water delivery occurred concurrently with a continuous reinforcement of leverpressing. On Day 3, noncontingent water was discontinued, and subjects were trained on the continuous reinforcement schedule alone. Subjects that did not finish this session with more than 50 responses were hand shaped through successive approximation on that day. On Days 4 and 5, a variable interval 20-sec (VI–20) schedule was imposed. This schedule of reinforcement prevailed during reshaping and testing. On Day 6, subjects were exposed to Context 2, in which water reinforcement was not available. The purpose of acclimating the subjects to two separate contexts was so that training and testing could occur in distinctly different contexts, thereby reducing the potential influence at test of contextual fear, which might be differentially supported by the two trace-conditioning intervals, summating with a fear of CS X. That is, the longer trace interval potentially allows the US to make the training context more excitatory—an effect similar to that when the US is presented alone in a context (i.e., contextual conditioning; see, e.g., Balsam & Schwartz, 1981)—which could confound any observed differences in responding to the target cue between groups if testing occurred in the same context.
Phase 1 (Days 7–11): Competing association training
Subjects in Groups 3–3 Block and 15–3 Block received four daily presentations of A–US, whereas subjects in Groups 3–3 No Block and 15–3 No Block received four daily presentations of B–US, with an intertrial interval (ITI) of 15 min in a 60-min session in Context 2. All CSs were 3 sec in duration. The CS presentations occurred 10, 25, 40, and 55 min into the session. The onset of the US occurred either 3 sec (Groups 3–3 Block and 3–3 No Block) or 15 sec (Groups 15–3 Block and 15–3 No Block) after CS termination. The footshock US presentation lasted 0.5 sec.
Phase 2 (Day 12): Target training
During a 60-min session in Context 2, all subjects received four AX–US pairings, with a 15-min ITI. All CSs were again 3 sec in duration. Groups 3–3 Block and 3–3 No Block received a simultaneous presentation of CSs A and X. Groups 15–3 Block and 15–3 No Block received a serial presentation of CSs A and X, with the termination of CS A occurring 9 sec prior to onset of Cue X. The onset of the US occurred either 3 sec after the termination of Cue AX (Groups 3–3 Block and 3–3 No Block) or 15 sec after Cue A termination and 3 sec after Cue X termination (Groups 15–3 Block and 15–3 No Block).
Reacclimation (Days 13 and 14)
All groups experienced daily 60-min sessions in Context 1 in order to restabilize leverpressing. Doing so was intended to minimize any fear of the test context that might have been generalized from training. The animals that registered less than 50 responses on Day 13 were given an extra 30-min session on that day.
Test (Day 15)
Subjects were tested on CS X in a 30-min test session in Context 1. During the session, there were four presentations of X alone, each being 30 sec in duration. The presentations of CS X occurred 5, 10, 15, and 20 min into the session. The number of leverpresses emitted both during the 60 sec immediately prior to each test-trial CS onset and during the presence of the 30-sec test-trial CS were recorded. In order to compensate for any potential differences in base-rate leverpressing, Kamin suppression ratios were calculated to assess conditioned suppression to the CS. The suppression ratio for each subject consisted of the total number of leverpresses made during all four presentations of the CS divided by the sum of that number, plus half of the total number of leverpresses made during all four of the 60-sec intervals that immediately preceded each 30-sec CS (i.e., leverpress CS/[leverpressCS + 0.5 leverpress pre-CS]). A 60-sec pre-CS period was used because it provided a less variable baseline than would have been provided by a 30-sec pre-CS period. Thus, one suppression ratio was calculated for each subject.
Results and Discussion
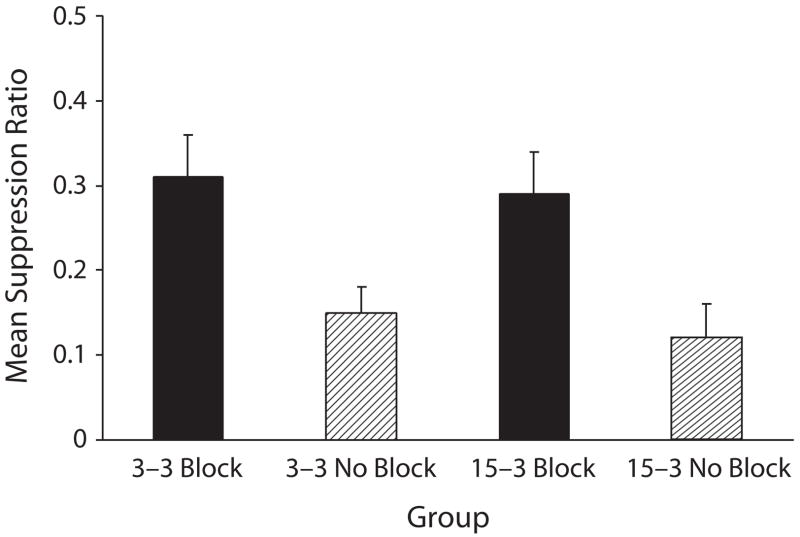
Experiment 1 failed to detect an attenuation of blocking when the blocked cue had a shorter trace interval than that used for the blocking cue, in comparison with blocking when both trace intervals were short (see Figure 1). This conclusion was confirmed by the following analyses. A 2 (blocking vs. no blocking) × 2 (3–3 trace intervals vs. 15–3 trace intervals) ANOVA was used to assess baseline lever-pressing just prior to onset of CS X during the test sessions. The means for Groups 3–3 Block, 3–3 No Block, 15–3 Block, and 15–3 No Block were 16.00, 16.98, 16.79, and 13.29 leverpresses per minute, respectively. The analysis of these means revealed no main effects or interactions (all ps > .05). A similar ANOVA of the suppression ratios was used to assess conditioned fear during the test presentations of CS X. This analysis revealed a main effect of blocking treatment [F(1,44) = 17.51, p < .01], but it did not reveal a main effect of the 3–3 versus 15–3 trace intervals or an interaction (Fs < 1). The lack of an interaction indicates that the difference between Groups 3–3 Block and 3–3 No Block in suppression to CS X did not differ significantly from the difference between Groups 15–3 Block and 15–3 No Block. A pairwise comparison between Groups 3–3 Block and 3–3 No Block revealed the occurrence of blocking when the trace intervals of the two phases were equally short [F(1,44) = 9.99, p < .01]. A second comparison between Groups 15–3 Block and 15–3 No Block determined that blocking also occurred in this condition [F(1,44) = 7.60, p < .01]. Thus, training CS A with a longer trace interval than that of CS X failed to appreciably attenuate blocking.
Figure 1.
Mean suppression ratio in Experiment 1 of the testing of Cue x (i.e., block of four trials). Group 3–3 Block was the group in which weak responding to x was expected because of the blocking of Cue x by Cue A. Conversely, Groups 15–3 Block, 3–3 no Block, and 15–3 no Block were those in which strong responding to Cue x was expected (see table 1). the error bars represent standard errors of the means.
EXPERIMENT 2
Experiment 1 demonstrated that making the trace interval between the blocked cue and US shorter than that between the blocking cue and US did not attenuate blocking. As was previously suggested, the temporal priority of the blocking cue (which might be expected to decrease suppression to CS X) seemingly trumped any possible attenuation of blocking because of the difference in temporal information provided by the blocking and blocked cue (which might be expected to increase suppression to CS X). If temporal priority is an important factor in serial blocking (i.e., A before X in Phase 2), then presenting the blocked cue with temporal priority over the blocking cue should prevent temporal priority from reducing suppression to CS X. This idea was examined in Experiment 2. Programming temporal priority for CS X with respect to the US in Phase 2 of Experiment 2 (see Table 2) could also result in temporal overshadowing of X by A, owing to A’s superior temporal contiguity with the US. However, since both Groups 3–15 Block and 3–15 No Block in Experiment 2 were subject to the same conditions with regard to temporal overshadowing, this possibility could be assessed. Group 15–15 Block was expected to exhibit more blocking than did Group 3–15 Block because the trace interval for CS A in Phases 1 and 2 was the same as that for CS X in Phase 2 for the former group. Group 3–15 Block was expected to display a reduction in blocking due to the trace interval for CS X in Phase 2 being different than that for CS A in Phases 1 and 2. Notably, an initial version of this experiment with n = 12 per group resulted in an interaction that approached significance (p < .13). A power analysis revealed that a significant interaction was likely if the sample size were doubled. Therefore, the experiment was replicated (n = 24 per group).
Table 2.
Design of Experiment 2
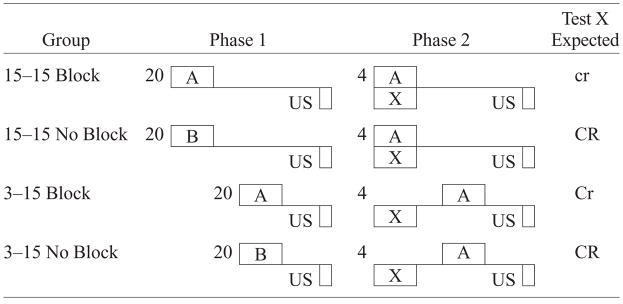
|
Note—The numbers 3 and 15 denote in seconds the time from CS termination to US onset (i.e., the trace interval). 15–15 indicates that CS A was trained with the same 15-sec trace interval in both phases, and 3–15 indicates that the CS X–US trace was longer (15 sec) than the CS A–US trace interval (3 sec) in Phase 2. The number preceding the letters in Phase 1 and Phase 2 denotes the number of trials. CS X was the target (blocked) cue; CS A was the blocking cue; CS B was distinctly different from CS A. The expected magnitude of conditioned suppression to CS X is indicated in the right-hand column in the degree of capitalization: CR > Cr > cr.
Method
Subjects and Apparatus
The subjects were 48 male (279–357 g) and 48 female (196–235 g) rats that were otherwise identical to those used in Experiment 1. The apparatus was the same as that used in Experiment 1.
Procedure
The procedure was the same as that in Experiment 1 except that instead of making the X–US trace interval short (3 sec) for all groups in Phase 2 (as was the case in Experiment 1), a longer trace interval (15 sec) was used for all groups, as indicated in Table 2.
Results and Discussion
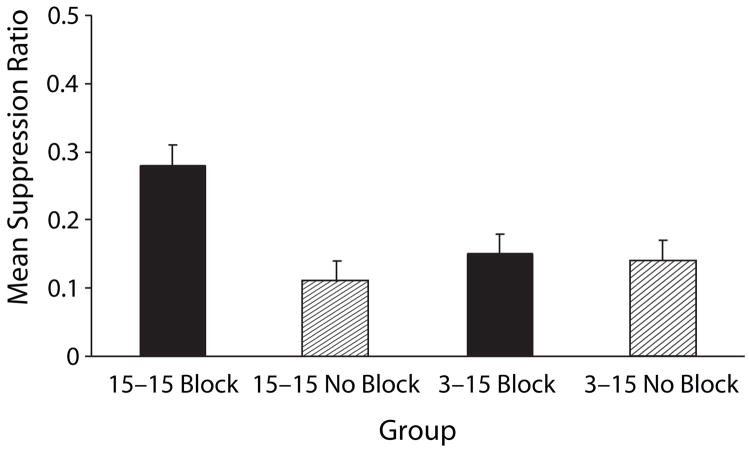
Experiment 2 determined that training the blocked cue with a longer trace interval in Phase 2 than that for the blocking cue attenuates blocking (see Figure 2). A 2 (blocking vs. no blocking) × 2 (15–15 trace intervals vs. 3–15 trace intervals) × 2 (replication) ANOVA was used to assess baseline leverpressing just prior to onset of CS X during the test sessions. The mean baselines for Groups 15–15 Block, 15–15 No Block, 3–15 Block, and 3–15 No Block were 18.90, 14.07, 16.31, and 14.01 leverpresses per minute, respectively. The analysis of these means revealed no main effects or interactions (all ps > .05). A similar ANOVA was conducted on the suppression ratios to assess conditioned fear during the test presentations of CS X. This analysis revealed a main effect of blocking treatment [F(1,88) = 10.39, p < .02] and, importantly, an interaction of blocking treatment and trace manipulation [F(1,88) = 7.02, p < .01], but no main effect of the trace manipulation [F(1,88) = 2.73, p > .10], replication [F(1,88) = 1.33, p > .25], or an interaction of replication with any other factor (all Fs < 0.45, ps > .50). The significant interaction between blocking treatment and trace manipulation suggests that the difference between Groups 15–15 Block and 15–15 No Block was greater than that between Groups 3–15 Block and 3–15 No Block. A pairwise comparison between Groups 15–15 Block and 15–15 No Block revealed blocking when the trace intervals of the two phases were equally long [F(1,88) = 17.24, p < .001]. A second comparison between Groups 3–15 Block and 3–15 No Block failed to detect significant blocking in this condition [F(1,88) = 0.16, p > .68]. Thus, the interaction in conjunction with the pairwise comparisons indicate that blocking was attenuated when the A–US trace interval was shorter than the X–US trace interval.
Figure 2.
Mean suppression ratio in Experiment 2 of the testing of Cue x (i.e., block of four trials). Group 15–15 Block was the group in which weak responding to x was expected because of the blocking of Cue x by Cue A. Conversely, Groups 3–15 Block, 15–15 no Block, and 3–15 no Block were those in which strong responding to Cue x was expected (see table 2). the error bars represent standard errors of the means.
EXPERIMENT 3
The results of Experiments 1 and 2 revealed contradictory effects of the blocked cue trace interval as compared with the blocking cue trace interval on the magnitude of blocking. Experiment 1 demonstrated that a shorter X–US trace interval in comparison with the A–US trace interval did not attenuate blocking. Experiment 2 demonstrated that a longer X–US trace interval in comparison with the A–US trace interval did attenuate blocking. The common factor of note in Experiments 1 and 2 was the opportunity for temporal priority. In Experiment 1, when the blocking stimulus had temporal priority in Phase 2 (i.e., the blocking stimulus occurred first during compound training), the difference in temporal information provided by X and A apparently became irrelevant, and blocking was observed. In Experiment 2, when the blocked cue had temporal priority in Phase 2 (i.e., the blocked cue occurred prior to the blocking cue during compound training), the temporal information provided by the blocked cue apparently was relevant, and blocking was attenuated. Hence, it is unclear whether the attenuated blocking in Experiment 2 was due to X’s temporal priority in Phase 2 or to the difference in the trace interval for A in Phase 1 as compared with that for X in Phase 2. However, the results of Experiment 1 implicate the temporal priority of X in Experiment 2 as being at least one critical factor. One way to differentiate between these two accounts would be to present A and X simultaneously in Phase 2, so there would be no difference in temporal priority. In this case, any difference in blocking would likely arise from the difference between A’s trace interval in Phase 1 and X’s trace interval in Phase 2. Experiments 3 and 4 were designed to answer this question. Specifically, Experiment 3 was conducted to assess the influence of a change from a long A–US trace interval in Phase 1 to a shorter A–US trace interval in Phase 2, with the onset of CSs A and X being synchronous during Phase 2 compound training. In order to make this assessment, subjects were pseudorandomly assigned to one of four groups: 3–3 Block, 3–3 No Block, 15–3 Block, and 15–3 No Block (see Table 3). It was expected that the difference between a long A–US trace interval in Phase 1 and a short X–US trace interval in Phase 2 would attenuate blocking of X by A so that stronger suppression to the blocked Cue X would be observed (Group 15–3 Block), as compared with a condition in which A’s trace interval in Phase 1 matched X’s trace interval in Phase 2 (Group 3–3 Block). That is, the attenuation of blocking was expected in the condition in which X in Phase 2 and A in Phase 1 had different temporal relationships with the US, but neither had temporal priority over the other in Phase 2.
Table 3.
Design of Experiment 3
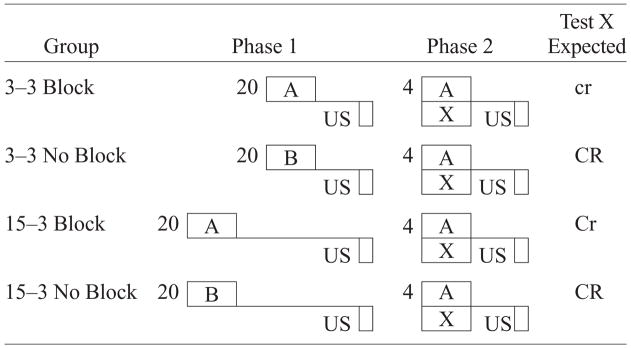
|
Note—The numbers 3 and 15 denote in seconds the time from CS termination to US onset (i.e., the trace interval). 3–3 indicates that subjects were trained with the same 3-sec trace interval in both phases, and 15–3 indicates that subjects were trained with a different trace interval in Phase 1 than in Phase 2. The number preceding the letters in Phase 1 and Phase 2 denotes the number of trials. CS X was the target (blocked) cue; CS A was the blocking cue; CS B was distinctly different from CS A. The expected magnitude of conditioned suppression to CS X is indicated in the right-hand column in the degree of capitalization: CR > Cr > cr.
Method
Subjects and Apparatus
The subjects were 24 male (269–329 g) and 24 female (188–246 g) rats that were similar to those used in Experiment 1. The apparatus was the same as that used in Experiments 1 and 2.
Procedure
The procedure was identical to that used in Experiment 1, except that in Phase 2, CS A was always simultaneous with CS X, which resulted in a shift between Phases 1 and 2 in the A–US trace interval for Groups 15–3 Block and 15–3 No Block, as indicated in Table 3.
Results and Discussion
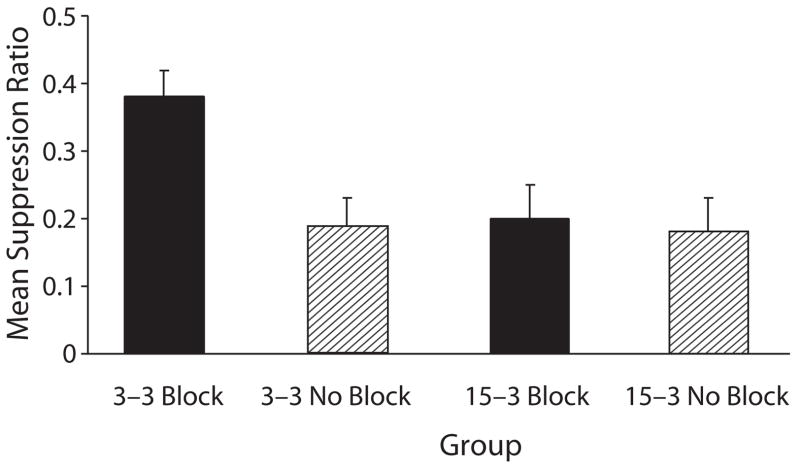
Experiment 3 revealed that a long A–US trace interval in Phase 1 and a shorter X (and A) trace interval in Phase 2 attenuated blocking (as compared with a condition in which all trace intervals were short), despite neither the blocking cue nor the blocked cue having temporal priority (see Figure 3). These conclusions were confirmed by the following analysis. A 2 (blocking vs. no blocking) × 2 (3–3 trace intervals vs. 15–3 trace intervals) ANOVA was used to assess baseline leverpressing just prior to onset of CS X during the test sessions. The means for Groups 3–3 Block, 3–3 No Block, 15–3 Block, and 15–3 No Block were 19.50, 17.96, 17.56, and 19.56 leverpresses per minute, respectively. The analysis of these means revealed no main effects or interactions (all ps > .05). A similar ANOVA was conducted on the suppression ratios to assess conditioned fear during the test presentation of CS X. This analysis revealed a main effect of blocking treatment [F(1,44) = 5.83, p < .05] and of the trace intervals differing between phases [F(1,44) = 4.80, p < .05], as well as an interaction [F(1,44) = 4.12, p < .05]. The interaction suggests that the difference between groups 3–3 Block and 3–3 No Block was different than that between Groups 15–3 Block and 15–3 No Block. A pairwise comparison between Groups 3–3 Block and 3–3 No Block revealed blocking when the trace intervals of the two phases were equally short [F(1,44) = 9.87, p < .01]. A second comparison between Groups 15–3 Block and 15–3 No Block found no significant blocking in this condition (F < 1). The interaction in conjunction with the pairwise comparisons indicate that blocking was attenuated by the A–US trace interval being longer in Phase 1 than the X–US (and A–US) trace interval in Phase 2.
Figure 3.
Mean suppression ratio in Experiment 3 of the testing of Cue x (i.e., block of four trials). Group 3–3 Block was the group in which weak responding to x was expected because of the blocking of Cue x by Cue A. Conversely, Groups 15–3 Block, 3–3 no Block, and 15–3 no Block were those in which strong responding to Cue x was expected (see table 3). the error bars represent standard errors of the means.
EXPERIMENT 4
Experiment 3 used a short trace interval for A in Phase 1 and a long trace interval for X in Phase 2, with the onsets of the pretrained and added stimuli being synchronous in the compound phase. Doing so resulted in the attenuation of blocking. However, in Experiment 3, the observed differences could be alternatively attributed to a difference in strength of the A–US association between the 3–3 and 15–3 trace interval conditions. Thus, the observed differences in responding to the blocked cue X between the two conditions were potentially a function of the stronger A–US association in the 3–3 trace interval condition producing better blocking. Experiment 4 was designed to address this alternative account of the attenuated blocking observed in Experiment 3. Specifically, in the 3–15 trace interval condition of Experiment 4, the blocking cue was trained with a short trace interval in Phase 1, whereas X (and A) were trained with a long trace interval in Phase 2. If blocking was found to be attenuated even when the A–US trace interval was short (i.e., with the blocking cue having superior temporal contiguity to the US), this would suggest that the attenuated blocking observed in both Experiments 3 and 4 reflected differences in the trace intervals between A in Phase 1 and X in Phase 2 rather than differences in the strength of the A–US association. The design is depicted in Table 4. The two control groups, 15–15 No Block and 3–15 No Block, controlled for possible overshadowing; no relevant Phase 1 treatment was administered to these subjects. Group 15–15 Block received blocking treatment with a 15-sec trace interval in both phases, and it was expected to exhibit maximal blocking because the trace interval for A in Phase 1 and the trace interval for X (and A) in Phase 2 were the same. Group 3–15 Block was expected to display a reduction in blocking due to the trace intervals being different in Phases 1 and 2 for CS A and CS X. Hence, the critical manipulation in Experiment 4 was the length of the trace interval for A in Phase 1, as compared with the long trace interval used for both X and A in Phase 2. Importantly, the short trace interval in Phase 1 for Group 3–15 Block should have—if anything—resulted in stronger learning with respect to CS X than for Group 15–15 Block. This factor would work against the prediction of less blocking in Group 3–15 Block on the basis of differences in the trace intervals for A and X. Assuming both factors were active, the question would be their relative magnitudes.
Table 4.
Design of Experiment 4
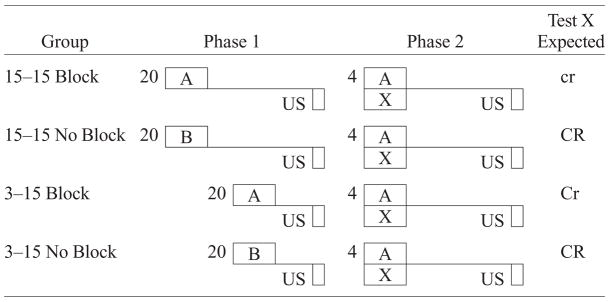
|
Note—The numbers 3 and 15 denote in seconds the time from CS termination to US onset (i.e., the trace interval). 15–15 indicates that subjects were trained with the same 15-sec trace interval in both phases, and 3–15 indicates that subjects were trained with a different trace interval in Phase 1 than in Phase 2. The number preceding the letters in Phase 1 and Phase 2 denotes the number of trials. CS X was the target (blocked) cue; CS A was the blocking cue; CS B was distinctly different from CS A. The expected magnitude of conditioned suppression to CS X is indicated in the right-hand column in the degree of capitalization: CR > Cr > cr.
Method
Subjects and Apparatus
The subjects were 24 male (208–312 g) and 24 female (182–225 g) rats that were otherwise identical to those used in Experiment 1. The apparatus was the same as that used in Experiments 1–3.
Procedure
The procedure was the same as that in Experiment 3 except for a change in the trace interval for both X and A during Phase 2. Instead of X and A being trained with a 3-sec trace interval in Phase 2, they were trained with a 15-sec trace interval, as indicated in Table 4. Thus, in the 3–15 trace interval condition, subjects were trained with a 3-sec trace interval in the elemental phase and a 15-sec trace interval in the compound training phase of the blocking procedure (i.e., Groups 3–15 Block and 3–15 No Block).
Results and Discussion
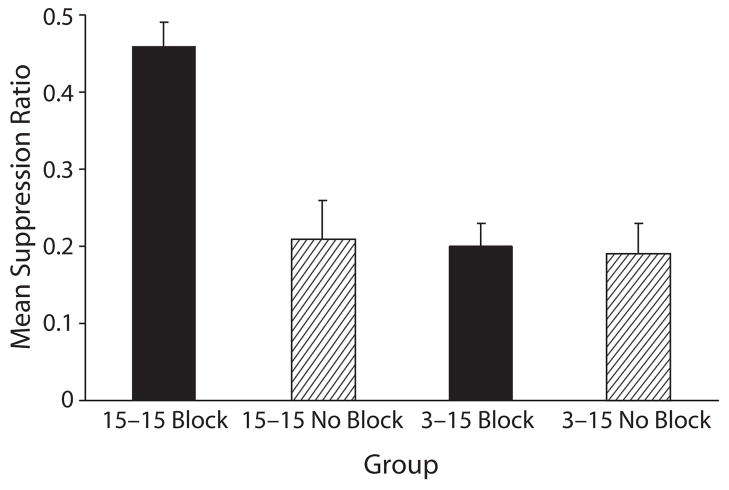
Experiment 4 determined that when X and A were trained with an equally long trace interval in Phase 2, less blocking resulted from A being trained in Phase 1 with a short trace interval than with a long trace interval (see Figure 4). This conclusion was confirmed by the following analysis. A 2 (blocking vs. no blocking) × 2 (15–15 trace intervals vs. 3–15 trace intervals) ANOVA was used to assess baseline leverpressing just prior to onset of CS X during the test sessions. The means for Groups 15–15 Block, 15–15 No Block, 3–15 Block, and 3–15 No Block were 16.69, 19.96, 17.65, and 18.27 leverpresses per minute, respectively. The analysis of these means revealed no main effects or interactions (all ps > .05). A similar ANOVA was used on the suppression ratios to assess conditioned fear during the test presentations of CS X. This analysis revealed main effects of blocking treatment [F(1,44) = 12.13, p < .01] and trace interval differences between Phase 1 and Phase 2 [F(1,44) = 14.80, p < .001], as well as an interaction [F(1,44) = 10.62, p < .01]. The interaction suggests that the difference between Groups 15–15 Block and 15–15 No Block differed from the difference between Groups 3–15 Block and 3–15 No Block. A pairwise comparison between Groups 15–15 Block and 15–15 No Block affirmed the blocking effect when the trace intervals for the two phases were equated [F(1,44) = 21.72, p < .001]. A second comparison between Groups 3–15 Block and 3–15 No Block found no significant blocking in this condition (F < 1). Thus, the interaction in conjunction with the pairwise comparisons indicate that blocking was attenuated when the trace interval for A in Phase 1 was shorter than the trace interval for X (and A) in Phase 2.
Figure 4.
Mean suppression ratio in Experiment 4 of the testing of Cue x (i.e., block of four trials). Group 15–15 Block was the group in which weak responding to x was expected because of the blocking of Cue x by Cue A. Conversely, Groups 3–15 Block, 15–15 no Block, and 3–15 no Block were those in which strong responding to Cue x was expected (see table 4). the error bars represent standard errors of the means.
GENERAL DISCUSSION
In the present experiments, an attenuation of blocking was expected when the trace intervals differed between the two phases of training as opposed to when they were equal. Experiments 1 and 2 assessed the blocking effect when the blocking cue trace interval in Phases 1 and 2 was different than that of the blocked cue trace interval in Phase 2. In Experiment 1, the blocking cue had a longer trace interval in both phases of training than did the blocked cue in Phase 2, but blocking was not attenuated. In Experiment 2, the blocking cue had a shorter trace interval in both phases than the blocked cue in Phase 2, and blocking was attenuated. One possible account of the results of Experiments 1 and 2 is that temporal priority in Phase 2 masked any possible effect on the blocking of differences between the A–US trace interval and the X–US trace interval. In Experiments 3 and 4, the trace intervals of the two cues during compound training were matched (i.e., neither had temporal priority). Blocking was attenuated when the blocking cue trace interval in the elemental phase was shorter (Experiment 3) or longer (Experiment 4) than the compound cue trace during compound training.
The lack of influence of the disparity in the trace intervals in Experiment 1 does not exclude the possibility that temporal relationships were encoded and had the potential to modulate blocking. It is possible that the X–US trace interval was encoded; however, presenting A prior to X during serial compound training gave A temporal priority over X, which encouraged blocking more than the discrepancy in the trace interval may have discouraged blocking. This view is consistent with the spirit (although not the specifics) of explanations of blocking by associative theories, such as that of Rescorla and Wagner (1972), which anticipate increased blocking when the competing cues convey greater amounts of redundant information—in this case, when the US will occur.
In Experiment 2, there had been a risk that attenuated blocking in Group 3–15 Block would not be observed because A likely had a stronger association with the US due to its superior contiguity with the US, thereby potentially leading to greater blocking. Additionally, the shorter A–US trace interval—in comparison with the X–US trace interval—favored overshadowing of X due to the superior temporal contiguity of A. However, strong responding to X in Group 3–15 No Block suggested that neither of these possibilities were obtained. Rather, attenuated overshadowing was observed in Group 3–15 Block, which could have resulted from temporal priority of X over A, or perhaps second-order conditioning in which suppression to X was augmented by X–A conditioning. That is, a second-order association could have developed in Experiment 2 between X and A because X occurred before A and because A presumably had a high associative strength due to its close temporal relationship with the US. The possibility of the temporal priority of X attenuating overshadowing in Experiment 2 is ruled out by the results of Experiment 3, and the results of Experiment 4 support the view that second-order conditioning is not the best explanation (these points are elaborated upon below). The findings of Jennings and Kirkpatrick (2006) provided an additional perspective on the results of the present Experiments 1 and 2. In their Experiments 2A and 2B, they manipulated the relative duration of the blocking CS in an appetitive blocking procedure with rats and found that a long CS blocked a short CS, but that a short CS did not block a long CS, regardless of relative CS duration. Jennings and Kirkpatrick interpreted their results based on the Sutton–Barto (1990) temporal difference model. This model proposes that CS onset produces a cascade of activation of individual representational units. The strength of activation of each unit at the time of reinforcement determines the strength of conditioning to that unit. Units that have closer temporal proximity to the US form the strongest association. Applied to the findings of Jennings and Kirkpatrick, their observed differences could have been due to the long CS providing novel units to be associated with the US. That is, these novel units may have occupied a temporal space not previously occupied by the short CS, and the new temporal information may have allowed the longer CS to become a better predictor of the US. It is possible that a similar mechanism was functioning in the present Experiments 1 and 2, although the CSs were of the same duration. For example, in Experiment 1, the shorter blocked cue–US trace interval—although it was closer to the US—did not occupy a novel temporal space. This may have allowed blocking to occur. Conversely, in Experiment 2, the longer blocked cue–US interval did occupy a novel temporal space, which may have allowed the blocked cue to be better conditioned. Hence, blocking was attenuated. In other words, the longer intervals may have made more representational units available to be conditioned, thereby resulting in better conditioning of that interval. However, this account, which actually provides a mechanistic basis for the previously mentioned temporal priority account of Experiments 1 and 2, is not readily applicable to Experiments 3 and 4 because A and X had the same relationship to the US in Phase 2.
In Experiments 3 and 4, A and X were presented simultaneously in Phase 2. Doing so eliminated differences between A and X in temporal priority, but introduced a difference in A’s trace interval between Phases 1 and 2. In Experiment 3, subjects in the condition with different trace intervals were trained with a longer A–US trace interval in the elemental phase and a shorter blocking cue A–US trace in the compound phase. The difference in A’s Phase 1 trace interval and X’s Phase 2 trace interval in Group 15–3 Block seemingly attenuated blocking, as compared with Group 3–3 Block, in which the trace interval associated with CS A was the same as that of CS X. However, an alternative explanation of Experiment 3 is that the observed differences between the two conditions could be attributed to stronger learning to the blocking cue with the same trace interval, because it had better contiguity to the US in Phase 1 than it did during Phase 1 of the condition with different trace intervals. Thus, the results of Experiment 3 suggest—but do not provide compelling evidence—that differences between A’s trace interval in Phase 1 and X’s trace interval in Phase 2 attenuated blocking. Notably, the Rescorla and Wagner (1972) model can explain such effects because the longer trace interval in Phase 1 of the 15–3 condition potentially made the association formed between A and the US weaker, which allowed X to acquire more associative strength during Phase 2.
Experiment 4 was designed to eliminate the potential confound arising from A’s superior contiguity to the US in Experiment 3. In Experiment 4, Group 3–15 Block CS A was trained with a shorter trace interval in Phase 1 than was the case in Group 15–15 Block. Thus, the view that more blocking should result from better contiguity between A and the US is diametrically opposed to the attenuation of blocking expected in the condition with the trace interval for A in Phase 1 differing from that for X in Phase 2. Nevertheless, attenuated blocking was observed, just as in Experiment 3. Apparently, the difference in trace intervals was sufficiently powerful to overcome the stronger blocking that might have been expected in the condition with different trace intervals on the basis of the greater contiguity of A to the US in Phase 1, as compared with the condition with trace intervals being the same. The findings of Experiment 4 suggest that the attenuated blocking observed in the 15–3 condition of Experiment 3 was caused at least in part by the difference in the trace interval between CS A in Phase 1 and CS X in Phase 2. If greater contiguity of A was solely responsible for the attenuated blocking observed in Experiment 3, then in Experiment 4, more blocking should have been observed in the 3–15 condition. Second-order conditioning provides an alternative account of the results of Experiment 4 as well as of Experiment 2. One could attribute the attenuated blocking of X in the 3–15 condition of Experiment 4 to higher order conditioning, similar to the 3–15 condition in Experiment 2. That is, the greater contiguity between A and the US in the 3–15 condition in comparison with the 15–15 condition potentially made the association between A and the US stronger. This would have allowed a stronger mediated X–A–US association to be formed during Phase 2 in the 3–15 condition than in the 15–15 condition. However, such an account of Experiment 4 is not applicable to Experiment 3, and it would be unparsimonious to provide different accounts of Experiments 3 and 4 when there are viable integrated accounts.
Most contemporary theories of Pavlovian learning assume that good temporal contiguity facilitates the development of an association but does not become part of that association. Thus, these theories cannot explain the effects of temporal dissimilarity observed here. The present findings suggest that such a theoretical perspective is not accurate. For example, an acquisition-focused account, such as the Rescorla and Wagner (1972) model, would not anticipate that a change in the temporal relations in the conditions with different trace intervals (such as in Experiment 4) would attenuate blocking, because the model does not presume that temporal information is encoded. Such models assume that for a CS to be effective it need only be a nonredundant event that predicts the occurrence of a US. The nonredundant requirement readily accounts for cue competition effects, such as blocking. However, if the information provided by the blocked cue is not redundant with the blocking cue, then blocking should not occur. In order for this to occur, the subject would need to detect the difference in the trace interval between the blocking cue and the blocked cue by encoding the temporal factors that are part of the A–US training and X–US training. Because models such as that of Rescorla and Wagner fail to consider the encoding of temporal information (e.g., the difference in the trace intervals between A and X), it is not possible for them to account for the observed effects in the present experiments. Moreover, evidence from studies of backward and simultaneous conditioning also supports the notion that the temporal information is encoded and that learning it is not just a matter of encoding what is going to occur (see, e.g., Barnet et al., 1991; Matzel et al., 1988).
In contrast to theories that focus on conditioning, most contemporary models of timing assume that animals encode temporal intervals between events and that a CS serves as a time marker, which indicates the start of a timed interval (see, e.g., Church & Broadbent, 1990; Machado, 1997; Moore & Choi, 1998; Schmajuk, 1997; Staddon & Higa, 1999; Sutton & Barto, 1990). These models do not explain how comparison of temporal information within a cue competition situation occurs. However, Gallistel and Gibbon’s (2000) rate expectancy theory allows such comparisons to occur. This theory postulates that organisms encode and compare temporal information. Specifically, the model emphasizes that encoded temporal intervals can be added and subtracted. In a blocking situation, for example, when the rate of reinforcement does not change when the blocked cue is present (in comparison with when the blocking cue is presented alone), the reinforcement rate attributed to the blocked cue is 0 (i.e., the additive combination of expected rates rule). In the case of the present Experiments 2–4, the difference in the temporal information provided by the blocking cue and the blocked cue allows for a different rate of reinforcement to be attributed to the blocked cue. Importantly, this rule could also account for the results of Experiment 1. In the case of Experiment 1, the reinforcement rate that was attributed to the blocked cue was part of the same reinforcement rate that was attributed to the blocking cue, because the shorter blocked cue–US interval was embedded in the longer blocking cue–US interval. Thus, the reinforcement rate attributed to the blocked cue should have been 0. The temporal coding hypothesis (Barnet et al., 1991; Matzel et al., 1988; Miller & Barnet, 1993; Savastano & Miller, 1998), as discussed in the introduction, focuses on how temporal information is expressed in behavior rather than how time is perceived. According to the temporal coding hypothesis, the attenuated blocking observed in the conditions with different trace intervals in Experiments 2–4 results from a mismatch in the temporal maps between the blocking and the blocked cue relative to the US. That is, when the temporal map for the A–US association formed during Phase 1 was compared with the temporal map for the X–US association formed during Phase 2 in the 15–3 and 3–15 conditions, the resultant expected temporal locations of the US were discrepant, thereby reducing redundancy either at the time of acquisition or at the time of testing. Thus, attenuated blocking would be expected. Conversely, in the conditions with the same trace intervals for A in Phase 1 and X in Phase 2, greater blocking would be expected because the temporal maps associated with the blocking cue and the blocked cue were not discrepant.
The importance of the present findings is twofold. First, they lend support to the view that CSs signal not only what is going to occur, but when it is going to occur. That is, temporal information is part of an association. Second, the present findings suggest that greater similarity between potentially competing associations fosters stronger competition (“similarity” in the present case referring to the trace intervals). Prior research failed to determine whether the critical factor in attenuating cue competition was qualitative differences between trace and delay CSs (or between simultaneous and forward CSs) or quantitative differences in qualitatively similar CS–US intervals between the competing cues. The present data suggest that purely quantitative differences in ISIs attenuate cue competition. The fact that temporally similar associations seem more apt to compete is congruent with reports that spatially and contextually similar associations are more apt to compete (see, e.g., Amundson & Miller, 2007; Bonardi, Honey, & Hall, 1990).
Acknowledgments
Support for this research was provided by NIMH Grant 33881. We thank Anne Clark, Patricia DiLorenzo, David Guez, Kenneth Kurtz, Alyssa Orinstein, Gonzalo Urcelay, Koji Urushihara, Daniel Wheeler, and James Witnauer for their comments on the manuscript.
Contributor Information
Jeffrey C. Amundson, University of Kansas, Lawrence, Kansas
Ralph R. Miller, State University of New York, Binghamton, New York
References
- Amundson JC, Miller RR. Similarity in spatial origin of information facilitates cue competition and interference. Learning & Motivation. 2007;38:155–171. doi: 10.1016/j.lmot.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcediano F, Escobar M, Miller RR. Temporal integration and temporal backward associations in human and nonhuman subjects. Learning & Behavior. 2003;31:242–256. doi: 10.3758/bf03195986. [DOI] [PubMed] [Google Scholar]
- Arcediano F, Miller RR. Some constraints for models of timing: A temporal coding hypothesis perspective. Learning & Motivation. 2002;33:105–123. [Google Scholar]
- Balsam PD, Drew MR, Yang C. Timing at the start of associative learning. Learning & Motivation. 2002;33:141–155. [Google Scholar]
- Balsam PD, Schwartz AL. Rapid contextual conditioning in autoshaping. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:382–393. [PubMed] [Google Scholar]
- Barnet RC, Arnold HM, Miller RR. Simultaneous conditioning demonstrated in second-order conditioning: Evidence for similar associative structure in forward and simultaneous conditioning. Learning & Motivation. 1991;22:253–268. [Google Scholar]
- Barnet RC, Grahame NJ, Miller RR. Temporal encoding as a determinant of blocking. Journal of Experimental Psychology: Animal Behavior Processes. 1993;19:327–341. doi: 10.1037//0097-7403.19.4.327. [DOI] [PubMed] [Google Scholar]
- Blaisdell AP, Denniston JC, Miller RR. Temporal encoding as a determinant of overshadowing. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:72–83. doi: 10.1037//0097-7403.24.1.72. [DOI] [PubMed] [Google Scholar]
- Bonardi C, Honey RC, Hall G. Context specificity of conditioning in flavor-aversion learning: Extinction and blocking tests. Animal Learning & Behavior. 1990;18:229–237. [Google Scholar]
- Church RM, Broadbent HA. Alternative representations of time, number, and rate. Cognition. 1990;37:55–81. doi: 10.1016/0010-0277(90)90018-f. [DOI] [PubMed] [Google Scholar]
- Church RM, Broadbent HA. A connectionist model of timing. In: Commons ML, Grossberg S, Staddon JER, editors. Neural network models of conditioning and action. Hillsdale, NJ: Erlbaum; 1991. pp. 225–240. [Google Scholar]
- Gallistel CR, Gibbon J. Time, rate, and conditioning. Psychological Review. 2000;107:289–344. doi: 10.1037/0033-295x.107.2.289. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Balsam PD. Spreading associations in time. In: Locurto CM, Terrace HS, Gibbon J, editors. Autoshaping and conditioning theory. San Diego: Academic Press; 1981. pp. 219–251. [Google Scholar]
- Gillan DJ, Domjan M. Taste-aversion conditioning with expected versus unexpected drug treatment. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:297–309. doi: 10.1037//0097-7403.3.4.297. [DOI] [PubMed] [Google Scholar]
- Goddard MJ, Jenkins HM. Blocking of a CS–US association by a US–US association. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:177–186. doi: 10.1037//0097-7403.22.3.258. [DOI] [PubMed] [Google Scholar]
- Jennings D, Kirkpatrick K. Interval duration effects on blocking in appetitive conditioning. Behavioural Processes. 2006;71:318–329. doi: 10.1016/j.beproc.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. Predictability, surprise, attention, and conditioning. In: Campbell BA, Church RM, editors. Punishment and aversive behavior. New York: Appleton-Century-Crofts; 1969. pp. 279–296. [Google Scholar]
- Killeen PR, Fetterman JG. A behavioral theory of timing. Psychological Review. 1988;95:274–295. doi: 10.1037/0033-295x.95.2.274. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K, Church RM. Tracking of the expected time to reinforcement in temporal conditioning procedures. Learning & Behavior. 2003;31:3–21. doi: 10.3758/bf03195967. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K, Church RM. Temporal learning in random control procedures. Journal of Experimental Psychology: Animal Behavior Processes. 2004;30:213–228. doi: 10.1037/0097-7403.30.3.213. [DOI] [PubMed] [Google Scholar]
- Kohler EA, Ayres JJ. Blocking with serial and simultaneous compounds in a trace conditioning procedure. Animal Learning & Behavior. 1982;10:277–287. [Google Scholar]
- Machado A. Learning the temporal dynamics of behavior. Psychological Review. 1997;104:241–265. doi: 10.1037/0033-295x.104.2.241. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82:276–298. [Google Scholar]
- Maleske RT, Frey PW. Blocking in eyelid conditioning: Effect of changing the CS–US interval and introducing an intertrial stimulus. Animal Learning & Behavior. 1979;7:452–456. [Google Scholar]
- Matzel LD, Held FP, Miller RR. Information and the expression of simultaneous and backward associations: Implications for contiguity theory. Learning & Motivation. 1988;19:317–344. [Google Scholar]
- Miller RR, Barnet RC. The role of time in elementary associations. Current Directions in Psychological Science. 1993;2:106–111. [Google Scholar]
- Miller RR, Matzel LD. The comparator hypothesis: A response rule for the expression of associations. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. San Diego: Academic Press; 1988. pp. 51–92. [Google Scholar]
- Moore JW, Choi J. Conditioned stimuli are occasion setters. In: Schmajuk NA, Holland PC, editors. Occasion setting: Associative learning and cognition in animals. Washington, DC: American Psychological Association; 1998. pp. 279–318. [Google Scholar]
- Pavlov IP. In: Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Anrep GV, editor. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM. A model for stimulus generalization in Pavlovian conditioning. Psychological Review. 1987;94:61–73. [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87:532–552. [PubMed] [Google Scholar]
- Rescorla RA. Simultaneous second-order conditioning produces S–S learning in conditioned suppression. Journal of Experimental Psychology: Animal Behavior Processes. 1982;8:23–32. [PubMed] [Google Scholar]
- Rescorla RA, Durlach PJ. Within-event learning in Pavlovian conditioning. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 81–111. [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Rick JH. Time in associative learning: Blocking, cue competition, and protection. Columbia University; 2005. Unpublished doctoral dissertation. [Google Scholar]
- Savastano HI, Miller RR. Time as content in Pavlovian conditioning. Behavioural Processes. 1998;44:147–162. doi: 10.1016/s0376-6357(98)00046-1. [DOI] [PubMed] [Google Scholar]
- Schmajuk NA. Animal learning and cognition: A neural network approach. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Schreurs BG, Westbrook RF. The effects of changes in the CS–US interval during compound conditioning upon an otherwise blocked element. Quarterly Journal of Experimental Psychology. 1982;34B:19–30. doi: 10.1080/14640748208400887. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Staddon JER, Higa JJ. Time and memory: Towards a pacemaker-free theory of interval timing. Journal of the Experimental Analysis of Behavior. 1999;71:215–251. doi: 10.1901/jeab.1999.71-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Time-derivative models of Pavlovian reinforcement. In: Gabriel M, Moore J, editors. Learning and computational neuroscience: Foundations of adaptive networks. Cambridge, MA: MIT Press; 1990. pp. 497–537. [Google Scholar]
- Wagner AR. Stimulus validity and stimulus selection in associative learning. In: Mackintosh NJ, Honig WK, editors. Fundamental issues in associative learning. Halifax, NS: Dalhousie University Press; 1969. pp. 90–122. [Google Scholar]
- Wagner AR. SOP: A model of automatic processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- Wagner AR, Logan FA, Haberlandt K, Price T. Stimulus selection in animal discrimination learning. Journal of Experimental Psychology. 1968;76:171–180. doi: 10.1037/h0025414. [DOI] [PubMed] [Google Scholar]